Abstract
Objectives
To compare detection of Salmonella species and antimicrobial‐resistant Escherichia coli in the faeces of dogs eating raw meat or non‐raw diets and examine risk factors for their carriage.
Materials and Methods
Canine faecal samples (raw fed n=114; non‐raw fed n=76) were collected from May to July 2015 from across the UK. Enrichment and selective culture and biochemical and PCR assays were used to identify isolates. Escherichia coli underwent susceptibility testing to a range of antimicrobials, including third‐generation cephalosporins; PCR assays were used to detect antimicrobial‐resistant genes. Questionnaires were used to collect data on independent variables as risks for antimicrobial‐resistant (resistant to ≥1 tested antimicrobial), multi‐drug‐resistant (resistant to ≥3 antimicrobial classes) and third‐generation cephalosporin resistant Escherichia coli.
Results
Antimicrobial‐resistant, multi‐drug‐resistant and third‐generation cephalosporin resistant Escherichia coli were significantly more likely to be detected in raw fed (54, 25 and 31%, respectively) compared to non‐raw fed (17, 4 and 4%, respectively) dogs; Salmonella species were detected in eight (4%) raw fed dogs only.
Clinical Significance
Raw fed dogs may be a source of Salmonella species and Escherichia coli, resistant to highest priority critically important antimicrobials, representing a potential animal welfare and public health issue. Owners should be aware of the risks, especially households with members, both human and canine, who are very young, elderly or immunocompromised.
INTRODUCTION
Escherichia coli are the most numerous facultative anaerobic Gram‐negative bacilli in mammalian gastrointestinal tracts and like many commensals, they are potentially pathogenic (Tenaillon et al. 2010). Gastrointestinal E. coli can also act as reservoirs of antimicrobial‐resistance (AMR) genes for pathogenic variants or other bacteria by horizontal transfer on mobile genetic elements, such as plasmids (Vila et al. 2016). AMR and multi‐drug resistance (MDR; resistance to three or more antimicrobial classes) are a growing threat to both human and animal health, increasing reliance on newer generation drugs, including those defined as the highest priority critically important antimicrobials, such as fluoroquinolones and third‐generation cephalosporins (Gould 2009, WHO 2019). Given the close contact between companion animals and their owners, there is an opportunity for the transmission of such bacteria between them (Guardabassi et al. 2004).
Extended‐spectrum beta‐lactamase (ESBL)‐producing E. coli are of concern as they are resistant to third‐generation cephalosporins (3GCR) and are often MDR (Bush 2018). The reported prevalence of ESBL‐producing E. coli in healthy dogs in the UK community is low at 0.5 to 4% (Schmidt et al. 2015, Wedley et al. 2017); the most commonly encountered β‐lactamases conferring 3GCR found in bacteria isolated commensally and clinically from companion animals are CTX‐M (bla CTX‐M‐15; bla CTX‐M group 1), TEM‐158, SHV‐12 and AmpC (bla CMY‐2; bla CITM) enzymes (Ewers et al. 2012, Tuerena et al. 2016, Wedley et al. 2017, Schmidt et al. 2018, Zogg et al. 2018). AmpC type β‐lactamases are a particular problem as they hydrolyse broad and extended‐spectrum cephalosporins and are not inhibited by β‐lactamase inhibitors such as clavulanic acid (Bush 2018). Transmissible low‐level fluoroquinolone resistance, conferred by qnr genes, may also be present in such bacteria (Zogg et al. 2018).
Salmonella is a significant cause of non‐typhoidal inflammatory gastroenteritis in people. Human illness associated with Salmonella is usually attributable to cross‐contamination in the kitchen from raw meat and/or the consumption of inadequately cooked meat (PHE 2013). The prevalence of Salmonella in a group of healthy UK dogs from midlands was reported to be low (0.23%; n=436 dogs) (Lowden et al. 2015). Similarly, a low Salmonella prevalence was reported in a large multi‐state, USA study (n=2422 dogs) in diarrhoeic (1.3%) and non‐diarrhoeic (1.1%) dogs (Reimschuessel et al. 2017) and in a UK study (n=160,427 samples) amongst laboratory submitted canine faecal samples (0.82%) (Arsevska et al. 2017). On the other hand, a Canadian study (n=138 dogs) reported the detection of faecal Salmonella in 23% of dogs where there was a positive association with eating raw meat diets or raw animal products (Leonard et al. 2011).
Raw meat diets are increasing in popularity (ACMSF 2018). Previous studies have shown that raw meat fed to dogs can be contaminated with E. coli and Salmonella species (Nilsson 2015, Baede et al. 2017, van Bree et al. 2018, Bacci et al. 2019, Hellgren et al. 2019) and feeding raw meat has been shown to be a risk factor for the detection of canine faecal Salmonella species and AMR E. coli (Schmidt et al. 2015, Wedley et al. 2017, Runesvard et al. 2020, Viegas et al. 2020). The consumption of raw meat by domestic pets may introduce AMR E. coli and other pathogenic bacteria into households (Johnson et al. 2007, Frost 2017, O'Halloran et al. 2020) where they can potentially colonise intestinal tracts and spread between household members by oro‐faecal contamination (Johnson et al. 2008). The aim of the present study was to investigate the prevalence of AMR E. coli and the enteric pathogen Salmonella species in dog faeces prospectively from pets fed raw or non‐raw diets in the UK and to determine risk factors associated with such carriage.
