Abstract
Objective
To assess long‐term efficacy and safety of guselkumab, an interleukin‐23 p19 subunit (IL‐23p19) inhibitor, in patients with active psoriatic arthritis (PsA) from the phase III DISCOVER‐2 trial.
Methods
In the DISCOVER‐2 trial, patients with active PsA (≥5 swollen joints and ≥5 tender joints; C‐reactive protein level ≥0.6 mg/dl) despite prior nonbiologic therapy were randomized to receive the following: guselkumab 100 mg every 4 weeks; guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks; or placebo with crossover to guselkumab 100 mg every 4 weeks, beginning at week 24. Efficacy assessments included American College of Rheumatology ≥20%/50%/70% improvement criteria (ACR20/50/70), Investigator’s Global Assessment (IGA) of psoriasis score of 0 (indicating complete skin clearance), resolution of enthesitis (Leeds Enthesitis Index) and dactylitis (Dactylitis Severity Score), and changes in the Sharp/van der Heijde modified radiographic scores for PsA. Clinical data (imputed as no response/no change from baseline if missing) and observed radiographic data were summarized through week 100; safety assessments continued through week 112.
Results
Of the 739 randomized and treated patients, 652 (88%) completed treatment through week 100. Across groups of guselkumab‐treated patients (including those in the placebo–guselkumab crossover group), the following findings at week 100 indicated that amelioration of arthritis signs/symptoms and extraarticular manifestations was durable through 2 years: ACR20 response (68–76%), ACR50 response (48–56%), ACR70 response (30–36%), IGA score of 0 (55–67%), enthesitis resolution (62–70%), and dactylitis resolution (72–83%). Mean changes in the Sharp/van der Heijde modified score for PsA from weeks 52 to week 100 (range 0.13–0.75) indicated that the low rates of radiographic progression observed among guselkumab‐treated patients at earlier time points extended through week 100. Through week 112, 8% (5.8 per 100 patient‐years) and 3% (1.9 per 100 patient‐years) of the 731 guselkumab‐treated patients had a serious adverse event or serious infection, respectively; 1 death occurred (road traffic accident).
Conclusion
In biologic‐naive PsA patients, guselkumab provided durable improvements in multiple disease domains with no unexpected safety findings through 2 years.
Short abstract
INTRODUCTION
Psoriatic arthritis (PsA), an inflammatory disorder primarily affecting the skin and joints, can present with a variety of manifestations including skin and nail lesions, peripheral joint pain, spondylitis, dactylitis, and enthesitis. Symptoms typically begin in early to mid‐adulthood, thus requiring long‐term treatment. Current treatment guidelines advise choosing therapeutics directed at specific PsA disease domains affected in individual patients (1, 2). Biologic therapies are often recommended for patients whose disease is not adequately controlled by conventional synthetic disease‐modifying antirheumatic drugs (DMARDs). In addition, it is not uncommon for PsA patients to switch biologic treatments due to loss of efficacy over time or intolerance (3, 4). Recent findings from an observational study of biologics in PsA patients show that treatment persistence and achieving low disease activity at 1 year was predictive of longer‐term persistence and remission at 12 years (5), highlighting the current unmet need for treatments exhibiting durable efficacy and safety (6, 7).
The Th17 cell line has been identified as a critical driver of skin inflammation in psoriasis (8, 9) and may also drive articular disease pathogenesis, given that interleukin‐17A (IL‐17A) inhibitors have demonstrated therapeutic benefits in this compartment (10). IL‐23 is known to promote differentiation and proliferation of Th17 cells in skin lesions from psoriasis patients (11, 12), and this pathway has been implicated in PsA pathogenesis (13). Guselkumab, a monoclonal antibody targeting the IL‐23p19 subunit, is approved both for adults with moderate‐to‐severe psoriasis and those with active PsA (14). In the phase III, randomized, placebo‐controlled DISCOVER‐1 (15) and DISCOVER‐2 (16) studies, patients treated with guselkumab 100 mg, either every 4 weeks or every 8 weeks, achieved greater improvements and higher response rates in several measures of joint and skin disease at week 24 compared to those receiving placebo. Radiographic progression, assessed only in the DISCOVER‐2 trial, was significantly lower in the guselkumab group treated every 4 weeks than in the placebo group at week 24. Improvements in the signs and symptoms of PsA and joint and skin response rates were maintained through 1 year, with safety findings consistent with the known profile of guselkumab (17, 18). The DISCOVER‐2 trial continued through 2 years, and the final clinical efficacy, radiographic progression, and safety results are reported herein.
PATIENTS AND METHODS
Patients
Patient eligibility criteria have been previously described (16). Briefly, the DISCOVER‐2 trial enrolled adults with active PsA (≥5 tender joints and ≥5 swollen joints; C‐reactive protein [CRP] level ≥0.6 mg/dl) despite standard nonbiologic treatment (DMARDs, apremilast, or nonsteroidal antiinflammatory drugs [NSAIDs]) who were naive to treatment with biologic agents and JAK inhibitors.
Study design
This phase III, randomized, double‐blind study was conducted at 118 sites across 13 countries. The trial included a 6‐week screening period, a 100‐week treatment phase (placebo‐controlled weeks 0–24, active treatment weeks 24–100), and 12 weeks of safety follow‐up (weeks 100–112) (16). Eligible patients were randomized (1:1:1) to receive subcutaneous injections of guselkumab 100 mg every 4 weeks; guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks; or placebo with crossover to guselkumab 100 mg every 4 weeks beginning at week 24. Patients had the option to self‐administer guselkumab in weeks 56–96. Central randomization and study blinding details through week 24 have been previously reported (16). After crossover to guselkumab, patients and investigators remained blinded with regard to dosing regimen. Patients could continue stable baseline use of selected nonbiologic DMARDs, oral glucocorticoids (≤10 mg/day of prednisone or equivalent), and NSAIDs/other analgesics up to regionally approved doses.
The DISCOVER‐2 trial (ClinicalTrials.gov identifier: NCT03158285) was conducted in accordance with Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by each site’s governing ethical body, and all patients provided written informed consent.
Procedures
Efficacy was assessed through week 100. American College of Rheumatology (ACR) response components (tender joint count [0–68], swollen joint count [0–66], pain [0–10 cm visual analog scale (VAS)], physician global assessment [0–10 cm VAS], patient global assessment [0–10 cm VAS], physical function as assessed by the Health Assessment Questionnaire disability index [HAQ DI; 0–3], and CRP level [mg/dl]) were determined as previously described (16, 17). Enthesitis (Leeds Enthesitis Index; total 0–6) (19) and dactylitis (Dactylitis Severity Score; total 0–60) (20) were also assessed.
Single radiographs of hands (posteroanterior) and feet (anteroposterior) were obtained at weeks 0, 24, 52, and 100 (or at discontinuation) and scored using the Sharp/van der Heijde modified scoring method for PsA (21). Findings previously described through week 24 and week 52 were derived from the first and second reading sessions, respectively (16, 17). The third reading session included radiographs from all time points and were independently evaluated by 2 central primary readers, with a third reader for adjudication, blinded with regard to treatment group and time point. Assignment of readers to primary reader/adjudicator roles was the same for reading sessions 1 and 3, and scores of the 2 primary readers in each session were averaged together (16, 17). Scores from a third adjudicator were utilized when the difference in change scores between the primary readers was >10 or if change scores from 1 primary reader were missing.
