Abstract
Background
The aim of this study was to assess the long‐term safety and efficacy of sapropterin in a real‐world setting in Japanese patients with tetrahydrobiopterin (BH4)‐responsive phenylketonuria.
Methods
This post‐marketing surveillance study enrolled all of the patients in Japan with confirmed BH4‐responsive PKU who were administrated sapropterin between July 2008 and October 2017. Patients were observed at least every 3 months during follow up, with key data collected on treatment exposure/duration, effectiveness according to physician’s judgement, serum phenylalanine levels, and adverse events.
Results
Of 87 enrolled patients, 85 patients (male, 42.4%; outpatients, 96.5%) were included in the safety and efficacy analysis sets. Treatment started at age <4 years in 43 (50.6%) patients and the most common starting daily dose was 5–10 mg/kg (n = 41, 48.2%) with the overall duration of treatment between 0.2 and 17.2 years. Serum phenylalanine levels, according to loading tests, reduced from a baseline level of 9.66 mg/dL (range 0.48–36.80 mg/dL) by >30% in 84 patients. Treatment was deemed effective in 79 of 85 patients (92.9%, 95% confidence interval: 85.3–97.4). One patient (1.2%) experienced an adverse drug reaction (alanine aminotransferase increased) 50 days after the start of administration, which resolved without complications with continued treatment.
Conclusions
Sapropterin appears well tolerated and highly effective in Japanese patients treated in a real‐world setting, including those who start treatment at age <4 years and pregnant women.
Keywords: hyperphenylalaninemia, Japanese, phenylketonuria, Sapropterin, tetrahydrobiopterin
In situ tetrahydrobiopterin (BH4) acts as a coenzyme for phenylalanine hydroxylase (PAH), tyrosine hydroxylase and tryptophan hydroxylase, which are enzymes involved in the biosynthesis of the catecholamine and serotonin neurotransmitters. Phenylketonuria (PKU) is a congenital metabolic disorder characterized by PAH deficiency and consequently elevated phenylalanine levels (hyperphenylalaninemia, HPA) of varying severity. 1 , 2 A small proportion of patients with HPA (3%) have BH4 deficiency due to congenital deficiency of the enzymes required for the synthesis of BH4 (atypical HPA). 1 Without treatment, HPA/PKU may lead to neuropsychological impairment and, in the case of a severe phenotype with pronounced HPA, to significant and irreversible mental disability. 1 , 3 , 4
The effectiveness of BH4 was first demonstrated in Japan for the treatment of patients with PAH deficiency who had at least one mutated allele of the PAH gene (e.g., R241C and P407S), which is associated with mild disease. 1 , 5 The development of sapropterin dihydrochloride, a synthetic preparation of the natural cofactor for PAH (6R‐BH4), for the treatment of atypical HPA (i.e., HPA caused by a BH4 deficiency) began in 1980 with a collaboration between the Japanese government and pharmaceutical companies. The chemical synthesis and granulation of sapropterin dihydrochloride for administration as granules was established by Suntory Co., Ltd. (Osaka, Japan), and the intellectual property was then transferred to Daiichi Sankyo Co. Ltd (Tokyo, Japan). The efficacy and safety of sapropterin dihydrochloride for the treatment of atypical HPA was demonstrated through clinical studies, showing reduction of serum phenylalanine levels, and the drug received regulatory approval for atypical HPA in March 1992 in Japan, 1 , 6 the first country to grant this. In 1999, Kure et al. identified a subtype of PAH deficiency that was distinct from atypical HPA, but was still responsive to BH4 treatment. 1 , 5 Subsequently, the labelling for sapropterin dihydrochloride was extended in Japan on July 16, 2008, to include BH4‐responsive HPA, acknowledging that it reduces “the serum level of phenylalanine in patients with HPA due to BH4‐responsive phenylalanine hydroxylase deficiency.” 1 , 7 Sapropterin dihydrochloride is currently used to treat BH4‐responsive HPA in more than 50 countries in the world and several follow‐up studies have been conducted.
The number of patients treated with sapropterin dihydrochloride at the time of approval was limited, 1 , 8 , 9 so the Japanese regulatory authority required that a drug use results surveillance study (i.e., a post‐marketing surveillance study) be conducted with all patients treated with the product after launch until data from a pre‐determined number of patients had been accumulated. In addition, the safety of sapropterin during pregnancy had not been established at the time of approval. According to the package insert in Japan, the drug should be used only when the benefits outweigh the risks, 1 , 7 so the study included patients who experienced pregnancy and delivery during the observation period.
The aim of this Japanese post‐marketing surveillance study was therefore to assess the safety and efficacy of sapropterin dihydrochloride over the long term in a real‐world setting in all treated patients with BH4‐responsive HPA in Japan.
Methods
Study design and patients
All patients diagnosed with BH4‐responsive HPA who were administered sapropterin dihydrochloride between July 2008 and October 2017 were included in this post‐marketing surveillance study. The diagnosis was confirmed by a BH4 single‐loading test, BH4 4‐times loading test, and BH4 1‐week loading test, at which time baseline data were collected and patients were enrolled.
