Abstract
Background
Cardiac perforation during leadless pacemaker implantation is more likely to require intervention than perforation by a transvenous lead. This study reports the consequences of Micra pacemaker perforations and related device and operator use problems based on information the manufacturer has submitted to the Food and Drug Administration (FDA).
Methods
FDA's Manufacturer and User Facility Device Experience (MAUDE) database was searched for Micra perforations. Data extracted included deaths, major adverse clinical events (MACEs), and device and/or operator use problems.
Results
Between 2016 and July 2021, 563 perforations were reported within 30 days of implant and resulted in 150 deaths (27%), 499 cardiac tamponades (89%), 64 pericardial effusions (11%), and 146 patients (26%) required emergency surgery. Half of perforations were associated with 139 (25%) device problems, 78 (14%) operator use problems, and 62 (11%) combined device and operator use problems. Inadequate electrical measurements or difficult positioning were the most frequent device problems (n = 129); non‐septal implants and perforation of other structures were the most frequent operator use problems (n = 69); a combined operator use and device problem resulted in 62 delivery system perforations. No device or operator use problem was identified for 282 perforations (50%), but they were associated with 78 deaths, 245 tamponades, and 57 emergency surgeries.
Conclusion
The Micra perforations reported in MAUDE are often associated with death and major complications requiring emergency intervention. Device and use problems account for at least half of perforations. Studies are needed to identify who is at risk for a perforation and how MACE can be avoided or mitigated.
Keywords: complication, leadless, pacemaker, perforation, pericardial effusion, tamponade
1. INTRODUCTION
The Micra™ leadless transcatheter pacing system (TPS) (Medtronic, Inc) was first implanted in 2013 and market‐released in the United States (USA) in 2016 after a successful multicenter clinical trial. 1 Subsequent studies suggested that Micra implant success rates were high and the incidence of major adverse clinical events (MACEs) was low. 2 , 3 , 4 , 5 , 6 Recently, we reported our analysis of MACE associated with Micra‐TPS implantation based on information obtained from the USA Food and Drug Administration (FDA) Manufacturer and User Facility Device Experience (MAUDE) database. 7 While their incidence appeared to be low, we found a concerning number of deaths and other MACE caused by Micra‐TPS cardiac perforations.
The present study was undertaken to identify the consequences of perforations that occurred during Micra implantation as described by the manufacturer in its MAUDE reports. We also sought to determine if a perforation was due to the Micra‐TPS or how it was used, or if it was caused by unidentified clinical, operator, or procedural factors.
2. METHODS
2.1. Study design
This is a retrospective study that includes Micra VR and Micra AV MAUDE reports submitted by Medtronic, Inc., and received by the FDA between June 9, 2016, and July 31, 2021. These reports were identified and downloaded to our database by proprietary software (Basil Systems). Excluded were duplicative reports for a single event and reports based on information communicated to the manufacturer by patients or their surrogates. Reports were not adjudicated or audited.
2.2. Devices and definitions
The Micra VR and Micra AV implantable pulse generator (IPG) models, delivery system, and the technique for implantation have been described. 8 Device problems include (1) failure to capture or an inadequate threshold, R‐wave, or impedance measurement; (2) unusual difficulty positioning the TPS; (3) a TPS material failure; (4) IPG dislodgement after fixation; (5) TPS not performing as expected. An operator use problem occurred if (1) the IPG was positioned in a location other than the right ventricular septum (non‐septal); (2) perforations of the atrium, inferior vena cava, or lung, 3) coronary sinus dissection; (4) a temporary pacing lead or ablation catheter that appeared to cause or contribute to a perforation; or, (4) an operator error. A device and operator. use problem was a (1) perforation that appeared to result from the combination of an operator error and a device problem; or, (2) a delivery system perforation that occurred before IPG deployment. A device problem was not a malfunction unless specified.
Micra received European approval in April 2015, followed by US FDA approval in April 2016, and approval in Japan in February 2017.
2.3. FDA MAUDE database
The FDA MAUDE database contains reports of adverse events involving marketed medical devices that are reported to USA manufacturers by users worldwide, and thus it captures real‐world events. 9 MAUDE reports contain no patient‐specific information. MAUDE medical device reports (MDRs) are available for the previous 10 years at www.fda.gov/cdrh/maude.html. Manufacturers must submit reports when they learn that a device may have caused or contributed to a death or serious injury or has malfunctioned. Reports include medical devices that remain implanted or have been explanted. MAUDE reports do not identify the implanting physician or hospital, and they provide no information on the experience or capabilities of the physician or hospital.
