Abstract
Objective
An exploratory analysis from a long‐term, phase 3, open‐label, repeat‐dose safety study of diazepam nasal spray for acute treatment of seizure clusters assessed the use of a second dose up to 24 hours after the initial dose and effectiveness in potentially reducing the number of seizures.
Methods
Seizures and doses were recorded in diaries.
Results
Of 175 patients enrolled, 163 received ≥1 dose of diazepam nasal spray and were included in the safety population; those patients received a total of 4390 doses for a total of 3853 seizure clusters. Less than half of these patients used a second dose a least once during the study (79 patients [48.5%]), with a total of 485 second doses for seizure clusters (12.6% of all seizure clusters). Among these 79 patients, 33 (41.8%) used only one second dose during the study (range: 1–82). The proportion of seizure clusters treated with a second dose over time was consistently low across 24 h: 0–4 h, 152 (3.9%); 4–6 h, 72 (1.9%); 6–8 h, 39 (1.0%); 8–12 h, 55 (1.4%); 12–16 h, 42 (1.1%); 16–20 h, 42 (1.1%); 20–24 h, 83 (2.2%). Rates of treatment‐emergent adverse events (TEAEs) and treatment‐related TEAEs occurring within 1 day of a second dose were low (15.2% and 5.1%, respectively).
Significance
Patients with epilepsy may experience seizure clusters lasting up to 24 hours, and little is known about the effectiveness of rescue therapies for that duration. The current labeling of the US Food and Drug Administration (FDA)–approved outpatient treatments for seizure clusters (rectal diazepam, intranasal midazolam, and diazepam nasal spray) allows for a second dose, if needed, for control. These findings support the safety profile of second doses, and the low use supports the effectiveness of diazepam nasal spray across 24 hours.
Keywords: acute repetitive seizures, diazepam, intranasal, rescue
Key Points.
Patients with intractable epilepsy are at high risk for seizure clusters, which may have a duration of 24 h or longer
Diazepam nasal spray is approved by the US Food and Drug Administration (FDA) for acute treatment of seizure clusters; a second dose may be used if needed to control a cluster
This analysis from a phase 3 safety study assesses use of a second dose of diazepam nasal spray within 24 h of the initial treatment
Of a total of 3853 seizure clusters, use of a second rescue dose of diazepam nasal spray was low overall (12.6% of seizure clusters)
The safety profile of the subgroup that used ≥1 second dose was consistent with that of the safety population for the overall study
1. INTRODUCTION
Intractable epilepsy is associated with a significant risk of seizure clusters, 1 , 2 which can be described as bouts of increased seizure activity 3 that pose a risk of progression to prolonged seizures or status epilepticus and potentially death. 1 , 4 The proportion of patients with epilepsy who experience seizure clusters has varied in published reports, as has the duration of seizure clusters, which may persist for 24 h or longer. 2
A large study using a web‐ and mobile‐based seizure diary with data from 28 697 patients with epilepsy from a period of ≥60 days found that 5018 patients (17.5%) experienced seizures occurring as clusters (29 341 events), and 1177 had seizure clusters defined as two or more seizures with specified timing within 24 h in a calendar day (12:00 am to 11:59 pm) (Fisher et al., 2015); 24.1% of the days with seizure clusters included 2–5 events. In that study, seizure clusters had varying durations, with more than half (58.5%) of patients having clusters lasting 6 h or longer. 2 This suggests that rescue therapies that provide both short‐term and longer‐term benefit are needed.
Response to rescue therapy can differ among patients, and some require a second dose of medication should seizures persist after administration of a first dose. The three US Food and Drug Administration (FDA)─approved rescue therapies for outpatient treatment of seizure clusters (ie, rectal diazepam, intranasal midazolam, and diazepam nasal spray) all allow for second doses if needed. 5 , 6 , 7 Examination of the use of second doses of rescue therapy for seizure‐cluster control may better characterize seizure clusters. Evaluating whether and when second doses are used may help illuminate the effectiveness of these therapies in controlling and potentially mitigating the severity and duration of seizure clusters.