MATERIALS AND METHODS
Sample population
Sample size estimates were based on the prevalence of 3GCR E. coli in canine faeces. Previous reported data (Wedley et al. 2011, Schmidt et al. 2015) and the author's unpublished pilot data suggested a prevalence of 40% versus <10% for dogs eating raw or non‐raw diets, respectively. To detect a difference between the groups, with 80% power and 95% confidence, it was determined that 32 dogs in each group were required. Based on previous studies, we expected a 75% response rate (Schmidt et al. 2015) and, therefore, aimed to recruit between 80 and 150 dogs in each group. Dogs were recruited via social media on a first‐response basis between May and July 2015. Participants were sent a sample pack with paperwork (consent form, information sheet and owner questionnaire), sample pot, gloves and biohazard bag. Samples were to be returned via prepaid first‐class post the same day as collection. Participation was restricted to one faecal sample per dog and a maximum of two dogs per household for recruitment. An owner questionnaire was administered, which had been used in previous studies (Wedley et al. 2011, Schmidt et al. 2015). Data were collected on the age, breed, gender, diet and reason for choice, health status (including any perceived health benefit due to diet) and number and type of in‐contact pets in the household. The foods listed on the questionnaire allowed the assignment of each dog to either raw or non‐raw fed groups. A dog was considered to eat raw diet if any component of the diet, fed on a regular basis (minimum once weekly), was not cooked; cooked included commercial kibble, tinned meat, treats and home‐cooked food. Ethical approval for the study was granted from the University's Veterinary Ethics Committee.
Escherichia coli isolation and identification
Faecal samples were tested immediately on receipt in the laboratory and processed as previously reported (Schmidt et al. 2015) for E. coli, including 3GCR isolates. Where present, isolates typical of E. coli were randomly selected and sub‐cultured onto nutrient agar: one colony from cefotaxime (CX) (1 mg/L) and/or ceftazidime (CZ) (1 mg/L) eosin methylene blue agar (EMBA) plates, and three colonies from plain EMBA. All isolates were confirmed biochemically as E. coli (Schmidt et al. 2015).
Salmonella species isolation and identification
Faeces (1 g) were incubated aerobically at 37°C overnight in 5 ml of buffered peptone water for pre‐enrichment and then 100 μl was inoculated into 10 ml of Rappaport‐Vassiliadis broth (RVB) and incubated aerobically at 42°C overnight. RVB cultures were inoculated onto xylose lysine deoxycholate and deoxycholate citrate agars and incubated aerobically at 37°C for 18 to 20 hours. Where present, isolates typical of Salmonella species were selected from each plate and sub‐cultured onto chromogenic agar for Salmonella esterase and incubated aerobically at 37°C for 20 to 24 hours. Isolates typical of Salmonella species were confirmed with Poly O and Poly H antisera as described by the manufacturer (ProLab, Wirral, UK) using a slide agglutination test.
Escherichia coli antimicrobial susceptibility testing
Antimicrobial susceptibility disc diffusion testing was performed according to the Clinical Laboratory Standards Institute (CLSI 2013) against: tetracycline 30 μg (T30), trimethoprim‐sulfamethoxazole 1.25/23.7 (TS25), ampicillin 10 μg (AP10), co‐amoxyclav 20 μg/10 μg (AUG30), ciprofloxacin 5 μg (CIP5), chloramphenicol 30 μg (C30) and gentamicin 10 μg (GM10). Escherichia coli ATCC® 25,922 (LGC Standards, Teddington, UK) was used as a control. Isolates selected from CX or CZ EMBA and isolates with phenotypic resistance to ampicillin or amoxicillin–clavulanate were further screened for 3GCR (cefpodoxime 10 μg) and phenotypic ESBL‐production, according to manufacturer instructions (Extended Spectrum Beta‐Lactamase Set D52C, MAST Group Ltd., Liverpool, UK) using the double‐disc test (M'Zali et al. 2000). Interpretation was based on the CLSI guidelines for animal species‐specific zone diameter (millimetre) interpretive standards for veterinary pathogens when available, otherwise human‐derived interpretive standards were used (CLSI 2015). As CLSI standards were not available for interpretation of ciprofloxacin, the European Committee on Antimicrobial Susceptibility Testing zone diameter interpretive standards were used (EUCAST 2015). All media was obtained from Lab M Ltd. (Bury, UK), the antibiotic powder from Sigma–Aldrich Company Ltd. (Gillingham, UK) and all antimicrobial discs from MAST Group Ltd. (Liverpool, UK).
DNA extraction
To extract DNA (E. coli), three colonies from each overnight culture were homogenised in 500 μl of sterile distilled water and heated at 100°C for 10 minutes before being centrifuged at 13,000 rpm for 2 minutes to retain the supernatant. All DNA extractions were stored at −80°C before use.
Genotypic characterisation of E. coli
A PCR assay was used to detect the presence of the uidA gene to confirm the identification of E. coli (McDaniels et al. 1996). Escherichia coli isolates with phenotypic ESBL‐production ±resistance to amoxicillin–clavulanate were tested for the presence of bla CTX‐M (Batchelor et al. 2005), bla SHV, bla TEM and bla OXA (Dallenne et al. 2010) and bla AmpC gene (Perez‐Perez & Hanson 2002) and the presence of qnrA, qnrB or qnrS genes (Robicsek et al. 2006), by PCR assay. Isolates positive for bla CTX‐M were tested by additional PCR assay to determine if they belonged to CTX‐M group 1, 2 and 9 genes (Batchelor et al. 2005, Hopkins et al. 2006). All PCR assays were performed with 5 μl of bacterial DNA, 5 pmol of each primer, 4 μl of 5xFIREPol® Master Mix (12.5 mM MgCl2), 0.5 μl of FIREPol® DNA Polymerase 5 U/μl (Solis‐Biodyne, Tartu, Estonia) and water, made up to a total reaction volume of 25 μl. Positive control strains were included, and molecular grade water (Sigma‐Aldrich Company Ltd.) was used as the negative control. PCR products were analysed by agarose gel (1.5%) electrophoresis and the DNA fragments were visualised under UV light after peqGREEN (peqlab, Fareham, UK) staining.
