Abstract
Objectives
Women are underrepresented in most HIV clinical trials in Western countries, but their participation remains crucial as the lack of information on sex‐ and gender‐specific effects may hinder the safety and efficacy of antiretroviral treatments. The aim of this study was to identify barriers to and facilitators of women's participation in HIV clinical trials in Switzerland.
Methods
We conducted semi‐structured interviews among 20 women with HIV to explore factors associated with non‐participation in clinical trials. The interviewer presented to participants a clinical trial's description and discussed it with them. Lexicometric analysis on transcribed interviews identified three themes and eight sub‐themes related to the pros and cons of participation in HIV clinical trials.
Results
Participants evoked mainly decision‐making drivers, concerns for women living with HIV and treatment side‐effects. They highlighted the need for extensive information provided by trusted healthcare professionals on the research process as central to the decision to enrol in HIV clinical trials. Familial responsibilities were clearly identified as barriers to their participation, but not pregnancy. Additional preoccupations were other health concerns and comorbidities and the consequences of stopping ongoing antiretroviral treatments.
Conclusions
To overcome the barriers to the participation of women living with HIV in clinical research in Western countries, healthcare professionals and researchers should increase women's research literacy by involving them in the study design and by tailoring clinical trials to their social roles and health concerns. Trust in professionals is a facilitator of enrolment of women living with HIV that should be maintained.
Keywords: gender perspective, HIV clinical trial, HIV women, people living with HIV, qualitative research
INTRODUCTION
Most HIV clinical trials in Western countries lack adequate participation of women, despite the fact that they represent one‐half of all people living with HIV worldwide [1, 2]. This situation is potentially worrisome as the safety and efficacy of treatments may vary according to biological and social factors associated with sex and gender (e.g. sex hormones or metabolism, access to healthcare institutions) [3, 4]. Past research with a focus on barriers to and facilitators of participation of people living with HIV in HIV research emphasized the underlying role of positive and trusting relationships with healthcare professionals, altruistic attitudes, and expectations of personal benefits (e.g. reduction of treatments, hope of being cured) in facilitating their participation in clinical trials [5, 6]. By contrast, negative attitudes towards research and perceived or actual health risks, as well as socioeconomic and cultural contexts, were described as barriers to participation [7, 8, 9, 10].
The main explanation given by researchers for the underrepresentation of women in HIV clinical trials is possible pregnancy and the potential risks to the foetus with a change or interruption of treatment [5]. However, beyond this argument, few studies have formally investigated the barriers and facilitators related to the enrolment of women living with HIV in such trials [6]. To our knowledge, this is particularly the case in European countries, with little consideration given to the women's own discourse. The aim of this study was to explore the knowledge gap related to women's underrepresentation and to approach the issue of participation in clinical trials by women living with HIV from their perspective. More specifically, we investigated the specific perceptions of women living with HIV on HIV research in order to determine the main drivers of participation or not in HIV clinical trials. We also explored the issue of pregnancy as a barrier to participation.
METHODS
Study design
We conducted a qualitative study using in‐depth semi‐structured interviews with women living with HIV under treatment at the HIV Unit of Geneva University Hospitals (Switzerland) between December 2020 and March 2021. Best practices for qualitative research requirements were followed (Supplemental content 1: COREQ checklist in Appendix S2).
Participants and recruitment
Eligible participants were cis‐gender women living with HIV, either included in the Swiss HIV Cohort Study (SHCS) network in Geneva or supported by a local association [Groupe sida (santé) Genève], aged > 18 years, receiving stable antiretroviral therapy (ART) and fluent in French. Members of the research teams of the HIV Unit of Geneva University Hospitals and the Groupe sida (santé) Genève recruited participants, provided information on the study, scheduled appointments and obtained informed consent to participate 72 h before the interview. Of the 40 patients screened and invited to participate to the study, 20 patients accepted to participate. Recruitment stopped when researchers reached data saturation. Interviewers did not have access to patients' names or medical information. Ethical approval was granted by the Ethics Committee of the Canton of Geneva (reference 2020‐02286).
