Abstract
Objectives
Age-related physiological changes, particularly immune system decline, may contribute to greater vulnerability to infectious diseases in older individuals. A growing body of evidence shows that both, acute, and chronic infections may be accompanied by cognitive disturbances as part of their manifestations. Given the importance of cognition in aging trajectories, the objective of this article was to review current knowledge on cognitive outcomes of infectious diseases in older adults, and to emphasize the importance of considering cognition as a domain of interest in its own rights in these diseases.
Methods
A MEDLINE/PubMed database search was conducted to identify articles reporting cognitive impairment associated with various severe acute infections and specific chronic infectious conditions such as human immune deficiency virus, the herpes virus family, hepatitis C virus, Lyme borreliosis, Helicobacter pylori, periodontitis, and emerging pathogens like SARS-CoV-2, as well as potentially preventive strategies like vaccination.
Results/ Conclusions
Taken together, the studies examined in the present review emphasize that numerous acute and chronic infectious diseases share mechanisms that, when added to specific risk factors frequently found in older persons, contribute to considerably increase the risk of cognitive outcomes such as cognitive decline and dementia. This review may help to appreciate the role that infectious diseases play in cognitive trajectories and thus promote further investigation on the topic.
Keywords: Infectious diseases, Cognitive decline, Dementia, Older persons
Introduction
Due to certain particularities of the aging process, such as the relative decline of the immune system (immunosenescence), the potentially accompanying chronic low-grade inflammation (inflammaging), and a higher prevalence of chronic comorbidities and their treatment, older adults are more vulnerable to infectious diseases. Moreover, they are more likely to develop severe forms of infections and have a higher risk of long-term exposure to chronic infections and repeated acute infections [1].
The role that some infectious diseases play in the development of adverse health outcomes, including increased short- and long-term mortality, is well known. However, a growing body of evidence in the field of infectious diseases points to another area of health that is frequently affected by these conditions and is highly relevant to the older population: cognition.
In a variety of chronic infections and acute infectious episodes, an impact on cognition is not uncommon [2, 3]. Moreover, cognitive decline may be part of the reported clinical manifestations of specific infections if appropriate interventions are not undertaken in a timely manner, and this observation is not only limited to those infections primarily affecting the central nervous system (CNS) [4, 5].
Given the relevance of mental health issues among older adults, along with their higher risk of contracting infectious diseases, the objective of this paper is to review not only the reasons but also the challenges for considering cognition as an outcome of interest in the field of infectious diseases in this population. For this, we address in this review several acute and chronic systemic diseases for which there is convincing epidemiological evidence showing an increased risk of adverse cognitive outcomes. Some pathophysiological mechanisms that these conditions may have in common or at the individual level potentially explaining the impact on cognition are developed; however, an in-depth or pathogen-dependent physio pathological review is beyond the scope of this publication. Similarly, this article does not cover those diseases that exclusively involve the CNS, since obviously, the cognitive manifestations in these infections are not new findings.
Specificities of the older persons that increase their risk of infectious diseases
The complex changes in the immune system that are associated with the aging process are collectively known as immunosenescence, and links between this phenomenon and potential higher risks of infectious diseases among older persons have been proposed since its earliest characterizations [6, 7]. Despite the absence of a formal consensus on the specific changes that encompass immunosenescence, some of its most frequently reported manifestations include a decline in cellular immunity that increases susceptibility to infections, cancer, and autoimmune diseases, as well as the development of a pro-inflammatory cellular phenotype, which has been proposed as the substrate for a chronic low-grade inflammatory state termed inflammaging (potentially linked to other age-related chronic conditions such as cardiovascular diseases, diabetes, sarcopenia, and frailty), and a relatively decreased antibody response (which may hamper for example, response to vaccinations) [1, 8]. Whilst this process has frequently been addressed from a predominantly negative perspective, recent publications propose that some components of both immunosenescence and inflammaging may not be solely detrimental or inappropriate. Still under debate, the hypothesis is that the reactive development of an increased inflammatory state due to a relatively reduced immune response would be an adaptive homeostatic mechanism or immune remodeling, which may be more suitable for the state of the organism in advanced age [9, 10].
Beyond these immunological changes, it is important to bear in mind (as usual in geriatric medicine) that it is not one factor, but the combination of several conditions that may render an individual more vulnerable to a given outcome. For most older adults, the decline or changes in their immune system during the aging process will not be significant enough to be considered pathological or the sole cause of increased infectious risk. In the same vein, such changes do not represent a state of immunodeficiency either. For many individuals, it is the multiplicative effect of the declining immune function and their individual morbidity risk profile that will trigger the risk of infectious diseases [11].
As previously stated, the immune system is not independent from the other components of the organism; thus, other age-related particularities also contribute to the increased risk of organ-specific infections. These changes include the waning of physical barriers (i.e., the skin), decreased cough reflex in frail individuals, urological alterations such as incomplete bladder voiding or mucosal atrophy in women, and changes in microbiota [6, 12, 13].
Common chronic conditions in older adults, such as diabetes (particularly long-standing poor glycemic control) and chronic kidney disease, can further compromise the immune system which increases the risk of infections [12, 14, 15]. Likewise, multimorbidity increases the likelihood of exposure to medical procedures (e.g., permanent venous accesses, pacemakers, urinary catheters, prosthetic joints), which may represent risk factors for specific infections [16]. In the same vein, geriatric syndromes as immobility, malnutrition, polypharmacy (e.g., sedatives, anticholinergic drugs, and proton pump inhibitors increase the risk for pneumonia), disabilities in activities of daily living, and frailty not only increase the risk of developing infectious diseases, but also of developing severe forms of diseases, as the organism is in a more vulnerable state [6, 17]. This is particularly relevant for the frailty syndrome, as this condition is characterized by poor resilience. In addition, severe forms of infectious diseases in this already vulnerable subgroup of the older population may lead to additional functional decline, reducing the likelihood of returning to a baseline state [18]. Consequently, this process may precipitate a spiral of various negative health-related outcomes (including further infections) such as institutionalization, permanent disability, and increased mortality [18–20]. Likewise, atypical presentations of infectious diseases (i.e., absence of fever, delirium as the sole manifestation) are more common in older adults, which may also contribute to delay the diagnosis of the underlying infection, and consequently, contribute to adverse outcomes [21, 22].