Statistical analysis
Outcome data for AMR were collapsed to the sample level such that a sample with at least one isolate that was resistant to a tested antimicrobial was classed as resistant for analysis. The prevalence of resistance was classified as AMR (resistance to at least one tested antimicrobial), MDR (resistance to three or more antimicrobial classes) and 3GCR. The percentage of samples with resistant E. coli was calculated with 95% confidence intervals (CI) for each group (raw fed and non‐raw fed) and compared using chi‐squared tests or Fisher's exact test if n ≤5. The detection of Salmonella species from raw versus non‐raw fed dogs was compared using Fisher's exact test (MedCalc© 2021). Independent variables were created from the owner questionnaires. Except for the age of the dog, all variables were categorical. The three binary considered outcomes were AMR, MDR and 3GCR, and univariable logistic regression analysis examined the association between independent variables and outcomes. All variables P‐value <0.25 were assessed in multi‐level, multi‐variable models with household included as a second‐level random intercept term to account for the clustering effect of sampling multiple dogs from common homes. Final multi‐variable multi‐level models were constructed by manual backwards stepwise procedures where variables with a P‐value <0.05 (calculated using the Wald test) were retained. Data were analysed using the MLwiN statistical software package (version 2.36, Centre for Multilevel Modelling, University of Bristol, UK) (Rabash et al. 2016).
RESULTS
Sample population and questionnaire data regarding owner's diet choice and reported health benefit
In total, 190 faecal samples were collected from dogs from n=140 households from across the UK: 114 raw fed and 76 non‐raw fed. From the owner‐compiled questionnaires, the most common reason for choosing a raw diet was adverts (28%), friends (26%) and dermatitis (11%). Reasons for choosing a non‐raw meat diet were friends (29%), veterinary advice (24%) and breeder advice (22%) (Table 1). Owners feeding raw diets perceived improvements in their dog's stool consistency (83%), oral hygiene and/or breath (65%) and demeanour/behaviour (39%), compared to a non‐raw diet. Owners feeding a non‐raw diet perceived improvement in their dog's coat quality (51%), stool consistency (41%) and oral hygiene and/or breath (16%) (Table 2). Most owners feeding raw, reported feeding chicken (86%), red meat (86%) and tripe (83.3%), but also bones (19.3%), kitchen leftovers (14.9%) or cured meats, for example, pig's ears (12.3%); most dogs were fed a mixture of meats with the majority (63%) being fed raw tripe, chicken and red meats. Only two dogs were fed a single type of raw food, one being fed raw tripe and another raw chicken.
Table 1.
Questionnaire data from owners of non‐raw fed (n=76) and raw fed (n=114) dogs: owner‐stated reasons for diet choice
| Current diet | Number and percentage of owners | |||
|---|---|---|---|---|
| Non‐raw (n) | % | Raw (n) | % | |
| Allergies | 5 | 6.6 | 4 | 3.5 |
| Dermatitis | 6 | 7.9 | 13 | 11.4 |
| Loose stools/colitis | 2 | 2.6 | 3 | 2.6 |
| Anal sacs | 0 | 0 | 0 | 0 |
| Weight gain | 3 | 3.9 | 0 | 0 |
| Renal problems | 1 | 1.3 | 0 | 0 |
| Pancreatitis | 1 | 1.3 | 0 | 0 |
| Sore ears | 0 | 0 | 1 | 0.9 |
| Hot spots | 0 | 0 | 1 | 0.9 |
| Dental | 0 | 0 | 0 | 0 |
| Joint problems | 2 | 2.6 | 0 | 0 |
| Seizures | 0 | 0 | 1 | 0.9 |
| Breeder advice | 17 | 22.4 | 11 | 9.6 |
| Vet advice | 18 | 23.7 | 6 | 5.3 |
| Advertisements | 2 | 2.6 | 32 | 28.1 |
| Social media | 2 | 2.6 | 4 | 3.5 |
| Friend's advice | 22 | 28.9 | 30 | 26.3 |
Table 2.
Questionnaire data from owners of non‐raw fed (n=76) and raw fed (n=114) dogs: owner's perceived benefits of the fed diet
| Current diet | Number and percentage of owners | |||
|---|---|---|---|---|
| Non‐raw (n) | % | Raw (n) | % | |
| Coat quality | 39 | 51.3 | 40 | 35.1 |
| Stool consistency | 31 | 40.8 | 95 | 83.3 |
| Demeanour/behaviour | 9 | 11.8 | 44 | 38.6 |
| Oral hygiene and/or breath | 12 | 15.8 | 74 | 64.9 |
Prevalence of Salmonella species and resistant E. coli
Salmonella species was detected from 4% of all dogs (8/190), all being raw fed. AMR E. coli (resistant to at least one tested antimicrobial) were detected from 40% (75/190) and MDR E. coli from 16% (31/190) of dogs. Resistance to antimicrobials was significant in raw fed, compared to non‐raw fed dogs, including MDR (Table 3). Resistance to four antibiotic classes was detected in a small number of dogs (n=4), all of which were raw fed dogs. ESBL‐producing E. coli (n=52) were detected from the faeces of 35 raw fed and three non‐raw fed dogs.