Administration method
Dosage and administration were determined according to the package insert in Japan, 7 which states: “Usually, sapropterin dihydrochloride is started at a dose of 10 mg/kg/day (in 1–3 divided oral doses), and the dose that can maintain the target serum level of phenylalanine corresponding to age, while observing clinical symptoms, etc., is defined as the effective maintenance dose.” In addition to the package insert description, the following instructions were added: (i) in principle, the dose should not exceed 20 mg/kg/day; and (ii) treat to a target serum phenylalanine level within the reference range for the corresponding age, as defined in Japanese treatment guidelines in 2012. 10
Observation period and assessments
All enrolled patients had a pre‐study visit and then one at least every 3 months during follow‐up, depending on the patient's condition. Patient data were censored at the time of the final follow‐up visit for any patients who discontinued the study drug prior to the end of December 2017 (which was the data cut‐off date for this analysis).
Regarding patient background characteristics, the following information was gathered at enrolment: sex, hospitalization/outpatient status, age, presence or absence of abnormal EEG, presence or absence of abnormal magnetic resonance imaging (MRI) findings, complications, family history of HPA, and the presence/absence of diet therapy. Information on sapropterin dihydrochloride treatment exposure was determined from the following data gathered at each study visit: daily dose, serum phenylalanine level. Treatment duration was calculated as the time from study drug initiation to sapropterin discontinuation or the last study visit prior to data cutoff.
In terms of outcome measures, treatment effectiveness was evaluated every 6 or 12 months as either “effective” or “ineffective” according to physician judgement based on a comparison of serum phenylalanine levels and neurocognitive development status before and after treatment. Whenever possible, serum phenylalanine levels were monitored at least once every 3 months (i.e., 4 times a year) until discontinuation or withdrawal. To allow comparison with a Phase 3 trial in non‐Japanese patients, data from patients with serum phenylalanine levels measured at 6 ± 1 weeks after treatment initiation were extracted. Considering the frequency of measurement of serum phenylalanine levels in daily clinical practice, serum phenylalanine levels measured 5–7 weeks after administration of the first dose of this drug were taken to be the “6‐week” levels. Baseline serum phenylalanine levels were those measured immediately before the BH4 single‐loading test, BH4 4‐times loading test, or BH4 1‐week loading test. Based on Japanese guidelines for the management of PKU in 2012, 10 the recommended maintenance phenylalanine serum levels were set according to age as follows: 0 to 3 years, 2–4 mg/dL; 4–9 years, 2–6 mg/dL; 10–11 years, 2–8 mg/dL; 12 years and older, 2–10 mg/dL.
Patient height and weight were measured at baseline, during the observation period (once every 3 months) and at last study visit in order to assess effects on growth. Phenylalanine intake, and the period over which it was assessed, were recorded in patients' case report forms, based on the patient's self‐report.
Adverse events (AEs) were classified according to the ICH Medical Dictionary for Regulatory Activities Japanese edition (MedDRA/J) Version 21.0. Adverse drug reactions were defined as AEs considered by the investigator to be treatment related.
Statistical analysis
The incidence of adverse drug reactions and corresponding two‐sided 95% confidence intervals (CI) were estimated for the safety analysis set. 11 Comparisons were tested by Fisher's exact test. The efficacy rate and its two‐sided 95% CI were estimated for the efficacy analysis set. 11 Summary statistics were used to describe serum phenylalanine levels at each year of age. SAS® Version 9.2 (SAS Institute Inc, Cary, NC, USA) was used for all statistical analyses.
Ethics
This study was conducted in accordance with Japanese good post‐marketing study practice (GPSP) regulations. The study protocol was reviewed and granted consent by the appropriate Japanese regulatory authority prior to study initiation.
Results
Patient disposition and characteristics
Sapropterin was administered to patients with BH4‐responsive HPA at 51 institutions in Japan between July 2008 and October 2017.
In total, 87 patients were enrolled; however, two patients were excluded from analysis because they were not definitively diagnosed with BH4‐responsive HPA. The remaining 85 patients were included in the safety and efficacy analysis sets (Fig. S1). No AEs were reported for the two excluded patients.
Baseline characteristics of the 85 patients are shown in Table 1. The study population included 36 male (42.4%) and 49 female (57.6%) patients, of whom 82 (96.5%) were outpatients and three (3.5%) were inpatients. Treatment started at younger than 4 years of age in 43 (50.6%) patients and at 4 years or older in 42 (49.4%) patients. At the start of treatment, complications were observed in 16 (18.8%) patients, and 64 (75.3%) patients were on diet therapy. None of the complications were related to BH4‐responsive HPA.
Table 1.