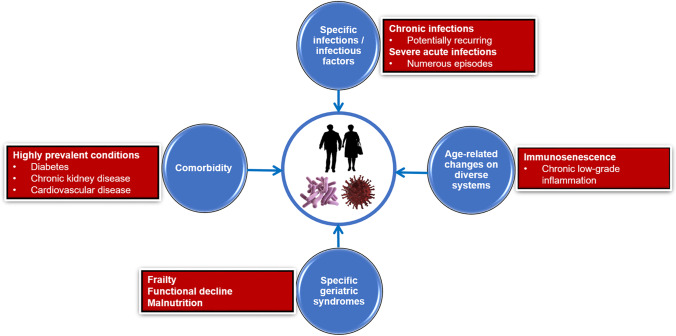
Figure 1 illustrates the constellation of factors contributing to the risk of infectious diseases in older adults.
Fig. 1.
Constellation of factors contributing to the risk of infectious diseases in older adults
As aging is a highly heterogeneous process, different aging trajectories are to be expected [23]. While some individuals maintain a good level of autonomy allowing them to continue to live in the community, others require the support of long-term care facilities, which will also increase their risk of contracting multidrug-resistant organisms [24]. Likewise, while some persons do not contract many infectious diseases throughout their lives, others develop repeated episodes of acute infections, or chronic (even lifelong) exposures to certain pathogens, and thus age with a significant burden of infectious diseases [25]. The cumulative effect of infectious diseases is a relevant dimension to consider, as both the higher burden of infections and living with specific chronic infectious diseases have been associated with cognitive decline [19, 25].
Cognition and cognitive decline as domains of interest in the field of infectious diseases
Knowing that older persons are at higher risk of infectious diseases, it may be relevant to pay a particular attention to mental health consequences, as mental health is not exclusively affected in the acute phase of infectious diseases (e.g., delirium) or by those that directly affect the CNS (vasculitis or neuro-infections). There is a growing evidence showing cognitive manifestations in a wide variety of systemic infections, and a long-term impact on cognitive function due to the exposure to certain others.
Severe acute infections and their impact on cognition
Severe infections are a common cause of emergency admissions in older adults (particularly pneumoniae, urinary tract infections, and bloodstream infections). They usually impose enormous physiological disturbances for the body to overcome, which in turn can lead to acute manifestations of disease such as delirium, short-term mortality, as well as long-term negative outcomes such as frailty, functional decline, institutionalization, and cognitive impairment [2, 3, 12, 19].
Delirium is one of the most frequent neuropsychiatric manifestations of acute infections in older persons. This syndrome is characterized by an acute onset of cognitive disturbances that fluctuates over time, with orientation in time and space, attention, and working memory being the most frequently affected cognitive domains, although delirium can affect almost any cognitive domain [22]. Delirium can be considered both as a marker of underlying cognitive vulnerability and a risk factor, as older individuals who develop delirium are at higher risk of subsequent cognitive decline, dementia, and worsened cognitive trajectories in individuals who already have dementia [22]. Therefore, delirium may be a warning sign of cognitive vulnerability.
Beyond delirium, recent evidence has shown that in older individuals, infections severe enough to require in-hospital treatment may be independent risk factors for cognitive decline and all-cause dementia [4, 26]. According to several epidemiological studies, frequent infections, regardless of etiology, such as pneumoniae (viral or bacterial etiology), urinary tract infections, skin, and soft tissue infections, as well as sepsis from diverse origins, are associated with an increased risk of cognitive decline, vascular dementia, and Alzheimer’s disease. Actually, the risk of unspecified dementia would be doubled compared with individuals without a history of severe infectious episodes [4, 26–28]. Moreover, a study suggested a risk gradient, with higher infectious burdens associated with higher cognitive risks: an all-cause dementia risk of about 1.4 for a history of one episode of hospital-treated infection, and about 2.5 for two episodes [27].
Specific chronic infections and cognition
Over the next section of the article, we describe specific examples of chronic infectious diseases and their associations with cognitive-related outcomes, as distinct changes in cognition may be part of their typical clinical manifestations.
Human immune deficiency virus
Human immune deficiency virus (HIV) represents one of the chronic infectious diseases with the largest evidence for cognitive outcomes. Almost from the beginning of the HIV pandemic, neurocognitive manifestations of the disease were identified, and eventually the term HIV-associated neurocognitive disorder (HAND) was coined [29]. This term has been used for more than fifteen years to describe a range of HIV-related cognitive changes, and the classification criteria proposed by Antinori et al. remain the most frequently used to date [30]. These clinical criteria, based on neuropsychological assessment (and its impact on daily functioning), classify HAND into three possible states: asymptomatic neurocognitive impairment (ANI), HIV-associated mild neurocognitive disorder (MND), and HIV-associated dementia (HAD).
In parallel, great advances have been made in the treatment of HIV, and the effect that combined antiretroviral therapy (cART) has had on minimizing the severe negative AIDS and HIV-related outcomes, including HAD is undeniable. Despite these advances in the cART era, HAND remains a frequent condition, particularly among the most vulnerable individuals, such as pre-frail or frail older adults [31]. However, relevant transitions in HAND subtypes and neuropsychological profiles have been reported [32]. Regarding HAND and its subtypes, the estimated prevalence for HAND ranges from 15 to 55%, and currently, the more common subtypes would be ANI and MND (with ANI accounting for approximatively 70% of cases), with HAD becoming a rare event [29]. In terms of the neuropsychological profiles of HAND, while a shift toward a more “cortical” profile has been suggested for persons under cART (potentially related to interactions between HIV and the aging process, as persons living with HIV live longer), evidence still supports a “subcortical” neuropsychological pattern in HAND characterized by impairments in tasks involving processing speed and executive functioning [33]. With respect to cognitive trajectories, the still scarce longitudinal data pertaining to the cART era suggests that ANI status is still associated with a higher risk of cognitive decline compared with cognitively normal peers without HIV. For instance, a cohort study reported that ANI was associated with a 2- to sixfold increased risk of developing symptomatic HAND (assessed by self-reported or objective performance), and another one reported that 13% of participants with HAND showed deterioration over 4 years of follow-up [34, 35]. Nonetheless, it seems that the largest proportion (61 to 77%) of persons on optimal HIV treatment remains stable in terms of cognitive functioning, and a small proportion may even improve (10 to 16.5%) [34, 36]. However, a limitation shared by many of these studies is that the proportion of older adults included is relatively small, as most focus on middle-aged persons living with HIV. As we currently have an ever-growing population of older adults living with HIV for whom these issues could overlap with age-related conditions (including neurodegenerative diseases), the issue of cognitive function in HIV is gaining momentum and a substantial amount of collaborative work between geriatrics and HIV-medicine is underway.
