Abstract
Delivery of biologics via cerebrospinal fluid (CSF) has demonstrated potential to access the tissues of the central nervous system (CNS) by circumventing the blood‐brain barrier and blood‐CSF barrier. Developing an effective CSF drug delivery strategy requires optimization of multiple parameters, including choice of CSF access point, delivery device technology, and delivery kinetics to achieve effective therapeutic concentrations in the target brain region, whereas also considering the biologic modality, mechanism of action, disease indication, and patient population. This review discusses key preclinical and clinical examples of CSF delivery for different biologic modalities (antibodies, nucleic acid‐based therapeutics, and gene therapy) to the brain via CSF or CNS access routes (intracerebroventricular, intrathecal‐cisterna magna, intrathecal‐lumbar, intraparenchymal, and intranasal), including the use of novel device technologies. This review also discusses quantitative models of CSF flow that provide insight into the effect of fluid dynamics in CSF on drug delivery and CNS distribution. Such models can facilitate delivery device design and pharmacokinetic/pharmacodynamic translation from preclinical species to humans in order to optimize CSF drug delivery to brain regions of interest.
INTRODUCTION
Delivery of biologic modalities to the central nervous system (CNS) via routine systemic administrations presents a challenge due to the blood‐brain barrier (BBB) and blood‐cerebrospinal fluid (CSF) barriers. 1 , 2 Preclinical and clinical data indicate that therapeutic antibodies have very limited brain partitioning when administered intravenously with less than 1% of antibody in plasma being delivered to the brain. 2 , 3 The CSF provides an access point to the brain and spinal cord by circumventing these barriers. The CSF is produced in the choroid plexus (at the blood‐CSF barrier), flows through ventricles, cisterns, and subarachnoid space in the brain and diffuses into interstitial fluid (ISF) in the brain bathing the brain parenchyma. 4 The CSF produced by choroid plexus also flows into the spinal canal along the spinal cord. 4 Access to CSF can be gained through different locations along the CSF flow path. 5 A balance of invasiveness and efficiency needs to be considered in selecting a suitable CSF access point. Advantages and limitations of the CSF access points are discussed in this review and need to be considered when setting a CSF delivery strategy.
Furthermore, this review discusses other factors that need to be considered when defining a CSF delivery strategy for a biologic modality. For instance, desired CNS distribution, targeted CNS regions of interest, mechanism of action, biologic modality of choice, duration of treatment, disease indication, and patient population. Key preclinical and clinical examples are described for various biologic modality formats, such as antibodies, enzymes, nucleic acid‐based therapeutics, and gene therapy. Understanding the impact of the CSF delivery access point, delivery parameters, and nature of drug modality on biodistribution of therapeutic in the brain is critical to inform the CSF delivery strategy. Novel biologic modalities, such as ribonucleic acid (RNA), gene, and cell therapeutics also require catheter and device‐assisted drug delivery at different CSF access locations for effective brain access. Ongoing innovation in this space will enable desired delivery rates, dosing frequency, patient safety, and comfort.
Quantitative models of CSF flow can provide insight into the effect of fluid dynamics in CSF on drug delivery and CNS distribution and facilitate pharmacokinetic/pharmacodynamic (PK/PD) and delivery translation from preclinical species to humans. 6 This review also discusses the computational fluid dynamics modeling approaches taken to optimize CSF drug delivery to brain regions of interest.
In summary, the focus of this review is brain delivery of biologic formats via CSF: comparison of CSF access locations, delivery needs for different biologic modalities with diverse mechanisms of actions, device‐based CSF delivery, and quantitative flow modeling to optimize delivery. In addition to device‐based delivery, novel formulation‐based approaches for targeting the CNS are being explored and reviewed elsewhere. 7 There have also been significant efforts to enhance brain delivery of biologics administered systemically by enhancing transport across the BBB. These have been reviewed elsewhere and are out of scope of this review. 8 , 9 , 10
CSF PHYSIOLOGY, FLOW, AND ACCESS LOCATIONS FOR DELIVERY
The CSF, primarily composed of water, bathes the CNS. Volume of CSF in an average adult is 250–400 mL with the majority of fluid bathing the brain in the cranial subarachnoid space (~ 2/3) and the remainder in ventricles, cisterns, and the spinal canal. 11 , 12 In humans, CSF is produced primarily in the choroid plexus. Classical theories suggest CSF flows through the ventricles, cisterns, and subarachnoid space to be primarily absorbed into venous blood via arachnoid villi. A minority portion was thought to drain into cervical lymph nodes. 4 , 13 Turnover of human CSF is rapid at about 3–4 times a day presenting a challenge for drug residence time due to rapid efflux out of CSF to plasma or lymph. 4 , 13 CSF flow is driven by forces generated due to cardiac pulsations, pulmonary respiration, and synchronous beating of ciliated ependymal. 14 With advancements in microscopic and live imaging techniques, newer theories on CSF production and absorption have emerged with a focus on the Virchow‐Robin perivascular spaces (VRS) and lymphatics for CSF drainage referred to as the glymphatic system. 4 , 13 , 15 , 16 , 17 The VRS is enclosed by glial and plial cells that control CSF exchange and drainage of CSF into lymphatics. 4 , 13 , 15 , 16 , 17 These theories highlight the complex role of the VRS that enables bidirectional fluid exchange among the VRS, brain ISF, and the subarachnoid CSF.
There are some known differences in ventricular physiology and CSF flow across species. In humans and monkeys, the fourth ventricle is aligned along the ventral‐to‐dorsal axis, whereas in rodents, it is aligned rostral‐to‐caudal (on the same axis as the lateral and third ventricles). 18 Because CSF moves by bulk flow, mainly driven by arterial pulsation and respiration, we speculate that ventricular orientation differences between species may not have significant implications to interpreting and translating PK/PD of intrathecally dosed biologics. The known CSF volume, production, and turnover differences across species parameters have been recently reviewed. 7 CSF volume appears to increase allometrically with species (~ 0.15 mL in rats, ~ 13–15 mL in monkeys, and ~ 100–400 mL in humans), whereas turnover is slower in higher species (~ 12–14 times/day in rats to 3–5 times/day in monkeys and humans). 7 The large variation in CSF volume and brain size, as well as surface area across species, means that the distance from the CSF space to the deeper brain compartments is different between species. This is likely to influence the exchange between these two compartments. These differences are expected to have a significant impact on PK/PD translation across species. Quantitative models of CSF flow discussed in subsequent section on “CSF flow modeling for drug delivery and CNS distribution” can account for such interspecies differences and facilitate translation of CSF delivery and CNS drug distribution across species.