Baseline characteristics of patients
| Category | Sapropterin (N = 85) |
|---|---|
| Sex, n (%) | |
| Male | 36 (42.4) |
| Female | 49 (57.6) |
| Inpatient/outpatient status, n (%) | |
| Inpatient | 3 (3.5) |
| Outpatient | 82 (96.5) |
| Age at the start of sapropterin†, year | |
| Mean (SD) | 6.2 (8.4) |
| Median (min, max) | 3.0 (0, 33) |
| Age range categories, years, n (%) | |
| <1 | 16 (18.8) |
| ≥1–<4 | 27 (31.8) |
| ≥4–<10 | 28 (32.9) |
| ≥10–<12 | 2 (2.4) |
| ≥12–<65 | 12 (14.1) |
| ≥65 | 0 |
| Presence/absence of abnormal electroencephalogram, n (%) | |
| Not tested | 66 (77.6) |
| Absence of abnormalities | 17 (20.0) |
| Presence of abnormalities | 1 (1.2) |
| Unknown/not detected | 1 (1.2) |
| Presence/absence of abnormal MRI findings, n (%) | |
| Not tested | 63 (74.1) |
| Absence of abnormalities | 19 (22.4) |
| Presence of abnormalities‡ | 2 (2.4) |
| Unknown/not detected | 1 (1.2) |
| Complications, n (%) | |
| Absent | 69 (81.2) |
| Present | 16 (18.8) |
| Family history of HPA, n (%) | |
| Absent | 71 (83.5) |
| BH4‐responsive HPA | 12 (14.1) |
| Others§ | 2 (2.4) |
| Diet therapy (phenylalanine‐restricted diet), n (%) | |
| Absent | 21 (24.7) |
| Present | 64 (75.3) |
†Age at the start of treatment written in the section on treatment in the survey sheet.
‡One patient had hyperintensity in the deep white matter around the bilateral ventricles, hyperintensity in the genu, body, and ampulla of the corpus callosum, and hyperintensity in the subcortical white matter of the bilateral frontoparietal lobes. The second patient had white matter lesions around the lateral ventricle triangle.
§Of these two cases, the detailed diagnosis was unknown for one case. The other case was diagnosed with phenylketonuria.
BH4, tetrahydrobiopterin; HPA, hyperphenylalaninemia; MRI, magnetic resonance imaging; SD, standard deviation.
Dosage and treatment exposure are shown in Table 2. The most common starting daily dose was 5–10 mg/kg (n = 41, 48.2%). Both the mean and median daily dose were approximately 10 mg/kg. The initial daily dose was <5 mg/kg in 10 (11.8%) patients, 10–15 mg/kg in 17 (20.0%), 15–20 mg/kg in 16 (18.8%), and >20 mg/kg in one (1.2%) patient. The daily dose at the time of the final study observation was <5 mg/kg in nine (10.6%) patients, 5–10 mg/kg in 26 (30.6%), 10–15 mg/kg in 21 (24.7%), 15–20 mg/kg in 24 (28.2%), and >20 mg/kg in five (5.9%) patients.
Table 2.
Dosage, administration, and treatment exposure of sapropterin dihydrochloride
| Sapropterin (N = 85) | |
|---|---|
| Daily dose at treatment initiation (mg/kg) | |
| Mean (SD) | 10.352 (4.879) |
| Median (min, max) | 9.620 (1.47, 20.06) |
| Dose categories, mg/kg/day, n (%) | |
| <5 | 10 (11.8) |
| ≥5–<10 | 41 (48.2) |
| ≥10–<15 | 17 (20.0) |
| ≥15–≤20 | 16 (18.8) |
| >20 | 1 (1.2) |
| Daily dose at the time of final observation (mg/kg) | |
| Mean (SD) | 12.036 (5.661) |
| Median (min, max) | 10.840 (1.54, 26.67) |
| Dose categories, mg/kg/day, n (%) | |
| <5 | 9 (10.6) |
| ≥5–<10 | 26 (30.6) |
| ≥10–<15 | 21 (24.7) |
| ≥15–≤20 | 24 (28.2) |
| >20 | 5 (5.9) |
| Treatment duration (years)† | |
| Mean (SD) | 5.75 (4.47) |
| Median (min, max) | 5.20 (0.2, 17.2) |
| Treatment duration categories, years, n (%) | |
| <1 | 14 (16.5) |
| ≥1–<4 | 24 (28.2) |
| ≥4–<7 | 11 (12.9) |
| ≥7–<10 | 26 (30.6) |
| ≥10 | 10 (11.8) |
†Period from the start date of treatment written in the section of treatment course in the survey sheet to the end date of observation (the end date of treatment if it is written).
SD, standard deviation.
The duration of treatment ranged from 0.2 to 17.2 years, with 26 (30.6%) patients receiving treatment for 7–10 years. Fourteen (16.5%) patients were treated for less than 1 year, 24 (28.2%) for between 1 and 4 years, 11 (12.9%) for between 4 and 7 years, and 10 (11.8%) patients for 10 years or more.
Discontinuation of treatment occurred in six of 85 cases. Five of the six patients discontinued due to a change in treatment policy related to the patient's condition, and one patient decided to withdraw from the study. No discontinuation due to AE was observed.