It is important to mention that HIV, and particularly AIDS, can cause a number of neurological complications like toxoplasmosis, Epstein-Barr virus-related lymphomas, and JC polyomavirus. They are not detailed in this review since, as mentioned in the introduction, conditions directly affecting the CNS are outside the scope of this review.
Herpesviridae
Infections from the herpes virus family have long been studied as potential risk factors for cognitive impairment, particularly infections with herpes simplex virus (HSV) type 1, a very frequent infection worldwide, with no curative treatment (i.e., viral eradication). The interest for cognitive issues is justified by the neurotropic properties of the virus leading to Herpes Simplex encephalitis, its recurring nature, and the biological and epidemiological associations between HSV reactivations/infection and cognitive decline, and the higher reported risks for Alzheimer’s disease [37–39].
Recent evidence emphasizes the neurotropism of HSV, and points to brain areas most vulnerable to this infection, such as the hippocampal region, a critical area for episodic memory [40]. In addition, a recent study reports that early imaging markers of Alzheimer’s disease such as microstructural alterations in para-hippocampal regions, and lower hippocampal volume, are more frequent in older adults with high anti-HSV 1 IgG titers. Additionally, the same work reports a higher risk of developing Alzheimer’s disease in APOE ε4 allele carriers with high IgG levels than in allele carriers with lower IgG titers [41]. These results are interesting in light of other findings from a population-based longitudinal study suggesting an association between HSV-1 carriage and episodic memory decline in older persons. Indeed, the proportion of participants with episodic memory decline was higher in those aged ≥ 65 years with HSV-1 IgG antibodies (9.8% vs 0.0% without anti-HSV IgG), and even higher among APOE ε4 allele carriers (23.5% vs 0.0% without anti-HSV IgG) [42]. Regarding the potential effect of antiviral treatment on cognitive outcomes, a retrospective analysis of Taiwan’s Health Insurance Research Database found that patients who received antiviral treatment during an active HSV episode had a reduced risk of developing all-cause dementia compared with patients who did not [43]. However, these findings need to be replicated in longitudinal studies.
Following the same trend, epidemiological studies have reported that previous varicella zoster virus (VZV) infection may also increase the risk for cognitive decline and dementia [39, 44, 45]. For example, a Taiwanese nationwide population-based survey reported a higher risk of incident all-cause dementia in individuals with a history of herpes zoster compared with those without [45]. Noteworthy, another retrospective cohort study reported an almost threefold increased risk of dementia in VZV infection with ophthalmologic involvement [44]. The proposed rationale for this association is lifelong viral latency, induction of chronic inflammation, direct neurotropic involvement, and the recurrent nature of the infection [39, 44].
Other herpes viruses such as cytomegalovirus and Epstein-Barr virus are also highly prevalent either alone, or as co-infections with other viruses like HIV. Their potential role on cognitive outcomes has been examined, but the evidence is inconclusive when considering their stand-alone effect [39].
Hepatitis C virus
In the last few years, significant progress has been made in the understanding of the pathophysiological mechanisms of hepatitis C virus (HCV) infection. Furthermore, we are witnessing the first generation of patients treated with potentially curative approaches such as the direct antiviral agents (DAAs). HCV is a primary hepatotropic virus that is relatively frequent in older adults. Although initially affecting the liver, this infection is considered a systemic condition owing to its many extra-hepatic manifestations, including cognitive impairment [46]. Mechanisms that induce CNS dysfunction such as neurotransmitter disturbances (particularly in the serotoninergic and dopaminergic systems of the midbrain and basal ganglia), direct viral damage (viral entry through the peripheral mononuclear system with subsequent infection of microvascular endothelial cells), and immune-mediated vascular damage (HCV-associated mixed cryoglobulinemia) are suspected to be the drivers of the cognitive manifestations of the disease [47–49]. Several cognitive functions can be affected by HCV including attention, working memory, episodic memory, psychomotor speed, and executive functions [47, 50, 51]. Given that the elderly population represents the main group affected by the infection, and that HCV may contribute to worsening cognitive decline, efforts have been made not only to include this group in clinical trials of DAAs (which have reported excellent responses to treatment), but also in studies with outcomes that focus on cognitive measures [52, 53]. As with all studies evaluating novel medical interventions, the results should be taken with some hindsight, but a new and exciting chapter is probably opening in the field of HCV infection, as the preliminary results from these studies suggest an improvement of cognitive functioning as assessed by a comprehensive neuropsychological evaluation following HCV treatment with DAAs [53]. If confirmed, these results would indicate a potential reversibility of cognitive deficits after treatment.
Lyme borreliosis
Lyme disease or Lyme borreliosis is a common tick-borne infection caused by a group of spirochetes of the Borrelia burgdorferi group [54]. Three clinical stages have been described for Lyme borreliosis (early-localized, early-disseminated, and late-disseminated), and systemic manifestations of the disease may develop during its course, including neurological symptoms [55]. The potential cognitive manifestations of Lyme borreliosis remain a rich and ongoing field of research. Indeed, due to the controversies surrounding definitions and classification criteria (for other states of disease out of the previously mentioned stages), and the different age groups considered in the studies, inconsistent results regarding the potential chronic manifestations of the disease are reported in the literature. Post-treatment Lyme disease syndrome (PTLDS) is a consensus-based classification created as a potential tool to detect individuals who have persistent symptomatology after a proper diagnosis and treatment of Lyme borreliosis, and for whom other common conditions have been excluded [56]. Memory complaints are frequent in PTLDS, and a few studies have suggested an association with cognitive decline; for instance, a case series of a well-characterized group of PTLDS patients reported that 26% of the participants endorsed a significant level of cognitive decline compared with an estimate of premorbid functioning, predominantly in measures of verbal memory and processing speed [57].However, when considering only prior positive serological status, a longitudinal population-based study showed no association with subsequent negative trajectories of cognitive aging, as no association was found with cognitive decline, functional decline or risk of dementia [58].