Table 3.
The percentage of dogs (n=190) with at least one faecal Escherichia coli isolate resistant to the tested antimicrobials or Salmonella species detected
| Antibiotic‐resistance phenotype | Raw (n=114) | % | 95% CI | Non‐raw (n=76) | % | 95% CI | P‐value |
|---|---|---|---|---|---|---|---|
| TS | 32 | 28.1 | 19.8 to 36.3 | 4 | 5.3 | 0 to 10.3 | <0.001* |
| Amp | 43 | 37.7 | 28.8 to 46.6 | 9 | 11.8 | 4.6 to 19.1 | <0.001** |
| AC | 4 | 3.5 | 0 to 6.9 | 3 | 3.9 | 0 to 8.3 | 0.45* |
| GM | 2 | 1.8 | 0 to 4.2 | 1 | 1.3 | 0 to 3.9 | 1.0* |
| Tet | 53 | 46.5 | 47.3 to 55.6 | 10 | 13.2 | 5.6 to 20.8 | <0.001** |
| Chlor | 8 | 7.0 | 2.3 to 11.7 | 2 | 2.6 | 0 to 6.2 | 0.32* |
| Cip | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| AMR | 62 | 54.4 | 45.2 to 63.5 | 13 | 17.1 | 8.6 to 25.6 | 0.001** |
| 3GCR | 35 | 30.7 | 22.2 to 39.2 | 3 | 3.95 | 0 to 8.3 | 0.001* |
| MDR | 28 | 24.6 | 16.7 to 32.4 | 3 | 3.95 | 0 to 8.3 | 0.001* |
| Salmonella | 8 | 7 | 3.6 to 13.2 | 0 | 0 | 0 | 0.025* |
95% CI Confidence interval, TS Trimethoprim‐sulfamethoxazole resistance, Amp Ampicillin resistance, AC Amoxicillin–clavulanate resistance, GM Gentamicin resistance, Tet, Tetracycline resistance, Chlor Chloramphenicol resistance, NA Not applicable, AMR Antimicrobial resistance (resistance to ≥1 tested antimicrobial), 3GCR Third‐generation cephalosporin resistance; MDR Multi‐drug resistance (resistance to ≥3 antimicrobial classes)
P‐values are from *Fisher's exact or
Chi‐squared P‐value; significant at P‐value <0.05
Resistance genes amongst phenotypic ESBL‐producing‐E. coli
The most common beta‐lactamase‐encoding genes were bla CTX‐M and bla TEM; however, no bla SHV genes were detected. Of the bla CTX‐M genes, group 1 genes were the most prevalent. Over a third of the tested isolates carried bla AmpC genes and low‐level plasmid‐mediated fluoroquinolone‐resistance genes (Table 4).
Table 4.
Antimicrobial resistance genes detected in canine faecal ESBL‐E. coli (n=52)
| Genes detected | Number (%) of isolates |
|---|---|
| bla TEM | 21 (40) |
| bla SHV | 0 |
| bla OXA | 11 (21) |
| bla CTX‐M | 25 (48) |
| bla CTX‐M uncharacterised | 5 (10) |
| bla CTX‐M group 1 | 19 (37) |
| bla CTX‐M group 2 | 0 |
| bla CTX‐M group 9 | 1 (2) |
| qnr | 20 (38) |
| qnrA | 10 (19) |
| qnrB | 0 |
| qnrS | 10 (19) |
| bla AmpC | 17 (33) |
| bla CITM | 13 (25) |
| bla ACC | 2 (4) |
| bla DHA | 2 (4) |
Multi‐level multi‐variable models
Final multi‐variable results (Table 5) showed that compared to non‐raw fed dogs, faecal E. coli from raw fed dogs were more likely AMR, MDR and 3GCR. Dogs receiving antimicrobials in the previous 3 months had increased odds of carriage of MDR E. coli.
Table 5.
Multi‐level multi‐variable logistic regression model results for the outcomes “MDR”, “3GCR” and “AMR” in faecal samples from n=190 [raw meat fed (n=114) and non‐raw meat fed (n=76)] dogs
| Final multi‐variable results | MDR | 3GCR | AMR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Feeds non‐raw | Ref | – | – | Ref | – | – | Ref | – | – |
| Feeds raw | 7.46 | 2.13 to 11.39 | 0.002 | 14.57 | 3.03 to 70.04 | 0.001 | 6.21 | 2.99 to 12.92 | <0.001 |
| AB not received in last 3 months | Ref | – | – | – | – | – | – | – | – |
| Received AB in last 3 months | 3.33 | 0.98 to 11.39 | 0.05 | – | – | – | – | – | – |
| Age (years) | – | – | – | 0.58 | 0.41 to 0.83 | 0.003 | – | – | – |
| Age (years) squared | – | – | – | 1.03 | 1.01 to 1.06 | 0.009 | – | – | – |
| Estimate of household variance (SE) | 0.28 (0.63) | 1.24 (0.82) | 0.22 (0.42) | ||||||
Household (n=140) was included as a second‐level random intercept term to account for the clustering effect of sampling multiple dogs from common homes. P‐values are from the Wald chi‐square test; significant at P‐value < 0.05 (odd ratio in bold text)
MDR Multi‐drug resistant (resistant to ≥3 antimicrobial classes), 3GCR Third‐generation cephalosporin resistant, AMR Antimicrobial resistant (resistant to at least one tested antimicrobial), OR Odds ratio, CI Confidence interval, Ref Reference category, AB Antibiotic, SE Standard error
Age of dog in years was associated with 3GCR‐resistant E. coli carriage, but this was not a linear relationship and was best explained by a quadratic polynomial. This indicated higher predicted probabilities in younger dogs followed by a decrease until approximately 12 years of age when the odds increased again (although confidence intervals here were wider) (Fig 1).
