Summary
As the central carbon uptake pathway in photosynthetic cells, the Calvin–Benson cycle is among the most important biochemical cycles for life on Earth. A carbon flux of anaplerotic origin (i.e. through the chloroplast‐localized oxidative branch of the pentose phosphate pathway) into the Calvin–Benson cycle was proposed recently.
Here, we measured intramolecular deuterium abundances in leaf starch of Helianthus annuus grown at varying ambient CO2 concentrations, C a. Additionally, we modelled deuterium fractionations expected for the anaplerotic pathway and compared modelled with measured fractionations.
We report deuterium fractionation signals at H1 and H2 of starch glucose. Below a C a change point, these signals increase with decreasing C a consistent with modelled fractionations by anaplerotic flux. Under standard conditions (C a = 450 ppm corresponding to intercellular CO2 concentrations, C i, of 328 ppm), we estimate negligible anaplerotic flux. At C a = 180 ppm (C i = 140 ppm), more than 10% of the glucose‐6‐phosphate entering the starch biosynthesis pathway is diverted into the anaplerotic pathway.
In conclusion, we report evidence consistent with anaplerotic carbon flux into the Calvin–Benson cycle in vivo. We propose the flux may help to: maintain high levels of ribulose 1,5‐bisphosphate under source‐limited growth conditions to facilitate photorespiratory nitrogen assimilation required to build‐up source strength; and counteract oxidative stress.
Keywords: Calvin–Benson cycle, flux estimation, glucose‐6‐phosphate shunt, hydrogen stable isotopes, nitrogen assimilation, oxidative pentose phosphate pathway, plant carbon metabolism, source–sink balance
Introduction
In photosynthetic cells, carbon is taken up primarily by the Calvin–Benson cycle (CBC). Thus, the CBC is among the most important biochemical cycles for life on Earth. Sharkey & Weise (2016) proposed the CBC may be anaplerotically refilled by carbon injection from the oxidative branch of the chloroplast‐localized pentose phosphate pathway (namely, glucose‐6‐phosphate (G6P) shunt, orange pathway in Fig. 1). This pathway liberates CO2 from metabolism and anaplerotically recovered energy (nicotinamide adenine dinucleotide phosphate, NADPH) is insufficient for quantitative refixation. Should this flux occur in vivo, it would therefore affect plant carbon and energy balances, plant performance, ecosystem productivity, and biosphere–atmosphere CO2 exchange. This includes the CO2 fertilization effect which has been identified as a major unknown in our current Earth system understanding (IPCC, 2013) as well as future atmospheric CO2 concentrations and crop yields (cf. Long et al., 2006).
Fig. 1.
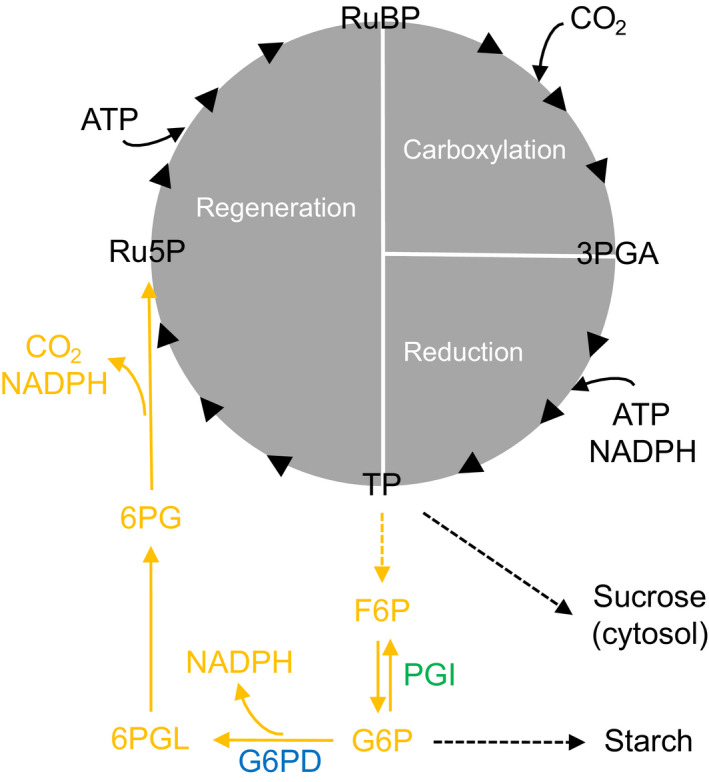
Oxidative branch of the chloroplast‐localized pentose phosphate pathway (in orange) carrying anaplerotic carbon flux into the Calvin–Benson cycle (in grey). Blue and green, enzyme reactions that may introduce deuterium fractionation signals at glucose H1 and H2, respectively. Dashed arrows, intermediate reactions not shown. Enzymes, G6PD, glucose‐6‐phosphate dehydrogenase; PGI, phosphoglucose isomerase. Metabolites: 3PGA, 3‐phosphoglycerate; 6PG, 6‐phosphogluconate; 6PGL, 6‐phosphogluconolactone; ATP, adenosine triphosphate; F6P, fructose 6‐phosphate; G6P, glucose 6‐phosphate; NADPH, nicotinamide adenine dinucleotide phosphate; Ru5P, ribulose 5‐phosphate; RuBP, ribulose 1,5‐bisphosphate; TP, triose phosphates (glyceraldehyde 3‐phosphate, dihydroxyacetone phosphate). Modified figure from Wieloch et al. (2022).