Helicobacter pylori
While the prevalence of H. pylori varies by geographic area, the infection remains common worldwide and affects approximately half of the world population [59]. Likewise, this infection represents one of the main causes of chronic gastritis, but this condition is not necessarily symptomatic, and can therefore remain undiagnosed or untreated throughout life, as it is usually contracted during childhood [59]. As for other chronic infectious diseases with systemic impact, extra-gastric manifestations of H. pylori are emerging as a topic of interest. Protracted H. pylori infections have been associated with chronic inflammatory states. This chronic inflammation along with other factors such as folate malabsorption, immune dysregulation, and bacteria entry into the CNS through the systemic circulation have been proposed as potential mechanisms linking H. pylori to neurodegeneration and Alzheimer’s disease [60–62]. A systematic review and meta-analysis conducted by Shindler-Itskovitch et al. reported that, compared with individuals without a history of H. pylori, patients with H. pylori infection have a 1.7 increased likelihood of developing all-cause dementia [60]. Specifically in older adults, a French longitudinal population-based cohort with a 20-year of follow-up found that after controlling for various conditions including APOE ε4 status, H. pylori infection remained a risk factor for incident all-cause dementia [63]. Similarly, an analysis of national surveys from the USA found an association between H. pylori seropositivity and incident Alzheimer’s disease and all-cause dementia [61]. Contrasting results have also been reported. For instance, a population-based cohort from the Netherlands found that IgG seropositivity to H.pylori was not associated with a higher risk of dementia in a follow-up of 13.3 years [64]. However, some methodological differences that may have contributed to these discrepant results have been suggested, such as a possible underestimation of the proportion of patients with a prior infection as complementary long-lasting antibodies were not assessed [65]. Given these findings, further longitudinal studies focusing on mechanistic or physiopathological associations between H. pylori infection and dementia, as well as clinical trials assessing the impact of H. pylori treatment on cognitive outcomes are expected, as this is a treatable condition, and hence a potentially modifiable risk factor.
Periodontitis
Periodontal disease, particularly chronic periodontitis, has gained ground as a health marker in older adults, because of its prevalence and its potential association with many negative outcomes, including neurocognitive disorders [66–68]. Various periodontal disease-causing microorganisms have been associated with these outcomes, with Porphyromonas gingivalis being one of the most frequently reported. A recent analysis of a national survey from the USA reported longitudinal associations between clustered bacterial periodontal markers (IgG) for Porphyromonas gingivalis and Campylobacter rectus with incident Alzheimer’s disease [69]. According to the systematic review and meta-analysis conducted by Hu et. al., irrespective of the specific microorganisms, periodontal disease would be associated with mild cognitive impairment (MCI) and Alzheimer’s disease with a 1.6 higher risk for MCI, and around 1.8 for Alzheimer’s disease in persons with a history of periodontal disease, and a nearly fivefold higher risk of Alzheimer’s disease in persons with severe periodontal disease [68].
The mechanisms behind the link between chronic periodontitis and cognitive disorders remain unclear; however, the induction of chronic inflammation and the potential ability of periodontal pathogens to cross the blood–brain barrier have been frequently suggested as potential drivers of this association [67, 68].
Emerging pathogens
Over the past 2 years, we have seen the impact of an emergent pathogen on basically all the domains of daily life. We still do not know for sure the role of SARS-CoV-2 and COVID-19 as a long-term chronic condition, but we cannot deny the enormous initial impact on the elderly population, nor can we deny the growing body of evidence for the persistent disease manifestations and their potential longitudinal implications. The World Health Organization has already developed a working clinical case definition for post COVID-19 conditions. Even though these conditions are referred-to by various terms (e.g., “post-acute COVID syndrome,” “post-acute sequelae of SARS-CoV-2 infection”), they share common elements such as the lack of return to a usual state of health after COVID-19 infection, and the fact that the symptoms cannot be better explained by an alternative diagnosis [70, 71]. Frequently reported persistent manifestations of COVID-19 include cognitive complaints, with attention difficulties (estimated frequency 27%) and memory loss (estimated frequency 16%) being the most frequently reported, along with fatigue, and respiratory symptoms [72]. Persistent hypo/anosmia and hypo/ageusia are thought to be predictive of future cognitive impairment [73–75]. Likewise, results from the first longitudinal case series focusing on brain metabolic correlates of persisting neuropsychiatric symptoms and sensory dysfunction report that distinct patterns of brain hypo metabolism can be distinguished over time after SARS-CoV-2 infection [76–78]. While hypometabolic changes in the frontal cortex, olfactory/rectus gyrus, anterior cingulate, and insular regions can be observed in the acute phase of disease, patients with persistent neuropsychiatric and olfactory symptoms show hypometabolic changes in areas involved in cognition such as the hippocampal, para-hippocampal, and fusiform gyri [76–78].
As with the other infections previously mentioned, systemic involvement, and direct neurotropism have been described for SARS-CoV-2. Direct invasion through the olfactory mucosa, disruption of the blood–brain barrier, or retrograde axonal transport have been proposed as potential routes of entry of the virus into the brain [79]. However, these assumptions are under debate. In particular, the neuro-invasive properties of the virus, and the precise mechanisms behind cognitive manifestations of the disease are yet to be fully understood [79, 80].
Vaccination and cognition
Knowing the potential impact of certain infectious diseases on cognitive decline or dementia, one may wonder whether specific public health interventions against those diseases could affect cognitive outcomes. Particularly, the hypothesis of a reduced risk of dementia in vaccinated older adults has been more thoroughly explored in recent years due to a growing body of epidemiological data on the topic. These studies have shown a consistent reduction of the risk of dementia among older adults with a history of vaccination against influenza, VZV, and tetanus, diphtheria, and pertussis (Tdap) [81–84]. For example, a recent analysis from a US veterans cohort reported a 42% reduction of the risk of incident dementia in individuals with a history of Tdap vaccination compared with those who did not receive the vaccine [82].
