Abstract
Background:
Kidney failure prevalence is increasing in older patients for whom dialysis initiation can be challenging. Assisted peritoneal dialysis (PD), where PD is performed with the help of a healthcare worker, can facilitate PD for frailer patients who may not be candidate otherwise.
Objectives:
This study aimed to assess the feasibility of implementing the first pilot assisted PD program in Quebec (Canada) and to evaluate the characteristics and outcomes of the PD cohort before and after assisted PD availability.
Design:
Observational retrospective cohort study.
Setting and Population:
All adult patients initiating PD between 2015 and 2020 in a single-center dialysis unit were included.
Measurements:
Incidence, characteristics, and outcomes of patients with PD were compared between (1) the “pre” (2015-2017) and the “post” assisted PD era (2018-2020) and (2) patients with assisted PD and independent PD in the more recent period.
Methods:
The primary outcome was peritonitis rate over the first year. Secondary outcomes included hospitalization, transfers to in-center hemodialysis (HD) and mortality.
Results:
Overall, 124 patients initiated PD with an annual incidence of 17 ± 3 patients during the “pre” and 24 ± 8 patients during the “post” assisted PD era (P = .18). First-year peritonitis rate was similar over the 2 eras. Years of PD initiation and use of assisted PD were not associated with risk peritonitis (over total follow-up) after adjustment. Adjusted hazard of transfer to HD or death was higher during the “post” era (hazard ratio [HR]: 2.77; 95% confidence interval [CI]: 1.42-5.58). Seventeen patients received assisted PD including 13 (18%) of the 72 patients initiated between 2018 and 2020. Patients with assisted PD were older than those with independent PD (72 [64-84] vs. 59 [47-67], P = .006) and received assistance for 0.8 (0.4-1.5) years. When comparing assisted and independent cohorts, there were no differences in crude rates of peritonitis or hospitalization.
Limitations:
Single-center study with small sample size.
Conclusion:
This study shows the feasibility of implementing an assisted PD program, with favorable overall outcomes including similar rates of peritonitis during the first year after PD initiation.
Keywords: peritoneal dialysis, assisted peritoneal dialysis, peritonitis, transfer to hemodialysis, mortality
Abrégé
Contexte:
La prévalence de l’insuffisance rénale augmente chez les patients plus âgés chez qui l’initiation de la dialyse peut être difficile. La dialyse péritonéale (DP) assistée, soit avec l’aide d’un professionnel de la santé, peut faciliter cette modalité chez les patients fragiles qui, autrement, ne seraient pas candidats.
Objectifs de l’étude:
Cette étude visait deux objectifs: 1) évaluer la faisabilité de la mise en œuvre du premier program pilote de DP assistée au Québec (Canada) et, 2) évaluer les caractéristiques et les résultats de la cohorte avant et après l’accès à la DP assistée.
Conception:
Étude de cohorte observationnelle rétrospective.
Cadre et participants:
Ont été inclus tous les patients adultes ayant initié une DP entre 2015 et 2020 dans l’unité de dialyse d’un center hospitalier.
Mesures:
L’incidence de la DP, ainsi que les caractéristiques et les résultats des patients sous DP ont été comparés entre [1] les patients « pré » (2015-2017) et « post » DP assistée (2018-2020) et entre [2] les patients sous DP assistée et sous DP autonome au cours de la période la plus récente.
Méthodologie:
Le principal critère d’évaluation était le taux de péritonite dans la première année. Les résultats secondaires comprenaient hospitalisation, les transferts à l’hémodialyse (HD) en centre et le taux de mortalité.
Résultats:
En tout, 124 patients ont amorcé un traitement de DP avec une incidence annuelle de 17 ± 3 patients au cours de la période « pré » et de 24 ± 8 patients au cours de la période « post » (p = 0,18). Le taux de péritonite dans la première année était semblable pour les deux périodes. Après ajustement, les années d’initiation et l’utilisation de la DP assistée n’étaient pas associées à un risque de péritonite accru (pour la période totale de suivi). Le risque ajusté de transfert à l’HD ou de décès était plus élevé durant la période « post » (RR 2,77; IC 95 %: 1,42-5,58). Dix-sept patients ont reçu la DP assistée, dont 13 (18 %) des 72 patients initiés entre 2018 et 2020. Les patients sous DP assistée étaient plus âgés que ceux sous DP autonome (72 [64-84] ans c. 59 [47-67] ans; p = 0,006) et ont reçu de l’aide pendant 0,8 (0,4-1,5) an. Aucune différence n’a été observée dans les taux bruts de péritonite ou d’hospitalization lors de la comparaison des cohortes assistée et autonome.
