Abstract
The influence of cell-bound microcystins on the survival time and feeding rates of six Daphnia clones belonging to five common species was studied. To do this, the effects of the microcystin-producing Microcystis strain PCC7806 and its mutant, which has been genetically engineered to knock out microcystin synthesis, were compared. Additionally, the relationship between microcystin ingestion rate by the Daphnia clones and Daphnia survival time was analyzed. Microcystins ingested with Microcystis cells were poisonous to all Daphnia clones tested. The median survival time of the animals was closely correlated to their microcystin ingestion rate. It was therefore suggested that differences in survival among Daphnia clones were due to variations in microcystin intake rather than due to differences in susceptibility to the toxins. The correlation between median survival time and microcystin ingestion rate could be described by a reciprocal power function. Feeding experiments showed that, independent of the occurrence of microcystins, cells of wild-type PCC7806 and its mutant are able to inhibit the feeding activity of Daphnia. Both variants of PCC7806 were thus ingested at low rates. In summary, our findings strongly suggest that (i) sensitivity to the toxic effect of cell-bound microcystins is typical for Daphnia spp., (ii) Daphnia spp. and clones may have a comparable sensitivity to microcystins ingested with food particles, (iii) Daphnia spp. may be unable to distinguish between microcystin-producing and -lacking cells, and (iv) the strength of the toxic effect can be predicted from the microcystin ingestion rate of the animals.
Apart from the significance of being widespread and often bloom forming, many cyanobacterial species share the ability to produce bioactive compounds (4, 6). The microcystins, mainly produced by Microcystis spp., are among the most dominant of these bioactive substances and have been characterized as cyclic heptapeptides. The bioactivity of microcystins is mainly based on an inhibition of eukaryotic protein phosphatases (12, 27). As a result, microcystins may, once they have entered tissues or cells, interfere with numerous processes essential for life and are thus potentially harmful for higher organisms and humans (19). However, despite the broad attention given them and many advances in their molecular and biochemical characterization, the ecological significance of microcystins is still controversial.
Microcystins are usually cell bound. Although Microcystis cells may have possibilities for an outward transport (22), the intracellular microcystin concentration is typically high. Microcystins may therefore harm organisms that feed on Microcystis cells. Special interest has been applied to the effects on Daphnia spp., which are a key component of freshwater food chains (e.g., see reference 35). Daphnia spp. can consume considerable amounts of the phytoplankton biomass and are large enough to feed on Microcystis colonies (e.g., see references 40 and 41).
The presence of Microcystis in the diet can affect Daphnia in different ways. Several Microcystis strains, for example, cause a nonmechanical feeding inhibition and, once ingested, direct toxic effects (7, 21, 25, 31, 42). Experiments performed to test whether or not microcystins are the cause of these effects have produced an array of inconsistent data. DeMott and Dhawale (8), for example, have shown that purified microcystins inhibit the in vitro activity of Daphnia's protein phosphatases 1 and 2A and may thus have various adverse effects if assimilated into the body of the animals. This is in good agreement with the finding that dissolved microcystins are toxic to several Daphnia spp. (reviewed in reference 5). Based on survival tests with cyanobacterial cells and cell extracts, Jungmann (20) and Reinikainen (34) have, on the other hand, suggested that toxicity to Daphnia is not due to microcystins. Matveev et al. (29) have even proposed an ineffectiveness of microcystins to harm Daphnia carinata, and Pflugmacher et al. (33) have described an in vitro detoxification mechanism that, if also active in living Daphnia, may result in a resistance to microcystins.
The role of microcystins in feeding inhibition is unclear as well. As mentioned, many Microcystis strains, though not all, slow down the feeding activity of Daphnia in a non mechanical way (7, 16, 21, 25, 31). The responsible factor is yet unknown, but it may be a perceptible structure or substance located in the outer cell compartments (26, 37). Since microcystins can possibly pass through the cell wall of Microcystis (22), they may well be involved in the feeding inhibition. Several studies have been conducted to test that idea, but the results obtained are, as for the case of the toxic effect, contrary (16, 21, 25, 26).
The main problem in studying the impact of microcystins seems to be their cell-bound character. Since Daphnia spp. ingest microcystins together with living cells, it is difficult to distinguish the potential microcystin effects from those caused by other components. To solve that problem, Rohrlack et al. (39) have compared the effects of the microcystin-producing Microcystis strain PCC7806 and its mutant, which has been genetically engineered to knock out microcystin production (11). That line of study has been combined with an analysis of the relationship between the microcystin ingestion rate by Daphnia and its survival time (38). It turned out that the toxic effect of Microcystis spp. could be explained by microcystins, while the feeding inhibition seems to be due to another factor. However, Rohrlack et al. (38, 39) have based their experiments on a single D. galeata clone only and so it is uncertain if the results obtained can be generalized and used to clarify the role of microcystins in the effects of Microcystis on other Daphnia spp. Furthermore, it is unknown if Daphnia spp. can differ in their sensitivity to cell-bound microcystins and if microcystins ingested with food particles are toxic to all species. Another important but still unanswered question is whether or not it is possible to find a simple dose-response relationship for microcystin effects. Such a relationship may be useful to estimate the possible impact of Microcystis on Daphnia populations and to distinguish microcystin effects from those of other bioactive compounds.