Given the consistent findings of risk reductions results for various types of vaccines and comorbidity profiles, these studies share the suggestion that the effects are not vaccine-specific, but rather a non-specific immune response to the vaccines, or that they represent general immune conditioning/training over time [81, 82, 85]. Even for infections for which there is no clear epidemiological evidence of an increased risk of dementia such as influenza, some studies have reported a decreased risk in post-vaccinated individuals [86]. Indeed, annual influenza vaccination may reduce the risk of dementia with a dose–response effect, with more annual doses resulting in lower risks in older adults, as shown in a Taiwanese nationwide retrospective cohort analysis (HR of about 0.8 for 2 to 3 prior years of vaccination, and HR of about 0.4 for 4 prior vaccinations in patients with chronic obstructive pulmonary disease) [83].
However, many of these studies share specific limitations; some of the vaccines considered are not mandatory. Therefore, the individuals receiving these vaccines may not be representative of the general population, and the reported risk reductions may be due to indirect associations, as vaccination could be a marker of a healthy lifestyle [87]. Nonetheless, studies regarding vaccination and reduced risks of dementia provide valuable information, as their findings are not only consistent with the reported associations between infectious diseases and cognitive outcomes but may also strengthen the role of vaccination as an already valuable public health intervention as well as a prevention strategy promoting healthy aging.
Mechanisms underlying brain dysfunction in infectious diseases
The role that infections play as precipitating and participating factors in neuropathology and neurodegeneration represents a vast field of investigation and a myriad of basic-research studies are available. However, frequently reported mechanisms that may ultimately induce neuropathology or neurodegeneration, and that various pathogens share, are the induction/interaction of systemic and neurologic inflammation, vascular damage, direct damage (neurotropism), hyperphosphorylation of tau protein, and abnormal aggregation of peptides and proteins such as beta-amyloid, and alpha-synuclein [88–91].
Associations between conditions that are associated with systemic inflammation and cognitive decline or dementia have been extensively studied through activation of brain microglia [88]. Given that severe acute infections and various chronic infectious diseases trigger systemic and neurologic inflammation, both have been proposed as potential contributors to cognitive decline, and a dose–response association between the severity of infections (i.e., the underlying severity of inflammation) and the risk of dementia has been suggested [26, 27]. This idea may be important to consider in the context of aging, as an individual may experience many infectious episodes over the course of a lifetime [92].Likewise, persistent exposure to inflammatory signals triggered by chronic infections may lead to an eventual decline in T cell function called T cell exhaustion; which may also participate in suboptimal responses against infections [93].
Beyond these studies, basic-research and animal-model studies have shown that systemic inflammation causes vascular (capillary) damage, and that severe infections induce blood–brain barrier dysfunctions (allowing transient exposure to neurotoxic substances and pathogens), which may contribute to the higher risks of vascular dementia observed in older adults with a history of severe infections [89, 94]. In addition, there is evidence that points towards a role of beta-amyloid, and alpha-synuclein as antimicrobial proteins. The underlying hypothesis is that both beta-amyloid peptide and alpha-synuclein protein have active properties against various pathogens, and that repeated infections throughout life could increase the production of beta-amyloid or alpha-synuclein, leading to the formation of pathologic aggregates. Such beta-amyloid and alpha-synuclein aggregates are thought to respectively promote the development of Alzheimer’s disease and alpha-synucleinopathies such as Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy, thereby explaining the potential link between infectious conditions and increased risk of neurodegenerative diseases [90, 95].
Conclusions
Numerous other microorganisms/infectious diseases than those mentioned in this article (i.e., Chlamydophila pneumonia, Syphilis, the viruses that cause dengue or Zika) have been associated with cognitive outcomes; alas, a pathogen-specific overview for all possible microorganisms was not the objective of this work. Nonetheless, it reminds us the tremendous variety of pathogens that may have an impact on cognition.
Should we be concerned about the relationship between infectious diseases and cognition? This mini review calls for a yes. A variety of interventions already exist among which vaccination, antivirals, anti-inflammatory therapy, but also healthy diet and physical activity which can potentially mitigate the cognitive impact of the infectious burden that older adults experience throughout their lives. For these reasons, but also given the relevance of cognitive issues in older adults, and their greater vulnerability to infectious diseases, the time has come to pay particular attention to the role of infectious diseases in age-related cognitive trajectories. As the global population is expected to grow older in the near future, this topic at the crossroads of Geriatrics, Neuropsychology, Infectious diseases, and Public Health is expected to develop.
Author contribution
V. Hernández-Ruiz, and H. Amieva designed the review and wrote the manuscript L. Letenneur, T. Fülöp, C. Helmer, C. Roubaud-Baudron, and J.A. Avila-Funes contributed equally to critical analysis and revising the manuscript.
Funding
VHR was supported by the National Council for Science and Technology (CONACYT) in Mexico.