Anaplerotic flux is believed to be controlled at phosphoglucose isomerase (PGI, EC 5.3.1.9; green in Fig. 1) which catalyses interconversions of fructose 6‐phosphate (F6P) and G6P (Sharkey & Weise, 2016). In the light, the reaction is strongly displaced from equilibrium on the side of F6P keeping chloroplastic G6P concentrations low (Dietz, 1985; Gerhardt et al., 1987; Kruckeberg et al., 1989; Schleucher et al., 1999) proposedly to restrict the anaplerotic flux (Sharkey & Weise, 2016). With decreasing PGI inhibitor concentrations (3‐phosphoglycerate, and especially erythrose 4‐phosphate), the PGI reaction shifts towards equilibrium (Dietz, 1985; Backhausen et al., 1997). Concomitantly increasing G6P concentrations (Dietz, 1985) may cause anaplerotic flux increases by the following mechanism (Sharkey & Weise, 2016). In the light, the first enzyme of the pentose phosphate pathway, glucose‐6‐phosphate dehydrogenase (G6PD, EC 1.1.1.49; blue in Fig. 1), is inhibited by redox regulation via thioredoxin (Née et al., 2009). However, inhibition can be reversed allosterically by increasing G6P concentrations (Cossar et al., 1984; Preiser et al., 2019).
While anaplerotic flux into the CBC seems biochemically feasible, so far, only three studies have reported flux‐level evidence. First, Wieloch et al. (2018) analysed intramolecular 13C/12C ratios in glucose extracted from an annually resolved Pinus nigra tree‐ring series (1961–1995). They reported 13C fractionation signals (i.e. systematic 13C/12C variation) at glucose C‐1 and C‐2. These signals respond to drought proposedly due to changes in anaplerotic flux by the following mechanism (Wieloch et al., 2018). As isohydric species, P. nigra responds to drought by stomatal closure which impedes CO2 uptake causing low intercellular CO2 concentrations, C i (Sade et al., 2012). With decreasing C i, PGI inhibitor concentrations reportedly decrease, and the chloroplastic PGI reaction shifts towards equilibrium (Badger et al., 1984; Dietz & Heber, 1984; Dietz, 1985). Concomitantly increasing G6P concentrations can be expected to cause increasing G6PD activity and anaplerotic flux (see last paragraph). Based on reported 13C isotope effects (Gilbert et al., 2012), Wieloch et al. (2018) predicted 13C/12C increases at C‐1 and C‐2 at a ratio of 2.25 in response to shifts of the PGI reaction towards equilibrium and found a ratio of 2.74 (+1.35SE, −0.60SE) confirming their prediction. Interestingly, the reported ratio is somewhat higher than the predicted ratio yet not significantly. Still, the offset may be explained by the 13C effect of G6PD (α = 1.0165; Hermes et al., 1982) which can be expected to cause an additional 13C/12C increase at C‐1 as anaplerotic flux increases.
Second, Wieloch et al. (2022) also analysed intramolecular deuterium (D) abundances across the P. nigra tree‐ring series. They reported two closely related D fractionation signals at glucose H1 and H2 which respond to drought and atmospheric CO2 concentrations. Interestingly, the response requires the crossing of a change point. Wieloch et al. (2022) hypothesize that shifts of the chloroplastic PGI reaction towards equilibrium contribute to the signal at H2 while chloroplastic G6PD contributes to the H1 signal as anaplerotic flux changes.
Third, Xu et al. (2021) analysed 13C enrichment patterns of central metabolites in Camelina sativa leaves by 13C isotopically nonstationary metabolic flux analysis. They reported estimates for anaplerotic flux (4.6 μmol CO2 g−1 fresh weight (FW) h−1, c. 3% of Rubisco carboxylation) and day respiration (5.2 μmol CO2 g−1 FW h−1). However, these estimates come from a physiologically unrealistic model and could not be confirmed by independent methods (Wieloch, 2021). In summary, Xu et al. (2021) reported evidence for anaplerotic flux under normal growth conditions while Wieloch et al. (2018, 2022) reported evidence for stress‐induced upregulation.
To explain the 13C and D fractionation signals observed in tree‐ring glucose of the gymnosperm P. nigra, Wieloch et al. (2022) proposed the following hypotheses. First, anaplerotic flux into the CBC is associated with D fractionation signals at H1 and H2 of chloroplastic G6P and its derivatives including leaf starch. Second, anaplerotic flux and associated D signals increase with decreasing C i. Third, increases occur below a C i change point. In addition, these authors discussed whether anaplerotic flux is a general feature of C3 metabolism. To test these hypotheses in combination, we analysed intramolecular D abundances in leaf starch of the angiosperm Helianthus annuus synthesized at varying C i. In addition, we discuss the anaplerotic flux in the context of plant physiology and climate change.