Serum phenylalanine levels at baseline and during the loading test are shown in Tables 3 and 4, respectively (efficacy analysis set). The mean serum level of phenylalanine at the pre‐study visit was 9.66 mg/dL (range 0.48–36.80 mg/dL), and the mean levels of phenylalanine at the time of newborn mass screening and before the BH4 loading test were both higher than 2 mg/dL. A BH4 single‐loading test, BH4 4‐times loading test or BH4 1‐week loading test was performed in 84 of 85 patients in the efficacy analysis set, and a mean decrease in serum phenylalanine of more than 30% was observed. One case in which loading test data were not available was later diagnosed with BH4‐responsive HPA based on clinical observation by the investigator, although no loading test data were available.
Table 3.
Serum phenylalanine level before the initiation of treatment
| Timing | n | Serum phenylalanine, mg/dL | |
|---|---|---|---|
| Mean (SD) | Min, Max | ||
| At newborn screening | 77 | 8.53 (3.86) | 2.07, 19.59 |
| At pre‐study visit | 83 | 9.66 (6.39) | 0.48, 36.80 |
| Before BH4 loading test | 84 | 12.00 (7.06) | 2.99, 40.00 |
BH4, tetrahydrobiopterin; SD, standard deviation.
Table 4.
Serum phenylalanine levels at BH4 loading tests and mean change in serum phenylalanine levels from baseline
| Test | Timing of serum measurement after loading | n | Serum phenylalanine, mg/dL | |
|---|---|---|---|---|
| Mean (SD) | Mean change (%) | |||
| BH4 single‐loading | 24 h | 65 | 8.70 (6.98) | –32.37 |
| BH4 4‐times loading | 4 or 8 h | 17 | 5.54 (1.83) | –20.43 |
| 24 h | 18 | 5.43 (2.39) | –22.01 | |
| 52 h | 18 | 4.93 (3.10) | –32.03 | |
| BH4 1‐week loading | 4 days | 47 | 5.70 (5.08) | –45.86 |
| 7 days | 48 | 6.09 (5.54) | –41.85 | |
BH4, tetrahydrobiopterin; SD, standard deviation.
Safety
Of 85 patients included in the safety analysis set, one patient (1.2%) experienced an increase in alanine aminotransferase level, which was designated as an adverse drug reaction (Table 5). This event occurred 50 days after the start of administration at 8 months of age, but was deemed not serious and was not associated with any particular clinical symptom. The patient recovered without complications with continued treatment. There were two serious AEs: nasopharyngitis (1.2%) and myocarditis (1.2%). Both AEs were observed in the same patient, who was younger than 4 years old, and were deemed unrelated to drug treatment.
Table 5.
Incidence of adverse drug reactions†
| Category | Sapropterin (N = 85) |
|---|---|
| Number of patients with adverse drug reactions | 1 |
| Number of adverse drug reactions (events) | 1 |
| Incidence of adverse drug reactions, % (95% CI) | 1.2 (0.03–6.38) |
| SOC: Investigations, n (%)† | 1 (1.2) |
| PT: Alanine aminotransferase increased | 1 (1.2) |
†SOC was used to calculate the number of patients who developed adverse drug reactions; PT was used to calculate the number of patients who developed adverse drug reactions (number of adverse drug reactions).
Adverse drug reactions were categorized per MedDRA/J Version 21.0.
CI, confidence interval; MedDRA/J, ICH Medical Dictionary for Regulatory Activities Japanese edition; PT, preferred term; SOC, System Organ Class.
Effectiveness
Effectiveness rate
The results of the treatment effectiveness analysis for the 85 patients included in the efficacy analysis set by investigators at the time of the final observation (at the end of December 2017 or at the final follow‐up visit for those who discontinued treatment before this date) are shown in Table 6. Treatment was deemed effective in 79 of 85 patients (92.9%, 95% CI: 85.3–97.4) and unevaluable in 6 (7.1%) patients; no patients were judged to have had ineffective treatment. The reasons for being unevaluable (according to the investigator) are listed in Table S1; the most common reasons were poor drug compliance and short observation period. No patients showed a lack of effectiveness of the drug. Neurocognitive function was not consistently reported by the study physicians, but where it was reported, it was considered to be unaffected. Analysis by age of sapropterin initiation found that treatment was effective in most patients (~93%) in the <4 years' group (40/43 patients) and ≥4 years' group (39/42 patients; Table S2).
Table 6.
Summary of effectiveness
| n | Sapropterin effectiveness rate, % (95% CI) | |
|---|---|---|
| Effective | 79 | 92.9 (85.3–97.4) |
| Ineffective | 0 | 0 |
| Unevaluable | 6 | 7.1 |
CI, confidence interval.
Serum phenylalanine levels by age
The mean serum phenylalanine levels according to age are shown in Figure 1. Serum phenylalanine levels during treatment were maintained below the upper limit of recommended levels in many of the age groups. None of the patients who had a serum phenylalanine level above the recommended range developed clinical symptoms associated with elevated serum phenylalanine levels.