2.4. Search software
In our previous study, 7 we inadvertently under‐reported the number of Micra implant‐related deaths and other adverse events because the FDA's MAUDE simple search tool did not identify all applicable reports. For this study, the search was conducted with Basil Systems software, a web‐based SaaS platform that contains all publicly available FDA data. The platform uses advanced machine learning to index, correlate, and cross‐reference data, enabling simultaneous natural language, and full‐text search of all FDA applications, regulations, recalls, and adverse events.
2.5. Statistics
Discrete variables are reported as counts and percentages and were compared across groups using Pearson's chi‐square or Fisher's exact tests. R version 4.0.1 (R Core Team, 2021) was used in the analysis.
3. RESULTS
The MAUDE search found 563 perforations that manifested clinically during Micra implantation (516; 92%) and up to 30 days post‐implant (47; 8%). Most MAUDE Micra perforation reports were from the USA (58%), followed by Japan (19%) and France (6%). The geographic distribution and consequences of perforation are shown in Figure 1.
Figure 1.
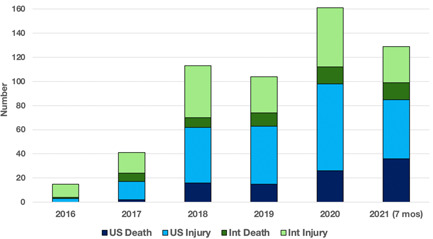
Year and geographic location of adverse events in MAUDE that were caused by Micra cardiac perforations. Int=international; MAUDE=Manufacturer and User Facility Device Experience; US=United States
The number of IPG deployments was available for 258 (46%) perforations. Of these, 159 (62%) IPGs were deployed more than once, 19 (7%) more than three times, and 29 (11%) perforations occurred after “multiple” IPG deployments. Sixty perforations were caused by the TPS delivery system before IPG deployment.
3.1. Clinical consequences
The reported perforations caused acute cardiac tamponade in 499 (89%) patients, and the remaining 64 (11%) patients had a pericardial effusion without tamponade (Figure 2). Mortality was significantly higher for patients who had tamponade compared to pericardial effusion (p < .001). Similarly, the incidences of cardiac arrest, shock or hypotension, and emergency surgery were significantly higher in the tamponade group (p < .001). A Micra IPG was implanted in at least 261 of the 413 perforation survivors; 40 patients ultimately received a transvenous or epicardial pacemaker.
Figure 2.
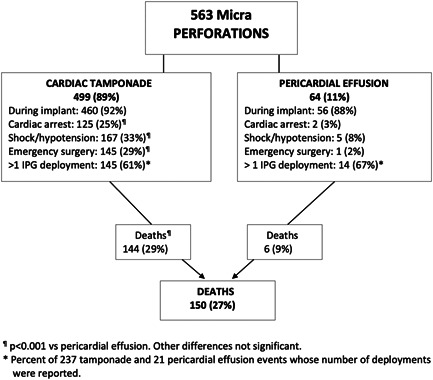
Micra perforation consequences
3.2. Device and use problems
A device and/or use problem was associated with 281 (50%) of the 563 Micra perforations (Figure 3). The most common device problem and reason for redeployment were non‐capture or inadequate electrical values (threshold, R‐wave, and impedance) that required IPG recapture and reimplantation, or replacement of the initial IPG with a second device. Clot or tissue on the cathode were frequent findings; in some cases, tissue fragments appeared to be from the encapsulating sheath of a retained or extracted lead.
Figure 3.