Therefore, aims of the present study were (i) to examine the effects of cell-bound microcystins on six Daphnia clones belonging to five common species, (ii) to compare the sensitivity of these Daphnia clones to microcystins ingested with food particles, and (iii) to test if it is possible to predict the strength of microcystin effects. To that end, feeding and survival of Daphnia were tested with either the microcystin-producing Microcystis strain PCC7806 or its genetically engineered, microcystin-lacking mutant (11) as sole food. Additionally, the relationship between the microcystin ingestion rate of the six Daphnia clones and their survival time was analyzed.
MATERIALS AND METHODS
Origin, description, and culturing of Microcystis and Scenedesmus.
The strain Microcystis aeruginosa PCC7806 was kindly provided by J. Weckesser (Albert-Ludwigs-University, Freiburg, Germany). It was originally isolated from the Braakman Reservoir (The Netherlands) in 1972 and grows as single cells without any sign of a mucilaginous envelope (transmission electron microscopic analysis by W. Bleiß and A. Marko, Humboldt-University, Berlin, Germany). Microcystis strain PCC7806 produces several bioactive compounds, mainly the microcystins MCYST-LR, (d-Asp3)MCYST-LR (10, 20), and cyanopeptolin depsipeptides (28). Cells of this strain usually cause a marked feeding inhibition in Daphnia (7) and have a moderate to strong toxic effect (21, 38, 39). The mutant cell line of PCC7806 was obtained by transformation of strain PCC7806 with a mutant version of one of the microcystin synthetase genes, including recombinative replacement of the wild-type copy of this gene. The peptide synthetase gene mcyB, which is known to be an essential part of the microcystin synthetase gene cluster (43), was insertionally inactivated using a chloramphenicol resistance cartridge. The insertion resulted in a highly specific and complete knockout of microcystin synthesis while it did not affect the production of other oligopeptides, such as cyanopeptolines (11). Wild-type and mutant cells of PCC7806 have the same genotype except for the insertion region.
The stock culture of Scenedesmus acutus was kindly supplied by W. Lampert (Max Planck Institute for Limnology, Plön, Germany). This green alga, which served as a food source for Daphnia cultures and a control in feeding experiments, grows in small aggregates consisting of up to 16 cells.
Both variants of Microcystis strain PCC7806 were grown in Z8 medium (24) as nonaxenic, semicontinuous cultures. The cultures were maintained under continuous light (25 μmol of photons m−2 · s−1) supplied by cool white fluorescent lamps and were shaken twice a day. The temperature was kept at 20 ± 1°C. The cultures were diluted daily at the same time to a final concentration of 100 mm3 liter−1 (a cell biovolume of 1 mm3 of PCC7806 [wild-type or mutant] is equal to 3.413 × 107 cells or 0.14 mg of C [calculated from reference 36], and under the described growth conditions the cell diameter of both PCC7806 variants was 3.83 ± 0.40 μm [mean ± standard deviation]). The cell density was determined using a calibration curve of the light absorbance at 800 nm and the biovolume concentration. Under these growth conditions the wild-type and mutant PCC7806 strains exhibited the same growth rate, which was about 0.30 day−1. This is comparable to the rates given for nutrient-saturated turbidostat cultures of Microcystis (30). Both variants of PCC7806 were clearly light- but not nutrient limited, since an increase in light intensity accelerated growth, while the addition of major nutrients (N, P) had no significant effect. Scenedesmus was grown using the same conditions and procedures except for the light intensity (32 μmol of photons m−2 · s−1) and the density (50 mm3 liter−1; 1 mm3 of Scenedesmus is equal to 5.53 × 106 cells and 0.14 mg of C [calculated from reference 36], and cell dimensions under the described growth conditions were 5.61 ± 0.65 by 10.85 ± 1.62 μm [means ± standard deviations]) to which the cultures were diluted daily. The mean growth rate was about 0.5 day−1.
Cyanobacterial and algal cultures were grown for at least 3 weeks at a constant rate before use in experiments. That time corresponds to nine or more cell divisions and should ensure a complete adaptation to the culture conditions described. Algae and cyanobacteria were harvested by means of centrifugation for 15 min at 500 × g. To produce radiolabeled Microcystis or Scenedesmus cells, 0.36 MBq of NaH14CO3 was added to 100-ml cultures, which were then grown under the described conditions for two further days.
Origin and culturing of Daphnia clones.
Six Daphnia clones belonging to five species were used in the experiments. They were isolated from different kinds of waters, ranging from small ponds to large lakes, and different locations, including the temperate zone of Central Europe and the high arctic region of Northeast Greenland. The main idea was to include animals that strongly differed in their genotype and that thus represent the diversity of the genus Daphnia, at least to some extent (Table 1).
TABLE 1.