Fig. 1.
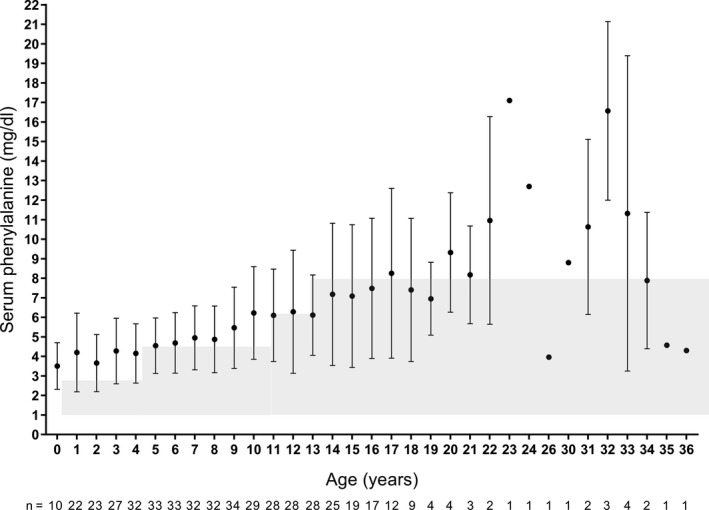
Mean (SD) serum phenylalanine levels according to age. Patient numbers at each age are shown below the graph. Data points for which the sample size was n = 1 do not have an SD value. Shading indicates recommended maintenance serum phenylalanine levels for each age group in Japan. 10 SD, standard deviation.
Comparison of current study results with Phase 3 study results
In Table 7 the change from baseline in serum phenylalanine levels were compared with the results from the Phase 3 study (PKU‐003). 4 The mean ± standard deviation (SD) change from before the loading test to 6 weeks after administration of this drug was −6.8 ± 5.5 mg/dL, showing no reduction in efficacy compared with the change of −3.9 ± 4.2 mg/dL at 6 weeks after administration in the Phase 3 study. In this study, the change at the time of the last observation was −6.3 ± 6.5 mg/dL, which was similar to the change observed at 6 weeks, indicating sustained efficacy.
Table 7.
Change from baseline in serum phenylalanine levels in this study and a Phase 3 clinical trial
| Study | Time point | Mean (SD) change from baseline, mg/dL |
|---|---|---|
| Current study† | Week 6 (n = 15) | –6.8 (5.5) |
| At final observation (n = 84) | –6.3 (6.5) | |
| PKU‐003‡ 4 | Week 6 (n = 41) | –3.9 (4.2) |
†Baseline was defined as the serum phenylalanine level at the BH4 loading test; ‡Conducted in patients with phenylketonuria.
SD, standard deviation.
Physical growth parameters
Patient height and bodyweight are plotted on a Japanese clinical growth chart 12 in Figure S2, shown by age of initiation of sapropterin. There were no issues with growth throughout the observation period.
Phenylalanine tolerance
Phenylalanine tolerance increased during treatment; the mean phenylalanine intake at baseline was 445.3 (SD: 356.5) mg/day (n = 29) and at last observation was 941.0 (SD: 759.7) mg/day (n = 15), and median phenylalanine intake increased from 320.0 to 800.0 mg/day during the study period.
Treatment outcomes in pregnant women
Two women in their early 30s were confirmed to be pregnant during treatment. Neither of them developed AEs during pregnancy or the perinatal period, and no AEs were observed in their newborns. Their physicians judged sapropterin dihydrochloride treatment to be effective in these patients.
Discussion
At the time of approval for BH4‐responsive HPA in Japan, the safety and efficacy of sapropterin dihydrochloride had been evaluated in an extremely small number of patients. This 9.5‐year study included all patients with BH4‐responsive HPA treated with sapropterin in Japan, to evaluate the long‐term safety and efficacy of this drug in real‐world clinical practice. This is the first report regarding the use of sapropterin dihydrochloride in patients with BH4‐responsive HPA followed for as long as 9.5 years. In total, 87 patients were enrolled, and sapropterin treatment was judged to be effective in 79 of the 85 (92.9%) patients in the efficacy analysis set. We confirmed the BH4‐responsive HPA diagnosis in our cohort using a full battery of BH4 loading tests, including a 1‐week test, which has been shown to be necessary for identifying all responders. 13
Adverse drug reactions occurred in 1.2% (1/85) of the patients in this study. Approximately one‐third (30.6%) of patients had received 7–10 years’ treatment and about half (50.6%) of patients were younger than 4 years old, suggesting an acceptable safety profile of the drug in young patients. This indicates that there were no safety issues regardless of age group or treatment duration.