FIG 1.
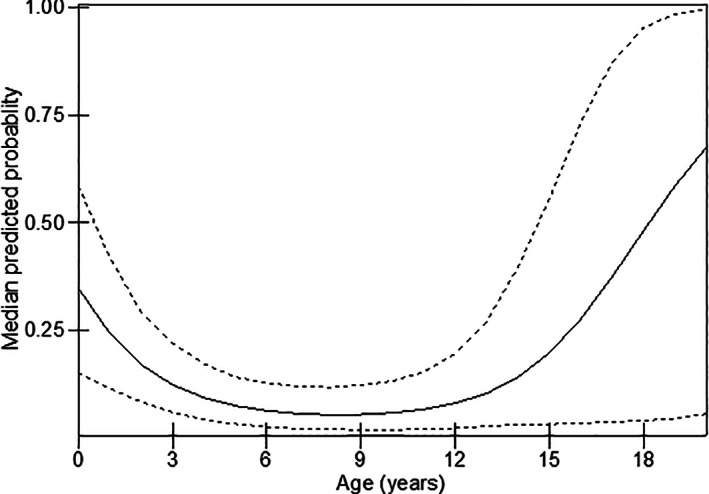
Predicted probabilities of 3GCR and dog age (in years) (predicted probabilities are from the multi‐level, multi‐variable model in Table 5). The solid line is the predicted probability, and the dotted lines are the 95% confidence intervals
DISCUSSION
The present study investigated whether feeding raw or non‐raw meat diets to pet dogs affected the detection of Salmonella species and/or AMR faecal E. coli as carriage of such bacteria may be a health risk to the pet, their owners, or in‐contact pets or people. A significantly higher prevalence of AMR, MDR and 3GCR/ESBL‐producing E. coli and Salmonella species were detected in dogs fed raw compared to non‐raw meat diets; Salmonella species were only found in the faeces of dogs eating raw meat diets.
The present study identified an overall prevalence of MDR E. coli of 16% out of 190 dogs (95% CI: 11.1 to 21.6), which was like the prevalence of MDR E. coli reported previously in a large cross‐sectional cohort of healthy UK‐based dogs, in which being fed raw poultry was reported as a risk factor for MDR E. coli (Wedley et al. 2017). Another UK study reported a higher prevalence (30%) of MDR faecal E. coli in healthy dogs (Schmidt et al. 2015) and again, raw meat diets were significant. In the present study, MDR E. coli was seven times more likely to be detected from dogs fed raw compared to non‐raw fed dogs.
Our study agreed with other reports from the UK (Wedley et al. 2011, Schmidt et al. 2015, Wedley et al. 2017, Wareham 2019) of common tetracycline and ampicillin resistance amongst canine faecal E. coli. In the present study, such phenotypes were significantly increased in raw fed dogs, with ampicillin resistance detected from 37.7% of raw and only 11.8% of non‐raw fed dogs and tetracycline resistance from 46.5% of raw and only 13.2% of non‐raw fed dogs. Wareham (2019) also reported raw meat fed puppies (n=223) had a greater risk of tetracycline‐resistant faecal E. coli. Tetracyclines and beta‐lactams (mainly penicillins) are first‐line antimicrobials and comprise most of the total tonnes of antibiotics sold for food‐producing animals in the UK (Eckford et al. 2013, UK‐VARSS 2020 2019).
Interestingly, ciprofloxacin resistance was not detected in any E. coli in the current study, despite finding genes that provide low‐level quinolone resistance (qnrA and S). The negative finding may be because fluoroquinolones are not as widely used in livestock, accounting for only 0.6% of total antibiotics sales in the UK over a similar time (Eckford et al. 2013) and even less so (0.4%), in 2019 (UK‐VARSS 2020 2019). A more recent study, however, reported that puppies fed raw meat diet had a greater risk of ciprofloxacin resistance (Wareham 2019). The difference in findings between the studies may be explained by the recruitment period (2015 versus 2017 to 2018) and/or the methodologies (disc diffusion versus minimum inhibitory concentration) used and/or the age group (pups versus adults) investigated.
Dogs eating raw meat diets had a higher prevalence for carriage of 3GCR/ESBL‐producing faecal E. coli (c.30.0%) compared to other UK‐based studies (Wedley et al. 2011, Schmidt et al. 2015); however, in previous studies, the prevalence was not differentiated based upon diet. Furthermore, the prevalence in the current study was even higher than that reported in hospitalised dogs in the UK, with a 14% (95% CI: 5.3 to 35.0) ESBL‐E. coli prevalence (Tuerena et al. 2016). Phenotypic 3GCR/ESBL‐producing E. coli isolates often carried bla CTX‐M, mainly bla CTX‐M group 1, in agreement with other UK studies (Schmidt et al. 2015, Tuerena et al. 2016, Wedley et al. 2017). ESBL‐producing E. coli are often associated with several infections in dogs (Zogg et al. 2018), which may decrease the success of treatment and increase the potential for morbidity and mortality (De Kraker et al. 2011); one recent USA study has confirmed similar strains in raw meat samples and companion animal clinical isolates (Jones et al. 2019).