The results presented here are from the completed long‐term, phase 3, open‐label, repeat‐dose, safety study of diazepam nasal spray (Valtoco). 8 Diazepam nasal spray is a proprietary intranasal formulation that is approved by the FDA for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (ie, seizure clusters, acute repetitive seizures) in patients with epilepsy 6 years of age and older. 7 In this study, second doses of diazepam nasal spray could be administered as needed to control a seizure cluster. This analysis assesses the use of a second dose of diazepam nasal spray in the first 24 h after initial treatment during a seizure cluster and reports treatment‐emergent adverse events (TEAEs).
2. MATERIAL AND METHODS
2.1. Study design
This was a long‐term, phase 3, repeat‐dose, open‐label, safety study of diazepam nasal spray that was completed in July 2020 (ClinicalTrials.gov identifier, NCT02721069). Overall results from this study have been published previously. 8 Study investigators obtained institutional review board or ethics committee approval of the protocol and other documents, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) according to the International Conference on Harmonization guidelines. Written consent was obtained from each patient prior to participation in the study in compliance with the Declaration of Helsinki, GCP, and federal and local regulations.
Diazepam nasal spray was administered as needed for seizure clusters during a treatment period of 12 months, with study visits on day 30 and every 60 days afterward. After day 365, patients could elect to continue on therapy. The primary purpose of the study was to assess the safety of diazepam nasal spray for treatment of seizure clusters. Use of a second dose, which was captured in patient diaries, was used as a proxy for effectiveness.
2.2. Patients
Male or female patients, 6–65 years of age, were enrolled if they had a diagnosis of epilepsy and their physician determined that rescue benzodiazepine therapy was warranted for management of seizure clusters that occurred at least once every other month on average despite use of chronic antiseizure medications. Patients had a diagnosis of either focal (localization‐related) or generalized epilepsy and still experienced clusters of seizures (eg, frequent breakthrough seizures or acute repetitive seizures) that produced either motor behavior or alteration of awareness. Other key inclusion criteria were the availability of a qualified care partner or medical professional who could administer study medication in the event of a seizure and no clinically significant abnormal findings in the medical history, physical examination, or electrocardiography.
Key exclusion criteria included history of active major depression, past suicide attempt, or suicidal ideation; history of clinically significant disease or medical condition that would jeopardize the safety of the patient; history of allergy or adverse response to diazepam; pregnancy; and positive blood screen for HIV, hepatitis B or C, or alcohol or drugs of abuse. Patients with a history of status epilepticus were not excluded, neither were patients with concomitant chronic or intermittent use of benzodiazepines or seasonal allergies/allergic rhinitis.
2.3. Administration and dosing
Care partners and patients were trained to administer age‐ and weight‐based doses (5, 10, 15, or 20 mg) of diazepam nasal spray, and were given instructions to administer a second dose 4 to 12 h after the first dose if needed to control a seizure cluster (Figure S1). Study investigators could adjust the dose of diazepam nasal spray as clinically warranted for effectiveness or safety, and they could adjust the timing of the second dose as well. Second doses for treatment of a single cluster were allowed throughout the study and were not necessarily administered to a patient on a regular basis.
Care partners and patients were given a study subject diary and instructions on how to record seizure and dosing information, including the time the seizure occurred, when it ended, the dose, and the date and time of the dosing. Treatment‐emergent adverse events (or TEAEs), including TEAEs at least possibly related to the study drug that occurred in the home setting after dosing were also recorded in the diary. During study visits, information from the diary was recorded on the patient's case report form.
2.4. Analysis
This analysis assessed how many times second doses were used per patient for a seizure cluster and the percentage of each patient's seizure clusters that were treated with second doses during the study. Seizure clusters were defined as two or more seizures in a 24‐h period. The percentage of each patient's seizure clusters treated with second doses was grouped in ranges from 1%–5%, 6%–10%, 11%–20%, 21%–40%, and >40% in the analyses. Times to the second dose in a seizure cluster were analyzed by grouping in categories of <4, >4–6, >6–8, >8–12, >12–16, and >16–24 h after the initial dose (use of second doses <4 or >12 h was not specified in the protocol but was allowed).