Materials and Methods
Samples
We used samples of the C3 plant H. annuus L. cv Zebulon from a previous study (Ehlers et al., 2015). These plants were raised in 1.4 l pots in a glasshouse at an ambient CO2 concentration of C a ≈ 450 ppm and a light intensity of 300–400 μmol photons m−2 s−1 (16 h photoperiod). They were watered daily, fertilized twice a week with Rika‐S (Weibulls, Hammenhög, Sweden) and transferred to a growth chamber in groups of eight after 7–8 wk. Growth chamber temperature and relative humidity was set to 22°C : 18°C and 60% : 70% (day : night), respectively. On the first day after transfer, the plants were kept in darkness at C a = 450 ppm to drain the starch reserves. During the following 2 days, the plant groups were grown at a C a of either 180, 280, 450, 700, or 1500 ppm (300–400 μmol photons m−2 s−1, 16 h photoperiod). On the second day, gas exchange measurements were performed (Supporting Information Notes S1), and leaves were harvested and stored at −20°C until starch extraction described in Ehlers et al. (2015). Reported C i values were estimated as described in the Notes S2.
Determination and expression of intramolecular deuterium abundances
Intramolecular D abundances in starch glucose were measured following published procedures (Betson et al., 2006; Ehlers et al., 2015). Proton nuclear magnetic resonance (1H‐NMR) spectroscopy was used to ensure sample purity of the glucose derivative used for quantification (3,6‐anhydro‐1,2‐O‐isopropylidene‐α‐d‐glucofuranose) of ≥ 99.5%. We recorded quantitative D NMR spectra on a DRX600 spectrometer with 5‐mm broadband observe probe and 19F lock (Bruker BioSpin GmbH, Rheinstetten, Germany). Relative intramolecular D abundances were determined by signal deconvolution using Lorentzian line shape fits in TopSpin 3.1 (Bruker BioSpin GmbH). To screen for intramolecular fractionations, we expressed the data as deviations from the molecular average as
| (Eqn 1) |
D i , denotes relative D abundances at specific glucose hydrogen positions. In this notation, whole‐molecule effects are not expressed. However, the size of any intramolecular fractionation effect is attenuated by its contribution to the denominator. To obtain estimates of effect sizes, we used as reference the average D abundance of the methyl‐group hydrogens of the glucose derivative (introduced during derivatization from a common batch of acetone) as
| (Eqn 2) |
DME, denotes relative D abundances of the methyl‐group hydrogens.
Results
Deuterium enrichments at glucose H1, and H2
Here, we analysed intramolecular D abundances in H. annuus leaf starch synthesized at different levels of C a (180, 280, 450, 700, and 1500 ppm). We found intramolecular fractionations at H1 and H2 (Fig. 2). With decreasing C a from 450 to 180 ppm (C i from 328 to 140 ppm), δD increases at H1 (210‰), and H2 (311‰, Fig. 3). Note that actual offsets may be somewhat larger than apparent offsets (Notes S3). Above C a = 450 ppm (C i ≈ 328 ppm), these relationships break, i.e. they exhibit a change point at C a ≈ 450 ppm. In general, intramolecular fractionations are caused by metabolic processes (see next paragraphs).
Fig. 2.
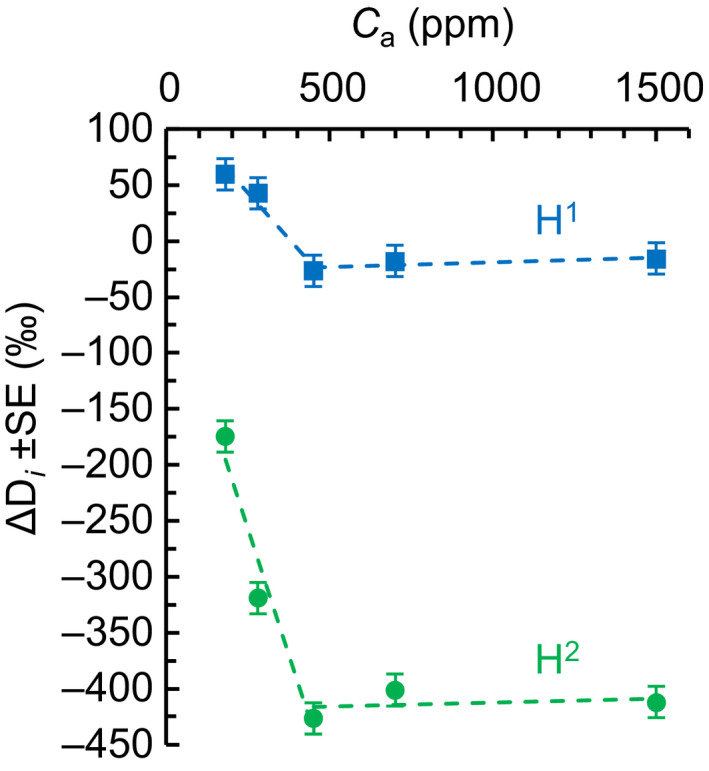
Relative deuterium (D) abundance at H1 (blue squares), and H2 (green circles) of Helianthus annuus leaf starch. Plants were grown in chambers at ambient CO2 concentration (C a) = 450 ppm. After a day in darkness to drain starch reserves, plants were grown at different levels of C a (180, 280, 450, 700, and 1500 ppm) corresponding to different levels of intercellular CO2 concentration (C i) (140, 206, 328, 531, and 1365 ppm) for 2 d. Data expressed in terms of the molecular average as ΔD i = D i /(ΣD i /7) − 1, where D i denotes relative D abundances at specific glucose hydrogen positions (±SE = 14.1‰). Figure shows discrete data. Dashed lines added to guide the eye.