Skin symptoms were assessed using the Investigator’s Global Assessment of psoriasis (IGA; 0 [cleared] to 4 [severe]) (22). The Psoriasis Area and Severity Index (PASI; 0–72) also assessed the extent (percentage body surface area affected) and degree of associated redness, thickness, and scaling (each graded from 0 [none] to 4 [maximum]) (23). Health‐related quality of life (HRQoL) was evaluated using Short Form 36 (SF‐36) physical component summary (PCS) and mental component summary (MCS) scores (24).
Adverse events (AEs) and routine hematology and chemistry parameters were monitored. Serum samples collected through week 112 were assayed to measure guselkumab concentrations and detect antibodies to guselkumab (15, 16).
Outcome measures
Outcome measures included the following: ACR ≥20%/50%/70% improvement criteria (ACR20/50/70) (25); IGA score 0/1 (score 0/1 and ≥2‐grade improvement); skin responses (IGA 0 and ≥75%, 90%, or 100% improvement in PASI [PASI75/90/100]) in patients with ≥3% body surface area affected with psoriasis and IGA score ≥2 at baseline; changes in total Sharp/van der Heijde modified scores for PsA derived from images read in the third session; changes from baseline in HAQ DI and proportions of patients with HAQ DI response (reduction ≥0.35 among patients with a baseline score ≥0.35) or normalized HAQ DI (≤0.5 among patients with a baseline score >0.5); changes from baseline in SF‐36 PCS and MCS scores and proportions of patients with a minimal clinically important difference (≥5) (26); resolution of enthesitis and dactylitis (score 0 among patients affected at baseline); and achievement of minimal disease activity (MDA) (27) or very low disease activity (VLDA) (28).
Safety outcomes included AEs, serious AEs (SAEs), AEs resulting in discontinuation of study drug, infections, serious infections, injection‐site reactions, malignancies, major adverse cardiovascular events (MACE; predefined as cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke), suicidal ideation or behavior (electronic Columbia‐Suicide Severity Rating Scale questionnaire or reported AEs), and clinical laboratory abnormalities classified by the National Cancer Institute’s Common Terminology Criteria for AEs.
Statistical analysis
DISCOVER‐2 sample size estimates have been previously reported (16). All patients continuing treatment at week 24 received guselkumab going forward; no formal hypothesis testing was planned after week 24.
As previously reported (16), treatment failure rules were applied to all clinical efficacy analyses through week 24: patients who discontinued study treatment, terminated study participation, initiated/increased doses of DMARDs or oral glucocorticoids, or initiated protocol‐prohibited PsA treatment were considered nonresponders for binary end points, or were considered to have no change from baseline for continuous end points. Missing data were imputed as nonresponse for categorical end points or using multiple imputation (assumed to be missing at random) for continuous end points. After week 24, the statistical analysis plan prespecified using observed data through week 100. Post hoc clinical efficacy (but not radiographic) analyses employed nonresponder imputation (NRI) in which patients with missing data were classified as nonresponders for categorical end points, and missing continuous end point data were imputed as having no change (for patients who discontinued study treatment), or were imputed using multiple imputation (assumed to be missing at random for patients with missing data for any other reason). Results of these post hoc analyses have been reported through week 52 (17) and are reported here through week 100. Additional post hoc analyses assessed the maintenance of ACR20/50/70 responses (NRI) at week 100 among patients with a response at week 52, the proportions of patients achieving ≥20% improvement in the individual ACR components (NRI) through week 100, and the median time to onset of treatment effect (for ACR20) using Kaplan–Meier curves. Least squares mean (LSM) changes in clinical efficacy and HRQoL measures were determined using analysis of covariance.
Observed changes in Sharp/van der Heijde scores from reading session 3 were summarized using descriptive statistics for patients who continued treatment at week 52. Cumulative probability plots show the observed cumulative distribution of these scores, ranked from lowest to highest, against the actual value according to study period (weeks 0–52 and weeks 52–100).
AEs were summarized by actual treatment received for patients who received ≥1 study agent administration. To account for the shorter placebo‐controlled period compared to active treatment, incidences of AEs, SAEs, AEs leading to discontinuation, infections, and serious infections are also reported as the number of events per 100 patient‐years of follow‐up with 95% confidence intervals (95% CIs).
RESULTS
Patient disposition and characteristics
A total of 739 patients were randomized and treated (guselkumab 100 mg every 4 weeks [n = 245], guselkumab 100 mg every 8 weeks [n = 248], or placebo [n = 246]). Baseline demographic and disease characteristics were generally well balanced among treatment groups, and disease activity measures were consistent with active PsA (16); 60% of patients were receiving concomitant methotrexate (MTX) at baseline.
Patient dispositions through week 24 (16) and week 52 (17) have also been reported. The robust patient retention seen through 1 year (93%) was durable through week 100, when nearly 90% of randomized and treated patients completed study treatment (89% of the every‐4‐weeks group; 90% of the every‐8‐weeks group; 85% of the placebo–guselkumab crossover group) (Supplementary Figure 1, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.42010). Among 687 patients who received ≥1 guselkumab administration at or after week 52, the most common reason for discontinuation was an AE (1.3% [3 of 227 patients], 2.2% [5 of 232 patients], and 3.1% [7 of 228 patients], respectively, in each of the aforementioned groups).
Efficacy
Clinical efficacy through week 24 and week 52 has been previously detailed (16, 17). Briefly, the primary end point was achieved, with 64% of patients in both guselkumab dosing groups achieving an ACR20 response at week 24 versus 33% of patients in the placebo group (16). At week 100, 76% of patients in the guselkumab every‐4‐weeks group and 74% in the every‐8‐weeks group had an ACR20 response, 56% and 55%, respectively, had an ACR50 response, and 35% and 36%, respectively, had an ACR70 response (all NRI accounting for ~12% of patients with missing data) (Figure 1). Trends in ACR response rates over time using observed data were consistent with those determined using NRI (Figure 1). When evaluating the time to ACR20 response, separation from placebo was observed at week 4, with continued increases in response rate through week 24 for patients in the guselkumab groups (Supplementary Figure 2, https://onlinelibrary.wiley.com/doi/10.1002/art.42010). Among ACR components, 45–62% of guselkumab‐treated patients achieved ≥20% improvement in tender and swollen joint counts, physician global assessment score, and CRP level by week 4 (Supplementary Figure 3, https://onlinelibrary.wiley.com/doi/10.1002/art.42010). In the guselkumab groups, the proportions of patients achieving ≥20% improvement were maintained or continued to increase through week 100 for all ACR components. In addition, at a group level, response rates for increasing levels of response (ACR50 and ACR70) increased over time through the second year of treatment. This suggests that individual patients may be improving over time and achieving higher levels of improvement with continued guselkumab treatment.
Figure 1.