Mechanisms of CSF egress from the subarachnoid space and contribution of venous blood vs. lymphatics in CSF drainage can also vary across species. 19 , 20 , 21 , 22 The complexity of the human arachnoid villi has been extensively reported and was shown to be different between primates and lower animals, with rat villi not penetrating the superior sagittal sinus in contrast to observations in humans and monkeys. 23 The intracellular transport mechanism has also been shown to differ between species: in primates, the bulk outflow between villi is via a dynamic system of transcellular pores formed by single cell membranous infoldings, termed giant vacuoles, whereas in lower animals it is primarily by pinocytosis. 24 Understanding of the relative contribution and mechanisms for the CSF outflow pathways and how that relates across species is an area of research that is evolving and needs to be considered for interspecies comparisons and drug delivery, PK/PD translations. Detailed perspectives on the same have been recently reviewed. 6
For drug delivery into the CSF, there is a need to balance invasiveness and efficiency. Three primary CSF access locations have been explored from the more invasive intracerebroventricular (ICV) and intrathecal‐cisterna magna (IT‐CM) to the less‐invasive intrathecal‐lumbar (IT‐L) (Figure 1 ). 5 Direct injections into brain parenchyma and ISF is an alternative location being investigated when local delivery to select brain regions is desired. 25 The intranasal (IN) access route to cranial CSF and brain parenchyma via the olfactory and trigeminal nerves has been used primarily for analgesics in the clinic, and is being explored for brain delivery of smaller biologic modalities, such as nucleic acid‐based therapeutics. 26 Table 1 compares and contrasts the advantages and limitations of each of these access points. Specific examples for CSF delivery of biologic modalities via these access points are discussed in subsequent section on “Drug delivery considerations for CNS delivery of biologic modalities via CSF.”
Figure 1.
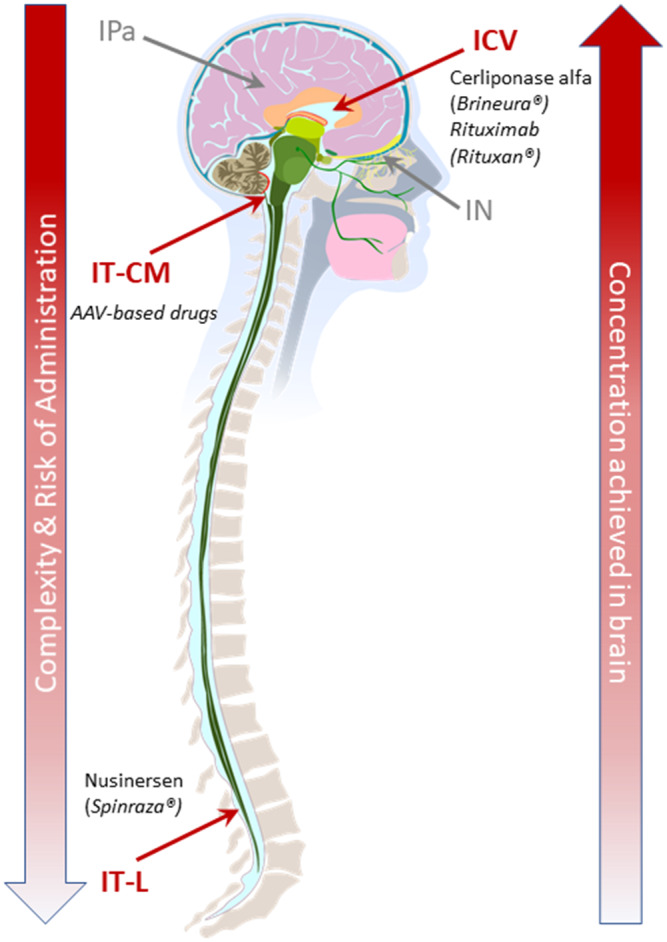
CNS delivery access locations require balancing effective delivery with invasiveness of the procedures. CSF access points shown in red (ICV, IT‐CM, and IT‐L); alternative access points to CNS not via CSF shown in grey (IN and IPa). Complexity and risk of administration, as well as achievable drug concentrations in the brain, are lowest for CSF access points that are more distal from the brain (e.g., IT‐L). CSF access points that are in closer proximity to the brain (e.g., IT‐CM) or within the brain (e.g., ICV) can achieve higher drug concentrations in brain, but are more invasive, with increased complexity and higher risks of complications. The IPa delivery can enable high drug exposure in regions of particular interest, but is highly invasive, whereas IN delivery is noninvasive, but so far has fewer applications due to limitations on achievable exposure and compatible drug types. Key examples of biologic modalities via CSF access points are highlighted in figure and further discussed in subsequent section on “Drug delivery considerations for CNS delivery of biologic modalities via CSF.” CNS, central nervous system; CSF, cerebrospinal fluid; ICV, intracerebroventricular; IN, intranasal; IPa, intraparenchymal; IT‐CM, intrathecal‐cisterna magna; IT‐L, intrathecal‐lumbar.
Table 1.
CNS delivery access locations: Advantages and Limitations. Expanded from 5 (Original publisher MDPI open access)
| CSF access location | Advantages | Limitations |
|---|---|---|
| IT‐L 30 , 35 , 38 , 39 , 56 |
|
|
| IT‐CM 53 , 54 , 57 |
|
|
| ICV 27 , 28 , 29 |
|
|
| IPa 25 , 49 , 50 , 52 , 58 |
|
|
| IN 26 |
|
|
BBB, blood‐brain barrier; CNS, central nervous system; CSF, cerebrospinal fluid; ICV, intracerebroventricular; IN, intranasal; IPa, intraparenchymal; IT‐CM, intrathecal‐cisterna magna; IT‐L, intrathecal‐lumbar.