Fig. 3.
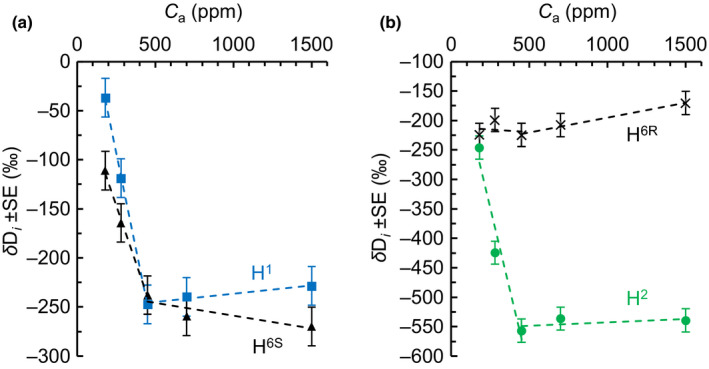
(a, b) Relative deuterium (D) abundance at H1 (blue squares), H6S (black triangles), H2 (green circles), and H6R (black crosses) of Helianthus annuus leaf starch. Plants were grown in chambers at ambient CO2 concentration (C a) = 450 ppm. After a day in darkness to drain starch reserves, plants were grown at different levels of C a (180, 280, 450, 700, and 1500 ppm) corresponding to different levels of intercellular CO2 concentration (C i) (140, 206, 328, 531, and 1365 ppm) for 2 d. Data expressed as δD i = D i /(ΣDME/6) − 1, with D i and DME denoting relative D abundances at specific glucose hydrogen positions and the methyl‐group hydrogens of the glucose derivative used for measurements, respectively (±SE = 19.7‰). Figure shows discrete data. Dashed lines added to guide the eye.
Photorespiratory deuterium fractionations occur at glucose H1, and H6S
With decreasing C a from 450 to 180 ppm (C i from 328 to 140 ppm), δD increases at H1 (210‰), and H6S (127‰) but not H6R (Fig. 3). Changing photorespiration‐to‐photosynthesis ratios reportedly has equal effects on both the H6R/H1 and H6R/H6S D abundance ratios (Schleucher, 1998). This was attributed to changes at H6R (Schleucher, 1998). However, δD6R is invariable between 450 and 180 ppm (Fig. 3b). Thus, photorespiratory fractionation likely occurs at glucose H1 and H6S but not H6R.
Metabolic origin of the deuterium enrichment at glucose H1
Starch biosynthesis from photosynthetic glyceraldehyde 3‐phosphate (GAP) proceeds via a multi‐step enzymatic pathway (Fig. 4). In the first step, triosephosphate isomerase converts GAP (glucose C‐4 to C‐6) to dihydroxyacetone phosphate (DHAP) (glucose C‐1 to C‐3). Thereby, the GAP hydrogen corresponding to glucose H6S becomes the DHAP hydrogen corresponding to glucose H1. These hydrogens are neither modified by the triosephosphate isomerase reaction, nor by any subsequent reaction leading to starch. Hence, starch glucose H6S and H1 can be expected to have equal D abundances. Evidently, this situation occurs at C a = 450 ppm (Fig. 3a). Thus, there is no evidence for anaplerotic fractionation and flux at C a = 450 ppm (under standard conditions) suggesting that G6PD is efficiently inhibited by thioredoxin despite the relatively low light intensity.
Fig. 4.
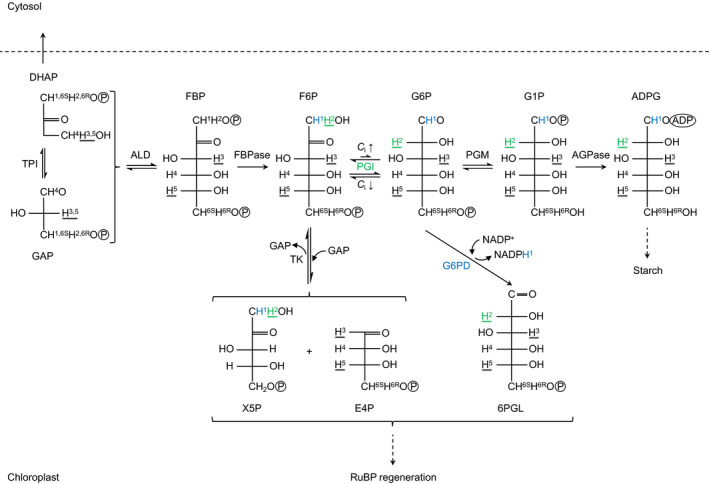
Hydrogen metabolism associated with starch biosynthesis. Hydrogens named according to their location(s) in starch glucose (indirect precursors not designated, see X5P). Green and blue hydrogens may be affected by isotope fractionation at PGI and G6PD, respectively. Wavy lines, hydrogen from water is fractionally introduced during the TPI and PGI reaction. Under standard C i conditions (C i↑), the chloroplastic PGI reaction is strongly displaced from equilibrium on the side of F6P. Under low C i conditions (C i↓), the chloroplastic PGI reaction is closer to or at equilibrium. Dashed arrows, lntermediate reactions not shown. Abbreviations: 6PGL, 6‐phosphogluconolactone; ADPG, ADP‐glucose; AGPase, glucose‐1‐phosphate adenylyltransferase; ALD, fructose‐1,6‐bisphosphate aldolase; C i, intercellular CO2 concentration; DHAP, dihydroxyacetone phosphate; E4P, erythrose 4‐phosphate; F6P, fructose 6‐phosphate; FBP, fructose 1,6‐bisphosphate; FBPase, fructose 1,6‐bisphosphatase; G1P, glucose 1‐phosphate; G6P, glucose 6‐phosphate; G6PD, glucose‐6‐phosphate dehydrogenase; GAP, glyceraldehyde 3‐phosphate; PGI, phosphoglucose isomerase; PGM, phosphoglucomutase; RuBP, ribulose 1,5‐bisphosphate; TK, transketolase; TPI, triose‐phosphate isomerase; X5P, xylulose 5‐phosphate.