TEAEs were recorded by Medical Dictionary for Regulatory Activities preferred terms. Summary data were provided for all patients and specifically for the subgroup of patients who received a second dose of diazepam nasal spray at least once during the study. In addition, for those receiving a second dose, TEAEs occurring within a day of that dose were summarized.
3. RESULTS
At the completion of the phase 3 safety study of diazepam nasal spray, 175 patients had been enrolled, and 163 patients were treated (54.6% female; mean age 23.1 years [range: 6–65]) and included in the safety population (Table 1). A total of 117 patients completed the study, resulting in an overall retention rate of 71.8% (117/163). No patients discontinued due to a treatment‐related TEAE or lack of efficacy. The majority of the 46 discontinuations (≥5 patients) were due to withdrawal by the patient (n = 19), lost to follow‐up (n = 11), and study closure (n = 7).
TABLE 1.
Demographics and exposure to diazepam nasal spray in the second‐dose subgroup and overall population
| Variable |
Second‐dose subgroup (n = 79) |
Overall population (N = 163) |
|---|---|---|
| Sex, n (%) | ||
| Male | 39 (49.4) | 74 (45.4) |
| Female | 40 (50.6) | 89 (54.6) |
| Age, years | ||
| Mean (SD) | 22.7 (15.1) | 23.1 (15.1) |
| Range | 6 to 59 | 6 to 65 |
| Weight, kg, mean (SD) | 60.0 (36.0) | 60.2 (33.6) |
| Duration of exposure, n (%) | ||
| <6 mo | 2 (2.5) | 9 (5.5) |
| 6 to <12 mo | 12 (15.2) | 21 (12.9) |
| ≥12 mo | 65 (82.3) | 133 (81.6) |
Most patients (81.6%) had a duration of exposure ≥12 months, and the median duration in the study was 15.0 months (mean, 17.4 months; range, 1.8–40.4 months). Based on total doses a patient received, including second doses, a majority of patients (52.8%) averaged ≥2 doses per month, and mean doses per patient per month were 2.3 (median, 2.0; range 1–10). During the duration of the study, 16 patients had their dose adjusted to the next higher dose (ie, an additional 5 mg to the prior dose), two had doses adjusted up two dose levels (10 mg added to the prior dose), and one had the dose reduced to the next lower level (decreased 5 mg from the prior dose).
In the overall safety population of 163 patients, a total of 4390 doses of diazepam nasal spray were administered for a total of 3853 seizure clusters. Among patients, slightly less than half of the safety population, 79 patients (48.5%) used a second dose of diazepam nasal spray within 24 h of the first dose on one occasion or more during the study; 84 patients (51.5%) did not use a second dose after receiving the initial dose. The percentage of patients using second doses was not consistent across the duration of this long‐term study, Patients included in the second dose group had used a second dose at any point during the study. As a proportion of treated seizure clusters, a second dose was administered within 24 h of the first dose for 485 (12.6%); thus, the large majority of seizure clusters in the study were controlled with one dose. For 52 doses, the patient diaries did not specify whether the doses were administered as the first or second dose within 24 h.
Baseline demographic characteristics for the subgroup who received second doses were similar to those of the overall population (Table 1). As in the overall population, most patients who received second doses (82.3%) had a duration of exposure of ≥12 months. The retention rate in the study for this subgroup was 73.4%. The number of second doses administered ranged from 1 to 82 per patient during the duration of the study (median, 2.0 second doses; mean, 6.1 second doses). Of the 79 patients using a second dose, 33 patients (41.8%) received a second dose only once during the study (Figure 1).
FIGURE 1.