Data collection and analysis
Three researchers (all three women; one PhD and one master’s degree in social psychology, and one master’s degree in health psychology) specialized in carrying out qualitative interviews conducted the interviews, which lasted approximately 90 min, and followed an interview guide developed on the basis of the published literature on barriers to and facilitators of participation in HIV clinical trials. After discussing women's views on HIV and research, the interviewer presented a clinical trial vignette (Supplemental content 2: vignette in Appendix S3) that described a fictitious clinical trial of a therapeutic vaccine. The hypothesis of the trial (e.g. duration, possible side‐effects, potential benefits and consequences on current treatment), as well as inclusion/exclusion criteria and safety aspects of the trial were explained. Participants were asked about their attitude towards their potential participation in the trial and the way in which they would decide to participate (i.e. motivations, barriers, necessary additional information). They were also invited to express in general the pros and cons of their participation in HIV clinical trials, independently of the vignette. Finally, women were asked to express their opinion on the underrepresentation of women in HIV clinical trials in general. Interviewers then asked participants whether pregnancy would be a barrier to their own participation in such trials, or if, from their point of view, they thought that this could be the case for other women living with HIV.
Due to COVID‐19 restrictions, interviews (n = 18) were conducted either face to face in the HIV Unit in Geneva, applying rules of distancing and hygiene recommended by the cantonal medical authorities. To identify barriers to and facilitators of the participation of women living with HIV in HIV clinical trials, a lexicometric analysis was performed by two researchers experienced in the use of IRaMuTeQ software (v.0.7 alpha 2, 2008–2014 Pierre Ratinaud; Toulouse, France), a computer‐assisted qualitative data analysis program specifically dedicated to conducting lexicographic content analysis and allowing the identification of recurring themes and their connections using word or expression co‐occurrences [11]. Following the Reinert method [12], texts were partitioned into elementary contextual units (i.e. sentences), which served as units for the analysis. Two hierarchical descending classifications of words by elementary contextual units were carried out to generate a classification of words in thematic classes, including a tree graph showing the associations between these classes in the texts. Each extracted thematic class was associated with a typical vocabulary and extracts. Once the classification was completed, the researchers identified and labelled the classes according to the typical words and extracts. Analyses of connections between classes were performed using a tree graph and a factor analysis of correspondence. Each researcher first conducted their analysis separately and then all researchers discussed the results together to reach a consensus.
RESULTS
All participants were treated with ART and all had undetectable viral load. Fifteen were HIV‐positive for more than 10 years, median age was 48 years, eight had already participated in HIV research, 14 had children, nine were of African origin, one was from South America and 10 were from Europe (Supplemental content 3: participants' characteristics in Appendix S1).
Lexicographic analysis
Lexicographic analysis identified eight sub‐themes grouped into three main themes: (1) decision‐making drivers; (2) living with HIV; and (3) treatments and side‐effects. Figure 1 presents themes, sub‐themes, typical vocabulary and extracts. Barriers and facilitators of the involvement of women living with HIV in HIV clinical trials derived from the eight sub‐themes are summarized in Table 1.
FIGURE 1.
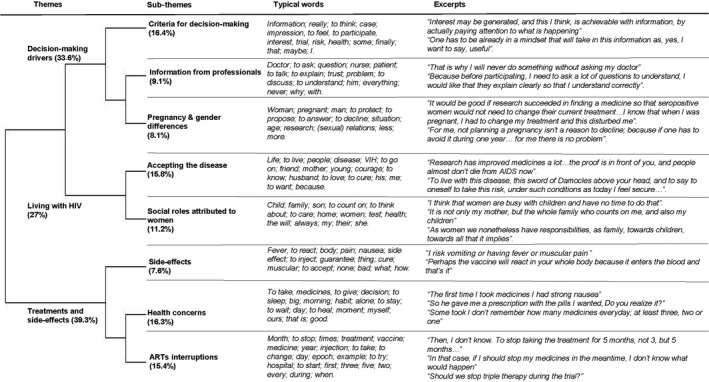
Themes, sub‐themes, typical words and excerpts from the lexicometric analysis. Percentages correspond to the proportion of text included in the analysis
TABLE 1.
Barriers and facilitators derived from the semi‐structured interviews for each of the eight sub‐themes
| Sub‐themes | Barriers | Facilitators |
|---|---|---|
| Criteria to decide about participation |
|
|
| Information from professionals |
|
|
| Pregnancy and gender differences |
|
|
| Accepting the disease |
|
|
| Social roles attributed to women |
|
|
| Side‐effects |
|
|
| Health issues |
|
|
| Antiretroviral treament (ART) |
|
|
Decision‐making drivers
This was composed of three sub‐themes. First, participants established their criteria to decide about participation. These concerned having enough information on HIV clinical trials and having a positive attitude towards the clinical trial proposed. This sub‐theme was the one with the highest amount of classified text, thus indicating that the need for information and the capacity to imagine and understand the trial were significant considerations regarding any potential participation. The second sub‐theme was specifically related to information provided by healthcare professionals who were their reference point to access information. Participants also mentioned the fundamental role of trust both in research and in the professionals to whom they would refer. In a third sub‐theme, participants discussed the issue of pregnancy and trial participation, as well as related gender specificities. Pregnancy as a barrier was rarely spontaneously expressed during interviews. Indeed, participants perceived pregnancy as a potential barrier to HIV clinical trial participation for women in general, without identifying it as a strong barrier for themselves. Some stated that participation was limited in time and that it would be conceivable for a woman to postpone pregnancy plans for this period. Others were confident in the fact that frequent controls during the trial would prevent pregnancy‐related problems. Finally, participants also expressed the view that underrepresentation could simply be due to the fact that women are less likely than men to be proposed for participation in these trials.