Limites:
Étude menée dans un seul center, sur un faible échantillon de patients.
Conclusion:
Cette étude montre que la mise en œuvre d’un program de DP assistée est faisable et qu’elle donne de bons résultats, notamment des taux similaires de péritonite dans l’année suivant l’initiation de la DP.
Introduction
In Canada, 55% of patients starting dialysis were 65 years and older in 2019 1 and among prevalent patients with kidney failure, the proportion of those 65 years and older increased from 34% to 45% between 2001 and 2019. 2 This demographic change may introduce barriers to kidney failure treatment with home dialysis.3,4 Peritoneal dialysis (PD) is an advantageous treatment compared to facility-hemodialysis (HD) on multiple levels including cost and quality of life, while having mostly similar clinical outcomes.5-12 Importantly, it allows patients to keep a certain level of autonomy. 13 Despite its benefits, PD can be challenging to initiate in specific groups of patients, such as the elderly and those with multiple comorbidities, physical limitations or psychosocial constraints. 14
Assisted PD programs can mitigate these barriers. Assisted PD is performed with the help of a caregiver or a healthcare worker, such as a nurse, who can assist with dialysis exchanges. Assisted PD has been introduced in many countries. It was shown to help overcome PD barriers without jeopardizing patient and technique survival nor peritonitis rates, 15 with equal or inferior cost than in-center HD 16 and improved treatment satisfaction. 17
In Canada, assisted PD is only available in a few provinces and until recently, was not an option for patients in the province of Quebec. 18 The aim of this study was to assess the feasibility of implementing the first pilot assisted PD program in Quebec and to compare the characteristics and outcomes of patients treated with PD before and after the assisted PD program was launched in 2018.
Methods and Material
Population
All adult patients initiating PD between January 2015 and December 2020 at the Maisonneuve-Rosemont hospital dialysis unit were included. Patients with PD catheter insertion without indication to start dialysis during the study period, those with training failure and those who died or received a kidney transplantation before starting PD were excluded. Independent PD was defined as PD performed at home without any nursing support, while assisted PD was defined as receiving aid from a home nurse for least one PD exchange per week. In our program, nursing assistance was provided by Registered Nurses from our provincial Home Support program (“Soutien à domicile”) through Center local de services communautaires (CLSC).
Outcomes, Exposures and Covariates
The primary outcome was peritonitis rate (first-year and total follow-up time). Secondary outcomes included hospitalization, transfers to in-center HD and mortality. The main exposure variables were as follows: (1) years of PD initiation, categorized as “pre” assisted PD (January 2015-December 2017) and “post” assisted PD (January 2018-December 2020), and (2) use of assisted PD versus independent PD in the 2018 to 2020 period.
Patients were offered assisted PD if (1) they were not considered eligible to independent PD based on nephrologist’s or nurse’s assessment either due to physical (eg, strength, dexterity, vision, frailty) or psychosocial limitation (eg, cognitive or psychiatric disease, language barrier) 19 or (2) if patients themselves were not confident they could perform independent PD. Patients who did not complete PD training successfully were also referred to our assisted PD program. The assisted program was limited to patients living in our center official geographical dialysis catchment area (representing >90%-95% of our PD population).
Descriptive data among the assisted PD cohort such as duration of assistance (in weeks), transfer to independent PD and number of nurse visits at home were assessed. Transfer to independent PD was defined as continuation of PD without any home nurse assistance, while semi-independent PD was defined as nursing assistance less than once per day (≤ 6 visits per week). Deaths occurring less than 30 days after transfer to HD were attributed to the PD period. Patients were followed until death, transfer to HD (defined >30 days on HD), kidney transplantation, or end of study follow-up (June 30, 2021).