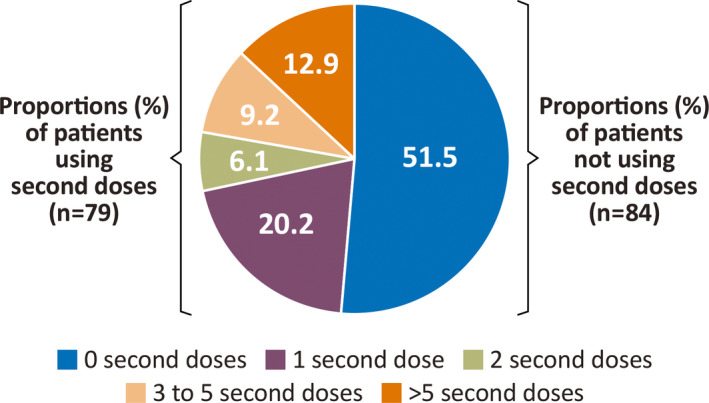
Frequency of second dose use of diazepam nasal spray in the safety population (N = 163 patients)
Of the patients who used at least one second dose, nearly half (36 of 79 [45.6%]) received a second dose for <11% of their seizure clusters, and only 5.1% (four patients) had >40% of their clusters treated with second doses (Figure 2). Among seizure clusters occurring in the 79 patients who ever used a second dose, a median proportion of 11.5% were treated with both a first and second dose for ≥1 seizure cluster.
FIGURE 2.
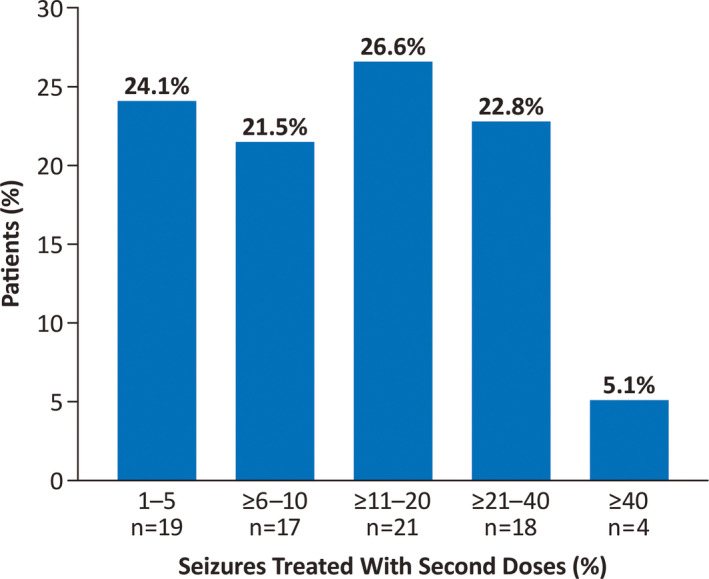
Percentage of an individual patient's total seizure clusters treated with a second dose of diazepam nasal spray (N = 79 patients)
Second doses were distributed relatively evenly across the time periods up to 24 h (Figure 3). Because patients could use second doses at different timepoints throughout the study, some patients were counted in multiple timing categories. Of the 152 second doses used within 4 h of the first dose, second doses for discrete periods were similar across the timepoints: 44 (11 patients) were administered in 0–10 min, 23 (13 patients) in >10–30 min, 16 (10 patients) in >30 min to 1 h, 28 (17 patients) in >1–2 h, 17 (n = 10 patients) in >2–3 h, and 24 (12 patients) in >3–4 h. A total of 485 second doses were used within 24 h (79 patients) of the first dose.
FIGURE 3.