Multi‐level modelling results confirmed that after adjusting for clustering and other factors, a raw fed diet was a significant risk factor for AMR, MDR and 3GCR E. coli. Eating raw chicken was also identified as a risk factor for the detection of ESBL‐producing E. coli in a large UK cross‐sectional study in healthy dogs (n=560) (Wedley et al. 2017). In addition, such bacteria have been detected in UK supermarket raw meats (Randall et al. 2017) and other studies have reported detection of ESBL‐producing E. coli in raw pet food products from the Netherlands, Italy and Sweden (Nilsson 2015, Baede et al. 2017, van Bree et al. 2018, Bacci et al. 2019). Although suppliers of raw meat pet food may advertise “human‐grade raw meat” (Ahmet 2017), it is still raw, and humans should not consume it without proper cooking to kill potentially harmful microorganisms (FSA 2019). Moreover, the packaging of these products generally lacks information on hygienic handling and preparation and in some cases may be damaged, resulting in contamination of hands and/or preparation and storage facilities (Bojanic et al. 2017, van Bree et al. 2018). Following a serious outbreak of shiga‐toxin‐producing E. coli O157, linked to feeding raw meat (PHE 2018), Public Health England has developed online raw meat handling guidelines for pet owners (PHE 2019).
The present study had several limitations. The answers from owner's questionnaires must be analysed with caution, as interpretation of questions and keywords, for example, diarrhoea may be different from veterinarians' definitions. Furthermore, recall may not be accurate when asking questions in terms of previous exposures such as prior antimicrobial treatments. As recruitment may have been biassed by participant's willingness to be involved, the study population may not be representative of all raw fed or non‐raw fed UK dogs. There is no standardised raw meat diet for dogs in the UK. The wide variation in raw diets with respect to composition, source, preparation, packaging and storage may alter the risk for each dog.
In conclusion, dogs fed a raw meat diet had an overall greater prevalence of faecal Salmonellae and AMR, MDR and 3GCR/ESBL‐producing E. coli than dogs fed non‐raw meat diets. Human exposure to pathogenic and/or AMR bacteria may occur through handling and preparation of raw meat. Strategies should be implemented to increase pet owners' awareness of the risks involved with feeding raw meat to their dogs and hence reduce any potential risk to themselves, their family and their pets. Hand hygiene should be strongly recommended after handling raw meat along with sanitisation of all in‐contact items as per normal handling of raw meat in a domestic kitchen. The current study provides evidence that can be used to educate owners on the risks of feeding raw meat diets to dogs.
Funding
Ellyn Groat was funded by PetSaver's 40th Anniversary Veterinary Student Grant and received a stipend for her summer project from the Institute of Veterinary Science, University of Liverpool.
Conflict of interest
None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgements
Stephanie Watkins, Ruth Ryvar and Jennifer Llewelyn for assistance with laboratory work.
References
- Advisory Committee on the Microbiological Safety of Food (ACMSF) . (2018) Raw pet food. https://acmsf.food.gov.uk/sites/default/files/acm_1270_annex_a.pdf. Accessed December 11, 2020
- Ahmet, S . (2017) An insight into raw dog food manufacture using human grade meats. https://www.naturaldogsdirect.co.uk/human‐grade‐raw‐dog‐food‐defra/. Accessed September 13, 2021
- Arsevska, E. , Singleton, D. , Sanchez‐Vizcaino, F. , et al. (2017) Small animal disease surveillance: GI disease and salmonellosis. Veterinary Record 181, 228‐232 [DOI] [PubMed] [Google Scholar]
- Bacci, C. , Vismarra, A. , Dander, S. , et al. (2019) Occurrence and antimicrobial profile of bacterial pathogens in former foodstuff meat products used for pet diets. Journal of Food Protection 82, 316‐324 [DOI] [PubMed] [Google Scholar]
- Baede, V. O. , Broens, E. M. , Spaninks, M. P. , et al. (2017) Raw pet food as a risk factor for shedding of extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae in household cats. PLoS One 12, e0187239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor, M. , Hopkins, K. , Threlfall, E. J. , et al. (2005) bla(CTX‐M) genes in clinical Salmonella isolates recovered from humans in England and Wales from 1992 to 2003. Antimicrobial Agents and Chemotherapy 49, 1319‐1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojanic, K. , Midwinter, A. C. , Marshall, J. C. , et al. (2017) Isolation of Campylobacter spp. from client‐owned dogs and dats, and retail raw meat pet food in the Manawatu, New Zealand. Zoonoses and Public Health 64, 438‐449 [DOI] [PubMed] [Google Scholar]
- Bush, K. (2018) Past and present perspectives on beta‐lactamases. Antimicrobial Agents and Chemotherapy 62, e01076‐18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (2013) Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. 4th edn., VET01‐A4. Clinical and Laboratory Standards Institute, Wayne, PA, USA: [Google Scholar]