Baseline characteristics were defined at PD initiation and included age, race, sex, chronic kidney disease etiology, duration of KRT, past kidney transplants and candidacy status, past dialysis modalities before transfer to PD (predialysis, in-center HD or home HD), and PD modality (continuous ambulatory peritoneal dialysis [CAPD] or automated peritoneal dialysis [APD]). Comorbidities (diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease [COPD], and malignancy) were also determined among each cohort of patients. Clinical data were collected using electronic and paper charts. The study was approved by the Research Ethic Board, in agreement with the Declaration of Helsinki.
Statistical Analysis
Baseline characteristics are presented as numbers and percentages for categorical variables, and median and interquartile ranges (IQRs) for continuous variables. Categorical variables were compared using χ2 test and continuous variables were compared using Wilcoxon tests.
Crude rates of peritonitis and hospitalization were evaluated in Poisson regressions. Adjusted risks of peritonitis and hospitalizations, adjusted for year of PD initiation, assisted PD and other confounding factors were assessed in a multivariable negative binomial model. Adjustment variables included age, sex, years of PD initiation and use of assisted PD as pre-specified variables, and additional variables with P < .2 on univariate analysis.
Time to transfer to HD or death was graphically assessed in a Kaplan-Meier graph and compared using log-rank p and a multivariable Cox proportional hazard model. A 2-tailed P-value <.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) to conduct all analyses. N.E. performed the analyses.
Results
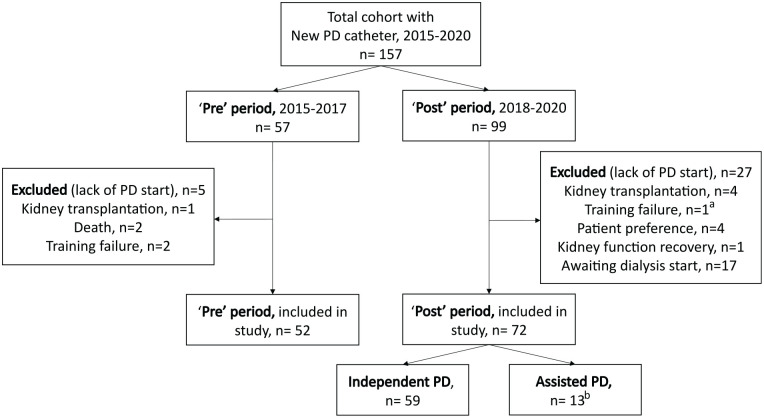
A total of 124 patients were included in the final study cohort. Fifty-two patients started PD during the “pre” period (2015-2017; annual incidence 17 ± 3), compared to 72 patients during the “post” period (2018-2020; annual incidence 24 ± 8, P = .18) (Figure 1). Most patients’ characteristics were similar in the 2 eras, including age, sex, and race. Cause of kidney failure differed between groups with higher prevalence of diabetic nephropathy (38% vs. 13%) and fewer glomerulonephritis (26% vs. 50%) in the “post” era than the “pre” era (P = .003). Concordantly, diabetes as a comorbidity was more common in the “post” group (56% vs. 36%, P = .02). Baseline characteristics of patients, stratified by pre/post era and PD assistance, are presented in Table 1. Of note, due to the Montreal location of our center, none of these patients were from rural communities.
Figure 1.
Flow chart—Study population.
Note. PD = peritoneal dialysis.
aArea without access to assisted PD.
bExcluding 4 patients with assisted PD and PD start before 2018.
Table 1.
Baseline Characteristics at Peritoneal Dialysis Initiation.