Declarations
Ethical approval and informed consent
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or Foes? Front Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandaker GM, Jones PB. Cognitive and functional impairment after severe sepsis. Jama. 2011;305(7):673–4. doi: 10.1001/jama.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muzambi R, Bhaskaran K, Brayne C, Davidson JA, Smeeth L, Warren-Gash C. Common bacterial infections and risk of dementia or cognitive decline: a systematic review. J Alzheimers Dis. 2020;76(4):1609–1626. doi: 10.3233/JAD-200303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mawanda F, Wallace RB, McCoy K, Abrams TE. Systemic and localized extra-central nervous system bacterial infections and the risk of dementia among US veterans: a retrospective cohort study. Alzheimers Dement (Amst) 2016;4:109–117. doi: 10.1016/j.dadm.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshikawa TT, Norman DC. Geriatric Infectious diseases: current concepts on diagnosis and management. J Am Geriatr Soc. 2017;65(3):631–641. doi: 10.1111/jgs.14731. [DOI] [PubMed] [Google Scholar]
- 7.Bandaranayake T, Shaw AC. Host resistance and immune aging. Clin Geriatr Med. 2016;32(3):415–432. doi: 10.1016/j.cger.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulop T, Dupuis G, Baehl S, Le Page A, Bourgade K, Frost E, et al. From inflamm-aging to immune-paralysis: a slippery slope during aging for immune-adaptation. Biogerontology. 2016;17(1):147–157. doi: 10.1007/s10522-015-9615-7. [DOI] [PubMed] [Google Scholar]
- 10.Pawelec G, Bronikowski A, Cunnane SC, Ferrucci L, Franceschi C, Fülöp T, et al. The conundrum of human immune system “senescence”. Mech Ageing Dev. 2020;192:111357. doi: 10.1016/j.mad.2020.111357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santoro A, Bientinesi E, Monti D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. 2021;71:101422. doi: 10.1016/j.arr.2021.101422. [DOI] [PubMed] [Google Scholar]
- 12.Alderman B, Frank LA, Khan SA. Infection in the older patient. Br J Hosp Med (Lond) 2017;78(8):C120–C123. doi: 10.12968/hmed.2017.78.8.C120. [DOI] [PubMed] [Google Scholar]
- 13.Hepper HJ, Sieber C, Walger P, Bahrmann P, Singler K. Infections in the elderly. Crit Care Clin. 2013;29(3):757–774. doi: 10.1016/j.ccc.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4(2):148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 15.Hine JL, de Lusignan S, Burleigh D, Pathirannehelage S, McGovern A, Gatenby P, et al. Association between glycaemic control and common infections in people with type 2 diabetes: a cohort study. Diabet Med. 2017;34(4):551–557. doi: 10.1111/dme.13205. [DOI] [PubMed] [Google Scholar]
- 16.Yahav D, Schlesinger A, Shaked H, Goldberg E, Paul M, Bishara J, et al. Clinical presentation, management and outcomes of Staph aureus bacteremia (SAB) in older adults. Aging Clin Exp Res. 2017;29(2):127–133. doi: 10.1007/s40520-016-0543-4. [DOI] [PubMed] [Google Scholar]
- 17.Tabue-Teguo M, Simo N, Gonzalez-Colaço Harmand M, Cesari M, Avila-Funes JA, Féart C, et al. Frailty in elderly: a brief review. Geriatr Psychol Neuropsychiatr Vieil. 2017;15(2):127–137. doi: 10.1684/pnv.2017.0670. [DOI] [PubMed] [Google Scholar]
- 18.High K, Bradley S, Loeb M, Palmer R, Quagliarello V, Yoshikawa T. A new paradigm for clinical investigation of infectious syndromes in older adults: assessing functional status as a risk factor and outcome measure. J Am Geriatr Soc. 2005;53(3):528–535. doi: 10.1111/j.1532-5415.2005.53240.x. [DOI] [PubMed] [Google Scholar]
- 19.Liang SY. sepsis and other infectious disease emergencies in the elderly. Emerg Med Clin North Am. 2016;34(3):501–522. doi: 10.1016/j.emc.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(12):1163–e1-e17. doi: 10.1016/j.jamda.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Hyernard C, Breining A, Duc S, Kobeh D, Dubos M, Prevel R, et al. Atypical presentation of bacteremia in older patients is a risk factor for death. Am J Med. 2019;132(11):1344–52.e1. doi: 10.1016/j.amjmed.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, et al. Delirium Nature Reviews Disease Primers. 2020;6(1):90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, et al. The Continuum of aging and age-related diseases: common mechanisms but different rates. Front Med (Lausanne) 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez-Villodres Á, Martín-Gandul C, Peñalva G, Guisado-Gil AB, Crespo-Rivas JC, Pachón-Ibáñez ME, et al (2021) Prevalence and risk factors for multidrug-resistant organisms colonization in long-term care facilities around the world: a review. Antibiotics (Basel) 10(6) [DOI] [PMC free article] [PubMed]
- 25.Lu Y, Tan CTY, Gwee X, Yap KB, Fulop T, Pan F, et al. Pathogen Burden, Blood biomarkers, and functional aging in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2021;76(10):1864–1873. doi: 10.1093/gerona/glab057. [DOI] [PubMed] [Google Scholar]
- 26.Muzambi R, Bhaskaran K, Smeeth L, Brayne C, Chaturvedi N, Warren-Gash C. Assessment of common infections and incident dementia using UK primary and secondary care data: a historical cohort study. Lancet Healthy Longev. 2021;2(7):e426–e435. doi: 10.1016/S2666-7568(21)00118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sipilä PN, Heikkilä N, Lindbohm JV, Hakulinen C, Vahtera J, Elovainio M, et al. Hospital-treated infectious diseases and the risk of dementia: a large, multicohort, observational study with a replication cohort. Lancet Infect Dis. 2021;21(11):1557–1567. doi: 10.1016/S1473-3099(21)00144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotstein A, Levine SZ. Childhood infectious diseases and old age cognitive functioning: a nationally representative sample of community-dwelling older adults. Int Psychogeriatr. 2021;33(1):75–82. doi: 10.1017/S1041610220001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234–248. doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamudio-Rodríguez A, Belaunzarán-Zamudio PF, Sierra-Madero JG, Cuellar-Rodríguez J, Crabtree-Ramírez BE, Alcala-Zermeno JL, et al. Association between frailty and HIV-associated neurodegenerative disorders among older adults living with HIV. AIDS Res Hum Retroviruses. 2018;34(5):449–455. doi: 10.1089/aid.2017.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clifford DB. HIV-associated neurocognitive disorder. Curr Opin Infect Dis. 2017;30(1):117–122. doi: 10.1097/QCO.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacktor N. Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol. 2018;24(2):141–145. doi: 10.1007/s13365-017-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86(4):334–340. doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant I, Franklin DR, Jr, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–2062. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heaton RK, Franklin DR, Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473–480. doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letenneur L, Pérès K, Fleury H, Garrigue I, Barberger-Gateau P, Helmer C, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer’s disease: a population-based cohort study. PLoS One. 2008;3(11):e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steel AJ, Eslick GD. Herpes viruses increase the risk of Alzheimer’s Disease: a meta-analysis. J Alzheimers Dis. 2015;47(2):351–364. doi: 10.3233/JAD-140822. [DOI] [PubMed] [Google Scholar]