- CLSI (2015) Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 3rd edn., CLSI supplement VET01S. Clinical and Laboratory Standards Institute, Wayne, PA, USA: [Google Scholar]
- Dallenne, C. , Da Costa, A. , Decre, D. , et al. (2010) Development of a set of multiplex PCR assays for the detection of genes encoding important beta‐lactamases in Enterobacteriaceae. Journal of Antimicrobial Chemotherapy 65, 490‐495 [DOI] [PubMed] [Google Scholar]
- De Kraker, M. E. , Wolkewitz, M. , Davey, P. G. , et al. (2011) Burden of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third‐generation cephalosporins. Journal of Antimicrobial Chemotherapy 66, 398‐407 [DOI] [PubMed] [Google Scholar]
- Eckford, S. , Grace, K. , Harris, C. , et al. (2013) UK Veterinary Antibiotic Resistance and Sales Surveillance: UK‐VARSS 2013. Addlestone, UK, UK, Veterinary Medicines Directorate (VMD) [Google Scholar]
- Ewers, C. , Bethe, A. , Semmler, T. , et al. (2012) Extended‐spectrum beta‐lactamase‐producing and AmpC‐producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clinical Microbiology and Infection 18, 646‐655 [DOI] [PubMed] [Google Scholar]
- Food Standards Agency (FSA) . (2019) How to cook your food to prevent food poisoning. https://www.food.gov.uk/safety‐hygiene/cooking‐your‐food. Accessed March 13, 2021
- Frost, A. (2017) Feeding of raw Brucella suis‐infected meat to dogs in the UK. Veterinary Record 181, 484 [DOI] [PubMed] [Google Scholar]
- Gould, I. M. (2009) Antibiotic resistance: the perfect storm. International Journal of Antimicrobial Agents 34(Suppl 3), S2‐S5 [DOI] [PubMed] [Google Scholar]
- Guardabassi, L. , Schwarz, S. & Lloyd, D. H. (2004) Pet animals as reservoirs of antimicrobial‐resistant bacteria. Journal of Antimicrobial Chemotherapy 54, 321‐332 [DOI] [PubMed] [Google Scholar]
- Hellgren, J. , Hasto, L. S. , Wikstrom, C. , et al. (2019) Occurrence of Salmonella, Campylobacter, Clostridium and Enterobacteriaceae in raw meat‐based diets for dogs. Veterinary Record 184, 442 [DOI] [PubMed] [Google Scholar]
- Hopkins, K. L. , Batchelor, M. J. , Liebana, E. , et al. (2006) Characterisation of CTX‐M and AmpC genes in human isolates of Escherichia coli identified between 1995 and 2003 in England and Wales. International Journal of Antimicrobial Agents 28, 180‐192 [DOI] [PubMed] [Google Scholar]
- Johnson, J. R. , Sannes, M. R. , Croy, C. , et al. (2007) Antimicrobial drug‐resistant Escherichia coli from humans and poultry products, Minnesota and Wisconsin, 2002‐2004. Emerging Infectious Diseases 13, 838‐846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J. R. , Owens, K. , Gajewski, A. , et al. (2008) Escherichia coli colonization patterns among human household members and pets, with attention to acute urinary tract infection. The Journal of Infectious Diseases 197, 218‐224 [DOI] [PubMed] [Google Scholar]
- Jones, J. L. , Wang, L. , Ceric, O. , et al. (2019) Whole genome sequencing confirms source of pathogens associated with bacterial foodborne illness in pets fed raw pet food. Journal of Veterinary Diagnostic Investigation 31, 235‐240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, E. K. , Pearl, D. L. , Finley, R. L. , et al. (2011) Evaluation of pet‐related management factors and the risk of Salmonella spp. carriage in pet dogs from volunteer households in Ontario (2005‐2006). Zoonoses and Public Health 58, 140‐149 [DOI] [PubMed] [Google Scholar]
- Lowden, P. , Wallis, C. , Gee, N. , et al. (2015) Investigating the prevalence of Salmonella in dogs within the Midlands region of the United Kingdom. BMC Veterinary Research 11, 239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniels, A. E. , Rice, E. W. , Reyes, A. L. , et al. (1996) Confirmational identification of Escherichia coli, a comparison of genotypic and phenotypic assays for glutamate decarboxylase and beta‐d‐glucuronidase. Applied and Environmental Microbiology 62, 3350‐3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MedCalc easy‐to‐use statistical software (2021) https://www.medcalc.org/calc/comparison_of_proportions.php. Accessed January 16, 2021
- M'Zali, F. H. , Chanawong, A. , Kerr, K. G. , et al. (2000) Detection of extended‐spectrum beta‐lactamases in members of the family enterobacteriaceae: comparison of the MAST DD test, the double disc and the Etest ESBL. Journal of Antimicrobial Chemotherapy 45, 881‐885 [DOI] [PubMed] [Google Scholar]
- Nilsson, O. (2015) Hygiene quality and presence of ESBL‐producing Escherichia coli in raw food diets for dogs. Infection Ecolology & Epidemiology 5, 28758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran, C. , Tørnqvist‐Johnsen, C. , Woods, G. , et al. (2020) Feline tuberculosis caused by Mycobacterium bovis infection of domestic UK cats associated with feeding a commercial raw food diet. Transboundary and Emerging Diseases 68, 2308‐2320 [DOI] [PubMed] [Google Scholar]
- Perez‐Perez, F. J. & Hanson, N. D. (2002) Detection of plasmid‐mediated AmpC beta‐lactamase genes in clinical isolates by using multiplex PCR. Journal of Clinical Microbiology 40, 2153‐2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England (PHE) . (2013) Summary of electronic foodborne and non‐foodborne gastrointestinal outbreak surveillance system (eFOSS) data. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/426019/eFOSS_surveillance_tables_for_Web.pdf. Accessed March 2, 2021