| PD initiation years | PD assistance 2018-2020 | |||||
|---|---|---|---|---|---|---|
| ‘Pre’ 2015-2017 (n = 52) |
‘Post’ 2018-2020 (n = 72) |
P-value | Independent PD (n = 59) | Assisted PD (n = 13) | P-value | |
| Age, in year | 60 (54-73) | 61 (52-69) | .45 | 59 (47-67) | 72 (64-84) | .006 |
| Female sex | 23 (44) | 24 (33) | .21 | 21 (35) | 3 (23) | .52 |
| Race | ||||||
| Black | 8 (15) | 12 (17) | .90 | 11 (19) | 1 (8) | .10 |
| Caucasian | 35 (67) | 47 (65) | 40 (68) | 7 (54) | ||
| Other | 9 (18) | 13 (19) | 8 (14) | 5 (38) | ||
| Cause of kidney failure | ||||||
| Glomerulonephritis | 26 (50) | 19 (26) | .003 | 15 (25) | 4 (31) | .99 |
| Diabetic nephropathy | 7 (13) | 27 (38) | 21 (36) | 6 (46) | ||
| Hypertension | 11 (21) | 9 (13) | 8 (14) | 1 (8) | ||
| Other | 8 (16) | 16 (22) | 14(24) | 2(15) | ||
| Comorbidities | ||||||
| Diabetes | 17 (36) | 40 (56) | .02 | 30 (51) | 10 (77) | .12 |
| CAD | 19 (37) | 26 (36) | .96 | 20 (34) | 6 (46) | .53 |
| PVD | 8 (15) | 14 (19) | .64 | 10 (17) | 4 (31) | .26 |
| CVD | 5 (10) | 11 (15) | .42 | 7 (12) | 4 (31) | .10 |
| COPD | 9 (17) | 8 (11) | .43 | 4 (7) | 4 (31) | .03 |
| Cancer | 20 (38) | 17 (24) | .07 | 15 (25) | 2 (15) | .72 |
| PD as first modality | 26 (50) | 38 (53) | .76 | 30 (51) | 8 (62) | .48 |
| KRT duration before PD, in year (n = 60) | 0.3 (0.2-0.5) | 0.5 (0.3-1.3) | .17 | 0 (0-0.6) | 0 (0-0.3) | .27 |
| Transplant candidate | 24 (46) | 45 (63) | .10 | 39 (66) | 6 (46) | .21 |
Note. PD = peritoneal dialysis; CAD = coronary artery disease; PVD = peripheral vascular disease; CVD = cerebrovascular disease; COPD = chronic obstructive pulmonary disease; KRT = kidney replacement therapy.
Over a mean follow-up period of 1.9 ± 1.5 years, patients experience 80 peritonitis episodes and 186 hospitalizations. Thirty-one patients were transferred to HD, 23 patients died during PD treatment, and 28 patients received a kidney transplantation (Table 2). Follow-up was longer in patients from the “pre” cohort (2.7 ± 1.8) than the “post” cohort (1.4 ± 1.0, P < .001).
Table 2.
Patient Outcomes at the End of Study Follow-Up.
| Assisted PD patients (n = 17) | Total cohort (n = 124) | |
|---|---|---|
| Independent dialysis status | ||
| Independent PD | 4 (24) | — |
| Semi-independent PD | 8 (47) | — |
| Ongoing fully assisted PD | 5 (29) | — |
| End of follow-up reason | ||
| Death | 4 (24) | 23 (19) |
| Transfer to HD | 5 (29) | 31 (25) |
| Kidney transplantation | 0 | 28 (23) |
| Ongoing PD | 8 (47) | 40 (32) |
| Other a | 0 | 2 (2) |
Note. PD = peritoneal dialysis; HD = hemodialysis.
Recovery of kidney function/change in dialysis unit. Data presented as frequency (%).
Peritonitis
Crude peritonitis rate was similar between the “pre” and “post” cohorts during the first PD year, while peritonitis rate was higher in patients initiated on PD during the “post” period when the total follow-up time was analyzed (0.26 [0.18-0.36] pre vs. 0.43 [0.32-0.58] post, per pt-year, P = .03) (Table 3). In adjusted models, year of PD initiation (adjusted incidence rate ratio [IRR] 0.68 [0.35-1.29] year “post” vs. “pre”) and use of assisted PD (adjusted IRR 0.93 [0.39-2.26]) were not associated with peritonitis risk over total study period (Table 4).
Table 3.
Rates of Peritonitis and Hospitalization, Stratified by the Year of PD Initiation and PD Assistance.