Origin and mean length of the Daphnia clones used in the experiment
| Daphnia clonea | Origin | Characteristics of original habitat | Length of 5- to 6-day-old animals (mm [mean ± SD]) |
|---|---|---|---|
| D. galeata (clone A) | Lake Müggelsee, Germany | Shallow, hypertrophic lake, with summer plankton dominated by cyanobacteria; microcystins are common | 1.60 ± 0.14 |
| D. galeata (clone B) | Alte Donau, Austria | Eutrophic water; potential microcystin producers (Microcystis) are present | 1.69 ± 0.15 |
| D. hyalina | Lake Zürichsee, Switzerland | Mesotrophic lake; potential microcystin producers (Oscillatoria rubescens) are present | 1.49 ± 0.15 |
| D. pulicaria | Lake Constance, Germany | Mesotrophic lake; cyanobacteria may be present | 1.93 ± 0.20 |
| D. pulex | Gadekæret, Zackenberg, Northeast Greenland | Ultra oligotrophic pond of the high arctic region; no cyanobacteria in the plankton | 1.72 ± 0.18 |
| D. magna | Langedam, Denmark | Eutrophic pond; cyanobacteria are present from time to time | 2.11 ± 0.22 |
Daphnia clones were kindly supplied by the following: D. galeata clone A, M. Henning (Humboldt-University, Berlin, Germany); D. galeata clone B and D. hyalina, R. Kurmayer (Institut für Wasser-, Boden- und Lufthygiene, Berlin, Germany); D. pulicaria, W. Lampert (Max Planck Institute for Limnology, Plön, Germany); D. pulex and D. magna, laboratory cultures of the Freshwater Biological Laboratory (Hillerød, Denmark).
All Daphnia clones were cultured under the same conditions in a synthetic zooplankton medium (23) with Scenedesmus as the sole food source. Prior to an experiment 7 to 10 newborn animals were harvested from well-fed stock cultures and transferred into 0.5-liter glass vessels completely filled with a suspension of 5 mm3 of Scenedesmus liter−1. The culture vessels were kept under indirect, continuous light (5 μmol of photons m−2 · s−1) and a constant temperature of 20 ± 1°C. The food suspension was changed and any offspring were removed every second day. The animals were maintained under these conditions for at least 2 weeks and then served as “defined mothers” for the animals used in the experiments. The experimental animals were taken as offspring born within 24 h from the mother cultures. They were kept under the described conditions for five further days and were then ready for use (see Table 1 for mean body lengths).
DNA isolation, PCR, and microcystin analyses.
As cyanobacteria are known to contain several genome copies (2), it was necessary to prove the homozygous genotype of the mutant before starting the experiments with Daphnia. Remaining wild-type copies of the mcyB gene might undergo amplification under the nonselective culture conditions (medium without chloramphenicol) applied during this study and lead to a restoration of microcystin production. The absence of all wild-type mcyB gene copies in the mutant was therefore checked by PCR using primers binding upstream and downstream of the mutated region. Genomic DNA of Microcystis strain PCC7806 was isolated as described previously (14). The PCR was performed using Goldstar thermostable DNA polymerase (Eurogentec) and primers Tox2p (5′GGAACAAGTTGCACAATCCGC3′) and Tox2m (5′CCAATCCCTATCTAACACAGTAACTCGG3′). The PCR procedure was initiated by a denaturation step (2 min, 95°C), followed by 30 cycles consisting of 20 s at 90°C, 30 s at 55°C, and 2 min at 72°C and by a final elongation step (5 min, 72°C).
Microcystins were extracted from cyanobacterial cells collected on glass fiber filters. A culture volume corresponding to 5 mm3 of cell biovolume was filtered through a GF/F filter (47 mm), which was then kept frozen at −18°C. Prior to the extraction procedure the filters were thawed and refrozen three times to break down the cell structures. Afterwards, each wet filter was transferred into a glass vial filled with 3 ml of 100% methanol, which was then sonicated (indirect sonication in a water bath in order to lyse and dislodge cyanobacterial cells; Bransonic model 2210, 20°C) for 15 min and shaken for a further 20 min. The filter was then squeezed repeatedly with a forceps and eventually removed from the extract. The whole procedure was repeated three times. The liquid phases from all extraction steps of a sample were combined, filtered (GF/F filter), and evaporated at 50°C overnight. The dried extract was reconstituted in 20% acetonitrile, shaken for 30 min, and transferred into a high-performance liquid chromatography (HPLC) autosampler vial.
The reversed-phase HPLC analysis of microcystins (Waters 600 PDA detector, 717 autosampler, 600E controller, symmetry C18 5-μm, 3.9- by 150-mm column) used a linear gradient starting with 20% acetonitrile in 10 mM ammonium acetate solution (pH 5.0) and ending after 30 min with 28% acetonitrile. That gradient allows a separation and quantification of all microcystin variants produced by Microcystis strain PCC7806. The column temperature was 40°C, the detector was set at 239 nm, and the flow rate was set at 1 ml min−1. The microcystins were quantified using a microcystin-LR standard supplied by G. Codd (University of Dundee, Dundee, Scotland). The microcystin content of the PCC7806 wild-type and mutant strains was analyzed for all cultures which were used in the experiments. The microcystin content is given as micrograms per cell biovolume (in cubic millimeters) of the cyanobacteria.