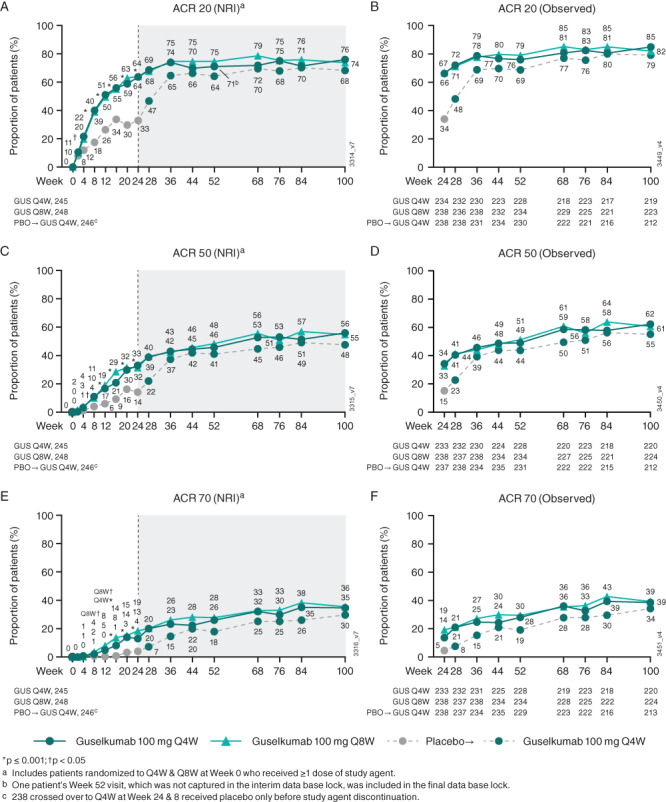
Proportions of patients achieving American College of Rheumatology ≥20% improvement criteria (ACR20) (A and B), ACR50 (C and D), and ACR70 (E and F) responses through week 100. Response rates derived using nonresponder imputation (NRI) for missing data (see Patients and Methods) are shown in panels A, C, and E; response rates from weeks 24–100 derived from observed data are shown in panels B, D, and F. The dashed vertical line at week 24 indicates placebo (PBO) crossover to guselkumab (GUS) administered every 4 weeks (Q4W); gray shading indicates post hoc NRI data. Q8W = every 8 weeks.
Among patients with available radiographs (reading session 3) in the guselkumab every‐4‐weeks and every‐8‐weeks groups, respectively, the observed mean changes in total Sharp/van der Heijde score were 0.48 and 0.68 from week 0 to week 24 (smallest detectable change [SDC] 2.18), 0.57 and 0.31 from week 24 to week 52 (SDC 2.25), and 0.75 and 0.46 from week 52 to week 100 (SDC 2.28) (Table 1). In the placebo crossover group, mean changes in Sharp/van der Heijde scores from week 24 to week 52 (0.34) and from week 52 to week 100 (0.13) indicated that, on average, patients in this group had less radiographic progression after initiating guselkumab compared to the 24‐week placebo‐controlled period (1.12). Low rates of radiographic progression were seen from week 0 to week 100 across both guselkumab dosing regimens (Figure 2). Mean changes in total Sharp/van der Heijde scores indicated less radiographic progression from week 52 to week 100 than from week 0 to week 52 in all 3 groups (Supplementary Figure 4, https://onlinelibrary.wiley.com/doi/10.1002/art.42010).
Table 1.
Extraarticular and HRQoL assessments, composite indices, and radiographic progression through week 100 in the DISCOVER‐2 study*
| Guselkumab Q4W | Guselkumab Q8W | Placebo–guselkumab crossover Q4W | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 24 | Week 52 | Week 100 | Week 24 | Week 52 | Week 100 | Week 24 | Week 52 | Week 100 | |
| No. patients with enthesitis at week 0 | 170 | 170 | 170 | 158 | 158 | 158 | 178 | 178 | 178 |
| Enthesitis resolution, % | 44 | 57 | 62 | 54 | 61 | 70 | 30 | 64 | 65 |
| LSM change (95% CI) | −1.5 (−1.8, −1.3) | −1.8 (−2.0, −1.6) | −1.9 (−2.1, −1.7) | −1.6 (−1.8, −1.4) | −1.9 (−2.1, −1.7) | −2.1 (−2.3, −1.8) | −1.0 (−1.3, −0.8) | −2.0 (−2.2, −1.8) | −2.1 (−2.3, −1.9) |
|
No. patients with dactylitis at week 0 |
121 | 121 | 121 | 111 | 111 | 111 | 99 | 99 | 99 |
| Dactylitis resolution, % | 64 | 74 | 72 | 57 | 78 | 83 | 38 | 74 | 73 |
| LSM change (95% CI) | −5.9 (−6.7, −5.0) | −6.5 (−7.2, −5.8) | −6.5 (−7.1, −5.8) | −6.0 (−6.8, −5.1) | −7.2 (−7.9, −6.5) | −7.5 (−8.1, −6.8) | −4.0 (−5.0, −3.1) | −6.9 (−7.6, −6.2) | −6.9 (−7.6, −6.2) |
| HAQ DI | |||||||||
| No. patients assessed | 245 | 245 | 245 | 248 | 248 | 248 | 246 | 246 | 246 |
| LSM change (95% CI) | −0.40 (−0.46, −0.34) | −0.49 (−0.56, −0.42) | −0.55 (−0.62, −0.48) | −0.37 (−0.43, 0.31) | −0.45 (−0.52, −0.38) | −0.53 (−0.59, −0.46) | −0.13 (−0.19, −0.07) | −0.35 (−0.42, −0.29) | −0.46 (−0.53, −0.40) |
|
No. patients with HAQ DI ≥0.35 at week 0 |
228 | 228 | 228 | 228 | 228 | 228 | 236 | 236 | 236 |
| Improvement ≥0.35, % | 56 | 59 | 63 | 50 | 58 | 64 | 31 | 48 | 56 |
|
No. patients with HAQ DI >0.5 at week 0 |
214 | 214 | 214 | 211 | 211 | 211 | 218 | 218 | 218 |
| HAQ DI score ≤0.5, % | 29 | 36 | 40 | 23 | 28 | 35 | 14 | 31 | 33 |
| SF‐36 | |||||||||
| No. patients assessed | 245 | 245 | 245 | 248 | 248 | 248 | 246 | 246 | 246 |
|
LSM change in PCS (95% CI) |
7.0 (6.1, 7.9) | 8.6 (7.6, 9.6) | 10.0 (8.9, 11.1) | 7.4 (6.5, 8.3) | 9.0 (7.9, 10.0) | 10.4 (9.3, 11.5) | 3.4 (2.5, 4.3) | 7.5 (6.5, 8.6) | 9.3 (8.2, 10.4) |
| Improvement ≥5, % | 56 | 61 | 62 | 60 | 63 | 63 | 40 | 59 | 63 |
|
LSM change in MCS (95% CI) |
4.2 (3.1, 5.3) | 4.5 (3.4, 5.5) | 4.9 (3.9, 6.0) | 4.2 (3.1, 5.2) | 4.3 (3.3, 5.4) | 4.2 (3.2, 5.3) | 2.1 (1.1, 3.2) | 4.0 (3.0, 5.1) | 3.9 (2.8, 4.9) |
| Improvement ≥5, % | 34 | 36 | 39 | 38 | 42 | 42 | 31 | 39 | 37 |
| Composite indices | |||||||||
| MDA, % | 19 | 34 | 38 | 25 | 31 | 40 | 6 | 30 | 37 |
| VLDA, % | 5 | 11 | 14 | 4 | 16 | 17 | 1 | 7 | 13 |
| Radiographic results† | |||||||||
| No. patients assessed | 221 | 221 | 211 | 228 | 228 | 216 | 215 | 213 | 202 |
|
Changes in Sharp/van der Heijde modified score for PsA, mean ± SD |
0.48 ± 2.70 | 0.57 ± 2.67 | 0.75 ± 4.02 | 0.68 ± 2.36 | 0.31 ± 1.57 | 0.46 ± 2.42 | 1.12 ± 3.80 | 0.34 ± 2.79 | 0.13 ± 3.74 |
Data are summarized by treatment group with application of missing data handling rules, with the exception of radiographic results (see Patients and Methods). Clinical efficacy and health‐related quality of life (HRQoL) results at week 24 (ref. 16) and week 52 (ref. 17) were previously published and are included here for reference. Q4W = every 4 weeks; Q8W = every 8 weeks; LSM = least squares mean; 95% CI = 95% confidence interval; HAQ DI = Health Assessment Questionnaire disability index; SF‐36 = Short Form 36; PCS = physical component summary; MCS = mental component summary; PsA = psoriatic arthritis; MDA = minimal disease activity; VLDA = very low disease activity.