| Events number | Rate per pt-yr | Rates difference | p-value | |
|---|---|---|---|---|
| ‘Pre’ 2015-2017 vs. ‘Post’ 2018-2020 | ||||
| Peritonitis (first-yr) | ||||
| 2015-2017 | 11 | 0.30 (0.15; 0.33) | 0.02 (-0.22; 0.23) | .98 |
| 2018-2020 | 15 | 0.30 (0.17; 0.49) | ||
| Peritonitis (total follow-up) | ||||
| 2015-2017 | 36 | 0.26 (0.18; 0.36) | ||
| 2018-2020 | 44 | 0.43 (0.32; 0.58) | 0.09 (0.02; 0.37) | .03 |
| Hospitalization | ||||
| 2015-2017 | 105 | 0.78 (0.64; 0.95) | ||
| 2018-2020 | 81 | 0.84 (0.67; 1.05) | 0.06 (-0.02; 0.16) | .62 |
| Assisted vs. independent PD patients (2018-2020) | ||||
| Peritonitis (first-year) | ||||
| Independent PD | 14 | 0.35 (0.20; 0.59) | -0.26 (-0.52; 0.003) | .06 |
| Assisted PD | 1 | 0.10 (0.24; 0.53) | ||
| Peritonitis (total follow-up) | ||||
| Independent PD | 34 | 0.43 (0.29; 0.58) | ||
| Assisted PD | 10 | 0.51 (0.24; 0.91) | 0.09 (-0.26; 0.44) | .63 |
| Hospitalization | ||||
| Independent PD | 60 | 0.78 (0.59; 1.00) | ||
| Assisted PD | 21 | 1.12 (0.69; 1.71) | 0.34 (-0.17; 0.86) | .20 |
Note. PD = peritoneal dialysis.
Table 4.
Adjusted Risk of Peritonitis Over Total Study Follow-Up Time.
| Characteristics | Adjusted IRR | P-value |
|---|---|---|
| Year of PD initiation 2018-2020 (vs. 2015-2017) | 0.68 (0.35; 1.29) | .24 |
| Assisted PD (vs. independent) | 0.93 (0.39; 2.26) | .88 |
| Age (per year) | 0.99 (0.97; 1.01) | .37 |
| Female (vs. male) | 1.10 (0.55; 2.21) | .78 |
| Diabetes | 0.90 (0.47; 1.74) | .76 |
| Coronary heart disease | 1.71 (0.84; 3.46) | .14 |
Note. IRR = incidence rate ratio; PD = peritoneal dialysis.
Hospitalization, Transfer to HD and Mortality
Crude and adjusted risk of hospitalization were similar during 2 periods (Table 3). Survival free of technique failure or death was not statistically different in patients initiated on PD during the “pre” or “post” years (log-rank P = .13) (Figure 2). However, after adjustment for main confounding factors (age, sex, use of assisted PD, diabetes, peripheral vascular disease and transplant candidacy), initiating PD in the “post” years was associated with a higher risk of transfer to HD or death (hazard ratio [HR]: 2.77; 95% confidence interval [CI]: 1.42-5.58).
Figure 2.
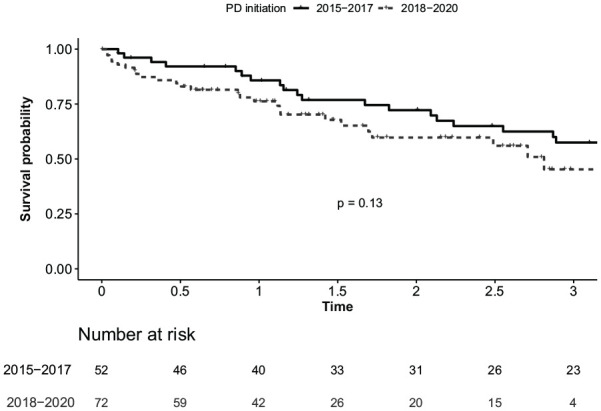
Survival free of technique failure or death in patients initiated on PD in 2015 to 2015 compared to 2018 to 2020.
Note. PD = peritoneal dialysis.
Assisted PD Program and Cohort Characteristics
In our pilot program, home nurses were trained by our PD program nurses during an 8-hour standardized session. Group sessions included 6 to 10 home nurses at the same time with printed material, oral presentation, and simulation content. If needed, home nurses could attend a second teaching session. They were trained to perform a range of activities including exchanges set up, blood pressure and volume assessment, administration of antibiotics in case of peritonitis, and PD catheter management. Home nurses could modify PD prescriptions based on a preformatted prescription integrating elements of the clinical assessment (eg, weight, blood pressure, volume, dyspnea). Home nurses could also reach out to PD program nurses from our dialysis program and Nephrologist if required based on PD program nurses’ assessment. Assisted PD patients attended our regular outpatient clinic for medical follow-up.
Of the 72 patients initiated between 2018 and 2020, 13 (18%) received assisted PD. Of note, an additional 4 patients were treated with assisted PD as prevalent patients (with PD initiated before 2018). Patients receiving assisted PD were older than independent patients (72 [47-67] vs. 59 [64-84], P = .006) and had more COPD (31% vs. 7%, P = .03) (Table 1).