Serum phenylalanine levels were maintained within the guideline‐recommended range in most patients. Those who showed elevated serum phenylalanine level outside the range did not develop clinical symptoms associated with elevated serum phenylalanine levels. Although the number of measured cases was not sufficient for evaluation, as only a few physicians reported their assessment of patient neurocognitive status before/during/after treatment, the study physicians concluded that intelligence, development, motor skills, and language ability were unimpaired even in the patients with elevated serum phenylalanine levels, and their school or employment condition was considered generally good. In addition, growth parameters were all within the normal range during the course of treatment. These results are consistent with prior findings from a long‐term study of pediatric BH4‐responsive patients who began treatment with sapropterin before 6 years of age, in conjunction with a phenylalanine‐restricted diet; this study reported that patients had intellectual ability and growth rates within normal ranges. 14 In 13 of 16 patients whose serum phenylalanine levels exceeded the upper limit of the recommended range, the dose of sapropterin had not been increased up to 20 mg/kg/day. Furthermore, the latest Japanese guidelines 15 also state that the target blood phenylalanine level should be 2–6 mg/dL regardless of age or sex, in line with the target blood phenylalanine level recommended in the USA and Europe. 16 , 17
It is important to consider increasing the dose of BH4 to obtain phenylalanine levels with the range recommended by the latest Japanese treatment guidelines in 2019. 15 Based on these guidelines, if the phenylalanine level does not decrease at BH4 doses below 20 mg/kg/day, an increase in BH4 should be considered, and when the phenylalanine level still does not fall within the maintenance range, continued dietary therapy (low‐protein diet, phenylalanine‐free milk) should be more strictly followed. 15 Approximately three‐quarters of patients in our study were also following dietary therapy, in addition to sapropterin treatment. Clinical studies with sapropterin have demonstrated that patients can eat a less restrictive diet while on this treatment, because sapropterin augments the reduction in phenylalanine levels. 9 , 18 , 19 , 20 Indeed, phenylalanine tolerance increased in the subgroup of patients in our study for whom these data were available, which supports findings from a previous long‐term study with 5–7 years' follow up, where oral BH4 treatment (followed by sapropterin) allowed for an increase in tolerance to phenylalanine while phenylalanine levels remained within the age‐group‐appropriate target range, although patients in this study were aged >4 years. 21 Our study extends these observations to patients initiating sapropterin treatment before 4 years of age. While protein restriction is a key element of dietary treatment for PKU, some protein intake from natural foods is also important because long‐term consumption of phenylalanine‐free milk and a low‐protein diet is associated with reduced vitamin and mineral intake. 22 , 23 Not only does the less restrictive diet offer psychosocial benefits, but it can also contribute to an improvement in nutritional status and bodyweight, which can be especially important for children. 18 , 19 , 20
In the present study, adverse drug reactions due to abnormal laboratory test values were observed in one of 85 patients, indicating good safety. Our study confirmed that no adverse drug reactions were observed in patients treated with sapropterin 20 mg/kg/day.
Special attention to metabolic control is needed for female patients with PKU, because HPA during pregnancy is associated with a risk of fetal malformations, such as microcephaly and heart malformation. 24 , 25 Treatment of PKU and HPA is especially important in pregnant women in order to prevent complications in newborns. 25 , 26 BH4 is a treatment option for pregnant women with PKU who have difficulty in controlling their serum phenylalanine level diet therapy alone. 26 , 27 In the present study, two women were confirmed to be pregnant during treatment; they continued with their treatment and reported no AEs during the pregnancy or perinatal period. There were also no AEs in their newborns. This is described in detail in a separate report. 27 , 28
In Europe and the USA, the approved indication for BH4 has been expanded to include use for children under 4 years of age, whereas in Japan, the 2009 Japanese Society for Inherited Metabolic Diseases interim guidelines for the diagnosis and treatment of BH4‐responsive HPA describe treatment for patients aged under 4 years as follows: “There is limited experience of use of sapropterin dihydrochloride in children less than 4 years of age. Sufficient informed consent should be obtained from parents before starting the drug after explaining that the safety has not been established. In particular, there is little experience of use of the drug in newborns or infants; therefore, careful attention is needed, and treatment needs to be started at a lower dose.” 29 Prior to our study, a study in Japan that included 43 pediatric patients, of whom 21 were aged <4 years at the initiation of sapropterin treatment, reported that sapropterin was both effective and well tolerated. 30 The present study included a larger number of patients, and also demonstrated that there were no safety or effectiveness issues over the long term in patients who started the treatment at <4 years of age. Our results show a comparable incidence of adverse drug reactions and a comparable proportion of patients for whom treatment was judged to be effective, between those who started sapropterin at <4 years versus ≥4 years, over a similar treatment duration (~6 years). The results of this study suggest that BH4 treatment is safe and effective in patients with BH4‐responsive HPA, even in patients of a young age.
Limitations
This study has a number of limitations. First, as an observational study, there was no control group. As BH4‐responsive HPA is a rare disease, only 85 patients were included in either the effectiveness or safety analysis, which on its own may be insufficient to fully assess the safety and effectiveness of sapropterin. However, we believe that 9.5 years of safety and efficacy data provide clinicians with clinically important information on the long‐term use of sapropterin in these patients.