Equal D enrichments at H1 and H6S can be attributed to photorespiratory fractionation (see previous paragraph). However, at C a = 180 ppm, H1 is 74‰ more enriched than H6S (Fig. 3a; δD1 = −37‰, δD6S = −111‰). This enrichment can be expected to be introduced beyond DHAP synthesis by a high‐flux carrying pathway branching off from the starch biosynthesis pathway. To our knowledge, there are only two possible candidates, the well‐established CBC‐regeneration pathway and the proposed anaplerotic pathway (Fig. 4). The former pathway starts with the conversion of F6P and GAP to erythrose 4‐phosphate and xylulose 5‐phosphate by transketolase. The hydrogen corresponding to starch glucose H1 is not modified by the transketolase reaction. By contrast, the anaplerotic pathway starts with the irreversible G6PD‐catalysed conversion of G6P to 6‐phosphogluconolactone which deprotonates the G6P hydrogen corresponding to starch glucose H1. This reaction reportedly has a D isotope effect in vitro (α D = k H/k D = 2.97) (Hermes et al., 1982). Thus, G6PD is a plausible candidate for D fractionation at H1.
Consistent with our observations, fractionation modelling shows that increasing anaplerotic flux, f, causes D increases at H1 of remaining G6P, δDG6P′ (Fig. 5; Notes S4). A 74‰ increase as observed in starch (Fig. 3a) indicates that c. 10% of the G6P entering the starch biosynthesis pathway is diverted into the anaplerotic pathway. However, G6P can be converted back to F6P by PGI and enter the CBC via transketolase (Fig. 4). This flux will be low under low chloroplastic G6P concentrations (i.e. under standard growth conditions) yet increase as the PGI reaction shifts towards equilibrium (with shifts towards low C a, see the ‘Introduction’ section). It can be expected to fractionally remove the G6PD‐fractionation signal from the starch biosynthesis pathway. Thus, at C a = 180 ppm, more than 10% of the G6P entering the starch biosynthesis pathway may be diverted into the anaplerotic pathway (to obtain a 74‰ increase at H1).
Fig. 5.
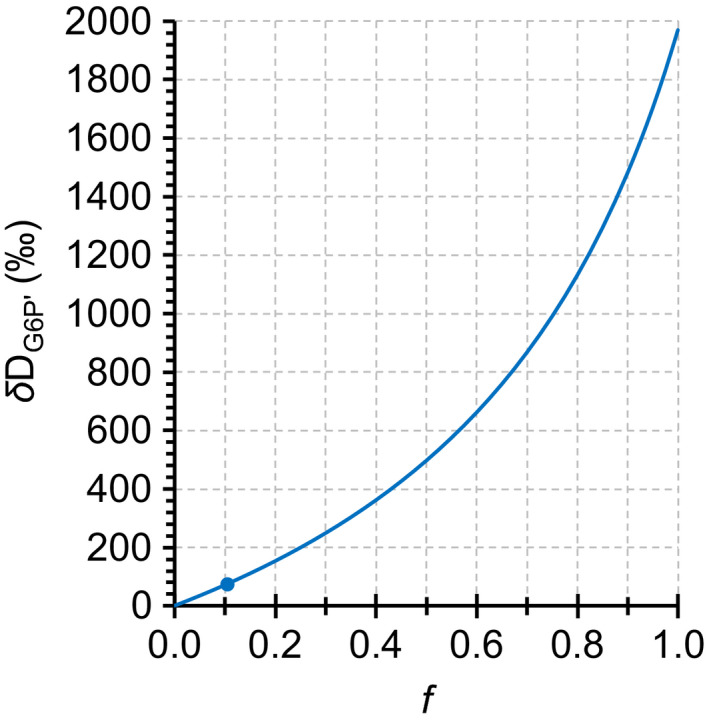
Modelled deuterium (D) fractionation by glucose‐6‐phosphate dehydrogenase (G6PD) at H1 of glucose 6‐phosphate (G6P), δDG6P′. Modelling assumed an open system at steady state. Incoming G6P has two fates, starch biosynthesis or anaplerotic reinjection into the Calvin–Benson cycle via G6PD (Fig. 4). Commitment of G6P to the anaplerotic pathway vs starch biosynthesis, f, is given on the abscissa (e.g. f = 1: all G6P enters the anaplerotic pathway, f = 0: all G6P enters starch biosynthesis). Blue dot, f = 0.1044, δDG6P′ = 74.41‰. G6PD has an in vitro kinetic isotope effect of α D = 2.97 (Hermes et al., 1982). G6P entering the system was assumed to have the same D : H ratio as Vienna Standard Mean Ocean Water (R VSMOW = 155.76 × 10−6) (Hagemann et al., 1970). Data expressed in terms of R VSMOW.