Among the 17 patients with PD assistance, CAPD was the most frequent modality (82%). CAPD was usually prescribed as a maximum of thrice-daily exchanges in patients receiving assisted PD to minimize the number of nursing visits. Median number of nursing visits at start of assisted PD was 21 per week (3 per day) and decreased to 7 per week by the end of PD assistance or the study follow-up period. Four (24%) patients reached complete independence and 8 (47%) were semi-independent (<1 nurse visit per day), with the majority of the latter receiving once-a-week visit (mostly for clinical assessment). Overall, patients stayed on assisted PD for a median duration of 138 days (IQR: 57-306) before reaching complete independence or ending PD. Four patients (24%) died while receiving PD assistance, 5 (29%) were transferred to HD and 8 (47%) were still on assisted PD (including semi-independent patients) at the end of the study (Table 2). There were no statistically significant differences in crude rates of peritonitis and hospitalization when comparing assisted and independent PD patients from the most recent cohort (2018-2020) (Table 3).
Discussion
In this study, we reported on the feasibility of implementing a pilot-assisted PD program, without compromising the quality of care. We found similar rates of peritonitis and hospitalization in incident patients PD during the “pre” years 2015 to 2017, before assisted PD was launched, and between 2018 and 2020 or “post” period, once assisted PD was offered. Nearly one-fifth of incident patients received PD assistance after the program launch in 2018 with 71% reaching partial or complete independence. Patients with assisted PD were older, had higher prevalence of COPD and fewer were transplant candidate than the independent cohort.
PD offers several advantages for frail or older patients compared to HD including less frequent travel to hospital, preservation of vascular access (along with its potential complications) and better hemodynamic stability.16,20,21 Assisted PD can directly increase PD eligibility for frail patients. 22 In addition, PD assistance may support home dialysis transition for patients with psychosocial barriers or fears. 19 In our cohort, the number of new yearly patients on PD increased after the launch of our assisted PD program, without reaching statistical significance, but leading to an actual expansion of our total cohort.
Unsurprisingly, patients who received assisted PD were older than patients on independent PD. Elderly patients are often frailer and more likely to lack the autonomy required to perform PD independently. COPD was also more common among the assisted PD group, which may be related to reduce autonomy for daily life activities in this population. 23
Other studies have found differences in risk of adverse outcomes with assisted PD compared to independent PD. In a Portuguese study, assisted patients were more likely to die or to be hospitalized compared to patients with independent PD. However, these authors found that patients with PD assistance had better outcomes regarding rates of peritonitis and technique survival, the latter potentially influencing mortality risk. 24 Another study from France showed an association between assisted PD and lower risk of technique failure. 25 Our study was not powered to directly compare assisted and independent patients, although crude rates of peritonitis and hospitalization were not statistically different.
Crude first-year peritonitis rate was similar for patients initiated before and after the launch of our assisted PD program in 2018. In contrast, when the entire follow-up time was analyzed, the more recent cohort was associated with a higher risk of peritonitis. The shorter follow-up time of the “post” period (1.4 vs. 2.7 years pre) may have introduce a bias whereby patients predispose to more favorable outcomes had a longer exposure in the “pre” cohort as patients with poorer outcomes may have cease PD earlier due to death or transfer to HD. In accordance to this assumption, year of PD initiation was not associated with peritonitis risk in the adjusted regression.