Drug delivery considerations for CNS delivery of biologic modalities via CSF
Novel biologic modalities, such as RNA and gene therapies, are tapping into previously undruggable target space in the CNS. Drug delivery considerations and strategies for these new modalities can differ from traditional biologic modalities, such as antibodies and proteins. Table 2 outlines the drug delivery considerations for the different modalities and lists the CSF delivery routes being explored. Subsequent to the table are described preclinical or clinical case examples of CSF delivery in each of the biologic modality categories. These case examples compare the distribution of biologic modalities via various CSF access locations while highlighting the diverse mechanism of action of the biologic modalities driving CSF delivery strategy and device‐based delivery.
Table 2.
Drug delivery considerations for biologics modalities and explored CSF delivery routes
| Antibody/proteins 1 , 30 | Nucleic acid‐based therapeutics 77 | Gene therapy 78 , 79 , 80 | |
|---|---|---|---|
| Mechanism of action | Neutralization/clearance of pathological extracellular proteins, cytokines, or cell surface receptors | RNA degradation, functional block, and splice modulation | Gene replacement, augmentation, silencing, editing, transgene for immunotherapy |
| Effect site | Brain ISF, cell membrane | Nucleus/cytosol | Nucleus |
| PK driver of effect |
Access to brain ISF Drug concentration in brain ISF Sustained target engagement |
Access to brain ISF Productive cellular uptake Endosomal escape |
Tissue/cell tropism of viral vector Endosomal escape Transgene expression Durable pharmacology |
| CSF delivery route |
Primary: i.v., s.c. Explored: ICV, IT‐L, IN |
IT‐L, IN | i.v., IT‐L; IT‐CM, IPa |
CSF, cerebrospinal fluid; ICV, intracerebroventricular; IN, intranasal; IPa, intraparenchymal; ISF, interstitial fluid; IT‐CM, intrathecal‐cisterna magna; IT‐L, intrathecal‐lumbar; PK, pharmacokinetic.
CNS distribution and CSF delivery of antibodies, proteins, and enzymes
Preclinical and clinical data indicate that brain delivery of antibodies via the intravenous (i.v.) route results in very limited partitioning to the brain (< 1%) due to the BBB. 1 The CSF is known to provide better access to the brain by bypassing the BBB, although location of access to the CSF and delivery parameters may affect the uptake and distribution of antibodies to the brain parenchyma. There has been no published systematic evaluation of the impact of these factors on brain tissue access and biodistribution of antibodies. Our in‐house comparisons of PK and PD data in the nonhuman primate (NHP) brain showed that in contrast to the ICV route, IT‐L administration of antibodies at sustained slow flow rates (0.4 mL/day, 5.6 mg/day) did not deliver therapeutic concentrations to brain regions of interest (unpublished data). Animals infused IT‐L with anti‐BACE1 antibody at a three‐fold higher dose and infusion rate (16.8 mg/day and flow‐rate of 1.2 mL/day) also did not deliver therapeutic concentrations to brain regions (unpublished data). Brain concentrations for the IT‐L route were comparable to a published report with the same antibody administered at a weekly high i.v. dose of 50 mg/kg. 27 Consistent with the published report on ICV administration, fast efflux of antibodies was noted from CSF to serum suggesting the majority of the IT‐L infused antibody also effluxed from CSF to serum. 27 There is therefore a need to explore clinically acceptable alternatives with device‐assisted sustained delivery flow rates and locations upstream of the IT‐L that may be able to provide better access to cranial CSF and brain parenchyma. Alternatively, bolus push administration through the IT‐L route could also be considered. However, this approach has limitations in maintaining steady‐state concentration of a therapeutic in the CSF and brain interstitial space. Frequent administration may not be safe or feasible to maintain drug levels. The bolus push and CSF flush approach is discussed for RNA therapeutics in a subsequent section where cellular uptake of the therapeutic post bolus administration can provide a drug depot (“CNS distribution and delivery of nucleotide therapeutics”).
Along with preclinical reports, such as those described above, there is clinical proof of concept for CSF delivery of antibodies. For brain metastasis, an ICV bolus of (Rituxan) rituximab in patients with CNS lymphoma that is refractory or unresponsive to i.v. treatment has demonstrated clear benefit in tumor reduction. 28 , 29 Intrathecal delivery of antibodies via the lumbar route has also been shown to benefit leptomeningeal metastasis that did not require deeper brain penetration. 30 In humans and monkeys, plasma and CSF concentrations of antibodies after administration to the CSF suggest a rapid efflux from CSF to plasma (elimination half‐life of just a few hours). 27 , 28 This highlights the need for a sustained delivery to the CSF of therapeutics that require sustained target engagement. The fast efflux of antibodies from CSF to serum also highlights a need to increase CSF retention of antibodies. Approaches to facilitate drug binding to neutral molecules within CNS matrix could potentially increase retention in CNS. There has been very limited scientific exploration of such approaches in literature. 31
For protein/enzyme therapeutics that require deep brain penetration and homogenous distribution, the recent US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval of BioMarin’s (Brineura) cerliponase alfa for enzyme replacement in a rare form of Battens disease has shown benefit with a slow ICV infusion in the pediatric population (Clinicaltrials.gov number: NCT01907087, EudraCT number 2014‐003480‐37).