Corresponding study periods for radiographic results are weeks 0–24, weeks 24–52, and weeks 52–100.
Figure 2.
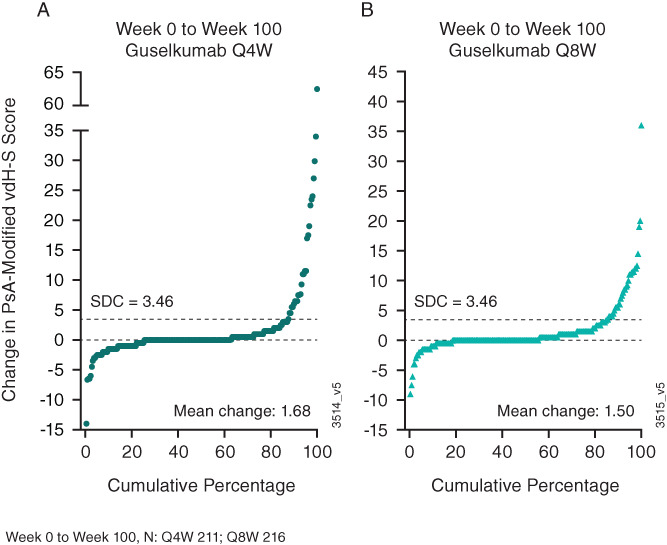
Cumulative probability plot of observed changes in Sharp/van der Heijde modified scores for psoriatic arthritis (PsA‐modified vdH‐S), from baseline to week 100, in patients randomized to receive guselkumab every 4 weeks (Q4W) (A) or every 8 weeks (Q8W) (B). SDC = smallest detectable change.
In prespecified pooled analyses of the DISCOVER‐1 and DISCOVER‐2 trials, guselkumab‐treated patients had greater improvements in enthesitis and dactylitis scores and higher rates of resolution at week 24 compared to placebo‐treated patients (16). In the DISCOVER‐2 study, among patients affected at baseline, 62% in the every‐4‐weeks group and 70% in the every‐8‐weeks group achieved complete resolution of enthesitis and 72% and 83%, respectively, achieved complete resolution of dactylitis by week 100 (Table 1). Additionally, LSM changes from baseline in enthesitis and dactylitis scores at week 100 in the every‐4‐weeks group (−1.9 and −6.5, respectively) and in the every‐8‐weeks group (−2.1 and −7.5, respectively) were consistent with those reported at week 52 (17).
Improvements in physical function and HRQoL at week 24 were also significantly greater in the 2 guselkumab groups compared to placebo (16). At week 100, LSM changes from baseline in HAQ DI in the every‐4‐weeks (−0.55) and every‐8‐weeks (−0.53) groups were consistent with those at week 52, and 63–64% of patients had a clinically meaningful improvement in HAQ DI (≥0.35). Additionally, 35–40% of patients in the guselkumab groups achieved normalized physical function (HAQ DI ≤0.5) by week 100. In the guselkumab groups, improvements in HRQoL by week 100 were consistent with those observed at week 52, with LSM changes in SF‐36 PCS and MCS scores ranging 10.0–10.4 and 4.2–4.9, respectively (Table 1).
At week 24, guselkumab‐randomized patients had higher response rates for skin assessments (IGA and PASI) compared to placebo (16). Among patients receiving guselkumab from week 0, 62% of those in the every‐4‐weeks group and 55% in the every‐8‐weeks group had an IGA score of 0 at week 100, and 76% and 72% had an IGA 0/1 response, respectively (Figure 3). In addition, 82–83% of patients in the guselkumab groups achieved PASI75 at week 100, 70–74% achieved PASI90, and 53–59% achieved PASI100 (Figure 3).
Figure 3.
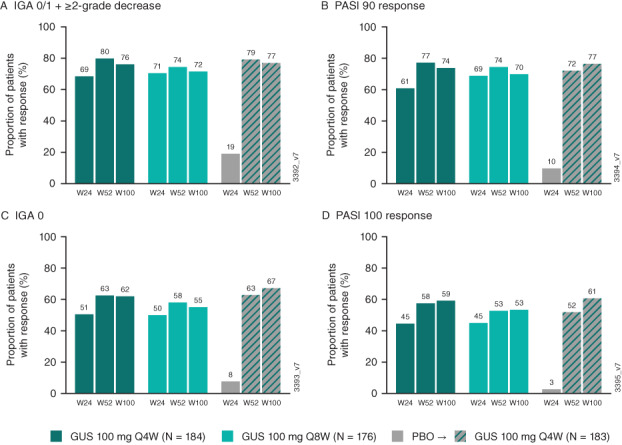
Proportions of patients achieving Investigator’s Global Assessment (IGA) 0/1 response (A), ≥90% improvement in the Psoriasis Area and Severity Index (PASI90) response (B), IGA score 0 (C), and PASI100 response (D) through week 100 (W100). IGA and PASI scores were assessed in patients with ≥3% body surface area with psoriasis involvement and an IGA score of ≥2 at baseline. Response rates were derived using NRI for missing data. IGA response was defined as score of 0/1 and ≥2‐grade improvement. See Figure 1 for other definitions.
Using composite measures of disease activity, 38% of those in the every‐4‐weeks group and 40% in the every‐8‐weeks group achieved MDA at week 100. Additionally, 14% and 17% of patients, respectively, achieved VLDA (Table 1).
For patients in the placebo–guselkumab crossover group, response rates for joint and skin manifestations and resolution of enthesitis and dactylitis, as well as improvements in these scores, at week 100 were similar to those at week 52. Low rates of radiographic progression and improvements in physical function and HRQoL also extended to week 100 in these patients. Also at week 100, 37% of placebo crossover patients achieved MDA and 13% achieved VLDA.