Hazard of transfer to HD or death was higher during the post-assisted PD period (than “pre”) after adjustment for confounding. This association may be related to changes in patient demographics or difference in clinical care and organization. First, although not apparent in our baseline characteristics assessment, the initiation of assisted PD after January 2018 may have facilitated the inclusion of frailer patients, at higher risk of death or transferred to HD. Second, as our PD program expanded, new nurses joined the program with less experienced compared to the former nursing team. This could have contributed to a slight increase in adverse outcomes. Finally, the COVID-19 pandemic starting in early 2020 decreased the number of in-person visits. Patient education and access to outpatient care were limited at that time (often due to fear from patient themselves), which could have made vulnerable patients more prone to adverse events. 26
In accordance to what has been described in previous studies, a large proportion of patients with assisted PD in our cohort gained autonomy with 24% reaching complete independence and 47% partial independence. Oliver and al. reported similar rates with 23% of assisted PD patients transferred to complete autonomous PD in a cohort from Ontario. 22 This suggests that our assisted PD program was successful at mitigating patients’ fears or barriers toward home dialysis transition, with added benefits on a psychosocial and financial level, and greater patient empowerment. 27 Of note, the proportion of patients using assisted PD remained modest at 18% in our center. This is less than in other regions, particularly France, where 37% of patients with PD were receiving assistance in a similar study. 28
Our study is limited by its small sample size, observational retrospective design and single-center population. This pilot assisted PD cohort was too small to compare outcomes with adequate power. Nonetheless, the addition of our pilot assisted PD program did not increase risk of peritonitis or hospitalization when comparing 2 eras before and after implantation. Additionally, detailed costs associated with our assisted PD program were not collected although this was previously assessed in other similar studies.16,29,30
Conclusion
This study shows the feasibility of implementing an assisted PD program, with favorable overall outcomes including similar rates of peritonitis and hospitalization before and after the launch of the assisted PD program. A high proportion of patients undergoing assisted PD successfully transferred to independent PD, in concordance with similar studies.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581221113387 for Assisted Peritoneal Dialysis Implementation: A Pilot Program From a Large Dialysis Unit in Quebec by Julien Melanson, Jessica Kachmar, Louis-Philippe Laurin, Naoual Elftouh and Annie-Claire Nadeau-Fredette in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the Hôpital Maisonneuve-Rosemont Research Ethics Board (2021-2547). Participant consent was not required due to the retrospective study design.
Consent for Publication: All authors provide consent for publication.
Availability of Data and Materials: This data is subject to REB restriction but reasonable requests sent to the corresponding author will be considered.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors have no conflicts of interests. LPL holds a Junior 2 scholarship from Fonds de recherche du Québec en santé (FRQS) and ACNF holds a Junior 1 scholarship from Fonds de recherche du Québec en santé (FRQS).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Louis-Philippe Laurin  https://orcid.org/0000-0003-1598-5562
https://orcid.org/0000-0003-1598-5562
Annie-Claire Nadeau-Fredette  https://orcid.org/0000-0002-7755-1404
https://orcid.org/0000-0002-7755-1404
References
- 1. Canadian Institute for Health Information. Trends in End-Stage Kidney Disease in Canada, 2019 [Infographic]. Ottawa, ON, Canada: Canadian Institute for Health Information; 2020. [Google Scholar]
- 2. Canadian Institute for Health Information. Treatment of End-Stage Organ Failure in Canada, Canadian Organ Replacement Register, 2011 to 2020: End-Stage Kidney Disease and Kidney Transplants — Data Tables. Ottawa, ON, Canada: Canadian Institute for Health Information; 2021. [Google Scholar]
- 3. Oreopoulos DG, Dimkovic N. Geriatric nephrology is coming of age. J Am Soc Nephrol. 2003;14(4):1099-1101. [DOI] [PubMed] [Google Scholar]
- 4. Auguste BL, Chan CT. Home dialysis among elderly patients: outcomes and future directions. Can J Kidney Health Dis. 2019;6:2054358119871031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klarenbach SW, Tonelli M, Chui B, Manns BJ. Economic evaluation of dialysis therapies. Nat Rev Nephrol. 2014;10(11):644-652. [DOI] [PubMed] [Google Scholar]