Living with HIV
This broad theme included two sub‐themes. The first related to HIV as a terminal illness and socially overwhelming, which they needed to deal with. Participants explained the importance of accepting the disease in order both to move forward despite HIV and to overcome HIV effects. Scientific research was perceived as one of the elements that allowed them to keep going: first, because some participants had personally experienced improvements in their everyday life due to research; and second, because research carries the hope of healing. The second sub‐theme concerned the role of women in society in general and of women living with HIV in particular. Parental responsibilities, as well as the central role participants played in their extended family, were identified as barriers to enrolment in HIV clinical trials. Constraints associated with participation (presented as consuming time and energy) were perceived as conflicting with their family role. In particular, side‐effects were considered a major risk for not being able to assume their familial responsibilities.
Treatments and side‐effects
This was divided into three sub‐themes. The first clearly referred to the side‐effects of HIV treatments, either in general or linked to the vignette. In fact, participants questioned their physical reaction to the vaccine. Whereas they were used to experiencing side‐effects and had a clear view on how to cope with them, side‐effects remained an aspect to consider carefully in the decision‐making process related to participation. The second sub‐theme referred to general health issues and treatment for conditions other than HIV. Several participants complained about the fact that some care providers placed too much emphasis on HIV and neglected other health issues. In addition, they questioned their eligibility to participate in HIV trials due to comorbidities. Finally, the third sub‐theme was related to ART. Participants related the history of their current ART and expressed the burden of remaining such a long‐term treatment, while some women even expressed the need to have pauses during ART. They then linked new long‐acting treatment administered by injection as proposed in the vignette to their current ART in terms of frequency of dosing. Thus, reduction of dosing appeared to be a clear benefit of participation in such a trial. However, despite that benefit, women expressed worry about the potential health consequences of interrupting their current treatment for several months.
DISCUSSION
Underrepresentation of women in clinical trials is a concern in many areas of medicine [13, 14], not only HIV research. Several policies and guidelines have been developed to increase women's participation in clinical research, without resulting in significant documented improvements overtime [13, 15]. This study investigated barriers and facilitators to engagement in HIV clinical trials of women living with HIV in Switzerland through their own discourse.
The women interviewed seemed to be particularly unfamiliar with research on HIV. Even those who had participated in clinical trials in the past had difficulties in explaining the aim of the research they had participated in. In fact, we found no differences in the perceptions of research between women who had already participated in clinical trials and those who had not. This suggests that the research process remains unclear even when explained to participants. Therefore, our results highlight that providing information is not sufficient but must be accompanied by an effort by researchers to make research familiar to participants. Together with the central role of healthcare providers, this need for a clear understanding of research by women living with HIV is a recurrent finding in the literature on research acceptability [8, 16]. To address this need, researchers should increase women's research literacy [17, 18]. As developed in community‐based strategies [19], this can be achieved by soliciting the active involvement of the women living with HIV community to help document specific information needed to make a decision on participation. In line with patient and public involvement approaches, including women living with HIV in pretrial preparation would have the following benefits: (1) increase awareness among women living with HIV of the active role they can play in HIV treatment development; (2) increase understanding of the relevance of trials to patients, thus making it a valued part of self‐care; and (3) allow women to obtain updated information on HIV [2, 20].