Metabolic origin of the deuterium enrichment at glucose H2
At the level of chloroplastic triose phosphates, the precursors of starch glucose H2 correspond to the precursors of starch glucose H6R (Fig. 4). In the biosynthetic pathway from triose phosphate to starch, the former is subject to modification by PGI while the latter is not modified. Therefore, we assume the D abundance at H6R is not modified during starch biosynthesis and can be used as reference for H2.
Under standard conditions (C a = 450 ppm), starch glucose H2 is 311‰ depleted compared to low C a conditions (= 180 ppm) and 332‰ depleted compared to H6R (Fig. 3b). This is consistent with reported glucose H2 depletions in leaf starch compared to sucrose (Phaseolus vulgaris, 333‰; Spinacia oleracea, 500‰) which were explained by different modus operandi of chloroplastic and cytosolic PGI (Schleucher et al., 1999). Reportedly, the chloroplastic PGI reaction is strongly displaced from equilibrium on the side of F6P (Dietz, 1985; Gerhardt et al., 1987; Leidreiter et al., 1995; Schleucher et al., 1999) while the cytosolic reaction is either somewhat displaced from equilibrium (Leidreiter et al., 1995; Schleucher et al., 1999) or at equilibrium (Gerhardt et al., 1987). Thus, in chloroplasts under standard conditions, the kinetic isotope effect of PGI is manifested. This effect can cause D depletions of up to c. 550‰ (Notes S5; hydrogen exchange with the medium not considered).
At low C a (= 180 ppm), starch glucose H2 is merely 21.5‰ depleted compared to H6R (Fig. 3b). This is consistent with a shift of the PGI reaction from kinetic to equilibrium conditions and thus upregulated anaplerotic flux (see the ‘Introduction’ section). Theoretically, equilibrium isotope fractionation by PGI causes a D depletion of ≈ 99‰ when fractionation related to hydrogen exchange with the medium is not considered (Notes S5). However, the offset between theoretical and observed values (c. 77.5‰) indicates that hydrogen exchange with the medium affects isotope fractionation by PGI.
Discussion
Consistent with isotope theory related to anaplerotic carbon flux into the CBC (see the ‘Introduction’ section; Wieloch et al., 2022), we found D fractionation signals at H1 and H2 of leaf starch which increase with decreasing C a (and C i) below a response change point (Figs 2, 3). Under standard conditions (C a = 450 ppm, C i = 328 ppm), we estimate negligible anaplerotic flux. This is in contrast to findings by Xu et al. (2021). At C a = 180 ppm (C i = 140 ppm), we estimate that more than 10% of the G6P entering the starch biosynthesis pathway is diverted into the anaplerotic pathway. Previously, we reported evidence for anaplerotic flux in P. nigra, a gymnosperm (Wieloch et al., 2018, 2022). Here, we report evidence for this flux in the angiosperm H. annuus (Figs 2, 3). Thus, anaplerotic flux into the CBC may be a general component of C3 metabolism. Lastly, our data suggest photorespiratory fractionation affects the D abundance at glucose H1 and H6S but not H6R.
Anaplerotic flux into the Calvin–Benson cycle: a leaf‐level response to source–sink imbalances?
In H. annuus, anaplerotic flux was upregulated under apparent steady‐state conditions at low C a concentrations (Figs 2, 3). Similarly, large anaplerotic isotope fractionations in P. nigra tree rings (formed over the course of several months) suggest that the anaplerotic flux can persist over long periods (Wieloch et al., 2018, 2022).
Helianthus annuus was raised at C a = 450 ppm over 7–8 wk. After a day in darkness, the plants were subjected to different C a treatments for 2 d. We found evidence for anaplerotic flux at C a < 450 ppm (Figs 2, 3). This may be explained by a shift from the plants' previously established source–sink balance at C a = 450 ppm to source‐limited conditions at C a < 450 ppm (where sink demands exceed the source strength). A concomitant downstream pull of carbohydrates may have caused excess export of triose phosphates from chloroplasts to the cytosol and shortage of chloroplastic triose phosphates for regeneration of ribulose 1,5‐bisphosphate (RuBP) (Fig. 4). To maintain the photosynthetic/photorespiratory capacity at the level of RuBP, anaplerotic flux may then have been upregulated by mechanisms described earlier (see the ‘Introduction’ section). Thus, we propose upregulation of the anaplerotic flux is a leaf‐level response to source‐limited growth conditions. It may be triggered by all environmental parameters that can cause source limitations such as low C a, low C i, and drought (as in P. nigra) (Wieloch et al., 2018, 2022). This may have implications for gas exchange studies aiming to investigate actual steady‐state conditions. To preclude errors due to anaplerotic CO2 liberation associated with disturbed source–sink balances, plants should be raised under the same conditions they are analysed.
Anaplerotic flux into the Calvin–Benson cycle: potential benefits for plant functioning and growth
Like photorespiration, anaplerotic flux has a negative carbon balance (Fig. 1) and seems, therefore, detrimental for plant functioning and growth but we propose two potential benefits.