More invasive intraparenchymal routes may be attempted when the brain target site of action is localized in a certain brain region and better accessed by direct parenchymal injection. This route requires sophisticated device‐catheter systems with image‐guided delivery. Renishaw’s Neuroinfuse drug delivery system was clinically evaluated in phases I and II for the delivery of glial‐cell derived neurotrophic growth factor in patients with Parkinson’s disease (PD; ClinicalTrials.gov number NCT03295786). The system consists of four catheters that are implanted through a transcutaneous port behind the patient’s ear opening into target regions within the brain. 32 , 33 The accessible port and accurately placed catheters allow for repeated, intermittent drug infusions into the parenchyma with magnetic resonance imaging (MRI) to monitor drug infusion lines. 32 , 33
Besides the intrathecal and intra‐parenchymal routes, there has been limited exploration of the IN route for biologics. 26 The CNS delivery of interferon‐beta, a 20 kDa protein, was investigated in NHPs with a bilateral bolus application to upper nasal passages. 34 The radiolabeled protein was tracked in the peripheral and CNS tissue. The highest concentration was noted in the olfactory bulb and trigeminal nerves, which have been shown to be the primary extracellular pathways to bypass the BBB via the IN routes. 34 Although distribution to CNS regions was limited to certain brain regions, such as the basal ganglia, this could be of significance for some neurological and immunological pathologies, such as multiple sclerosis. 34
CNS distribution and CSF delivery of nucleotide therapeutics
Antisense oligonucleotides (ASO), chemically modified single strand DNA, or RNA, are emerging as a popular class of nucleotide therapeutics for neurological indications requiring CSF delivery. ASOs have been developed to complementarily bind to the target mRNA in the cells and moderate its expression or splicing. Due to its hydrophilic and charged physical chemical properties, an ASO is not able to penetrate the BBB efficiently via systemic administration. Therefore, ASO therapeutics for CNS indications are usually administered intrathecally via lumbar puncture. For example, (Spinraza) nusinersen, which is an ASO targeting survival of motor neuron 2 (SMN2) and modulating the differential splicing of SMN2, has been widely approved in multiple countries for the treatment of genetic motor neuron disease named spinal muscular atrophy (SMA). Nusinersen is administered intrathecally to pediatric and adult patients with 4 loading doses at 12 mg/5 mL injection followed by a maintenance dose every 4 months. 35 Milasen, an ASO approved for the treatment of Batten disease due to CLN mutation, is administered via biweekly IT administration at the beginning followed by a reduction in dosing frequency to every 3 months during the maintenance treatment. 36 Besides the clinically approved ASO therapeutics, IT administration has been or is being extensively explored for ASOs under clinical investigation for CNS diseases. Examples include Tominersen (anti‐HTT ASO) for Huntington’s disease, 37 tofersen (anti‐SOD1 ASO) for amyotrophic lateral sclerosis, 38 , 39 and BIIB080 (anti‐Tau ASO) for frontotemporal dementia and Alzheimer’s disease (AD). 40
The PKs of ASOs in CNS following IT administration has been investigated in both cynomolgus monkeys and humans. ASOs exhibited a biphasic or triphasic concentration‐time profile in CSF from cynomolgus monkeys after single IT administration, with a very rapid reduction at the beginning followed by a slow terminal elimination phase. 41 , 42 , 43 The rapid reduction of ASO concentration in CSF after IT administration is believed to be due to the fast turnover rate of CSF and the distribution to the spinal cord tissues. In contrast to CSF, a sustained exposure of ASO was observed in the spinal cord and brain tissues throughout the study likely due to a combination of nonspecific membrane interaction and intracellular uptake. 41 , 42 , 43 Post IT administration, ASO levels were observed in systemic circulation suggesting that ASOs may distribute to systemic circulation when CSF is absorbed through blood vessels or the lymphatic system. 41 , 42 , 43 However, the relative systemic exposure was lower compared to CSF. In addition to cynomolgus monkeys, the concentrations of ASOs in CSF and brain tissues after IT administration were also evaluated in rats. 44 However, for comparison across species, the CSF turnover rate differences need to be accounted for. Quantitative CSF flow modeling approaches discussed in subsequent section on “CSF flow modeling for drug delivery and CNS distribution” can provide helpful insight in interspecies translation.
Compared with the amount of data obtained in nonclinical species, clinical trial data on the concentrations of ASO in human CSF is usually sparse and variable due to the challenge of obtaining frequent lumbar CSF samples from patients. Nonetheless, dose‐dependent increases in ASO concentrations in human CSF have been reported in the clinical trials of multiple ASO therapeutics. 37 , 39 To evaluate the spatial distribution of ASO in human brain tissue, imaging approaches are being considered. In a clinical study by Biogen, 99mTc‐labeled ASO was in CSF and tissues monitored via single photon emission computed tomography) imaging (ClinicalTrials.gov number NCT03764488).
Although IT‐L administration as a high‐volume bolus is the common CSF delivery procedure for commercially available ASO products, an implantable subcutaneous port connected to a permanent intrathecal catheter system is being investigated as an alternative, for patients with severe scoliosis, spinal fusion, or comorbidities that make serial interlaminar punctures complicated and risky. 45 These studies were done as off‐label use for the port catheter system. Feasibility, safety, and tolerability of the intrathecal port and catheter‐ assisted administration were assessed in eight patients with SMA II/SMA III receiving nusinersen. 45 The commercially available intrathecal port catheter (Ascenda, Medtronic Germany or Celsite Safety, Braun, Germany) was implanted subcutaneously and the intrathecal catheter was placed via microsurgical hemilaminectomy at L4. 45 Although leakage of the port catheter occurred in two patients, promptly resolved after re‐suturing, no further complications, such as infection, dislocation, kinking, or obstruction of the port, were noted in any of the patients. 45 An IT port and catheter was found to be a safe and feasible administration option for nusinersen treatment in subjects with SMA, however, longer‐term assessment in a larger study cohort is still needed.
Although the primary route of delivery for ASOs used clinically has been to the CSF given IT‐L, recently, there have been a few noteworthy preclinical examples of successful delivery of nucleotide therapeutics using delivery systems, such as nanoplexes and targeted conjugates to the brain via IN administration. 26 , 34 , 46 , 47 , 48 In one case, an anti‐SNCA ASO was selectively targeted to monoamine neurons in mice using an indatraline‐ASO conjugate. 47 Confocal fluorescence microscopy demonstrated that functional monoamine transporters served to enable selective oligonucleotide uptake and internalization in monoamine neurons along with target mRNA and protein knockdown. In another case, the biodistribution and efficacy of IN delivery of small interfering RNA against the Beclin1 gene nanoplexed with cationic polyethyleneimines was investigated in an adult mouse brain. 46 Successful delivery of siRNA in different cell types of the prefrontal cortex (cytoplasm of neurons and glial cells) was noted along with functional benefit post repeat dosing with minimal off‐target effects in the lung. In a more recent example, a study compared IT‐ICV and IN delivery of anti‐HuR ASO in mice. 48 The effect was comparable between the two routes with similar extents of spinal HuR protein reduction and inhibition of microglial‐mediated spinal neuroinflammation suggesting the potential for effective access of nucleotide therapeutics by the IN route.
CNS distribution and CSF delivery of gene therapy
CSF delivery presents an attractive route for in vivo gene therapy due to the potential for better access to the brain, reduced systemic exposure and immunogenicity, compared with systemic administration. More invasive delivery routes can be attempted with gene therapy products due to their durable pharmacology and the requirement of only a single administration.