Conclusion
The results from this study supporting the long‐term safety and effectiveness of sapropterin in all treated patients with BH4‐responsive HPA in a real‐world setting in Japan are clinically important. The cumulative results of this study are expected to contribute significantly to real‐world evidence for the lifelong treatment of BH4‐responsive HPA.
Funding
Financial support for this study and the current manuscript was provided by Daiichi Sankyo Company Limited.
Disclosures
All authors are employees of Daiichi Sankyo Company Limited. As such, the sponsor was involved in the development of this manuscript, including study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the manuscript for publication.
Author contributions
T.M., S.S., and Y.K. contributed to the conceptualization, design, and methodology of the study. T.M. and S.S. also contributed to the investigation, data curation, and writing of the initial manuscript. Y.K. contributed to the formal analysis and reviewing/editing of the manuscript during development. S.C. and K.W. provided study supervision/oversight and allocation of resourcing for the study as well as reviewing/editing of the manuscript during development. K.W. also contributed to funding acquisition for the study. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors read and approved the final manuscript.
Supporting information
Fig. S1. Patient study populations.
Fig. S2. Patient height and weight plotted on growth standard charts for Japanese children in (A) males and (B) females. Growth standard charts show mean, ±1 SD, and ±2 SD lines (Z‐score lines) based on the year 2000 national survey, which were constructed by LMS method. SD, standard deviation; y.o., year old.
Table S1. Reasons for cases considered to be unevaluable.
Table S2. Treatment duration, safety and effectiveness of initiating sapropterin dihydrochloride treatment before or after 4 years of age.
Acknowledgments
The authors would like to thank Professor Haruo Shintaku, Emeritus Professor of Osaka City University Graduate School of Medicine for his guidance and advice in conducting the sapropterin survey and in writing this paper. The authors thank all of the clinicians for their involvement and contributions to the study. The authors also thank Yoshiko Okamoto, PhD, and Tracy Harrison of inScience Communications, Springer Healthcare, for editing the manuscript. This medical writing assistance was funded by Daiichi Sankyo, Co. Ltd. This study was funded by Daiichi Sankyo, Co. Ltd.
Prior presentation of study data as an abstract or poster: An interim report for this study was presented previously at the following conference: 13th International Congress of Inborn Errors of Metabolism (ICIEM 2017), Sep 2017, Rio de Janeiro, Brazil.
References
- 1. Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010; 376(9750): 1417–27. [DOI] [PubMed] [Google Scholar]
- 2. Kure S, Shintaku H. Tetrahydrobipterin‐responsive phenylalanine hydroxylase deficiency. J. Hum. Genet. 2019; 64: 67–71. [DOI] [PubMed] [Google Scholar]
- 3. Opladen T, López‐Laso E, Cortès‐Saladelafont E et al. Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies. Orphanet J. Rare Dis. 2020; 15(1): 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levy HL, Milanowski A, Chakrapani A et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R‐BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: A phase III randomised placebo‐controlled study. Lancet. 2007; 370: 504–10. [DOI] [PubMed] [Google Scholar]
- 5. Kure S, Hou DC, Ohura T et al. Tetrahydrobiopterin‐responsive phenylalanine hydroxylase deficiency. J. Pediatr. 1999; 135: 375–8. [DOI] [PubMed] [Google Scholar]
- 6. Kitagawa T, Owada M, Arashima S. Clinical results of sapropterin hydrochloride (R‐tetrahydrobiopterin) (R‐type BH4) for atypical hyperphenylalaninemia. [in Japanese] Syouni‐naika 1990; 22: 1737–50. [Google Scholar]
- 7. Daiichi‐Sankyo . Biopterin 2.5%/10.0% granules. Japanese Product Information [In Japanese]. Available from https://pins.japic.or.jp/pdf/newPINS/00061995.pdf. Accessed 16 December 2019. [Google Scholar]
- 8. Matsubara Y, Kure S, Ohura T et al. Study report by the expert committee for treatment criteria for tetrahydrobiopterin (BH4)‐reactive hyperphenylalaninemia 2. [in Japanese] Tokushu Milk Joho 2002; 38: 44–59. [Google Scholar]
- 9. Shintaku H, Kure S, Ohura T et al. Long‐term treatment and diagnosis of tetrahydrobiopterin‐responsive hyperphenylalaninemia with a mutant phenylalanine hydroxylase gene. Pediatr. Res. 2004; 55: 425–30. [DOI] [PubMed] [Google Scholar]
- 10. Kitagawa T, Matsuda I, Aoki K, Owada M, Okano Y, Ohura T. Details of the 2nd revision of the treatment guideline for phenylketonuria (Include a part of hyperphenylalaninemia) and the revised recommended treatment guideline (Fiscal year 2012). [in Japanese] Tokushu Milk Joho 2012; 48: 82–4. [Google Scholar]