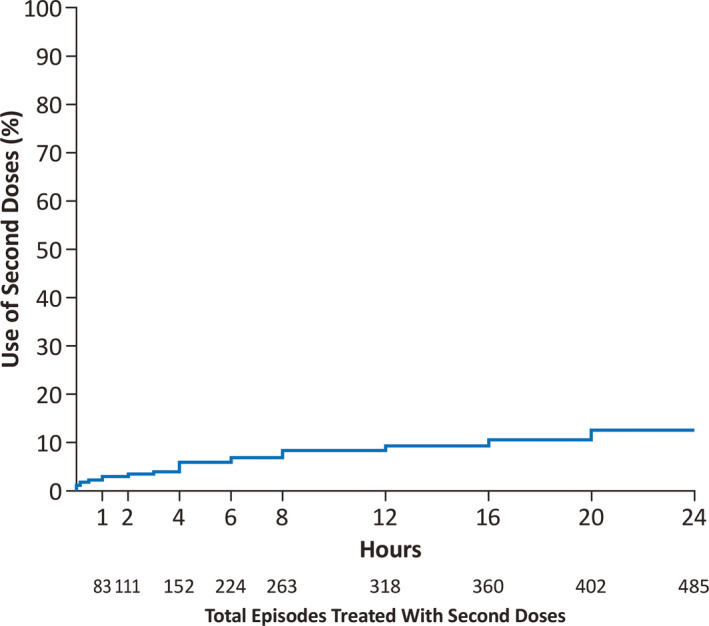
Time to second dose when used
3.1. Safety
TEAEs were reported for 134 patients (82.2%) in the overall safety population (163 patients), with events considered to be at least possibly treatment‐related (TR‐TEAEs) reported in 18.4% of patients (Table 2). Of the 79 patients using at least one second dose during the study, 67 (84.8%) had TEAEs, and 19 (24.1%) had TR‐TEAEs. Twenty‐eight patients (35.4%) had serious TEAEs, including 6 (7.6%) with important medical events and 25 (31.6%) requiring or prolonging hospitalization; no serious TEAE was considered treatment related. Among 163 patients, “seizure cluster” specifically was listed as a serious TEAE in two patients. One death (sudden unexpected death in epilepsy) and one discontinuation due to a TEAE (major depression) was recorded for patients in the subgroup that used second doses; neither event was considered to be treatment‐related.
TABLE 2.
Treatment‐emergent adverse events (TEAEs) in the second‐dose subgroup and overall population
| Category, n (%) |
Second‐dose subgroup (n = 79) |
Overall population (N = 163) |
|---|---|---|
| Number of patients with TEAEs | 67 (84.8) | 134 (82.2) |
| Number of patients with SAEs | 28 (35.4) | 50 (30.7) |
| Death | 1 (1.3) a | 1 (0.6) a |
| Important medical events | 6 (7.6) | 10 (6.1) |
| Requires/prolongs hospitalization | 25 (31.6) | 44 (27.0) |
| Number of patients who discontinued due to a TEAE | 1 (1.3) a | 1 (0.6) a |
| Number of patients with treatment‐related TEAEs | 19 (24.1) | 30 (18.4) |
| Most common TEAEs (>5% in the second dose group) | ||
| Seizure | 16 (20.3) | 31 (19.0) |
| Nasopharyngitis | 15 (19.0) | 20 (12.3) |
| Upper respiratory tract infection | 10 (12.7) | 20 (12.3) |
| Influenza | 9 (11.4) | 13 (8.0) |
| Pyrexia | 8 (10.1) | 17 (10.4) |
| Somnolence | 8 (10.1) | 11 (6.7) |
| Vomiting | 7 (8.9) | 9 (5.5) |
| Diarrhea | 6 (7.6) | 9 (5.5) |
| Headache | 6 (7.6) | 7 (4.3) |
| Nausea | 6 (7.6) | 8 (4.9) |
| Constipation | 5 (6.3) | 8 (4.9) |
| Epilepsy | 5 (6.3) | 6 (3.7) |
| Nasal discomfort | 5 (6.3) | 10 (6.1) |
| Status epilepticus | 5 (6.3) | 7 (4.3) |
| Urinary tract infection | 5 (6.3) | 11 (6.7) |
| Cough | 4 (5.1) | 8 (4.9) |
| Ear infection | 4 (5.1) | 7 (4.3) |
| Pneumonia | 4 (5.1) | 12 (7.4) |
Abbreviation: SAE, serious adverse event.