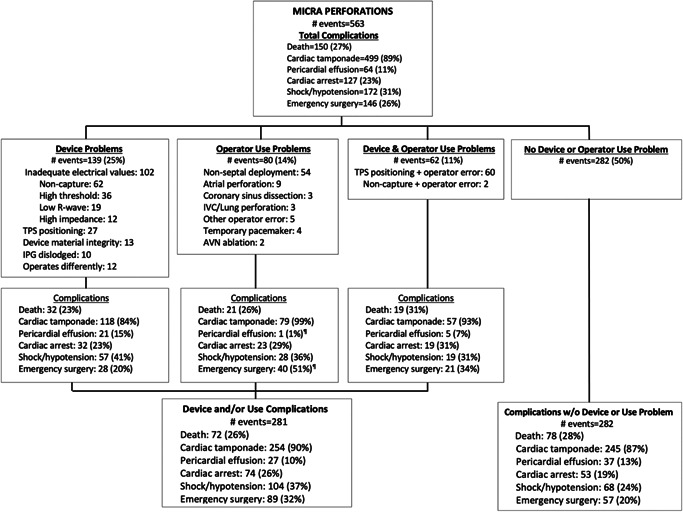
Device and/or operator use problems associated with Micra perforations
TPS positioning was unexpectedly difficult in 34 patients who had perforations; examples are provided in the online supplement, as are descriptions of material integrity and dislodgement issues associated with perforation.
A TPS use problem was significantly more likely to result in tamponade or need for emergency surgery (Figure 3). The most common use problem was non‐septal IPG deployment and fixation in the free wall of the right ventricle. Fifteen perforations of other cardiovascular structures by the delivery system or introducer also occurred. Six perforations may have been caused in part by the presence of a temporary pacing lead or ablation catheter.
The most common device and use problem was TPS positioning difficulty that together with an operator error resulted in a perforation by the delivery system before IPG deployment. Some perforations appeared to be the result of catheter recoil or slippage in the right ventricle, while others were caused by anatomic factors including small right ventricle and vascular tortuosity. Illustrative cases are provided in the online supplement.
No device or use problem could be identified for 282 perforations (Figure 3). These patients suffered similar complications, although proportionately fewer had shock/hypotension or required emergency surgery. No significant differences were found in the proportion of device and/or use problems reported for the USA compared to other countries.
3.3. Malfunctions and returned product analyses
The manufacturer classified 2 of the 563 perforations as malfunction events; one was a positioning device problem without evidence of a malfunction, and the second was a knotted tether use problem that was consistent with operator error. No returned product analysis (RPA) was available for either malfunction.
RPAs were available for 57 Micra systems (10.1%). No device failures or manufacturing defects were found. However, extensive procedure‐related damage to the delivery system was described. Kinking and buckling of the delivery system shaft were common findings. Representative examples are provided in the online supplement.
4. DISCUSSION
The results of this study show that Micra leadless pacemaker perforation is a complication that may result in death and other major complications requiring resuscitation and emergency surgery. Recently, Piccini et al. 10 found in a study of Micra patients that they were twice as likely to suffer a perforation/effusion event (P/E) than patients who received a transvenous pacemaker. There was a nonsignificant but slightly higher percentage (1.6% vs. 1.1%) of cardiac injuries such as perforation in the Micra pivotal trial. 1 In the 1817 patient Micra Post‐Approval Registry (MPAR) study 4 there were 14 Micra P/E adverse events (0.8%); eight of these patients underwent pericardiocentesis and two patients needed surgical repair but did not survive. In the Micra acute performance study, 11 there were 9 pericardial effusions among 926 implants (0.97%). Thus, while the incidence of leadless pacemaker perforation in these published studies 1 , 4 , 12 may be low (≈1%), its causes should be understood so that deaths and other MACEs can be avoided or mitigated. Solutions are needed because the number of leadless pacemaker implants is increasing, and new and novel leadless intracardiac electronic devices are in various stages of development and evaluation.
Based on data from two Micra clinical trials and MPAR, Mont et al. 12 reported that P/E events occurred in patients who were significantly older, had a lower body mass index, were more likely female, and had a higher incidence of chronic obstructive pulmonary disease. These data and the results of our study suggest that clinical risk factors for perforation should be confirmed, and a P/E risk score developed and validated.
Leadless pacemaker perforations are not confined to Micra devices. In the Nanostim LEADLESS II trial, 13 there were eight perforations, and the LEADLESS Observational Study was paused after two deaths occurred due to perforation. 14
We found that 281 of the 563 (49.9%) perforations were associated with one or more device and/or operator use problems. Two‐thirds of device‐only problems were electrical, namely non‐capture, high threshold or impedance, and low R‐wave amplitude. In some cases, high thresholds occurred after a complicated tether removal or because clots formed and adhered to the IPG's cathode, requiring IPG removal and cleaning or replacement with a new Micra‐TPS. Inadequate electrical values necessitated IPG recapture, redeployment, and re‐fixation, and often these maneuvers had to be repeated, further increasing the risk of perforation. A fundamental problem appears to be the need to deploy and actively fixate the Micra IPG before measuring threshold, R‐wave, and impedance.