Among patients in the guselkumab groups who achieved an ACR20, ACR50, or ACR70 response at week 52, 91% in the every‐4‐weeks group and 87% in the every‐8‐weeks group maintained an ACR20 response, 83% and 79%, respectively, maintained an ACR50 response, and 72% and 80%, respectively, maintained an ACR70 response at week 100 (Figure 4). Among patients in these 2 groups who achieved the more stringent MDA criteria at week 52, 81% and 83%, respectively, maintained MDA at week 100.
Figure 4.
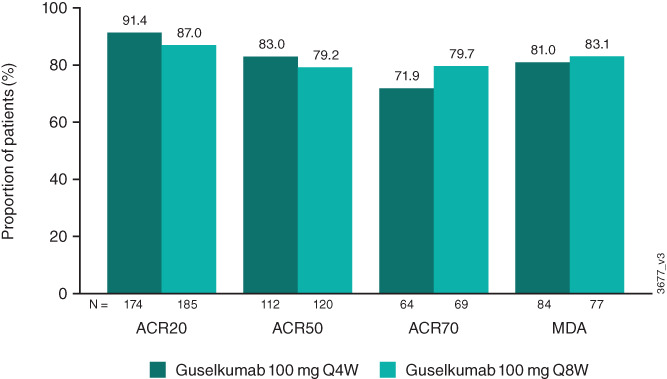
Proportions of patients maintaining ACR20, ACR50, or ACR70 responses or minimal disease activity (MDA) at week 100 among those who achieved these responses at week 52. Response rates were derived using NRI for missing data. See Figure 1 for other definitions.
AEs
Detailed safety results through week 24 and week 52 have been previously reported (16, 17). Through week 112, a total of 731 patients received ≥1 administration of guselkumab, including patients initially randomized to placebo who crossed over to guselkumab at week 24, for a total of 1,392 patient‐years of follow‐up.
Through week 112, as with earlier time points, infections were the most common type of AE reported in guselkumab‐treated patients (Table 2). The most common infections were upper respiratory tract infection (8.5%) and nasopharyngitis (7.5%). Among all guselkumab‐treated patients, 21 patients (every‐4‐weeks group [n = 5; 2%]; every‐8‐weeks group [n = 8; 3%]; placebo–guselkumab crossover group [n = 8; 3%]) reported a serious infection through week 112. Of these, 6 reported pneumonia (every‐4‐weeks group [n = 2]; every‐8‐weeks group [n = 3]; placebo crossover group [n = 1]), and 2 had diverticulitis (every‐4‐weeks group [n = 1, with perforation]; every‐8‐weeks group [n = 1]). Other serious infections that occurred included acute hepatitis B and oophoritis (in the every‐4‐weeks group); appendicitis, herpes zoster, cystitis, 1 patient with bacterial vaginosis and trichomoniasis, and 1 patient with pyrexia and urinary tract infection (in the every‐8‐weeks group); and acute hepatitis C, bacterial meningitis, costochondritis, dengue fever, infective periostitis, influenza, pericarditis, and tracheitis (in the placebo crossover group). The number of infections per 100 patient‐years was 37.3 among guselkumab‐treated patients compared to 50.5 among placebo‐treated patients; the respective numbers of serious infections were 1.9 and 0.9 per 100 patient‐years (Table 2).
Table 2.
AEs through week 112 of the DISCOVER‐2 study*
| Placebo (weeks 0–24) (n = 246) | Placebo–guselkumab Q4W crossover(weeks 24–112)(n = 238) | Guselkumab Q4W (weeks 0–112) (n = 245) | Guselkumab Q8W (weeks 0–112) (n = 248) | All guselkumab (n = 731)† | |
|---|---|---|---|---|---|
|
Duration of follow‐up, weeks |
24.4 | 84.2 | 106.4 | 107.1 | 99.4 |
|
Patient‐years of follow‐up |
115 | 384 | 499 | 509 | 1,392 |
| AEs | |||||
| No. patient‐years | 85 | 240 | 225 | 224 | 690 |
| Patients | 101 (41) | 126 (53) | 172 (70) | 178 (72) | 476 (65) |
|
No. events per 100 patient‐ years (95% CI) |
188.9 (164.6, 215.8) | 110.7 (100.5, 121.8) | 121.2 (111.7, 131.2) | 158.0 (147.3, 169.3) | 131.7 (125.8, 137.9) |
| Serious AEs | |||||
| No. patient‐years | 113 | 368 | 476 | 487 | 1,330 |
| Patients | 7 (3) | 16 (7) | 22 (9) | 22 (9) | 60 (8) |
|
No. events per 100 patient‐ years (95% CI) |
6.1 (2.5, 12.6) | 6.0 (3.8, 9.0) | 5.2 (3.4, 7.6) | 6.1 (4.1, 8.7) | 5.8 (4.6, 7.2) |
|
AEs leading to study discontinuation |
|||||
| No. patient‐years | 114 | 381 | 496 | 507 | 1,383 |
| Patients | 4 (2) | 10 (4) | 13 (5) | 8 (3) | 31 (4) |
|
No. events per 100 patient‐ years (95% CI) |
3.5 (1.0, 8.9) | 2.9 (1.4, 5.1) | 3.2 (1.8, 5.2) | 1.6 (0.7, 3.1) | 2.5 (1.8, 3.5) |
| Infections | |||||
| No. patient‐years | 104 | 315 | 378 | 381 | 1,075 |
| Patients | 45 (18) | 61 (26) | 82 (34) | 94 (38) | 237 (32) |
|
No. events per 100 patient years (95% CI) |
50.5 (38.3, 65.3) | 34.9 (29.3, 41.4) | 35.8 (30.8, 41.5) | 40.5 (35.1, 46.4) | 37.3 (34.1, 40.6) |
| Serious infections | |||||
| No. patient‐years | 115 | 378 | 496 | 504 | 1,378 |
| Patients | 1 (0.4) | 8 (3) | 5 (2) | 8 (3) | 21 (3) |
|
No. events per 100 patient‐ years (95% CI) |
0.9 (0.02, 4.9) | 2.6 (1.3, 4.8) | 1.0 (0.3, 2.3) | 2.2 (1.1, 3.9) | 1.9 (1.2, 2.7) |
Except where indicated otherwise, values are the number (%) of patients. AEs = adverse events; 95% CI = 95% confidence interval; Q4W = every 4 weeks; Q8W = every 8 weeks.
Includes all patients who received ≥1 administration of guselkumab, including patients who crossed over from placebo at week 24.
One death occurred during the study (road traffic accident in the placebo crossover group post–week 52). Two malignancies occurred, both before week 24 (melanoma in situ in the every‐8‐weeks group and renal clear cell cancer in the placebo group) (16). Three patients experienced MACE (all nonfatal), including 2 patients in the every‐4‐weeks group who had an ischemic stroke: 1 had a history of hypertension, hyperlipidemia, and diabetes (16), and the second had a history of hypertension, stroke, and smoking. The third patient (also in the every‐4‐weeks group), who had a history of smoking, hypertension, and hyperlipidemia, experienced a myocardial infarction. Opportunistic infections occurred in 3 guselkumab‐treated patients (all post–week 52): fungal esophagitis (concomitant MTX, longstanding history of gastroesophageal reflux disease and recent course of antibiotics) and herpes zoster disseminated (no concomitant DMARDs, history of diabetes mellitus, and no zoster vaccination) in the every‐8‐weeks group, and meningitis listeria (concomitant MTX) in the placebo–guselkumab crossover group. No patients developed active tuberculosis. One patient in the every‐8‐weeks group reported unilateral iridocyclitis, which resolved following steroid and NSAID treatment. While no case of inflammatory bowel disease occurred in guselkumab‐treated patients, 1 was suspected in a patient receiving placebo (16).