The gene therapy product for SMA, (Zolgensma) onasemnogene abeparvovec‐xioii, is an adeno‐associated virus AAV9‐based delivery currently approved for intravenous administration in the pediatric population under 2 years of age by the FDA and EMA (Clinicaltrials.gov numbers NCT02122952, NCT03306277, NCT03461289, and EudraCT numbers 2020‐001235‐27 and 2020‐000095‐38). Potential for CSF delivery of Zolgensma is being evaluated in the clinical setting in the older pediatric population to enable better spinal access (ClinicalTrials.gov number NCT03381729).
CNS distribution of AAV‐based gene therapy has been evaluated in NHPs using different CSF access points as well as serotypes. 5 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 In Gray et al., both IT‐CM and IT‐L routes were able to achieve broad transduction of green fluorescent protein in the NHP brain using the natural AAV9 serotype as well as a recombinant AAV2.5 serotype. 51 Peripheral organ distribution was reduced compared to the i.v. route and CNS transduction was effective in the presence of neutralizing antibodies. 51 However, Hinderer et al. reported that delivery of AAV9 to an NHP brain is far more efficient via IT‐CM than the IT‐L route. 55 These studies used slightly different bolus push volumes (1 mL in Hinderer et al. vs. 2 mL in Gray et al.), which may contribute to less efficient delivery via the IT‐L route. Subsequently, Hinderer et al. also reported that both IT‐CM and ICV administration yielded similarly effective AAV9 brain distribution in dogs, but encephalitis occurred only in the ICV group. 56 The report also highlighted that IT‐CM continued to be superior for NHP brain access compared with IT‐L in Trendelenburg position—speculated to improve cranial flow of CSF. 56 Their findings highlight the potential of the IT‐CM route for AAV‐based gene therapy with advantages of improved brain tissue access and reduced immune responses.
Clinical safety for the novel IT‐CM route of administration of gene therapies is being evaluated in a few phase I clinical trials and may be suitable for one‐time administration of therapeutics (ClinicalTrials.gov numbers: NCT04713475, NCT04408625, and NCT04127578). However, IT‐CM administration is not a commonly utilized clinical procedure and significant procedural development is required due to high risks with medullary injury and related complications, which could be fatal. 54 The IT‐CM route can benefit from fluoroscopic image‐guided delivery for controlled administration. Taghian et al. developed a catheter‐mediated IT‐CM delivery for AAV vectors using an intravascular microcatheter navigated in the spinal canal from the lumbar region to the cisterna magna. 57 The safety, reproducibility, vector distribution, and transduction of an (scAAV9)‐GFP vector was evaluated in sheep using this catheter system. Broad distribution of AAV and transduction of GFP was observed in the brain. The technique was also experimentally tested in two infants with Tay‐Sachs disease with AAV gene therapy. No adverse effects were noted during infusion or post‐treatment. This catheter‐based delivery technique through the IT‐L access point is a minimally invasive alternative to direct infusion into the cisterna magna. 57
When delivered via CSF, significant spinal cord and dorsal root ganglion transduction has been observed for AAV gene therapies in NHP resulting in neuroinflammatory response, which may limit the therapeutic window and high dose that can be utilized. 5 However, our understanding of the translatability of this finding to humans is evolving. Other practical limitations to dosing high can be due to concentration limits on AAV formulations (~ 1013 vg/mL), small volume administration via IT‐CM, or low flow‐rate infusions via ICV.
Gene therapy that targets restricted brain regions could benefit from a direct intraparenchymal delivery. Although more invasive, this route is being explored with image‐guided delivery for precision. A real‐time MRI‐guided SmartFlow neuro‐ventricular cannula is being investigated for VY‐AADC gene therapy in the case of PD programs (clinicaltrials.gov numbers NCT03562494 and NCT01973543). An MRI‐guided cannula preferentially targets the putamen with convection‐enhanced infusion of about 1 mL of transgene solution for one time administration. Phase I and II trials reported some brain abnormalities but acceptable tolerability overall. Dose‐dependent increases in transduction and clinical measures were noted along with persistence of benefit. 58
Cell therapies utilizing hematopoietic stem cells, adult‐tissue derived mesenchymal stem cells, or induced pluripotent stem cells have therapeutic potential for neurological diseases, such as AD, PD, amyotrophic lateral sclerosis, brain tumors, stroke, spinal cord injury, and traumatic brain injury. 59 Such therapies may also benefit from a direct injection into injured tissues of the CNS utilizing device‐catheter systems being developed for RNA and gene therapies. The less invasive, clinically acceptable IT‐L route has been explored for cell therapies in animal models and human with potential for benefit in spinal cord injury, amyotrophic lateral sclerosis, and AD. 60 , 61 , 62 , 63 , 64 (ClinicalTrials.gov numbers NCT01933321, NCT01363401, NCT04821479, NCT01254539, and NCT03828123).
CSF flow modeling for drug delivery and CNS distribution
Our scientific understanding of how CSF dynamics affects CSF drug distribution is evolving and is described in reports of varying model‐complexity to describe CSF flow. 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 Computational fluid dynamic (CFD) modeling is a great tool that provides additional insight into CSF dynamics, identifies delivery parameters to ensure targeted delivery to the intended site in the brain, and facilitates translation from animal to human. CFD modeling of CSF flow involves a computer‐aided design model of the anatomic compartments of CSF, spine, and brain (informed by anatomic MRI) and CSF flow data (informed by Phase Contrast MR techniques). Well‐developed and validated CSF flow models can serve as great tools to replace/reduce costly animal experiments, facilitate translation to humans, and/or test scenarios in humans where conducting invasive experiments are not possible.