- 6. Karopadi AN, Mason G, Rettore E, Ronco C. The role of economies of scale in the cost of dialysis across the world: a macroeconomic perspective. Nephrol Dial Transplant. 2014;29(4):885-892. [DOI] [PubMed] [Google Scholar]
- 7. Fan SL, Sathick I, McKitty K, Punzalan S. Quality of life of caregivers and patients on peritoneal dialysis. Nephrol Dial Transplant. 2008;23(5):1713-1719. [DOI] [PubMed] [Google Scholar]
- 8. Kutner NG, Zhang R, Barnhart H, Collins AJ. Health status and quality of life reported by incident patients after 1 year on haemodialysis or peritoneal dialysis. Nephrol Dial Transplant. 2005;20(10):2159-2167. [DOI] [PubMed] [Google Scholar]
- 9. Hsu CC, Huang CC, Chang YC, Chen JS, Tsai WC, Wang KY. A comparison of quality of life between patients treated with different dialysis modalities in Taiwan. PLoS ONE. 2020;15(1):e0227297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant. 2012;27(9):3568-3575. [DOI] [PubMed] [Google Scholar]
- 11. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med. 2011;171(2):110-118. [DOI] [PubMed] [Google Scholar]
- 12. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20(1):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sauve C, Vandyk AD, Bourbonnais FF. Exploring the facilitators and barriers to home dialysis: a scoping review. Nephrol Nurs J. 2016;43(4):295-308. [PubMed] [Google Scholar]
- 14. Mann BS, Manns BJ, Barnieh L, et al. Peritoneal dialysis: a scoping review of strategies to maximize PD utilization. Perit Dial Int. 2017;37(2):159-164. [DOI] [PubMed] [Google Scholar]
- 15. Povlsen JV, Ivarsen P. Assisted automated peritoneal dialysis (AAPD) for the functionally dependent and elderly patient. Perit Dial Int. 2005;25(suppl 3):S60-S63. [PubMed] [Google Scholar]
- 16. Bechade C, Lobbedez T, Ivarsen P, Povlsen JV. Assisted peritoneal dialysis for older people with end-stage renal disease: the French and Danish experience. Perit Dial Int. 2015;35(6):663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iyasere OU, Brown EA, Johansson L, et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol. 2016;11(3):423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliver MJ, Salenger P. Making assisted peritoneal dialysis a reality in the United States: a Canadian and American viewpoint. Clin J Am Soc Nephrol. 2020;15(4):566-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliver MJ, Garg AX, Blake PG, et al. Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrol Dial Transplant. 2010;25(8):2737-2744. [DOI] [PubMed] [Google Scholar]
- 20. Brown EA. Should older patients be offered peritoneal dialysis? Perit Dial Int. 2008;28(5):444-448. [PubMed] [Google Scholar]
- 21. Blake PG. Peritoneal dialysis: a “kinder, gentler” treatment for the elderly? Perit Dial Int. 2008;28(5):435-436. [PubMed] [Google Scholar]
- 22. Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ. Home care assistance and the utilization of peritoneal dialysis. Kidney Int. 2007;71(7):673-678. [DOI] [PubMed] [Google Scholar]
- 23. Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Heijdra YF. Physiologic limitations during daily life activities in COPD patients. Respir Med. 2010;104(8):1152-1159. [DOI] [PubMed] [Google Scholar]
- 24. Querido S, Branco PQ, Costa E, Pereira S, Gaspar MA, Barata JD. Results in assisted peritoneal dialysis: a ten-year experience. Int J Nephrol. 2015;2015:712539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lobbedez T, Verger C, Ryckelynck JP, Fabre E, Evans D. Is assisted peritoneal dialysis associated with technique survival when competing events are considered. Clin J Am Soc Nephrol. 2012;7(4):612-618. [DOI] [PubMed] [Google Scholar]
- 26. Yerram P, Misra M. Home dialysis in the coronavirus disease 2019 era. Adv Chronic Kidney Dis. 2020;27(5):442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Francois K, Bargman JM. Evaluating the benefits of home-based peritoneal dialysis. Int J Nephrol Renovasc Dis. 2014;7:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lobbedez T, Moldovan R, Lecame M, Hurault de Ligny B, El Haggan W, Ryckelynck JP. Assisted peritoneal dialysis. Experience in a French renal department. Perit Dial Int. 2006;26(6):671-676. [PubMed] [Google Scholar]
- 29. Laplante S, Krepel H, Simons B, Nijhoff A, van Liere R, Simons M. Offering assisted peritoneal dialysis is a cost-effective alternative to the current care pathway in frail elderly Dutch patients. Int J Healthc Manag. 2013;6(1):27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bevilacqua MU, Turnbull L, Saunders S, et al. Evaluation of a 12-month pilot of long-term and temporary assisted peritoneal dialysis. Perit Dial Int. 2017;37(3):307-313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581221113387 for Assisted Peritoneal Dialysis Implementation: A Pilot Program From a Large Dialysis Unit in Quebec by Julien Melanson, Jessica Kachmar, Louis-Philippe Laurin, Naoual Elftouh and Annie-Claire Nadeau-Fredette in Canadian Journal of Kidney Health and Disease