Four patients (1 receiving placebo and 3 receiving guselkumab) experienced suicidal ideation; 3 of these events occurred prior to week 52 (16, 17). All 4 events were classified as level 1; no events of suicidal behavior or self‐injurious behavior without suicidal intent were reported.
Among all patients who received ≥1 administration of guselkumab, 20 (2.7%) had an injection site reaction, with no apparent difference between the every‐4‐weeks regimen (12 of 483 patients [2.5%], including patients who crossed over from placebo) and the every‐8‐weeks regimen (8 of 248 patients [3.2%]). Most reactions were considered mild. Two patients discontinued treatment, prior to week 52, due to an injection site reaction (moderate injection site erythema/rash and erythema/swelling/warming) (17). No cases of anaphylaxis or serum sickness were reported.
A total of 727 patients received ≥1 administration of guselkumab and had available serum samples through week 112. Fifty‐three guselkumab‐treated patients (7.3%) tested positive for antibodies to guselkumab; of these, 3 (5.7%) were positive for neutralizing antibodies. Among 22 guselkumab‐randomized patients who tested positive for antibodies through week 100 and had ACR evaluations at week 100, 18 (81.8%) achieved ACR20 response and 12 (54.5%) achieved ACR50 response. Median steady‐state trough guselkumab concentrations were maintained from week 52 to week 100, with both the every‐4‐weeks regimen (4.53 to 3.86 μg/ml) and every‐8‐weeks regimen (1.15 to 0.97 μg/ml).
Through week 112, grade 2 and grade 3 decreased neutrophil counts occurred in ~4% and 0.7%, respectively, of all guselkumab‐treated patients, with no apparent differences between the 2 dosing regimens (Supplementary Table 1, https://onlinelibrary.wiley.com/doi/10.1002/art.42010). One patient (in the every‐4‐weeks group) had a grade 4 decreased neutrophil count (17). Generally, these decreased levels were transient and resolved spontaneously without discontinuation of study treatment. One infection (mild nasopharyngitis) was associated with a grade 2 decreased neutrophil count (17).
Among 725 patients who received guselkumab and had postbaseline samples available, grade 2 or 3 increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were seen in ~5% of guselkumab‐treated patients through week 112 (Supplementary Table 1, https://onlinelibrary.wiley.com/doi/10.1002/art.42010); no grade 4 ALT or AST elevation occurred. Grade 2 ALT elevations were reported in 7.0% of those in the every‐4‐weeks group and 2.4% in the every‐8‐weeks group; grade 3 ALT elevations were reported in 2.1% and 1.6% of these patients, respectively. Grade 2 increased AST levels occurred in 4.5% and 3.6%, of patients in these 2 groups, respectively, and grade 3 AST elevations occurred in 3.3% and 1.2% of patients. Most increased ALT and AST levels were transient and resolved without discontinuation of guselkumab, with few exceptions: 3 patients in the every‐4‐weeks group (1 each with acute hepatitis B, isoniazid‐induced liver injury, and hepatic steatosis [this patient had a history of chronic liver disease]) discontinued the study, and another in the every‐4‐weeks group had an extended interruption in treatment primarily due to investigator concerns of alcohol use, hepatic steatosis, and chronic cholecystitis with persistently elevated transaminase levels (16, 17), and discontinued due to investigator decision.
Three patients in the placebo–guselkumab crossover group discontinued guselkumab after week 52 (1 each with nonalcoholic fatty liver disease, acute hepatitis C, and grade 2 ALT/grade 3 AST elevations [AST‐dominant in patient reporting alcohol use]) (16, 17). Through week 112, ALT and AST elevations occurred in 48% and 34% of patients receiving concomitant MTX, respectively, and in 40% and 31% of patients without concomitant MTX. Increased bilirubin levels in guselkumab‐treated patients were limited to grade 1 (6.3%) and grade 2 (1.8%) elevations (Supplementary Table 1, https://onlinelibrary.wiley.com/doi/10.1002/art.42010), which was consistent with results through week 52 (17). No elevation met the criteria for Hy’s law (total bilirubin >2 × upper limit of normal [ULN] and either ALT or AST ≥3 × ULN).
DISCUSSION
Results through 2 years of the phase III DISCOVER‐2 study demonstrated robust and sustained joint and skin response rates among biologic‐naive patients with active PsA receiving guselkumab 100 mg every 4 weeks or every 8 weeks. At week 24, ACR, IGA, and PASI response rates and the proportions of patients achieving resolution of enthesitis and dactylitis and meaningful improvements in physical function and HRQoL were significantly greater in patients receiving guselkumab at either frequency compared to placebo. These response rates were sustained through week 52 (17) and through week 100 and were generally similar between the 2 dosing regimens. At week 24, patients in the every‐4‐weeks guselkumab group had significantly less radiographic progression compared to the placebo group (16). After week 24, when all patients were receiving guselkumab, further radiographic progression was limited across the 3 treatment groups through week 100.
The proportion of guselkumab‐treated patients achieving MDA increased over time, with ~40% of patients meeting this treatment target at week 100, and ~80% of guselkumab‐randomized patients who achieved MDA at week 52 maintained low levels of disease activity across disease domains at week 100. In an open‐label study, a treat‐to‐target approach utilizing the MDA criteria was associated with greater improvements across joint and skin assessments, as well as patient‐reported outcomes compared to a standard care approach (29). Because it assesses multiple disease domains, the MDA criteria can be used in all PsA patients regardless of their disease pattern, and the MDA criteria have been recommended by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis/Outcome Measures in Rheumatology Group to assess treatment target goals (30). With the growing number of treatment options for PsA, achieving and maintaining low disease activity in several disease domains should be an attainable goal for many patients.
Response rates for achieving PASI100 (53–59%) and achieving an IGA score of 0 (55–62%) at week 100 were similar to those observed at week 52. Guselkumab has consistently demonstrated a high level of efficacy in treating psoriatic skin lesions both in patients with PsA and those with plaque psoriasis. The PASI and IGA response rates observed in the DISCOVER‐2 trial are consistent with those seen in phase III trials of psoriasis patients (31, 32, 33).
The IL‐23/Th17 axis is thought to play a central role in the pathogenesis of both psoriasis and PsA (10). By specifically inhibiting IL‐23 upstream in this pathway, guselkumab has demonstrated efficacy in a broad range of skin and articular symptoms, including enthesitis and dactylitis. Based on findings reported through 2 years of the DISCOVER‐2 trial, this mechanism of action appears to provide durable improvements across disease domains, an important feature for a therapy in the heterogeneous patient population with frequently treatment‐resistant disease (1, 2). In a pharmacodynamic analysis of patients from the DISCOVER‐1 and 2 trials, guselkumab treatment was associated with marked decreases in acute phase proteins and IL‐23/Th17 effector cytokines through week 24 (34). Although these decreases did not directly correlate with clinical response, this biomarker analysis was limited to the placebo‐controlled period, and longer‐term pharmacodynamic evaluations may provide additional insight into maintenance of response to guselkumab. In a separate analysis of whole‐blood transcriptome profiling through week 24 in a subgroup of patients from the DISCOVER‐1 and 2 studies, the majority of the disease‐associated genes evaluated were modulated by guselkumab treatment, resulting in a transcriptome profile closer to that of healthy controls, with little change in the placebo group. Greater changes in the level of expression of disease‐associated genes were observed in ACR20 responders than in nonresponders in both guselkumab groups (35).