- Public Health England (PHE) . (2018) Investigation into an outbreak of Shiga toxin producing Escherichia coli (STEC) O157 PT 21/28 Stx2 in England, August 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/765498/STEC_O157_PT21.28_Outbreak_Report.pdf. Accessed March 2, 2021
- Public Health England (PHE) . (2019) Raw pet foods: handling and preventing infection. https://www.gov.uk/guidance/raw‐pet‐foods‐handling‐and‐preventing‐infection. Accessed March 2, 2021
- Rabash, J. , Charlton, C. , Browne, W. J. , et al. (2016) MLwiN Version 2.36. http://www.bristol.ac.uk/cmm/media/software/mlwin/downloads/manuals/2‐36/manual‐print.pdf. Accessed January 16, 2021
- Randall, L. P. , Lodge, M. P. , Elviss, N. C. , et al. (2017) Evaluation of meat, fruit and vegetables from retail stores in five United Kingdom regions as sources of extended‐spectrum beta‐lactamase (ESBL)‐producing and carbapenem‐resistant Escherichia coli . International Journal of Food Microbiology 241, 283‐290 [DOI] [PubMed] [Google Scholar]
- Reimschuessel, R. , Grabenstein, M. , Guag, J. , et al. (2017) Multilaboratory survey to evaluate Salmonella prevalence in diarrheic and nondiarrheic dogs and cats in the United States between 2012 and 2014. Journal of Clinical Microbiology 55, 1350‐1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robicsek, A. , Strahilevitz, J. , Sahm, D. F. , et al. (2006) qnr prevalence in ceftazidime‐resistant Enterobacteriaceae isolates from the United States. Antimicrobial Agents and Chemotherapy 50, 2872‐2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runesvard, E. , Wilkstrom, C. , Fernstrom, L. L. , et al. (2020) Presence of pathogenic bacteria in faeces from dogs fed raw meat‐based diets or dry kibble. Veterinary Record 187, e71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, V. M. , Pinchbeck, G. L. , Nuttall, T. , et al. (2015) Antimicrobial resistance risk factors and characterisation of faecal E. coli isolated from healthy Labrador retrievers in the United Kingdom. Preventative Veterinary Medicine 119, 31‐40 [DOI] [PubMed] [Google Scholar]
- Schmidt, V. M. , Pinchbeck, G. , McIntyre, K. M. , et al. (2018) Routine antibiotic therapy in dogs increases the detection of antimicrobial‐resistant faecal Escherichia coli . Journal of Antimicrobial Chemotherapy 73, 3305‐3316 [DOI] [PubMed] [Google Scholar]
- Tenaillon, O. , Skurnik, D. , Picard, B. , et al. (2010) The population genetics of commensal Escherichia coli . Nature Reviews Microbiology 8, 207‐217 [DOI] [PubMed] [Google Scholar]
- The European Committee on Antimicrobial Susceptiblity Testing (EUCAST) . (2015). Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0, 2015. https://eucast.org. Accessed December 31, 2015
- Tuerena, I. , Williams, N. J. , Nuttall, T. , et al. (2016) Antimicrobial‐resistant Escherichia coli in hospitalised companion animals and their hospital environment. Journal of Small Animal Practice 57, 339‐347 [DOI] [PubMed] [Google Scholar]
- UK‐VARSS 2020 . (2019) Veterinary antibiotic resistance and sales surveillance report. https://www.gov.uk/government/publications/veterinary‐antimicrobial‐resistance‐and‐sales‐surveillance‐2019. Accessed February 2, 2021
- van Bree, F. P. J. , Bokken, G. , Mineur, R. , et al. (2018) Zoonotic bacteria and parasites found in raw meat‐based diets for cats and dogs. Veterinary Record 182, 50 [DOI] [PubMed] [Google Scholar]
- Viegas, F. M. , Ramos, C. P. , Xavier, R. G. C. , et al. (2020) Fecal shedding of Salmonella spp., Clostridium perfringens, and Clostridioides difficile in dogs fed raw meat‐based diets in Brazil and their owners' motivation. PLoS One 15, e0231275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila, J. , Saez‐Lope, E. , Johnson, J. R. , et al. (2016) Escherichia coli: an old friend with new tidings. FEMS Microbiology Reviews 40, 437‐463 [DOI] [PubMed] [Google Scholar]
- Wareham, K. (2019) Risk factors for the carriage of antimicrobial‐resistant Escherichia coli in puppies and adult dogs. Master's Thesis, University of Bristol
- Wedley, A. L. , Maddox, T. W. , Westgarth, C. , et al. (2011) Prevalence of antimicrobial‐resistant Escherichia coli in dogs in a cross‐sectional, community‐based study. Veterinary Record 168, 354 [DOI] [PubMed] [Google Scholar]
- Wedley, A. L. , Dawson, S. , Maddox, T. W. , et al. (2017) Carriage of antimicrobial resistant Escherichia coli in dogs: prevalence, associated risk factors and molecular characteristics. Veterinary Microbiology 199, 23‐30 [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) . (2019) Critically important antimicrobials for human medicine. https://www.who.int/foodsafety/publications/antimicrobials‐sixth/en/. Accessed January 23, 2021
- Zogg, A. L. , Simmen, S. , Zurfluh, K. , et al. (2018) High prevalence of extended‐spectrum beta‐lactamase producing Enterobacteriaceae among clinical isolates from cats and dogs admitted to a veterinary hospital in Switzerland. Frontiers in Veterinary Science 5, 62 [DOI] [PMC free article] [PubMed] [Google Scholar]