The principal operator use problem was non‐septal IPG deployment. In some cases, the operator did not appear to know that the IPG was being fixated to the free wall, while in others the site was selected because the septum could not be accessed or only a non‐septal location provided acceptable electrical values. A learning curve and degree of operator variability exists with any complex invasive procedure including leadless pacemaker implantation. 15 , 16 , 17 Operator errors are more common and amplified when implant techniques lack precision. Improved navigation technology and contrast‐enhanced imaging beforand/or during implant may help guide accurate and safe IPG deployment.
TPS positioning was the second most common device problem (Figure 3). When accompanied by user (operator) errors, positioning problems resulted in delivery system perforations that typically occurred before the IPG was deployed. Many perforations appeared to happen when the TPS first entered the right ventricle after crossing the tricuspid valve. El‐Chami et al. 8 have emphasized the importance of not allowing the cup holding the IPG to “pop” through the valve, and the operator should release the deflection button once across the valve to avoid directing the delivery system toward the inferior free wall. Initial contrast injections often identified these perforations. Another critical stage is the application of correct tip pressure before IPG deployment. Some perforations occurred when the delivery system slipped off the septum or recoiled after pressure was applied to the insertion tool.
Half of perforations were not associated with a device or user problem; however, these patients suffered similar complications. It is possible that device or user data for these cases were unreported or not available. But importantly, it also suggests that there may be patient‐specific or other procedural or operator factors that are unknown or have not been characterized.
No perforation appeared to be the result of a Micra‐TPS malfunction. We found no evidence of intrinsic device failure or manufacturing defects in the returned product reports. Rather, the RPAs revealed procedure‐related damage to the delivery system, and the most prominent was kinking and buckling of the distal shaft that could have compromised navigation and deployment. This finding suggests that the delivery system performance may degrade with continued use.
The number of deaths associated with Micra perforation is concerning. Unlike transvenous lead perforations, Micra perforations are often large and catastrophic, and only prompt intervention that may include cardiac surgery is required to rescue the patient. It seems clear that leadless pacemaker implants should be performed only in centers capable of managing all complications, and especially perforation.
Leadless technology is at an evolutionary stage where operator skill, experience, and attention to detail are critical. Optimum caseloads for individual operators and implant centers should be determined. Professional societies should develop guidelines for the implantation and follow‐up of leadless cardiac electronic devices.
5. LIMITATIONS
This study has the same limitation as all investigations using MAUDE data: without a denominator, the incidence of an adverse event is unknown. Our study may have been impacted by ascertainment bias in that the number of Micra MAUDE reports could be higher than usual because of the ongoing claims‐based longitudinal surveillance study. It is possible that the manufacturer may have received relatively more Micra adverse events reports than is usual for an implantable device.
It is likely that some MACE, especially those that occurred post‐discharge, were not reported to the manufacturer or to the FDA and are not in the MAUDE database. For example, some perforations may not have been reported, thereby increasing the percentage of perforations resulting in tamponade. It is possible that the high proportion of USA events is due to underreporting in other countries or local patient confidentiality regulations. Since MAUDE reports contain no patient information, we do not know how clinical risk factors have impacted the results. Similarly, we do not know the qualifications, experience, or caseload of the implanting physicians or hospitals. It is possible that adverse events due to a device or use problem were not communicated to Medtronic and thus were not included in the manufacturer's MAUDE reports.
6. CONCLUSION
The Micra leadless perforations reported in MAUDE are associated with severe adverse events, including cardiac tamponade, cardiac arrest, shock, and death. Many patients required emergency intervention including pericardial drainage and surgical repair. Micra implants should be confined to centers capable of managing these complications. Half of perforations in MAUDE are due to device and/or operator use problems, and the balance are due to unidentified patient, operator, or procedural issues. No perforation was the result of a confirmed device defect or malfunction. Risk factors for perforation should be identified, and a risk score developed and validated. Professional societies should publish guidelines for leadless cardiac electronic device implantation.