- 11. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26: 404–13. [Google Scholar]
- 12. Isojima T, Kato N, Ito Y, Kanzaki S, Murata M. Growth standard charts for Japanese children with mean and standard deviation (SD) values based on the year 2000 national survey. Clin. Pediatr. Endocrinol. 2016; 25: 71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Wegberg AM, Evers RA, van Dam E et al. Does the 48‐hour BH4 loading test miss responsive PKU patients? Mol. Genet. Metab. Rep. 2020; 129(3): 186–92. [DOI] [PubMed] [Google Scholar]
- 14. Waisbren S, Burton BK, Feigenbaum A et al. Long‐term preservation of intellectual functioning in sapropterin‐treated infants and young children with phenylketonuria: A seven‐year analysis. Mol. Genet. Metab. 2021; 132: 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Japanese Society of Inherited Metabolic Diseases . New Born Mass Screening Disease Practice Guideline [in Japanese]. Tokyo: Shindan to Chiryo Sha, Inc., 2019. [Google Scholar]
- 16. Camp KM, Parisi MA, Acosta PB et al. Phenylketonuria Scientific Review Conference: State of the science and future research needs. Mol. Genet. Metab. Rep. 2014; 112: 87–122. [DOI] [PubMed] [Google Scholar]
- 17. van Wegberg AMJ, MacDonald A, Ahring K et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017; 12(1): 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambruschini N, Pérez‐Dueñas B, Vilaseca MA et al. Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol. Genet. Metab. 2005; 86(Suppl 1): S54–60. [DOI] [PubMed] [Google Scholar]
- 19. Qu J, Yang T, Wang E et al. Efficacy and safety of sapropterin dihydrochloride in patients with phenylketonuria: A meta‐analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2019; 85: 893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trefz FK, Scheible D, Frauendienst‐Egger G, Korall H, Blau N. Long‐term treatment of patients with mild and classical phenylketonuria by tetrahydrobiopterin. Mol. Genet. Metab. 2005; 86(Suppl 1): S75–80. [DOI] [PubMed] [Google Scholar]
- 21. Scala I, Concolino D, Della Casa R et al. Long‐term follow‐up of patients with phenylketonuria treated with tetrahydrobiopterin: A seven years experience. Orphanet J. Rare Dis. 2015; 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ilgaz F, Pinto A, Gökmen‐Özel H et al. Long‐term growth in phenylketonuria: A systematic review and meta‐analysis. Nutrients. 2019; 11: 2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okano Y, Hattori T, Fujimoto H et al. Nutritional status of patients with phenylketonuria in Japan. Mol. Genet. Metab. Rep. 2016; 8: 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lenke RR, Levy HL. Maternal phenylketonuria and hyperphenylalaninemia. An international survey of the outcome of untreated and treated pregnancies. N. Engl. J. Med. 1980; 303(21): 1202–8. [DOI] [PubMed] [Google Scholar]
- 25. Wang L, Ye F, Zou H et al. The first study of successful pregnancies in Chinese patients with phenylketonuria. BMC Pregnancy Childbirth. 2020; 20: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prick BW, Hop WC, Duvekot JJ. Maternal phenylketonuria and hyperphenylalaninemia in pregnancy: Pregnancy complications and neonatal sequelae in untreated and treated pregnancies. Am. J. Clin. Nutr. 2012; 95: 374–82. [DOI] [PubMed] [Google Scholar]
- 27. Nyuzuki H, Yamazaki T, Saito M, Ohtake A. First Japanese case of maternal phenylketonuria treated with sapropterin dihydrochloride and the normal growth and development of the child. Mol. Genet. Metab. Rep. 2019; 21: 100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakamoto O, Arai‐Ichinoi N, Murayama K, Kure S. Successful control of maternal phenylketonuria by tetrahydrobiopterin. Pediatr. Int. 2018; 60: 985–6. [DOI] [PubMed] [Google Scholar]
- 29. Ohura T, Shintaku H, Takayanagi M et al. Provisional guidelines for the proper use of natural BH4 preparations sapopterin hydrochloride for tetrahydrobiopterin (BH4) ‐reactive hyperphenylalaninemia. [in Japanese] Nihon Shonikagakkaishi 2009; 113: 649–53. [Google Scholar]
- 30. Shintaku H, Ohura T. Sapropterin is safe and effective in patients less than 4‐years‐old with BH4‐responsive phenylalanine hydrolase deficiency. J. Pediatr. 2014; 165(6): 1241–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Patient study populations.
Fig. S2. Patient height and weight plotted on growth standard charts for Japanese children in (A) males and (B) females. Growth standard charts show mean, ±1 SD, and ±2 SD lines (Z‐score lines) based on the year 2000 national survey, which were constructed by LMS method. SD, standard deviation; y.o., year old.
Table S1. Reasons for cases considered to be unevaluable.
Table S2. Treatment duration, safety and effectiveness of initiating sapropterin dihydrochloride treatment before or after 4 years of age.