Determination of feeding rate and calculation of microcystin ingestion rate.
Feeding rates were measured by using a radioisotope technique and 14C-labeled Microcystis or Scenedesmus cells. The experiments were run in 300-ml glass vessels at 20 ± 1°C and a constant light intensity of about 5 μmol of photons m−2 · s−1. At the beginning of an experiment each container received 200 ml of an unlabeled suspension of either wild-type Microcystis strain PCC7806, the PCC7806 mutant, or Scenedesmus and up to 10 animals of one of the Daphnia clones. The food suspensions were prepared with synthetic zooplankton medium. The particle concentration was always 10 mm3 liter−1. After an adaptation period of 1 h, 14C-labeled material of the respective food source was added at a ratio of 1:3 to the unlabeled food. The animals were exposed to the radioactive food suspension for 10 min, after which they were removed from the containers, washed with culture medium, and measured to calculate their biovolume (1). All animals from one incubation vessel were then collected in a scintillation vial to which 100 μl of a tissue solubilizer was added. These vials were then incubated at room temperature overnight. To measure the specific radioactivity of the food, two 10-ml samples of food suspension were taken from each container at the beginning of the 10-min feeding time. These samples were individually filtered through 0.45-μm-pore-size cellulose nitrate filters, which were then separately transferred into scintillation vials. To all vials (animal and food samples) 10 ml of a liquid scintillation cocktail (Ultima-Gold; Packard) were added and the vials were then shaken for 12 h. The radioactivity of the samples was measured using a liquid scintillation counter (Rackbeta 1219; LKB Wallac) and external standards. The whole experimental procedure was repeated five times for all food types and Daphnia clones. Feeding rates were calculated as biovolume of ingested food (in cubic millimeters) per biovolume of Daphnia (in cubic millimeters) per hour.
The microcystin ingestion rate describes the amount of microcystins which the animals have taken in together with food particles per time unit (see reference 38). The value was calculated by multiplying the cellular microcystin content of a Microcystis strain (sum over all microcystin variants) with the feeding rate of Daphnia on that particular cyanobacterial strain. The microcystin ingestion rate is given in nanograms of microcystin per cubic millimeter of animal biovolume per hour.
Survival experiments.
Survival experiments were carried out in autoclaved 300-ml glass bottles (Schott, Mainz, Germany) at 20 ± 1°C and a mean constant light intensity of about 5 μmol of photons m−2 · s−1. The bottles were placed on a plankton wheel (one rotation per minute) to ensure a homogenous distribution of the food particles. At the beginning of an experiment each bottle received 300 ml of a suspension of either wild-type Microcystis strain PCC7806 or its mutant and 10 animals of one of the Daphnia clones. The food suspensions were prepared using synthetic zooplankton medium. The food particle concentration was always 10 mm3 liter−1. In addition to the Microcystis suspensions, nonfood controls were run to evaluate the effect of starvation. Survivors were counted every 12 h. Animals were considered dead if they did not show any movement during 30 s of intensive disturbance. The food suspensions (or medium in the case of nonfood controls) and bottles were changed daily. The whole procedure was repeated three (D. magna, D. galeata, D. hyalina) or four (D. pulex, D. pulicaria) times for all food types, the nonfood control, and the Daphnia clones. The experiments were terminated when all animals were dead or after 10 days.
Statistics.
Student's t test was used to compare the means of feeding rates for the different food types (data showed normal distribution). The significance of possible differences between survival functions was tested by the log-rank test. In order to quantify the effect of Microcystis or starvation on survival, the time needed to kill 50% of the animals (LT50) was calculated as the median of the Kaplan-Meier survival function estimation (44). Correlation and regression analyses were carried out using the SPSS regression program package. All statistical tests were performed at the 95% level of significance unless something different is stated.
RESULTS
Characterization of the PCC7806 mutant and microcystin analysis.
Before any experiment was started, the absence of wild-type gene copies in the PCC7806 mutant was checked by the described PCR procedure. The results clearly proved the lack of wild-type mcyB gene copies in the mutant cells and thus their inability to produce microcystins (data not shown). In addition, all cultures of the PCC7806 mutant used in the experiments were analyzed by HPLC. The lack of any microcystin was evident in all cases. The wild-type strain PCC7806, on the other hand, produced considerable amounts of MCYST-LR (0.27 ± 0.04 μg mm−3 [mean ± standard deviations]; n = 22) and (d-Asp3)MCYST-LR (0.60 ± 0.06 μg mm−3). Other microcystin variants were not detected. The total microcystin content was the same in all cultures used in the experiments.
Feeding rates.