First, by maintaining RuBP levels, anaplerotic flux maintains photorespiration (both fluxes increase with decreasing C a and C i). Reportedly, photorespiration is positively correlated with de novo nitrate assimilation into protein (Bloom, 2015). Thus, anaplerotic flux may help to increase photosynthetic capacity at the enzyme level, and it seems plausible that plants would try to increase this capacity under source‐limited conditions. Additionally, increased nitrogen assimilation may support the biosynthesis of nitrogen‐containing compounds used for maintenance and repair processes. Overall, it seems plausible that metabolism would shift towards nitrogen assimilation when carbon metabolism is substrate limited.
Second, optimal plant functioning requires a balance between energy supply and consumption (Huner et al., 1996). Upregulation of the anaplerotic flux occurs at low C i (Figs 2, 3) which can cause energy imbalances in chloroplasts (Huner et al., 1996; Wilhelm & Selmar, 2011; Vanlerberghe et al., 2015). This involves decreased consumption of adenosine triphosphate (ATP) and NADPH by the CBC but constant electron input by light‐harvesting complexes. The resulting lack of electron acceptors (NADP+ and adenosine diphosphate (ADP)) leads to the generation of reactive oxygen species (Vanlerberghe et al., 2015). Futile carbon cycling involving CO2 liberation by the anaplerotic flux and refixation by Rubisco has a negative NADPH and especially ATP balance (Fig. 1). Thus, anaplerotic flux may help to dissipate excess energy and counteract oxidative stress and photoinhibition at low C i.
In P. nigra, upregulation of the anaplerotic pathway was reportedly associated with below‐average yet not exceptionally low growth (Wieloch et al., 2022). This is surprising since anaplerotic flux increases with decreasing C i and liberates CO2 (Figs 1, 2, 3). Principally, inputs of storage carbohydrates from previous years may have rescued growth rates but such inputs were shown to be negligible (Wieloch et al., 2018). By contrast, physiological benefits proposed earlier may have contributed to maintaining growth rates.
Anaplerotic flux into the Calvin–Benson cycle and climate change
In P. nigra, anaplerotic flux reportedly correlates positively with drought and negatively with C a (Wieloch et al., 2018, 2022). This is consistent with findings reported here. The Intergovernmental Panel on Climate Change (IPCC) predicts a higher frequency of drought events in already dry regions towards the end of the 21st century (RCP8.5) (IPCC, 2013). Simultaneously, C a will increase globally. Therefore, it is unclear whether photosynthesis involving an upregulated anaplerotic flux will become more prevalent.
Despite potentially significant impacts on carbon, nitrogen, and energy metabolism, the anaplerotic pathway is currently neither well understood nor considered in models of photosynthesis, biosphere–atmosphere CO2 exchange, and plant growth. Stable isotope tools utilized here may enable comprehensive flux analyses across spatiotemporal scales.
Author contributions
TW conceived the study and led the research. AA and JS prepared the samples and acquired the data. TW analysed and interpreted the data. TW wrote the article with input from AA and JS.
Supporting information
Notes S1 Leaf gas exchange measurements.
Notes S2 Estimation of intercellular CO2 concentrations during growth chamber experiments.
Notes S3 Dilution of isotope signals by remnant starch.
Notes S4 Deuterium fractionation by glucose‐6‐phosphate dehydrogenase.
Notes S5 Deuterium fractionation by phosphoglucose isomerase.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Backhausen JE, Jöstingmeyer P, Scheibe R. 1997. Competitive inhibition of spinach leaf phosphoglucose isomerase isoenzymes by erythrose 4‐phosphate. Plant Science 130: 121–131. [Google Scholar]
- Badger MR, Sharkey TD, von Caemmerer S. 1984. The relationship between steady‐state gas exchange of bean leaves and the levels of carbon‐reduction‐cycle intermediates. Planta 160: 305–313. [DOI] [PubMed] [Google Scholar]
- Betson TR, Augusti A, Schleucher J. 2006. Quantification of deuterium isotopomers of tree‐ring cellulose using nuclear magnetic resonance. Analytical Chemistry 78: 8406–8411. [DOI] [PubMed] [Google Scholar]
- Bloom AJ. 2015. Photorespiration and nitrate assimilation: a major intersection between plant carbon and nitrogen. Photosynthesis Research 123: 117–128. [DOI] [PubMed] [Google Scholar]
- Cossar JD, Rowell P, Stewart WDP. 1984. Thioredoxin as a modulator of glucose‐6‐phosphate dehydrogenase in a N2‐fixing cyanobacterium. Microbiology 130: 991–998. [Google Scholar]
- Dietz K‐J. 1985. A possible rate‐limiting function of chloroplast hexosemonophosphate isomerase in starch synthesis of leaves. Biochimica et Biophysica Acta 839: 240–248. [Google Scholar]
- Dietz K‐J, Heber U. 1984. Rate‐limiting factors in leaf photosynthesis. I. Carbon fluxes in the Calvin cycle. Biochimica et Biophysica Acta 767: 432–443. [Google Scholar]