Other barriers identified were concrete and anchored in gender stereotypes attributed to women, especially the role of care within the family context and in their preoccupations with health concerns other than HIV. The participation of women living with HIV in HIV clinical trials was presented as potentially in conflict with familial responsibilities and treatment for other conditions. Women feared that trials required time and energy that they would usually devote to their family and, more importantly, that potential strong side‐effects linked to trials could compromise their ability to assume these responsibilities. This is consistent with research suggesting that women downplay considerations regarding their own health because of their ‘primary care roles in their families’ [18]. They feared also that interactions between trial treatments and those they take for other health concerns would compromise their participation in the trial or the efficacy of other treatments, or both. These aspects highlight that recruitment into research studies needs to be more sensitive to the circumstances of participants' everyday life. This could be overcome by ensuring a certain flexibility and by adapting to a woman's personal organization or health needs (e.g. by providing childcare services or transportation arrangements or discussing other treatments) [16, 21]. However, we believe that a more comprehensive strategy of integrating research into the everyday life of all participants would benefit the enrolment of both men and women.
Although prospective pregnancy was cited among the excluding conditions of the fictitious trial vignette, it is striking that among participants of child‐bearing age, none spontaneously expressed prospective pregnancy as a reason for underrepresentation of women in general in HIV clinical trials. This confirms the literature on women's enrolment in HIV research, which reports that pregnancy issues are not among the strongest barriers according to women [1]. When evoked, it was often linked to cultural aspects or social roles attributed to women [22]. This is particularly interesting, as investigators and sponsors in HIV clinical research, as in other fields of medicine [13, 14], frequently avoid recruiting women of childbearing age to protect them and their babies from possible complications [13]. As the involvement of women of childbearing age in research is necessary [13, 22, 23], associating women and proposing research protocols tailored to their specific needs, either in general or during specific life episodes, represent a crucial step towards increasing their participation in HIV trials and should be endorsed by all those planning phase 3 studies in HIV research.
Strengths and limitations
The main strength of this study is that it complements the understanding of barriers to and facilitators of participation in HIV clinical trials by women living with HIV from a European perspective. However, the following limitations must be considered in interpreting the results. First, our study focused on cis‐gender women attending a Swiss HIV consultation. Generalization of our results to all women living with HIV, especially those belonging to minority or vulnerable groups, is limited. Nevertheless, our findings are coherent with studies conducted among the latter groups. Second, the median age of our sample was 48 years, which is close to the median age of the SHCS (51 years), but implies that less than half of the women interviewed were or might be concerned about a potential pregnancy. The fact that the participants did not spontaneously mention pregnancy might be related to this limitation. Although our finding is consistent with some current research, further studies should be conducted to specifically explore the perceptions of women of childbearing age compared with older women regarding pregnancy as a barrier to participation in clinical studies. Third, the trial proposed in our study focused on a vaccine curing HIV. Some participants expressed their concerns about vaccine safety, also in relation to the controversy around COVID‐19 vaccines. Studies presenting clinical trials on other treatments, such as dosing frequency, could be considered in order to confirm the central role of the research literacy theme.
CONCLUSIONS
Women living with HIV are not very familiar with HIV research and they clearly expressed the need for personalized and accurate information about HIV clinical trials, combined with a consideration of the social roles they endorse in everyday life and other health concerns. Pregnancy and anxiety over the baby's health as an obstacle to women's inclusion in research do not appear to be a major barrier. Involving women living with HIV in the study design and tailoring HIV clinical trials according to their social roles and actual health concerns would facilitate their enrolment in HIV clinical trials.
AUTHOR CONTRIBUTIONS
IG, IPB and AC designed the study. CS, SL, LV and CB contributed to the acquisition and transcription of interviews. IG, CS and NC performed the analyses and drafted the manuscript. All authors gave final approval for all aspects of work, ensuring its accuracy.
Supporting information
App S1
App S2
App S3
ACKNOWLEDGEMENTS
The authors thank the participants for their kind participation in the study, and the physicians and nurses of the HIV units for their help with recruitment: Clarisse Gaude, Olivier Nawej, Hélène Buvelot, Vera Portillo, Pierre‐David Kamoun, Chiara Fedeli and Yvan Gosmain. In addition, the authors thank the Groupe santé Genève for its involvement in this study and essential help in recruiting participants. This study received a research grant from Merck Sharp and Dohme AG (MSD). Open Access Funding provided by Universite de Lausanne.
Courvoisier N, Storari C, Lesage S, et al. Facilitators and barriers of women's participation in HIV clinical research in Switzerland: A qualitative study. HIV Med. 2022;23:441–447. doi: 10.1111/hiv.13259
Nelly Courvoisier, Chiara Storari are co‐first authors.
Ingrid Gilles, Alexandra Calmy are co‐last authors.