Not deemed possibly or probably treatment related.
Treatment‐emergent adverse events within 1 day of a second dose were reported for 12 patients (15.2%) (Table 3). The most common TEAEs within 1 day of a second dose (≥2% of patients) were seizure, epistaxis, and vomiting (each 2.5%). Four patients (5.1%) had TEAEs within 1 day of a second dose that were TR‐TEAEs; the most common and only event in ≥2% of these patients was epistaxis (2.5%).
TABLE 3.
Treatment‐emergent adverse events (TEAEs) within 1 day of second dose
| Category, n (%) |
Second‐dose subgroup (n = 79) |
|---|---|
| Number of patients with TEAEs | 12 (15.2) |
| Seizure | 2 (2.5) |
| Epistaxis | 2 (2.5) |
| Vomiting | 2 (2.5) |
| Administration site pain | 1 (1.3) |
| Dysgeusia | 1 (1.3) |
| Gastrointestinal reflux disease | 1 (1.3) |
| Headache | 1 (1.3) |
| Hematuria | 1 (1.3) |
| Infected cyst | 1 (1.3) |
| Nausea | 1 (1.3) |
| Pyrexia | 1 (1.3) |
| Sedation | 1 (1.3) |
| Somnolence | 1 (1.3) |
| Status epilepticus | 1 (1.3) |
| Thrombocytopenia | 1 (1.3) |
| Tooth infection | 1 (1.3) |
| Number of patients with treatment‐related TEAEs | 4 (5.1) |
| Epistaxis | 2 (2.5) |
| Administration site pain | 1 (1.3) |
| Dysgeusia | 1 (1.3) |
| Somnolence | 1 (1.3) |
4. DISCUSSION
Second doses of rescue medication may be needed to control seizure clusters when seizures persist after an initial dose of rescue drug. In this cohort with intractable epilepsy, the use of a second rescue dose of diazepam nasal spray within a seizure cluster was relatively low overall (12.6% of seizure clusters) and relatively uncommon in most who used a second dose. Most patients who required a second dose only used that dose one or two times, for a small proportion of their clusters over the duration of the study. The amount of diazepam was adjusted upward in only a small number of patients during the study, and this had a modest effect on the need for second doses; the ability to safely increase the initial dose represents a benefit of the drug, allowing physicians to accommodate patient need. Finally, the rate of TEAEs for patients using second doses was similar to that in the overall population, so there appears to be no significant added risk when a second dose is used in a seizure cluster.
The rate of use of a second dose by timepoints was consistently low throughout the 24‐h period, with relatively similar use between early and late timepoints after initial dosing, suggesting that there is no loss of effectiveness of the first dose over time. In a separate study that used diaries to characterize untreated seizure clusters, one‐third of second seizures occurred within 3 h of the initial seizure and two‐thirds of second seizures occurred within 6 h of the initial seizure in the cluster, with the number of seizures at later timepoints increasing over time (ie, <10 min, 5 second seizures; 11–30 min, 438 second seizures; 31–60 min, 409 second seizures; 1–2 h, 818 second seizures; 2–3 h, 852 second seizures; 3–6 h, 1929 second seizures). 2 A historically controlled study in patients with seizure cluster or status epilepticus measured seizure duration prior to the introduction of intermittent home therapy. During the period without treatment, 611 events were reported, and 75.6% had a duration longer than 30 min, most lasting hours to days. 9 In the present study, the effectiveness of the first dose of diazepam nasal spray is supported by the reduced need for second doses over 24 h; this suggests that the first dose of diazepam nasal spray alters the natural history of the cluster. This may occur because of the persistent bioavailability of diazepam over 24 h, or more likely, because the first diazepam dose “resets” the system and abolishes the tendency for additional seizures. The time‐limited duration of some clusters undoubtedly contributes to the lack of need for a second dose in some patients as well.