- Ehlers I, Augusti A, Betson TR, Nilsson MB, Marshall JD, Schleucher J. 2015. Detecting long‐term metabolic shifts using isotopomers: cO2‐driven suppression of photorespiration in C3 plants over the 20th century. Proceedings of the National Academy of Sciences, USA 112: 15585–15590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW. 1987. Subcellular metabolite levels in spinach leaves: regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiology 83: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A, Robins RJ, Remaud GS, Tcherkez G. 2012. Intramolecular 13C pattern in hexoses from autotrophic and heterotrophic C3 plant tissues. Proceedings of the National Academy of Sciences, USA 109: 18204–18209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann R, Nief G, Roth E. 1970. Absolute isotopic scale for deuterium analysis of natural waters. Absolute D/H ratio for SMOW. Tellus 22: 712–715. [Google Scholar]
- Hermes JD, Roeske CA, O'Leary MH, Cleland WW. 1982. Use of multiple isotope effects to determine enzyme mechanisms and intrinsic isotope effects. Malic enzyme and glucose 6‐phosphate dehydrogenase. Biochemistry 21: 5106–5114. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Maxwell DP, Gray GR, Savitch LV, Krol M, Ivanov AG, Falk S. 1996. Sensing environmental temperature change through imbalances between energy supply and energy consumption: redox state of photosystem II. Physiologia Plantarum 98: 358–364. [Google Scholar]
- IPCC . 2013. Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner G‐K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, eds, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, USA: Cambridge University Press. [Google Scholar]
- Kruckeberg AL, Neuhaus HE, Feil R, Gottlieb LD, Stitt M. 1989. Decreased‐activity mutants of phosphoglucose isomerase in the cytosol and chloroplast of Clarkia xantiana . Biochemical Journal 261: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidreiter K, Kruse A, Heineke D, Robinson DG, Heldt H‐W. 1995. Subcellular volumes and metabolite concentrations in potato (Solanum tuberosum cv. Désirée) leaves. Botanica Acta 108: 439–444. [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. 2006. Food for thought: lower‐than‐expected crop yield stimulation with rising CO2 concentrations. Science 312: 1918–1921. [DOI] [PubMed] [Google Scholar]
- Née G, Zaffagnini M, Trost P, Issakidis‐Bourguet E. 2009. Redox regulation of chloroplastic glucose‐6‐phosphate dehydrogenase: a new role for f‐type thioredoxin. FEBS Letters 583: 2827–2832. [DOI] [PubMed] [Google Scholar]
- Preiser AL, Fisher N, Banerjee A, Sharkey TD. 2019. Plastidic glucose‐6‐phosphate dehydrogenases are regulated to maintain activity in the light. Biochemical Journal 476: 1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade N, Gebremedhin A, Moshelion M. 2012. Risk‐taking plants: anisohydric behavior as a stress‐resistance trait. Plant Signaling & Behavior 7: 767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleucher J. 1998. Intramolecular deuterium distributions and plant growth conditions. In: Griffiths H, ed. Stable isotopes – integration of biological, ecological and geochemical processes. Oxford, UK: Bios Scientific Publishers, 63–73. [Google Scholar]
- Schleucher J, Vanderveer P, Markley JL, Sharkey TD. 1999. Intramolecular deuterium distributions reveal disequilibrium of chloroplast phosphoglucose isomerase. Plant, Cell & Environment 22: 525–533. [Google Scholar]
- Sharkey TD, Weise SE. 2016. The glucose 6‐phosphate shunt around the Calvin–Benson cycle. Journal of Experimental Botany 67: 4067–4077. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Wang J, Cvetkovska M, Dahal K. 2015. Modes of electron transport chain function during stress: does alternative oxidase respiration aid in balancing cellular energy metabolism during drought stress and recovery? In: Gupta KJ, Mur LAJ, Neelwarne B, eds. Alternative respiratory pathways in higher plants. Oxford, UK: John Wiley & Sons, 157–183. [Google Scholar]
- Wieloch T. 2021. The next phase in the development of 13C isotopically nonstationary metabolic flux analysis. Journal of Experimental Botany 72: 6087–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieloch T, Ehlers I, Yu J, Frank D, Grabner M, Gessler A, Schleucher J. 2018. Intramolecular 13C analysis of tree rings provides multiple plant ecophysiology signals covering decades. Scientific Reports 8: 5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieloch T, Grabner M, Augusti A, Serk H, Ehlers I, Yu J, Schleucher J. 2022. Metabolism is a major driver of hydrogen isotope fractionation recorded in tree‐ring glucose of Pinus nigra . New Phytologist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C, Selmar D. 2011. Energy dissipation is an essential mechanism to sustain the viability of plants: the physiological limits of improved photosynthesis. Journal of Plant Physiology 168: 79–87. [DOI] [PubMed] [Google Scholar]
- Xu Y, Fu X, Sharkey TD, Shachar‐Hill Y, Walker BJ. 2021. The metabolic origins of non‐photorespiratory CO2 release during photosynthesis: a metabolic flux analysis. Plant Physiology 186: 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Notes S1 Leaf gas exchange measurements.
Notes S2 Estimation of intercellular CO2 concentrations during growth chamber experiments.
Notes S3 Dilution of isotope signals by remnant starch.
Notes S4 Deuterium fractionation by glucose‐6‐phosphate dehydrogenase.
Notes S5 Deuterium fractionation by phosphoglucose isomerase.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