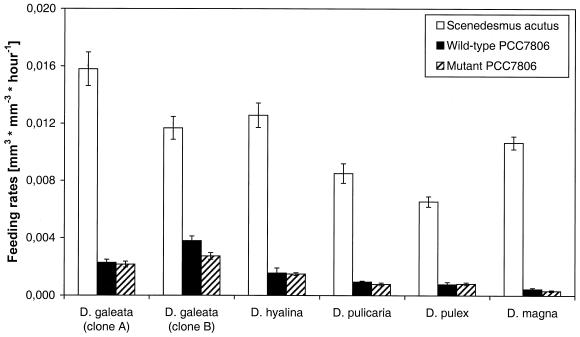
All six Daphnia clones ingested the wild-type strain PCC7806 at rates that were 75 to 95% less than those measured for Scenedesmus (Fig. 1). It is thus obvious that the animals somehow avoid feeding on PCC7806 cells. The strength of that effect differed slightly among the Daphnia clones tested. D. magna showed the strongest effect, and although the feeding rate was different from zero, the animals of this clone almost refused to feed on Microcystis strain PCC7806. D. galeata clone B, on the other hand, exhibited a comparatively high feeding activity on wild-type PCC7806 cells. The most important finding is, however, that the microcystin-lacking cells of the PCC7806 mutant were ingested at the same low rate as the microcystin-producing cells of the wild-type PCC7806 (Fig. 1). A slight, though significant, difference was only found for D. galeata clone B, which ingested wild-type cells at a higher rate than the microcystin-lacking cells of the mutant.
FIG. 1.
Feeding rates of Daphnia clones on Scenedesmus, wild-type PCC7806, and mutant PCC7806. The data represent mean values of five replicates and the respective standard errors (SEs).
Survival tests.
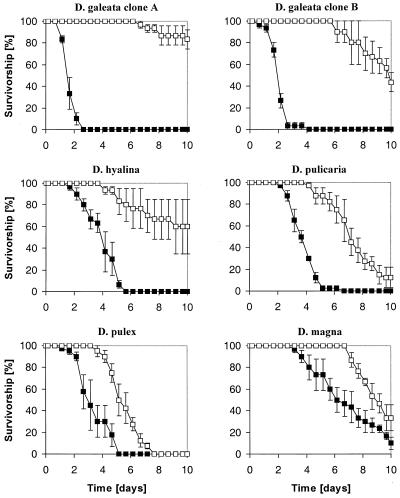
Life table experiments showed that all Daphnia clones survived significantly longer in suspensions of the microcystin-lacking mutant than in those of the microcystin-producing wild-type strain PCC7806 (Fig. 2). The differences in the median survival time (LT50) between animals fed with either mutant or wild-type cells ranged from 2 days (D. pulex) to more than 8.5 days (D. galeata clone A) (Table 2).
FIG. 2.
Survivorship of Daphnia clones fed with either the wild-type PCC7806 (black squares) or the mutant (open squares). The data represent mean values of three (D. galeata clone A and B, D. hyalina, D. magna) or four (D. pulex, D. pulicaria) replicates and the respective SEs.
TABLE 2.
LT50s of animals fed with either the wild-type PCC7806, mutant PCC7806, or nothing at all (nonfood control)
| Daphnia clone | LT50 values (days [± SE])
|
||
|---|---|---|---|
| Wild-type PCC7806 | Mutant PCC7806 | Nonfood control | |
| D. galeata (clone A) | 1.48 ± 0.09 | >10 | 5.49 ± 0.40 |
| D. galeata (clone B) | 1.91 ± 0.10 | 9.12 ± 1.74 | 5.88 ± 0.17 |
| D. hyalina | 3.87 ± 0.18 | >10 | 5.15 ± 0.26 |
| D. pulicaria | 3.58 ± 0.28 | 6.89 ± 0.22 | 5.24 ± 1.73 |
| D. pulex | 2.91 ± 0.32 | 5.07 ± 0.17 | 4.78 ± 1.70 |
| D. magna | 5.83 ± 0.52 | 8.29 ± 0.43 | 7.41 ± 0.27 |
Animals fed with the wild-type PCC7806 died significantly faster than the starved animals of the nonfood controls. The only exception was D. magna, which showed no significant differences in survivorship when fed either with wild-type PCC7806 or nothing at all (nonfood control). Animals fed with the mutant, on the other hand, survived either longer (D. galeata, D. hyalina, D. magna) or as long (D. pulex, D. pulicaria) as starving animals (Table 2). Before death all animals fed with the wild type showed malfunctional symptoms such as sudden stops in swimming and filtering activity, remaining quiet at the bottom of the bottle unless touched, and incomplete molting. In contrast, animals fed with the mutant exhibited clear starvation symptoms such as getting pale, lack of oil drops in the body, and a gradual decrease in swimming and filtering activity.