Supporting information
Supplementary information.
ACKNOWLEDGMENT
This study was funded entirely by the Minneapolis Heart Institute Foundation.
Hauser RG, Gornick CC, Abdelhadi RH, et al. Leadless pacemaker perforations: Clinical consequences and related device and user problems. J Cardiovasc Electrophysiol. 2022;33:154‐159. 10.1111/jce.15343
Disclosures: Robert G. Hauser, MD, FHRS: Cardiac Insight Inc, Scientific Advisory Board. Other authors: No disclosures.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533‐541. [DOI] [PubMed] [Google Scholar]
- 2. Ritter P, Duray GZ, Zhang S, et al. The rationale and design of the Micra Transcatheter Pacing Study: safety and efficacy of a novel miniaturized pacemaker. Europace. 2015;17:807‐813. [DOI] [PubMed] [Google Scholar]
- 3. Roberts PR, Clementy N, Al Samadi F, et al. A leadless pacemaker in the real‐world setting: The Micra Transcatheter Pacing System Post‐Approval Registry. Heart Rhythm. 2017;14:1375‐1379. [DOI] [PubMed] [Google Scholar]
- 4. El‐Chami MF, Al‐Samadi F, Clementy N, et al. Updated performance of the Micra transcatheter pacemaker in the real‐world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018;15:1800‐1807. [DOI] [PubMed] [Google Scholar]
- 5. Valiton V, Graf D, Pruvot E, et al. Leadless pacing using the transcatheter pacing system (Micra TPS) in the real world: initial Swiss experience from the Romandie region. Europace. 2019;21:275‐280. [DOI] [PubMed] [Google Scholar]
- 6. Zucchelli G, Tolve S, Barletta V, et al. Comparison between leadless and transvenous single‐chamber pacemaker therapy in a referral center for lead extraction. J Interv Card Electrophysiol. 2020;61:395‐404. 10.1007/s10840-020-00832-9 [DOI] [PubMed] [Google Scholar]
- 7. Hauser RG, Gornick CC, Abdelhadi RG, Tang C, Casey SA, Sengupta JD. Major adverse clinical events associated with implantation of a leadless intracardiac pacemaker. Heart Rhythm. 2021;18:1132‐1139. [DOI] [PubMed] [Google Scholar]
- 8. El‐Chami MF, Roberts PR, Kypta MD, et al. How to implant a leadless pacemaker with tine‐based fixation. J Interv Card Electrophysiol. 2016;27:1495‐1501. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration MAUDE‐Manufacturer and User Facility Device Experience. Accessed September 6, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm
- 10. Piccini JP, El‐Chami M, Wherry K, et al. Contemporaneous comparison of outcomes among patients implanted with a leadless vs transvenous single‐chamber ventricular pacemaker. JAMA Cardiol. 2021;6:1187‐1195. 10.1001/jamacardio.2021.2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garweg C, Clementy N, Mondoloy P, et al. A leadless pacemaker in the real‐world setting: Patient profile and performance over time. Europace. 2021;23(S3):euab116.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mont L, Cunnane R, El‐Chami MF, et al. Risk factors for cardiac perforation/effusion in leadless pacemaker patients: experience with the Micra transcatheter pacemaker. Heart Rhythm. 2018;15:S119‐S675. [Google Scholar]
- 13. Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125‐1135. [DOI] [PubMed] [Google Scholar]
- 14. Sperzel J, Defaye P, Delnoy P‐P, et al. Primary safety results from the LEADLESS Observational Study. Europace. 2018;20:1491‐1497. [DOI] [PubMed] [Google Scholar]
- 15. Haeberlin A, Kozhuharov N, Knecht S, et al. Leadless pacemaker implantation quality: importance of the operator's experience. Europace. 2020;22:939‐946. [DOI] [PubMed] [Google Scholar]
- 16. Tjong FVY, Beurskens NEG, Neuzil P, et al. The learning curve associated with the implantation of the Nanostim leadless pacemaker. J Interv Card Electrophysiol. 2018;53:239‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garweg C, Vandenberk B, Foulon S, et al. Determinants of the difficulty of leadless pacemaker implantation. Pacing Clin Electrophysiol. 2020;43:552‐557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
Data available on request from the authors.