Second dose use in large (≥100 patients with ≥1000 seizure clusters), long‐term (≥12 months), open‐label studies with generally similar designs evaluating rectal diazepam and intranasal midazolam also can provide context to the results of this study of diazepam nasal spray. Together, these studies may offer insights into the need for multiple doses of rescue therapy for controlling a seizure cluster and the timing associated with those doses. The three studies all included populations with intractable epilepsy and seizure clusters that were treated with rescue therapy by nonmedical caregivers in the community setting. The rectal diazepam study included 149 patients 2 to 76 years of age who were treated for a total of 1578 seizure clusters defined by 12 h; although second doses of rectal diazepam were not provided for all patients per protocol, 363 of the initial administrations (23%) lacked efficacy, suggesting that a second dose may have been appropriate. 10 The open‐label extension trial of midazolam nasal spray study included 161 patients 12 to 62 years of age who were treated for 1998 total seizure clusters; a second dose was used for 38.5% of administrations within 6 h. In the present study, second doses of diazepam nasal spray were administered at a lower rate than reported for rectal diazepam and intranasal midazolam. In this study, second doses of diazepam nasal spray were used for 5.8% of administrations in 6 h, 8.3% in 12 h, and 12.6% in 24 h. However, our cohort may have differed in its cluster characteristics from those in previous studies, and a randomized comparison trial would be required to definitively assess differences in need for a second dose.
4.1. Limitations
Seizure diaries were used to collect patient‐ and care partner–reported outcomes. Diary use has limitations, as it depends on such factors as understanding of instructions, completeness of reporting, and awareness of seizures. 11 In addition, as a safety study with no controls, this study was not powered to test statistical differences between groups of patients, thereby limiting the ability to make strong conclusions. However, controls were not required in the diazepam nasal spray approval pathway due to the use of the proven agent (diazepam) and bioavailability comparable to that of the reference formulation (diazepam rectal gel).
5. CONCLUSIONS
Second doses of diazepam nasal spray are infrequently needed in seizure clusters, suggesting that a sustained treatment effect is obtained after administration of the first dose. Because there is no increasing need for the second dose over 24 h, this suggests that the natural history of clusters is altered by therapy. Should a second dose be needed, the data support safety and tolerability of this dose.
CONFLICT OF INTEREST
Dr. Sperling has received compensation for speaking at continuing medical education (CME) programs from Medscape, Projects for Knowledge, International Medical Press, Eisai, and UCB Pharma. He is an advisor for scientific publications for Neurelis. He consults for Medtronic with payments to Thomas Jefferson University. He has received research support from Eisai Inc.; Medtronic; Neurelis, Inc.; SK Life Science; Takeda; Xenon; Cerevel; UCB Pharma; and Engage Pharmaceuticals. He has received royalties from Oxford University Press. Dr. Wheless has served as an advisor or consultant for CombiMatrix; Eisai Inc.; GW Pharmaceuticals; Lundbeck, Inc.; Neurelis, Inc.; NeuroPace, Inc.; Supernus Pharmaceuticals, Inc.; and Upsher‐Smith Laboratories, Inc. Dr. Wheless has served as a speaker or a member of a speakers bureau for Cyberonics, Inc.; Eisai Inc.; Lundbeck, Inc.; Mallinckrodt; Neurelis, Inc.; Supernus Pharmaceuticals, Inc.; and Upsher‐Smith Laboratories, Inc., and has received grants for clinical research from Acorda Therapeutics; GW Pharmaceuticals; and INSYS. Dr. Hogan has received research support from UCB Pharmaceuticals, Neurelis, Inc; and Biogen Inc, and is an advisor for Neurelis, Inc. Dr. Dlugos receives salary support from The Epilepsy Study Consortium. His institution receives research support for protocol development or studies from Zogenix; Greenwich Biosciences; Neurelis, Inc.; Aquestive; Bio‐Pharm; Stoke Therapeutics; SK Life Science; and Encoded Therapeutics. He received travel expenses for protocol development or investigator meetings from Marinus, Ovid/Takeda, and Zogenix. Dr. Cascino has nothing to disclose. Dr. Liow has received research support from Intra‐Cellular Therapies, SK Life Science, Genentech, Biotie Therapies, Monosol, Aqestive Therapeutics, Engage Therapeutics, Xenon, Lundbeck, Biogen, Eli Lilly, Pfizer, Novartis, Sunovion, Acorda, Eisai, UCB Pharma, LivaNova, Axsome, and Acadia. Dr. Rabinowicz is an employee and has received stock options from Neurelis, Inc. Dr. Carrazana is an employee of and has received stock and stock options from Neurelis, Inc. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1
ACKNOWLEDGMENTS
Medical writing support was provided at the direction of the authors by Laura J. Herold, MA, of The Curry Rockefeller Group, LLC (Tarrytown, NY), which also provided additional editorial assistance including formatting and proofreading. This support was funded by Neurelis, Inc. (San Diego, CA).