In general, AEs in DISCOVER‐2 patients were consistent with those reported in DISCOVER‐1 (1‐year study) and in the 5‐year VOYAGE 1 and 2 studies in psoriasis patients (18, 31, 32, 36). Safety results reported in the present study, through 2 years in the DISCOVER‐2 trial, represent the most comprehensive results for an IL‐23p19 subunit inhibitor in PsA patients, who often receive concomitant therapy with MTX and oral glucocorticoids, in contrast with psoriasis patients. The majority of ALT and AST elevations were generally mild and transient, and patients receiving MTX had numerically higher rates of ALT and AST elevations than patients not receiving MTX. Among all patients, there were no cases of active tuberculosis, and in guselkumab‐treated patients, there were no cases of inflammatory bowel disease.
Among treated patients in the DISCOVER‐2 study, 89% randomized to receive guselkumab every 4 weeks and 90% randomized to receive guselkumab every 8 weeks completed treatment through 2 years; few guselkumab‐treated patients discontinued due to inadequate efficacy. Given the chronic and progressive nature of PsA, maintaining long‐term treatment persistence is critical to controlling disease activity and inhibiting radiographic progression to achieve optimal response. Achieving low disease activity at 1 year has been shown to be predictive of long‐term treatment persistence, which, in turn, is predictive of achieving long‐term remission (5).
Limitations of the DISCOVER‐2 study include enrollment restricted to biologic‐naive patients, thus potentially limiting the generalizability of the results. However, findings through 1 year were consistent with those in the DISCOVER‐1 study, in which 31% of patients had previously received ≥1 tumor necrosis factor inhibitor (18). While no unexpected safety signals were identified through 2 years, this study was not powered to detect rare events. Patient retention was high through 2 years (88% completed study treatment) resulting in a relatively small number of patients with missing data. Furthermore, it should be noted that clinical efficacy analyses were conducted using a rigorous NRI approach for patients with missing data after week 24 to account for any effects of discontinuations over time.
Taken together, findings from the DISCOVER‐2 trial demonstrate the robust and sustained efficacy of guselkumab in improving the signs and symptoms of PsA, including enthesitis and dactylitis, inhibiting radiographic progression, and decreasing the effects of PsA on physical function and HRQoL, with a safety profile through 2 years that is consistent with the known safety profile of guselkumab.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. McInnes had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Hsia, Kollmeier, Xu.
Acquisition of data
Hsia, Kollmeier, Xu.
Analysis and interpretation of data
McInnes, Rahman, Gottlieb, Hsia, Kollmeier, Xu, Jiang, Sheng, Shawi, Chakravarty, van der Heijde, Mease.
ROLE OF THE STUDY SPONSOR
Janssen Research & Development, LLC facilitated the study design, provided writing assistance for the manuscript, and reviewed and approved the manuscript prior to submission. The authors independently collected the data, interpreted the results, and had the final decision to submit the manuscript for publication. Writing assistance was provided by Janssen Scientific Affairs, LLC. Publication of this article was not contingent upon approval by Janssen Research & Development, LLC.
Supporting information
Disclosureform
Supplemental Figure 1 Patient disposition through Week 100 of the DISCOVER‐2 study. PsA = psoriatic arthritis; Q4W = every 4 weeks; Q8W = every 8 weeks
Supplemental Figure 2. Median time to achieving ACR20 response in DISCOVER‐2. The intersection of the horizontal and vertical dashed lines denotes the time at which 50% of patients in the treatment group achieved an ACR20 response. ACR20 = ≥20% improvement in American College of Rheumatology criteria; GUS = guselkumab; PBO = placebo; Q4W = every 4 weeks; Q8W = every 8 weeks
Supplemental Figure 3. The proportions of patients achieving an ACR20 response (A) and achieving at least 20% improvement in the individual components of HAQ‐DI (B), SJC (C), TJC (D), PhGA (E), PGA (F), Pain VAS (G), and CRP (H) through Week 100 in DISCOVER‐2. Response rates derived employing nonresponder imputation for missing data (described in Patients and Methods). ACR20 = ≥20% improvement in American College of Rheumatology criteria; CRP = C‐reactive protein; HAQ‐DI = Health Assessment Questionnaire‐Disability Index; PGA = Patient’s Global Assessment; PhGA = Physician’s Global Assessment; SJC = swollen joint count; TJC = tender joint count; VAS = visual analog scale
Supplemental Figure 4. Cumulative probability plot of observed changes from Week 0 to Week 52 (A‐C) and from Week 52 to Week 100 (D‐F) in PsA‐modified vdH‐S scores. PsA‐modified vdH‐S = van der Heijde‐Sharp score modified for patients with psoriatic arthritis; Q4W/Q8W = every 4 weeks/every 8 weeks; SDC = smallest detectable change
Supplemental Table 1 Patients with post‐baseline laboratory values by maximum NCI‐CTCAE Grade through Week 112 in DISCOVER‐2.
ACKNOWLEDGMENTS
The authors thank Rebecca Clemente, PhD and Mona Patel, PharmD of Janssen Scientific Affairs, for writing support, and Diane Harrison, MD, MPH, a consultant funded by Janssen Research & Development, LLC, for substantive review.
A video abstract of this article can be found at https://players.brightcove.net/3806881048001/default_default/index.html?videoId=6295462884001
Supported by Janssen Research & Development.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.42010&file=art42010‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta‐Felquer ML, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 2. Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mateo Soria L, Prior‐Espanol A, Grigorov MM, Holgado‐Perez S, Aparicio‐Espinar M, Martinez‐Morillo M, et al. Long‐term survival of biological therapy in psoriatic arthritis: 18‐year analysis of a cohort in a tertiary hospital. Rheumatol Int 2021. doi: 10.1007/s00296-021-04928-x. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4. Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum 2017;47:29–37. [DOI] [PubMed] [Google Scholar]
- 5. Murray K, Turk M, Alammari Y, Young F, Gallagher P, Saber T, et al. Long‐term remission and biologic persistence rates: 12‐year real‐world data. Arthritis Res Ther 2021;23:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrold LR, Stolshek BS, Rebello S, Collier DH, Mutebi A, Wade SW, et al. Impact of prior biologic use on persistence of treatment in patients with psoriatic arthritis enrolled in the US Corrona registry. Clin Rheumatol 2017;36:895–901. [DOI] [PubMed] [Google Scholar]
- 7. Mease PJ, Accortt NA, Rebello S, Etzel CJ, Harrison RW, Aras GA, et al. Persistence of tumor necrosis factor inhibitor or conventional synthetic disease‐modifying antirheumatic drug monotherapy or combination therapy in psoriatic arthritis in a real‐world setting. Rheumatol Int 2019;39:1547–58. [DOI] [PubMed] [Google Scholar]
- 8. Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol 2009;129:2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Lagowski JP, Gao S, Raymond JH, White CR, Kulesz‐Martin MF. Regulation of the psoriatic chemokine CCL20 by E3 ligases Trim32 and Piasy in keratinocytes. J Invest Dermatol 2010;130:1384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blauvelt A, Chiricozzi A. The immunologic role of IL‐17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 2018;55:379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med 2004;199:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yawalkar N, Tscharner GG, Hunger RE, Hassan AS. Increased expression of IL‐12p70 and IL‐23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J Dermatol Sci 2009;54:99–105. [DOI] [PubMed] [Google Scholar]
- 13. Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. [DOI] [PubMed] [Google Scholar]
- 14. Tremfya [package insert]. Horsham (PA): Janssen Biotech; 2020.