Several studies have investigated the effect of the injection method and injection parameters on CSF drug distribution. For example, Kuttler et al. compared drug distribution following bolus injection versus IT infusion of the same dose in a 3D computational model of the spinal canal. 65 Haga et al. developed a subject‐specific numerical model of the cervical subarachnoid space and conducted simulations to investigate the effect of anatomy, catheter position, and angle and flow rate of injection on solute distribution. 70 Among the parameters investigated, they found that catheter position and angle could alter the spread up to 86%. 70 Pizzichelli et al. investigated the effect of catheter position and angle on local spinal cord drug concentration and found that lateral injection perpendicular to the cord was the more effective method among the scenarios tested. 72 Khani et al. investigated the impact of neurapheresis therapy on tracer removal from CSF compared with lumbar drain over a 24‐hour period using a subject‐specific CFD model of CSF solute transport and found neurapheresis therapy to substantially increase tracer clearance compared to lumbar drain. 76 Finally, Tangen et al. leveraged the CFD model to identify parameters to achieve drug distribution to specific tissues in the CNS by varying infusion settings, drug chemistry, and subject‐specific anatomy accounting for kinetics of tissue uptake. 71 As illustrated by the examples above, CFD models of CSF flow can be used as a framework to find suitable parameters (Injection volume, injection location, ...) for intended drug distribution by integrating fluid mechanics, drug transport, and potentially biochemical reaction kinetics. Better understanding and optimization of drug distribution in CSF can directly enhance preclinical and clinical development of drugs delivered via CSF and interspecies translation. The computational framework reviewed here enables testing of different drugs, devices used for injection, and location of injection to find the optimum scenario for effective drug delivery to the region of interest and improve probability of success.
CONCLUSION
Delivery of biologics and nucleic acid therapeutics via CSF has demonstrated potential to access CNS tissues. Access to CSF can be via multiple locations along the CNS‐spinal canal and ventricular space. Choice of CSF access location is a balance of invasiveness, clinical practice, safety, and efficacy. Developing a CSF drug delivery strategy includes defining a CSF access point and a drug delivery device to enable the desired dosing parameters, duration, and frequency of delivery. Factors determining CSF delivery strategy will include the drug modality, mechanism of action, target dose, brain region of interest, disease indication, and patient population. Novel biologic modalities, such as RNA and gene therapies, are opening up druggable target space in the CNS. CSF delivery is a promising approach to enable efficient delivery of novel biologics and ensure their technical and clinical success. An understanding of CSF flow, physiological differences across species, and drug distribution in brain regions is critical to enable successful translation of CSF delivery in humans. Innovation in CSF delivery devices is key to enable efficient and safe brain delivery of therapeutics via CSF.
FUNDING
This research was funded by Genentech, Inc.
CONFLICT OF INTEREST
The authors were affiliated with Genentech, Inc. when performing this work.
ACKNOWLEDGMENTS
The authors acknowledge Northern Biomedical Research for in‐life conduct of NHP study discussed in section on “CNS distribution and CSF delivery of antibodies, proteins, and enzymes,” Paul Nauleau and Darren Doud for characterizing CSF delivery pumps for NHP study discussed in “CNS distribution and CSF delivery of antibodies, proteins, and enzymes,” and Kathleen Farinas for engaging in discussions on CSF delivery of therapeutics.
- 1. Pardridge, W.M. CSF, blood‐brain barrier, and brain drug delivery. Expert Opin. Drug Del. 13, 963–975 (2016). [DOI] [PubMed] [Google Scholar]
- 2. Pardridge, W.M. Blood‐brain barrier and delivery of protein and gene therapeutics to brain. Front. Aging Neurosci. 11, 373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kouhi, A. et al. Brain disposition of antibody‐based therapeutics: dogma, approaches and perspectives. Int. J. Mol. Sci. 22, 6442 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brinker, T. , Stopa, E. , Morrison, J. & Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11, 10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez, B.A. , Shutterly, A. , Chan, Y.K. , Byrne, B.J. & Corti, M. Management of neuroinflammatory responses to AAV‐mediated gene therapies for neurodegenerative diseases. Brain Sci. 10, 119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naseri Kouzehgarani, G. et al. Harnessing cerebrospinal fluid circulation for drug delivery to brain tissues. Adv. Drug Deliver. Rev. 173, 20–59 (2021). [DOI] [PubMed] [Google Scholar]
- 7. Fowler, M.J. et al. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliver. Rev. 165, 77–95 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pardridge, W.M. Delivery of biologics across the blood‐brain barrier with molecular Trojan horse technology. Biodrugs 31, 503–519 (2017). [DOI] [PubMed] [Google Scholar]
- 9. Xiao, G. & Gan, L.‐S. Receptor‐mediated endocytosis and brain delivery of therapeutic biologics. Int. J. Cell Biol. 2013, 1–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lajoie, J.M. & Shusta, E.V. Targeting receptor‐mediated transport for delivery of biologics across the blood‐brain barrier. Annu. Rev. Pharmacol. 55, 613–631 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sass, L.R. et al. A 3D subject‐specific model of the spinal subarachnoid space with anatomically realistic ventral and dorsal spinal cord nerve rootlets. Fluids Barriers CNS 14, 36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chazen, J.L. et al. Automated segmentation of MR imaging to determine normative central nervous system cerebrospinal fluid volumes in healthy volunteers. Clin. Imag. 43, 132–135 (2017). [DOI] [PubMed] [Google Scholar]