Sperling MR, Wheless JW, Hogan RE, Dlugos D, Cascino GD, Liow K, et al. Use of second doses of Valtoco® (diazepam nasal spray) across 24 hours after the initial dose for out‐of‐hospital seizure clusters: Results from a phase 3, open‐label, repeat‐dose safety study. Epilepsia. 2022;63:836–843. 10.1111/epi.17177
Funding information
This study was funded by Neurelis, Inc. (San Diego, CA).
REFERENCES
- 1. Jafarpour S, Hirsch LJ, Gainza‐Lein M, Kellinghaus C, Detyniecki K. Seizure cluster: definition, prevalence, consequences, and management. Seizure. 2019;68:9–15. [DOI] [PubMed] [Google Scholar]
- 2. Fisher RS, Bartfeld E, Cramer JA. Use of an online epilepsy diary to characterize repetitive seizures. Epilepsy Behav. 2015;47:66–71. [DOI] [PubMed] [Google Scholar]
- 3. Penovich PE, Buelow J, Steinberg K, Sirven J, Wheless J. Burden of seizure clusters on patients with epilepsy and caregivers: survey of patient, caregiver, and clinician perspectives. Neurologist. 2017;22(6):207–14. [DOI] [PubMed] [Google Scholar]
- 4. Haut SR, Shinnar S, Moshe SL, O'Dell C, Legatt AD. The association between seizure clustering and convulsive status epilepticus in patients with intractable complex partial seizures. Epilepsia. 1999;40(12):1832–4. [DOI] [PubMed] [Google Scholar]
- 5. Bausch Health US, LLC . Diastat ® C‐IV (diazepam rectal gel). Full prescribing information. Bridgewater, NJ: Bausch Health US, LLC; 2021. [Google Scholar]
- 6. UCB, Inc . NAYZILAM® (midazolam nasal spray). Full prescribing information. Smyrna, GA: UCB, Inc.; 2021. [Google Scholar]
- 7. Neurelis, Inc . Valtoco (diazepam nasal spray). Full prescribing information. San Diego, CA: Neurelis, Inc.; 2021. [Google Scholar]
- 8. Wheless JW, Miller I, Hogan RE, Dlugos D, Biton V, Cascino GD, et al. Final results from a Phase 3, long‐term, open‐label, repeat‐dose safety study of diazepam nasal spray for seizure clusters in patients with epilepsy. Epilepsia. 2021;62(10):2485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lombroso CT. Intermittent home treatment of status and clusters of seizures. Epilepsia. 1989;30(Suppl 2):S11–4. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell WG, Conry JA, Crumrine PK, Kriel RL, Cereghino JJ, Groves L, et al. An open‐label study of repeated use of diazepam rectal gel (Diastat) for episodes of acute breakthrough seizures and clusters: safety, efficacy, and tolerance. North American Diastat Group. Epilepsia. 1999;40(11):1610–7. [DOI] [PubMed] [Google Scholar]
- 11. Fisher RS, Blum DE, DiVentura B, Vannest J, Hixson JD, Moss R, et al. Seizure diaries for clinical research and practice: limitations and future prospects. Epilepsy Behav. 2012;24(3):304–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