- 39.Warren-Gash C, Forbes HJ, Williamson E, Breuer J, Hayward AC, Mavrodaris A, et al. Human herpesvirus infections and dementia or mild cognitive impairment: a systematic review and meta-analysis. Sci Rep. 2019;9(1):4743. doi: 10.1038/s41598-019-41218-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong SJ, Yong MH, Teoh SL, Soga T, Parhar I, Chew J, et al. The hippocampal vulnerability to herpes simplex virus type I infection: relevance to Alzheimer’s disease and memory impairment. Front Cell Neurosci. 2021;15:695738. doi: 10.3389/fncel.2021.695738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linard M, Baillet M, Letenneur L, Garrigue I, Catheline G, Dartigues JF, et al. Herpes simplex virus, early neuroimaging markers and incidence of Alzheimer’s disease. Transl Psychiatry. 2021;11(1):414. doi: 10.1038/s41398-021-01532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lövheim H, Norman T, Weidung B, Olsson J, Josefsson M, Adolfsson R, et al. Herpes simplex virus, APOEɛ4, and cognitive decline in old age: results from the Betula Cohort Study. J Alzheimers Dis. 2019;67(1):211–220. doi: 10.3233/JAD-171162. [DOI] [PubMed] [Google Scholar]
- 43.Tzeng NS, Chung CH, Lin FH, Chiang CP, Yeh CB, Huang SY, et al. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections-a nationwide, population-based cohort study in Taiwan. Neurotherapeutics. 2018;15(2):417–429. doi: 10.1007/s13311-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai MC, Cheng WL, Sheu JJ, Huang CC, Shia BC, Kao LT, et al. Increased risk of dementia following herpes zoster ophthalmicus. PLoS One. 2017;12(11):e0188490. doi: 10.1371/journal.pone.0188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen VC, Wu SI, Huang KY, Yang YH, Kuo TY, Liang HY, et al (2018) Herpes zoster and dementia: a nationwide population-based cohort Study J Clin Psychiatry 79(1) [DOI] [PubMed]
- 46.Reid M, Price JC, Tien PC. Hepatitis C virus infection in the older patient. Infect Dis Clin North Am. 2017;31(4):827–838. doi: 10.1016/j.idc.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faccioli J, Nardelli S, Gioia S, Riggio O, Ridola L. Neurological and psychiatric effects of hepatitis C virus infection. World J Gastroenterol. 2021;27(29):4846–4861. doi: 10.3748/wjg.v27.i29.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathew S, Faheem M, Ibrahim SM, Iqbal W, Rauff B, Fatima K, et al. Hepatitis C virus and neurological damage. World J Hepatol. 2016;8(12):545–556. doi: 10.4254/wjh.v8.i12.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fletcher NF, Wilson GK, Murray J, Hu K, Lewis A, Reynolds GM, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142(3):634–43.e6. doi: 10.1053/j.gastro.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amirsardari Z, Rahmani F, Rezaei N. Cognitive impairments in HCV infection: From pathogenesis to neuroimaging. J Clin Exp Neuropsychol. 2019;41(10):987–1000. doi: 10.1080/13803395.2019.1652728. [DOI] [PubMed] [Google Scholar]
- 51.Yarlott L, Heald E, Forton D. Hepatitis C virus infection, and neurological and psychiatric disorders - A review. J Adv Res. 2017;8(2):139–148. doi: 10.1016/j.jare.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villani R, Monami M, Di Cosimo F, Fioravanti G, Mannucci E, Vendemiale G, et al. Direct-acting antivirals for HCV treatment in older patients: a systematic review and meta-analysis. J Viral Hepat. 2019;26(11):1249–1256. doi: 10.1111/jvh.13169. [DOI] [PubMed] [Google Scholar]
- 53.Ibáñez-Samaniego L, Rapado-Castro M, Cabrero L, Navarrete C, García-Mulas S, Ahumada A, Marquez L, Pérez MD, Rincon D, Bañares R, Garcia-Martinez R (2022) Hepatitis C eradication improves cognitive function in patients with or without cirrhosis: a prospective real-life study. Eur J Neurol 29(2):400–412. 10.1111/ene.15138 [DOI] [PubMed]
- 54.Ross Russell AL, Dryden MS, Pinto AA, Lovett JK. Lyme disease: diagnosis and management. Pract Neurol. 2018;18(6):455–464. doi: 10.1136/practneurol-2018-001998. [DOI] [PubMed] [Google Scholar]
- 55.Koedel U, Fingerle V, Pfister HW. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol. 2015;11(8):446–456. doi: 10.1038/nrneurol.2015.121. [DOI] [PubMed] [Google Scholar]
- 56.Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW (2020) Lyme borreliosis: diagnosis and management. BMJ 369:m1041. 10.1136/bmj.m1041 [DOI] [PubMed]
- 57.Touradji P, Aucott JN, Yang T, Rebman AW, Bechtold KT. cognitive decline in post-treatment lyme disease syndrome. Arch Clin Neuropsychol. 2019;34(4):455–465. doi: 10.1093/arclin/acy051. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz VH, Edjolo A, Roubaud-Baudron C, Jaulhac B, Avila-Funes JA, Dartigues JF, et al. Association of seropositivity to borrelia burgdorferi with the risk of neuropsychiatric disorders and functional decline in older adults: the Aging Multidisciplinary Investigation Study. JAMA Neurol. 2020;77(2):210–214. doi: 10.1001/jamaneurol.2019.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: a review. World J Gastroenterol. 2018;24(29):3204–3221. doi: 10.3748/wjg.v24.i29.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shindler-Itskovitch T, Ravona-Springer R, Leibovitz A, Muhsen K. A systematic review and meta-analysis of the association between Helicobacterpylori infection and dementia. J Alzheimers Dis. 2016;52(4):1431–1442. doi: 10.3233/JAD-160132. [DOI] [PubMed] [Google Scholar]
- 61.Beydoun MA, Beydoun HA, Elbejjani M, Dore GA, Zonderman AB. Helicobacter pylori seropositivity and its association with incident all-cause and Alzheimer’s disease dementia in large national surveys. Alzheimers Dement. 2018;14(9):1148–1158. doi: 10.1016/j.jalz.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol Aging. 2012;33(5):1009.e11–9. doi: 10.1016/j.neurobiolaging.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Roubaud Baudron C, Letenneur L, Langlais A, Buissonnière A, Mégraud F, Dartigues JF, et al. Does Helicobacter pylori infection increase incidence of dementia? The Personnes Agées QUID Study. J Am Geriatr Soc. 2013;61(1):74–78. doi: 10.1111/jgs.12065. [DOI] [PubMed] [Google Scholar]