- 13. Khasawneh, A.H. , Garling, R.J. & Harris, C.A. Cerebrospinal fluid circulation: What do we know and how do we know it? Brain Circul. 4, 14–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dreha‐Kulaczewski, S. et al. Respiration and the watershed of spinal CSF flow in humans. Sci. Rep‐UK 8, 5594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hladky, S.B. & Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11, 26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mestre, H. , Mori, Y. & Nedergaard, M. The brain’s glymphatic system: current controversies. Trends Neurosci. 43, 458–466 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mantovani, G. , Menegatti, M. , Scerrati, A. , Cavallo, M.A. & De Bonis, P.D. Controversies and misconceptions related to cerebrospinal fluid circulation: a review of the literature from the historical pioneers’ theories to current models. Biomed. Res. Int. 2018, 1–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snyder, J.M. 20 – Nervous System. In Comparative Anatomy and Histology. 2nd edn. (eds. Treuting, P.M. , Dintzis, S.M. & Montine, K.S. ), pp. 403–444. (Academic Press, San Diego, CA, 2018). [Google Scholar]
- 19. Pollay, M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 7, 9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boulton, M. , Flessner, M. , Armstrong, D. , Hay, J. & Johnston, M. Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am. J. Physiology‐regulatory Integr. Comp. Physiol. 274, R88–R96 (1998). [DOI] [PubMed] [Google Scholar]
- 21. Bradbury, M.W. & Cole, D.F. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J. Physiol. 299, 353–365 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma, Q. , Ineichen, B.V. , Detmar, M. & Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8, 1434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Upton, M.L. & Weller, R.O. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J. Neurosurg. 63, 867–875 (1985). [DOI] [PubMed] [Google Scholar]
- 24. Tripathi, B.J. & Tripathi, R.C. Vacuolar transcellular channels as a drainage pathway for cerebrospinal fluid. J. Physiol. 239, 195–206 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lonser, R.R. , Sarntinoranont, M. , Morrison, P.F. & Oldfield, E.H. Convection‐enhanced delivery to the central nervous system. J. Neurosurg. 122, 697–706 (2015). [DOI] [PubMed] [Google Scholar]
- 26. Lochhead, J.J. & Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliver. Rev. 64, 614–628 (2012). [DOI] [PubMed] [Google Scholar]
- 27. Yadav, D.B. et al. Widespread brain distribution and activity following I.C.V. infusion of anti‐β‐secretase (BACE1) in nonhuman primates. Brit. J. Pharmacol. 174, 4173–4185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubenstein, J.L. et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood 101, 466–468 (2003). [DOI] [PubMed] [Google Scholar]
- 29. Rubenstein, J.L. et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood 121, 745–751 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calias, P. , Banks, W.A. , Begley, D. , Scarpa, M. & Dickson, P. Intrathecal delivery of protein therapeutics to the brain: A critical reassessment. Pharmacol. Therapeut. 144, 114–122 (2014). [DOI] [PubMed] [Google Scholar]
- 31. Nakano, R. et al. A new technology for increasing therapeutic protein levels in the brain over extended periods. PLoS One 14, e0214404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis, O. et al. Chronic, intermittent convection‐enhanced delivery devices. J. Neurosci. Meth. 259, 47–56 (2016). [DOI] [PubMed] [Google Scholar]
- 33. Whone, A.L. et al. Extended treatment with glial cell line‐derived neurotrophic factor in Parkinson’s disease. J. Park Dis. Preprint 9, 301–313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thorne, R.G. , Hanson, L.R. , Ross, T.M. , Tung, D. & Frey, W.H. Delivery of interferon‐β to the monkey nervous system following intranasal administration. Neuroscience 152, 785–797 (2008). [DOI] [PubMed] [Google Scholar]
- 35. Wurster, C.D. et al. Intrathecal administration of nusinersen in adolescent and adult SMA type 2 and 3 patients. J. Neurol. 266, 183–194 (2019). [DOI] [PubMed] [Google Scholar]
- 36. Kim, J. et al. Patient‐customized oligonucleotide therapy for a rare genetic disease. New Engl. J. Med. 381, 1644–1652 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tabrizi, S.J. et al. Targeting huntingtin expression in patients with Huntington’s disease. New Engl. J. Med. 380, 2307–2316 (2019). [DOI] [PubMed] [Google Scholar]
- 38. Miller, T.M. et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first‐in‐man study. Lancet Neurol. 12, 435–442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller, T. et al. Phase 1–2 trial of antisense oligonucleotide tofersen for SOD1 ALS. New Engl. J. Med. 383, 109–119 (2020). [DOI] [PubMed] [Google Scholar]
- 40. Ljubenkov, P.A. & Boxer, A.L. Frontotemporal dementias, emerging milestones of the 21st century. Adv. Exp. Med. Biol. 1281, 297–310 (2021). [DOI] [PubMed] [Google Scholar]
- 41. Geary, R.S. , Norris, D. , Yu, R. & Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliver. Rev. 87, 46–51 (2015). [DOI] [PubMed] [Google Scholar]
- 42. Luu, K.T. et al. Population pharmacokinetics of nusinersen in the cerebral spinal fluid and plasma of pediatric patients with spinal muscular atrophy following intrathecal administrations. J. Clin. Pharmacol. 57, 1031–1041 (2017). [DOI] [PubMed] [Google Scholar]
- 43. Biliouris, K. et al. A Semi‐mechanistic population pharmacokinetic model of nusinersen: an antisense oligonucleotide for the treatment of spinal muscular atrophy. CPT Pharmacometrics Syst. Pharmacol. 7, 581–592 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Butler, M. et al. Spinal distribution and metabolism of 2′‐O‐(2‐methoxyethyl)‐modified oligonucleotides after intrathecal administration in rats. Neuroscience 131, 705–715 (2005). [DOI] [PubMed] [Google Scholar]
- 45. Flotats‐Bastardas, M. et al. multicenter experience with nusinersen application via an intrathecal port and catheter system in spinal muscular atrophy. Neuropediatrics 51, 401–406 (2020). [DOI] [PubMed] [Google Scholar]
- 46. Rodriguez, M. et al. Intranasal drug delivery of small interfering RNA targeting Beclin1 encapsulated with polyethylenimine (PEI) in mouse brain to achieve HIV attenuation. Sci. Rep‐UK 7, 1862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alarcón‐Arís, D. et al. Selective α‐Synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: potential therapy for Parkinson’s disease. Mol. Ther. 26, 550–567 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Borgonetti, V. & Galeotti, N. Intranasal delivery of an antisense oligonucleotide to the RNA‐binding protein HuR relieves nerve injury‐induced neuropathic pain. Pain 162, 1500–1510 (2020). [DOI] [PubMed] [Google Scholar]