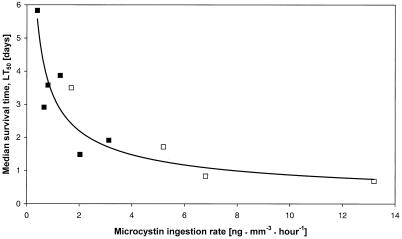
The Daphnia clones differed in their survival times when fed with wild-type cells. The LT50 values range from approximately 1.5 days (D. galeata clone A) to almost 6 days (D. magna) (Table 2). Furthermore, the LT50 is closely related to the feeding rate on wild-type PCC7806 cells and thus is also related to the respective microcystin ingestion rate. The relationship between microcystin ingestion rate and LT50 follows a reciprocal power function (Fig. 3). A regression analysis of this function revealed that 71% of the variation in LT50 among Daphnia clones can be explained by differences in the microcystin ingestion rate of the animals. Moreover, the reciprocal power function also describes the results of experiments performed with D. galeata and four Microcystis strains which produce different microcystin variants (data taken from reference 38). The equation for the whole data set is LT50 = 3.28 · microcystin ingestion rate−0.58 (r = 0.92, F = 42.1, P < 0.001).
FIG. 3.
Regression analyses of the relationship between microcystin ingestion rate and LT50. Black squares represent data obtained with different Daphnia clones, with the wild-type PCC7806 as food (r = 0.84, F = 9.3, P < 0.04). The open squares correspond to data obtained with D. galeata and four microcystin-producing Microcystis strains (data from reference 38). The equation for the whole data set is LT50 = 3.28 · microcystin ingestion rate−0.58 (r = 0.92, F = 42.1, P < 0.001).
There were also clone-specific differences in the survival time of animals fed with the mutant of PCC7806. Animals which exhibited a higher feeding rate tended to live longer (D. galeata clone A and B, D. hyalina) than those which ingested the mutant with a comparatively low rate (D. magna, D. pulex, D. pulicaria) (Table 2). However, this tendency was not statistically significant.
DISCUSSION
Most Microcystis strains can poison Daphnia spp. in a way that usually causes a die-off faster than that due to starvation (21, 25, 31). Based on the data presented here, it seems evident that microcystins are the major source of acute Daphnia toxicity caused by Microcystis. It seems furthermore obvious that microcystins ingested with living cyanobacterial cells are poisonous to most or maybe all Daphnia spp. The main support for these conclusions comes from experiments performed with the microcystin-producing Microcystis strain PCC7806 and its mutant. Toxicity tests with both variants of PCC7806 have clearly shown that wild-type cells are acutely toxic to clones of five common Daphnia spp. while cells of the mutant allow a significantly better survival. The most likely explanation for these different effects on survival is the lack of any microcystins in the mutant. A further clue to the significance of microcystins comes from the analysis of the relationship between survival time and microcystin ingestion rate of the Daphnia clones. Both values are closely correlated, which probably means that daphnids die faster the more microcystins they ingest with food particles.
According to widespread opinion, Daphnia spp. and clones differ strongly in their sensitivity to microcystins. Several authors reported, for example, striking species- or clone-specific variations in the 50% lethal concentration or 50% effective concentration for dissolved, purified microcystins (reviewed in reference 5). Others found differences in survival and growth among species and clones fed with microcystin-containing Microcystis cells (7, 13, 15). At least one paper reported an apparent resistance to microcystins (29). However, the observed differences in response to microcystins contained in solutions or cyanobacterial cells can have various causes. These differences can be due to variations in sensitivity to microcystins but also to variations in toxin uptake. Our studies with the wild-type PCC7806 strongly suggest the latter. The results clearly show that 71% of the differences in survival among six Daphnia clones can be explained by differences in microcystin intake which are due to variations in feeding activity on the microcystin-producing cells. The microcystins themselves, once ingested, probably affect all Daphnia clones in a comparable way. This is somewhat surprising, since the tested daphnids belong to different species, they were isolated from various habitats and geographical regions, and not all of them came from waters with toxic cyanobacteria. Explanations for this may include a loss of resistance or detoxification mechanisms during the culturing process or the notion that the microcystins affect Daphnia in a way which limits the possible extent of an adaptation to the toxin. It is also possible that the toxic effect of microcystins is based on an interference with life processes, which are expressed in all Daphnia spp. in a similar way. The latter idea finds some support in the fact that microcystins inhibit protein phosphatases 1 and 2A (8), which contribute to many basic and essential life functions (e.g., see references 32 and 45).
Since microcystins are ingested together with living cyanobacterial cells, the toxicity of a Microcystis strain depends, as shown, not only on its cellular microcystin content but also on the rate with which Daphnia feeds on that particular strain. Thus, variations in feeding activity will in turn influence the toxic effect of Microcystis. In short-term experiments, for example, different Microcystis strains usually are ingested at different rates. Strains like PCC7806 cause a feeding inhibition and are thus only slightly ingested (7, 21, 25, 31), while other strains are consumed at high rates (16). However, our data strongly suggest that the feeding inhibition and the resulting Microcystis strain-specific differences in consumption by Daphnia are neither caused by nor related to microcystins. The feeding experiments with the wild-type PCC7806 and its mutant have indeed shown that Daphnia ingests Microcystis strain PCC7806 cells at low rates regardless of whether the cyanobacterial cells contain microcystins or not. The microcystin content of Microcystis cells and the feeding activity of Daphnia on these cells are thus independent values which nevertheless both determine the rate of microcystin intake. This may explain why several authors failed to find a correlation between microcystin content and toxicity of Microcystis cells (21, 31, 38). Furthermore, it emphasizes the fact that differences in ingestion rate must always be considered when evaluating the toxicity of Microcystis cells.