- 64.Fani L, Wolters FJ, Ikram MK, Bruno MJ, Hofman A, Koudstaal PJ, et al. Helicobacter pylori and the risk of dementia: a population-based study. Alzheimers Dement. 2018;14(10):1377–1382. doi: 10.1016/j.jalz.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Roubaud-Baudron C, Mégraud F, Salles N, Dartigues JF, Letenneur L. Detecting both current and prior Helicobacter pylori infection is important to assess its impact on dementia. Alzheimers Dement. 2019;15(5):721–722. doi: 10.1016/j.jalz.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 66.Werber T, Bata Z, Vaszine ES, Berente DB, Kamondi A, Horvath AA. The association of periodontitis and Alzheimer’s Disease: how to hit two birds with one stone. J of Alzheimer’s Dis. 2021;84(1):1–21. doi: 10.3233/JAD-210491. [DOI] [PubMed] [Google Scholar]
- 67.Borsa L, Dubois M, Sacco G, Lupi L. Analysis the link between periodontal diseases and Alzheimer’s disease: a systematic review. Int J Environ Res Public Health. 2021;18(17):9312. doi: 10.3390/ijerph18179312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu X, Zhang J, Qiu Y, Liu Z. Periodontal disease and the risk of Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Psychogeriatrics. 2021;21(5):813–825. doi: 10.1111/psyg.12743. [DOI] [PubMed] [Google Scholar]
- 69.Beydoun MA, Beydoun HA, Hossain S, El-Hajj ZW, Weiss J, Zonderman AB. Clinical and bacterial markers of periodontitis and their association with incident all-cause and Alzheimer’s disease dementia in a large national survey. J Alzheimers Dis. 2020;75(1):157–172. doi: 10.3233/JAD-200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joan B Soriano MA, Carine Alsokhn, Nisreen A Alwan, Lisa Askie, Hannah E Davis, Janet V Diaz, Tarun Dua, Wouter de Groote, Robert Jakob, Marta Lado, John Marshall, Srin Murthy, Jacobus Preller, Pryanka Relan, Nicoline Schiess, Archana Seahwag (2021) A clinical case definition of post COVID-19 condition by a Delphi consensus [updated 6 October 2021 Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
- 72.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pilotto A, Cristillo V, Cotti Piccinelli S, Zoppi N, Bonzi G, Sattin D, et al 2021 Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurol Sci 1–5 [DOI] [PMC free article] [PubMed]
- 74.Xydakis MS, Albers MW, Holbrook EH, Lyon DM, Shih RY, Frasnelli JA, et al. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021;20(9):753–761. doi: 10.1016/S1474-4422(21)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guedj E, Million M, Dudouet P, Tissot-Dupont H, Bregeon F, Cammilleri S, et al. (18)F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging. 2021;48(2):592–595. doi: 10.1007/s00259-020-04973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kas A, Soret M, Pyatigoskaya N, Habert MO, Hesters A, Le Guennec L, et al. The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging. 2021;48(8):2543–2557. doi: 10.1007/s00259-020-05178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sollini M, Morbelli S, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, et al. Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging. 2021;48(10):3187–3197. doi: 10.1007/s00259-021-05294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donegani MI, Miceli A, Pardini M, Bauckneht M, Chiola S, Pennone M, et al (2021) Brain metabolic correlates of persistent olfactory dysfunction after SARS-Cov2 infection. Biomedicines 9(3) [DOI] [PMC free article] [PubMed]
- 79.Baig AM (2022) Counting the neurological cost of COVID-19. Nat Rev Neurol 18(1):5–6. 10.1038/s41582-021-00593-7 [DOI] [PMC free article] [PubMed]
- 80.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al (2021) Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 218(3) [DOI] [PMC free article] [PubMed]
- 81.Lophatananon A, Mekli K, Cant R, Burns A, Dobson C, Itzhaki R, et al. Shingles, Zostavax vaccination and risk of developing dementia: a nested case–control study—results from the UK Biobank cohort. BMJ Open. 2021;11(10):e045871. doi: 10.1136/bmjopen-2020-045871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scherrer JF, Salas J, Wiemken TL, Jacobs C, Morley JE, Hoft DF. Lower risk for dementia following adult tetanus, diphtheria, and pertussis (Tdap) vaccination. J Gerontol A Biol Sci Med Sci. 2021;76(8):1436–1443. doi: 10.1093/gerona/glab115. [DOI] [PubMed] [Google Scholar]
- 83.Luo CS, Chi CC, Fang YA, Liu JC, Lee KY. Influenza vaccination reduces dementia in patients with chronic obstructive pulmonary disease: a nationwide cohort study. J Investig Med. 2020;68(4):838–845. doi: 10.1136/jim-2019-001155. [DOI] [PubMed] [Google Scholar]
- 84.Wiemken TL, Salas J, Hoft DF, Jacobs C, Morley JE, Scherrer JF. Dementia risk following influenza vaccination in a large veteran cohort. Vaccine. 2021;39(39):5524–5531. doi: 10.1016/j.vaccine.2021.08.046. [DOI] [PubMed] [Google Scholar]
- 85.Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Liu JC, Hsu YP, Kao PF, Hao WR, Liu SH, Lin CF, et al. Influenza vaccination reduces dementia risk in chronic kidney disease patients: a population-based cohort study. Medicine (Baltimore) 2016;95(9):e2868. doi: 10.1097/MD.0000000000002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lower risk of dementia adds to lengthy list of reasons to be vaccinated. J Gerontol A Biol Sci Med Sci 2021 76(8):1435 [DOI] [PubMed]
- 88.Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, Hofman A. Inflammatory markers and the risk of dementia and Alzheimer’s disease: a meta-analysis. Alzheimers Dement. 2018;14(11):1450–1459. doi: 10.1016/j.jalz.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 89.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fulop T, Witkowski JM, Bourgade K, Khalil A, Zerif E, Larbi A, et al. Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer’s disease? Front Aging Neurosci. 2018;10:224. doi: 10.3389/fnagi.2018.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Linard M, Ravier A, Mougué L, Grgurina I, Boutillier A-L, Foubert-Samier A, Blanc F and Helmer C (2022) Infectious agents as potential drivers of α-synucleinopathies. Mov Disord 10.1002/mds.28925 [DOI] [PubMed]
- 92.Bu XL, Yao XQ, Jiao SS, Zeng F, Liu YH, Xiang Y, et al. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol. 2015;22(12):1519–1525. doi: 10.1111/ene.12477. [DOI] [PubMed] [Google Scholar]
- 93.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, et al. Microbes and Alzheimer’s disease. J Alzheimers Dis. 2016;51(4):979–984. doi: 10.3233/JAD-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]