- 49. Su, X. et al. Real‐time MR imaging with gadoteridol predicts distribution of transgenes after convection‐enhanced delivery of AAV2 vectors. Mol. Ther. 18, 1490–1495 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. San Sebastian, W. et al. Safety and tolerability of magnetic resonance imaging‐guided convection‐enhanced delivery of AAV2‐hAADC with a novel delivery platform in nonhuman primate striatum. Hum. Gene Ther. 23, 210–217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gray, S.J. , Nagabhushan Kalburgi, S. , McCown, T.J. & Jude Samulski, R. Global CNS gene delivery and evasion of anti‐AAV‐neutralizing antibodies by intrathecal AAV administration in non‐human primates. Gene Ther. 20, 450–459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sebastian, W.S. et al. Safety and tolerability of MRI‐guided infusion of AAV2‐hAADC into the mid‐brain of nonhuman primate. Mol. Ther. Methods Clin. Dev. 1, 14049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hinderer, C. et al. Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna. Mol. Ther. Methods Clin. Dev. 1, 14051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Samaranch, L. et al. Cerebellomedullary cistern delivery for AAV‐based gene therapy: a technical note for nonhuman primates. Hum. Gene Ther. Method 27, 13–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hinderer, C. et al. Evaluation of intrathecal routes of administration for adeno‐associated viral vectors in large animals. Hum. Gene Ther. 29, 15–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hinderer, C. et al. Translational feasibility of lumbar puncture for intrathecal AAV administration. Mol. Ther. Methods Clin. Dev. 17, 969–974 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taghian, T. et al. A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna. Mol. Ther. 28, 411–421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christine, C.W. et al. Magnetic resonance imaging–guided phase 1 trial of putaminal AADC gene therapy for Parkinson’s disease. Ann. Neurol. 85, 704–714 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yasuhara, T. et al. Cell therapy for central nervous system disorders: Current obstacles to progress. CNS Neurosci. Ther. 26, 595–602 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bakshi, A. , Hunter, C. , Swanger, S. , Lepore, A. & Fischer, I. Minimally invasive delivery of stem cells for spinal cord injury: advantages of the lumbar puncture technique. J. Neurosurg. Spine 1, 330–337 (2004). [DOI] [PubMed] [Google Scholar]
- 61. Lepore, A.C. , Bakshi, A. , Swanger, S.A. , Rao, M.S. & Fischer, I. Neural precursor cells can be delivered into the injured cervical spinal cord by intrathecal injection at the lumbar cord. Brain Res. 1045, 206–216 (2005). [DOI] [PubMed] [Google Scholar]
- 62. Callera, F. & do Nascimento, R.X. Delivery of autologous bone marrow precursor cells into the spinal cord via lumbar puncture technique in patients with spinal cord injury: A preliminary safety study. Exp. Hematol. 34, 130–131 (2006). [DOI] [PubMed] [Google Scholar]
- 63. Vaquero, J. et al. Progressive increase in brain glucose metabolism after intrathecal administration of autologous mesenchymal stromal cells in patients with diffuse axonal injury. Cytotherapy 19, 88–94 (2017). [DOI] [PubMed] [Google Scholar]
- 64. Zakerinia, M. et al. Intrathecal autologous bone marrow‐derived hematopoietic stem cell therapy in neurological diseases. Int. J. Organ Transplant Med. 9, 157–167 (2018). [PMC free article] [PubMed] [Google Scholar]
- 65. Kuttler, A. et al. Understanding pharmacokinetics using realistic computational models of fluid dynamics: biosimulation of drug distribution within the CSF space for intrathecal drugs. J. Pharmacokinet. Phar. 37, 629–644 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yiallourou, T.I. et al. Comparison of 4D phase‐contrast MRI Flow measurements to computational fluid dynamics simulations of cerebrospinal fluid motion in the cervical spine. PLoS One 7, e52284 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heidari Pahlavian, S. et al. The impact of spinal cord nerve roots and denticulate ligaments on cerebrospinal fluid dynamics in the cervical spine. PLoS One 9, e91888 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Heidari Pahlavian, S. et al. Accuracy of 4D flow measurement of cerebrospinal fluid dynamics in the cervical spine: an in vitro verification against numerical simulation. Ann. Biomed. Eng. 44, 3202–3214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martin, B.A. et al. Inter‐operator reliability of magnetic resonance image‐based computational fluid dynamics prediction of cerebrospinal fluid motion in the cervical spine. Ann. Biomed. Eng. 44, 1524–1537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haga, P.T. et al. A numerical investigation of intrathecal isobaric drug dispersion within the cervical subarachnoid space. PLoS One 12, e0173680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tangen, K.M. , Leval, R. , Mehta, A.I. & Linninger, A.A. Computational and in vitro experimental investigation of intrathecal drug distribution. Anesthesia Analgesia 124, 1686–1696 (2017). [DOI] [PubMed] [Google Scholar]
- 72. Pizzichelli, G. et al. Numerical study of intrathecal drug delivery to a permeable spinal cord: effect of catheter position and angle. Comput. Method Biomec. 20, 1599–1608 (2017). [DOI] [PubMed] [Google Scholar]
- 73. Khani, M. , Sass, L.R. , Xing, T. , Sharp, M.K. , Balédent, O. & Martin, B.A. Anthropomorphic model of intrathecal cerebrospinal fluid dynamics within the spinal subarachnoid space: spinal cord nerve roots increase steady‐streaming. J. Biomech. Eng. 140, 0810121‐08101215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kurtcuoglu, V. , Jain, K. & Martin, B.A. Biomechanics of the Brain. Biol. Med. Phys. Biomed. https://doi.org/ 10.1007/978-3-030-04996-6_9. [DOI] [Google Scholar]
- 75. Khani, M. et al. In vitro and numerical simulation of blood removal from cerebrospinal fluid: comparison of lumbar drain to Neurapheresis therapy. Fluids Barriers CNS 17, 23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Khani, M. et al. Impact of Neurapheresis system on intrathecal cerebrospinal fluid dynamics: a computational fluid dynamics study. J. Biomech. Eng. 142, 0210061‐0210069 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Roovers, J. , De Jonghe, P. & Weckhuysen, S. The therapeutic potential of RNA regulation in neurological disorders. Expert Opin. Ther. Targets 22, 1017–1028 (2018). [DOI] [PubMed] [Google Scholar]
- 78. Ojala, D.S. , Amara, D.P. & Schaffer, D.V. Adeno‐associated virus vectors and neurological gene therapy. Neuroscience 21, 84–98 (2015). [DOI] [PubMed] [Google Scholar]
- 79. Deverman, B.E. , Ravina, B.M. , Bankiewicz, K.S. , Paul, S.M. & Sah, D.W.Y. Gene therapy for neurological disorders: progress and prospects. Nat. Rev. Drug Discov. 17, 641–659 (2018). [DOI] [PubMed] [Google Scholar]
- 80. Lykken, E.A. , Shyng, C. , Edwards, R.J. , Rozenberg, A. & Gray, S.J. Recent progress and considerations for AAV gene therapies targeting the central nervous system. J. Neurodev. Disord. 10, 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