This can be done by calculating the microcystin ingestion rate, which may serve as a gross estimation of the maximal microcystin dose taken up per time. As long as it is impossible to determine the microcystin assimilation directly, the microcystin ingestion rate is actually one of the easiest ways to estimate microcystin uptake and maybe to predict the microcystin-based toxicity of Microcystis. As shown here, the relationship between microcystin ingestion rate and survival time of Daphnia fits well to a reciprocal power function. That function describes the effects of ingested microcystins and may thus help to distinguish these effects from those of additional toxins. The function may also help to understand the mechanisms of microcystin intoxication. It turns out that survival of Daphnia is strongly affected if the microcystin ingestion rate is higher than approximately 0.4 ng mm−3 · h−1. Microcystins are thus effective even at low intake rates. The toxins can possibly accumulate in the animals until a lethal dose is reached. Microcystin ingestion rates higher than 6 ng mm−3 · h−1, however, do not further accelerate the time of death and so LT50 values lower than approximately half a day seem unlikely. This may indicate that intoxication by microcystins is based on an interference with life processes, the malfunction of which does not kill Daphnia immediately.
The results presented here indicate that microcystins may have an ecological significance to Microcystis. Microcystins are, as shown, effective toxins which most likely affect not only daphnids but also other grazers of Microcystis. The toxins are efficient at low intake rates and kill, once ingested, within hours or a few days. Furthermore, the occurrence of microcystin-producing Microcystis cells may induce shifts in the zooplankton community, since grazers of Microcystis eventually die or are impaired, while animals that can select other food sources survive and reproduce. Future studies should show if these frequently observed changes in the zooplankton community (e.g., see reference 26) could play a decisive role in the formation of Microcystis blooms by shifting the grazing pressure from the cyanobacterium to its potential competitors.
The present study suggests that microcystins are very effective and potent Daphnia toxins produced by Microcystis, but there may be additional poisonous substances produced. Microcystis produces many other bioactive compounds (4, 6) which could potentially harm Daphnia. Jungmann (20), for instance, isolated and partially characterized a toxic substance from cell extracts of Microcystis that was not a microcystin. Another toxin has been found by Reinikainen (34). Protease inhibitors like cyanopeptolines (17, 28) frequently occur in Microcystis and may interfere with the digestion process of Daphnia. At least one such substance has been shown to be toxic to Daphnia (18). However, all these compounds have been tested in a purified form only. It remains unknown if these compounds are also effective when taken in with living cyanobacterial cells as has so far been shown exclusively for microcystins.
The occurrence of additional toxins would, nevertheless, explain why some Daphnia clones fed with the microcystin-free PCC7806 mutant died almost as fast as animals of the nonfood controls. A more likely explanation is that PCC7806 does not provide enough resources for the survival of the animals. The feeding rate on PCC7806 is, indeed, very low, and as cyanobacteria are also of poor nutrient value (3, 9, 26), Daphnia may thus die of starvation. The observation of clear hunger effects supports this hypothesis.
In summary, the present study strongly suggests that microcystins ingested with living cyanobacterial cells are acutely toxic to Daphnia spp. in general. This shows that our previously published findings of experiments performed with a single D. galeata clone (38, 39) can be generalized. Moreover, the present study demonstrates that toxicity of Microcystis to several Daphnia spp. and clones can be estimated from a simple parameter like the microcystin ingestion rate. Microcystin-based toxicity may thus be predictable not only for laboratory Daphnia cultures but also for heterogeneous mixtures of different clones and species. The results and methods of this study may furthermore help to perform experiments to clarify the ecological role of microcystins in Microcystis-Daphnia interactions in nature.
ACKNOWLEDGMENTS
We thank Nils Willumsen for his laboratory assistance. We thank W. Lampert, J. Weckesser, M. Henning, and R. Kurmayer for providing stock cultures of Microcystis, Scenedesmus, and Daphnia.
This study was supported by grants FMRX-CT97-0097 and FMRX-CT98-0246 from the European Community to T.R., K.C., and T.B, respectively.