REFERENCES
- 1. Curno MJ, Rossi S, Hodges‐Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr (1999). 2016;71(2):181‐188. [DOI] [PubMed] [Google Scholar]
- 2. Dubé K, Hosey L, Starr K, et al. Participant perspectives in an HIV cure‐related trial conducted exclusively in women in the United States: results from AIDS clinical trials group 5366. AIDS Res Hum Retroviruses. 2020;36(4):268‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merkatz RB, Temple R, Subel S, Feiden K, Kessler DA. Women in clinical trials of new drugs. A change in food and drug administration policy. The working group on women in clinical trials. N Engl J Med. 1993;329(4):292‐296. [DOI] [PubMed] [Google Scholar]
- 4. Gianella S, Tsibris A, Barr L, Godfrey C. Barriers to a cure for HIV in women. J Int AIDS Soc. 2016;19(1):20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berlin JA, Ellenberg SS. Inclusion of women in clinical trials. BMC Med. 2009;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sullivan KA, Little MO, Rosenberg NE, et al. Women’s views about contraception requirements for biomedical research participation. PLoS One. 2019;14(5):e0216332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Power J, Westle A, Dowsett GW, et al. Perceptions of HIV cure research among people living with HIV in Australia. PLoS One. 2018;13(8):e0202647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubé K, Evans D, Sylla L, et al. Willingness to participate and take risks in HIV cure research: survey results from 400 people living with HIV in the US. J Virus Erad. 2017;3(1):40‐50.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asiki G, Abaasa A, Ruzagira E, et al. Willingness to participate in HIV vaccine efficacy trials among high risk men and women from fishing communities along Lake Victoria in Uganda. Vaccine. 2013;31(44):5055‐5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macphail C, Delany‐Moretlwe S, Mayaud P. ‘It's not about money, it's about my health’: determinants of participation and adherence among women in an HIV‐HSV2 prevention trial in Johannesburg, South Africa. Patient Prefer Adherence. 2012;6:579‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stoneman P, Sturgis P, Allum N. Exploring public discourses about emerging technologies through statistical clustering of open‐ended survey questions. Public Underst Sci. 2013;22(7):850‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reinert M. Alceste une méthodologie d'analyse des données textuelles et une application: Aurelia De Gerard De Nerval. Bull Sociol Methodol. 1990;26(1):24‐54. [Google Scholar]
- 13. Phelan AL, Kunselman AR, Chuang CH, Raja‐Khan NT, Legro RS. Exclusion of women of childbearing potential in clinical trials of type 2 diabetes medications: a review of protocol‐based barriers to enrollment. Diabetes Care. 2016;39(6):1004‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vitale C, Fini M, Spoletini I, Lainscak M, Seferovic P, Rosano GM. Under‐representation of elderly and women in clinical trials. Int J Cardiol. 2017;232:216‐221. [DOI] [PubMed] [Google Scholar]
- 15. Yakerson A. Women in clinical trials: a review of policy development and health equity in the Canadian context. Int J Equity Health. 2019;18(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Webster K, Carter A, Proulx‐Boucher K, et al. Strategies for recruiting women living with human immunodeficiency virus in community‐based research: lessons from Canada. Prog Community Health Partnersh. 2018;12(1):21‐34. [DOI] [PubMed] [Google Scholar]
- 17. Isler MR, Brown AL, Eley N, et al. Curriculum development to increase minority research literacy for HIV prevention research: a CBPR approach. Prog Community Health Partnersh. 2014;8(4):511‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falcon R, Bridge DA, Currier J, et al. Recruitment and retention of diverse populations in antiretroviral clinical trials: practical applications from the gender, race and clinical experience study. J Womens Health (Larchmt). 2011;20(7):1043‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Germino BB, Mishel MH, Alexander GR, et al. Engaging African American breast cancer survivors in an intervention trial: culture, responsiveness and community. J Cancer Surviv. 2011;5(1):82‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greenhalgh T, Hinton L, Finlay T, et al. Frameworks for supporting patient and public involvement in research: systematic review and co‐design pilot. Health Expect. 2019;22(4):785‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mills E, Nixon S, Singh S, Dolma S, Nayyar A, Kapoor S. Enrolling women into HIV preventive vaccine trials: an ethical imperative but a logistical challenge. PLoS Med. 2006;3(3):e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartz DA. Clinical trials and administration of Zika virus vaccine in pregnant women: lessons (that Should Have Been) learned from excluding immunization with the Ebola vaccine during pregnancy and lactation. Vaccines. 2018;6(4):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brogly S, Read JS, Shapiro D, Stek A, Tuomala R. Participation of HIV‐infected pregnant women in research in the United States. AIDS Res Hum Retroviruses. 2007;23(1):51‐53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1
App S2
App S3