- 15. Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic‐naive or had previously received TNFα inhibitor treatment (DISCOVER‐1): a double‐blind, randomised, placebo‐controlled phase 3 trial. Lancet 2020;395:1115–25. [DOI] [PubMed] [Google Scholar]
- 16. Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, et al. Guselkumab in biologic‐naive patients with active psoriatic arthritis (DISCOVER‐2): a double‐blind, randomised, placebo‐controlled phase 3 trial. Lancet 2020;395:1126–36. [DOI] [PubMed] [Google Scholar]
- 17. McInnes IB, Rahman P, Gottlieb AB, Hsia EC, Kollmeier AP, Chakravarty SD, et al. Efficacy and safety of guselkumab, an interleukin‐23p19–specific monoclonal antibody, through one year in biologic‐naive patients with psoriatic arthritis. Arthritis Rheumatol 2021;73:604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ritchlin CT, Helliwell PS, Boehncke WH, Soriano ER, Hsia EC, Kollmeier AP, et al. Guselkumab, an inhibitor of the IL‐23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic‐naive or TNFα inhibitor‐experienced. RMD Open 2021;7. 10.1136/rmdopen-2020-001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 20. Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227–36. [DOI] [PubMed] [Google Scholar]
- 21. Van der Heijde D, Sharp J, Wassenberg S, Gladman DD. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64 Suppl:ii61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langley RG, Feldman SR, Nyirady J, van de Kerkhof P, Papavassilis C. The 5‐point Investigator's Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat 2015;26:23–31. [DOI] [PubMed] [Google Scholar]
- 23. Fredriksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica 1978;157:238–44. [DOI] [PubMed] [Google Scholar]
- 24. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 25. Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 26. Lubeck DP. Patient‐reported outcomes and their role in the assessment of rheumatoid arthritis. Pharmacoeconomics 2004;22:27–38. [DOI] [PubMed] [Google Scholar]
- 27. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 28. Coates LC, Helliwell PS. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol 2016;43:371–5. [DOI] [PubMed] [Google Scholar]
- 29. Coates LC, Moverley AR, McParland L, Brown S, Navarro‐Coy N, O'Dwyer JL, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open‐label, randomised controlled trial. Lancet 2015;386:2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coates LC, FitzGerald O, Merola JF, Smolen J, van Mens LJ, Bertheussen H, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis/Outcome Measures in Rheumatology consensus‐based recommendations and research agenda for use of composite measures and treatment targets in psoriatic arthritis. Arthritis Rheumatol 2018;70:345–55. [DOI] [PubMed] [Google Scholar]
- 31. Griffiths CE, Papp KA, Song M, Miller M, You Y, Shen YK, et al. Continuous treatment with guselkumab maintains clinical responses through 4 years in patients with moderate‐to‐severe psoriasis: results from VOYAGE 1. J Dermatolog Treat 2020. doi: 10.1080/09546634.2020.1782817. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32. Reich K, Armstrong AW, Foley P, Song M, Miller M, Shen YK, et al. Maintenance of response through up to 4 years of continuous guselkumab treatment of psoriasis in the VOYAGE 2 phase 3 study. Am J Clin Dermatol 2020;21:881–90. [DOI] [PubMed] [Google Scholar]
- 33. Reich K, Armstrong AW, Langley RG, Flavin S, Randazzo B, Li S, et al. Guselkumab versus secukinumab for the treatment of moderate‐to‐severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet 2019;394:831–9. [DOI] [PubMed] [Google Scholar]
- 34. Sweet K, Song Q, Loza MJ, McInnes IB, Ma K, Leander K, et al. Guselkumab induces robust reduction in acute phase proteins and type 17 effector cytokines in active psoriatic arthritis: results from phase 3 trials. RMD Open 2021;7:e001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siebert S, Sweet K, Ritchlin CT, Hsia EC, Kollmeier A, Xu XL, et al. Guselkumab treatment modulates core psoriatic arthritis gene expression in two phase 3 clinical trials (DISCOVER‐1 and ‐2) Ann Rheum Dis 2021;80:312.33272960 [Google Scholar]
- 36. Blauvelt A, Tsai TF, Langley RG, Miller M, Shen YK, You Y, et al. Consistent safety profile with up to 5 years of continuous treatment with guselkumab: pooled analyses from the phase 3 VOYAGE 1 and VOYAGE 2 trials of patients with moderate‐to‐severe psoriasis. J Am Acad Dermatol 2021. doi: 10.1016/j.jaad.2021.11.004. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosureform
Supplemental Figure 1 Patient disposition through Week 100 of the DISCOVER‐2 study. PsA = psoriatic arthritis; Q4W = every 4 weeks; Q8W = every 8 weeks
Supplemental Figure 2. Median time to achieving ACR20 response in DISCOVER‐2. The intersection of the horizontal and vertical dashed lines denotes the time at which 50% of patients in the treatment group achieved an ACR20 response. ACR20 = ≥20% improvement in American College of Rheumatology criteria; GUS = guselkumab; PBO = placebo; Q4W = every 4 weeks; Q8W = every 8 weeks
Supplemental Figure 3. The proportions of patients achieving an ACR20 response (A) and achieving at least 20% improvement in the individual components of HAQ‐DI (B), SJC (C), TJC (D), PhGA (E), PGA (F), Pain VAS (G), and CRP (H) through Week 100 in DISCOVER‐2. Response rates derived employing nonresponder imputation for missing data (described in Patients and Methods). ACR20 = ≥20% improvement in American College of Rheumatology criteria; CRP = C‐reactive protein; HAQ‐DI = Health Assessment Questionnaire‐Disability Index; PGA = Patient’s Global Assessment; PhGA = Physician’s Global Assessment; SJC = swollen joint count; TJC = tender joint count; VAS = visual analog scale
Supplemental Figure 4. Cumulative probability plot of observed changes from Week 0 to Week 52 (A‐C) and from Week 52 to Week 100 (D‐F) in PsA‐modified vdH‐S scores. PsA‐modified vdH‐S = van der Heijde‐Sharp score modified for patients with psoriatic arthritis; Q4W/Q8W = every 4 weeks/every 8 weeks; SDC = smallest detectable change
Supplemental Table 1 Patients with post‐baseline laboratory values by maximum NCI‐CTCAE Grade through Week 112 in DISCOVER‐2.