REFERENCES
- 1.Balushkina E W, Vinberg G G. Relation between length and body weight of plankton crustacean. In: Vinberg G G, editor. Biological base in lake productivity determination. Zoological Institute Press, Leningrad, Union of Soviet Socialist Republics; 1979. pp. 58–79. [Google Scholar]
- 2.Barry B A, Boerner R J, de Paula J C. The use of cyanobacteria in the study of the structure and function of photosystem II. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 217–257. [Google Scholar]
- 3.Brett M T, Mueller-Navarra D C, Park S K. Empirical analysis of the effect of phosphorus limitation on algal food quality for freshwater zooplankton. Limnol Oceanogr. 2000;45:1564–1575. [Google Scholar]
- 4.Carmichael W W. Cyanobacteria secondary metabolites—the cyanotoxins. J Appl Bacteriol. 1992;72:445–459. doi: 10.1111/j.1365-2672.1992.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 5.Christoffersen K. Ecological implications of cyanobacterial toxins in aquatic food webs. Phycologia. 1996;35:42–50. [Google Scholar]
- 6.Codd G A. Cyanobacterial toxins: occurrence, properties and biological significance. Water Sci Technol. 1995;32:149–156. [Google Scholar]
- 7.DeMott W R. Foraging strategies and growth inhibition in five daphnids feeding on mixtures of a toxic cyanobacterium and a green alga. Freshw Biol. 1999;42:263–274. [Google Scholar]
- 8.DeMott W R, Dhawale S. Inhibition of in vitro protein phosphatase activity in three zooplankton species by microcystin-LR, a toxin from cyanobacteria. Arch Hydrobiol. 1995;134:417–424. [Google Scholar]
- 9.DeMott W R, Mueller-Navarra D C. The importance of highly unsaturated fatty acids in zooplankton nutrition: evidence from experiments with Daphnia, a cyanobacterium and lipid emulsions. Freshw Biol. 1997;38:649–664. [Google Scholar]
- 10.Dierstein R, Kaiser I, Weckesser J, Matern U, König W A, Krebber R. Two closely related peptide toxins in axenically grown Microcystis aeruginosa PCC 7806. Syst Appl Microbiol. 1990;13:86–91. [Google Scholar]
- 11.Dittmann E, Neilan B A, Erhard M, von Döhren H, Börner T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol Microbiol. 1997;26:779–787. doi: 10.1046/j.1365-2958.1997.6131982.x. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson J E, Toivola D, Meriluoto J A O, Karaki H, Han Y-G, Hartshorne D. Hepatocyte deformation induced by cyanobacterial toxins reflects inhibition of protein phosphatases. Biochem Biophys Res Commun. 1990;173:1347–1353. doi: 10.1016/s0006-291x(05)80936-2. [DOI] [PubMed] [Google Scholar]
- 13.Ferrao A S, Azevedo S M F O, DeMott W R. Effects of toxic and non-toxic cyanobacteria on the life history of tropical and temperate cladocerans. Freshw Biol. 2000;45:1–19. [Google Scholar]
- 14.Franche C, Damerval T. Tests on nif probes and DNA hybridizations. Methods Enzymol. 1988;167:803–808. [Google Scholar]
- 15.Hairston N G, Lampert W, Caceres C E, Holtmeier C I, Weider L J, Gaedke U, Fischer J M, Fox J A, Post D M. Lake ecosystems—rapid evolution revealed by dormant eggs. Nature. 1999;401:446. [Google Scholar]
- 16.Henning M, Hertel H, Wall H, Kohl J-G. Strain-specific influence of Microcystis aeruginosa on food ingestion and assimilation of some cladocerans and copepods. Int Rev Gesamten Hydrobiol. 1991;76:37–45. [Google Scholar]
- 17.Jakobi C, Oberer L, Quiquerez C, König W A, Weckesser J. Cyanopeptolin S, a sulphate containing depsipeptide from a water bloom of Microcystis sp. FEMS Microbiol Lett. 1995;129:129–133. doi: 10.1111/j.1574-6968.1995.tb07569.x. [DOI] [PubMed] [Google Scholar]
- 18.Jakobi C, Rinehart K L, Neuber R, Mez K, Weckessser J. Cyanopeptolin SS, a disulfated depsipeptide from a water bloom in Leipzig (Germany): structural elucidation and biological activities. Phycologia. 1996;35:111–116. [Google Scholar]
- 19.Jochimsen E M, Carmichael W W, An J S, Cardo D M, Cooksen S T, Holmes C E M, Antunes M B D, de Melo D A, Lyra T M, Barreto V S T, Azevedo S M F O, Jarvis W R. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N Engl J Med. 1998;338:873–878. doi: 10.1056/NEJM199803263381304. [DOI] [PubMed] [Google Scholar]
- 20.Jungmann D. Toxic compounds isolated from PCC7806 that are more active to Daphnia than two microcystins. Limnol Oceanogr. 1992;37:1777–1793. [Google Scholar]
- 21.Jungmann D, Henning M, Jüttner F. Are the same compounds in Microcystis responsible for toxicity to Daphnia and inhibition of its filtering rate? Int Rev Gesamten Hydrobiol. 1991;76:47–56. [Google Scholar]
- 22.Kaebernick M, Neilan B A, Börner T, Dittmann E. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl Environ Microbiol. 2000;66:3387–3392. doi: 10.1128/aem.66.8.3387-3392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klüttgen B, Dulmer U, Engels M, Ratte H T. ADaM, an artificial fresh-water for the culture of zooplankton. Freshw Biol. 1994;28:743–746. [Google Scholar]
- 24.Kotai J. Instructions for the preparation of modified nutrient solution Z8 for algae. Publication B-11/69. Oslo, Norway: Norsk institutt for vannforskning; 1972. [Google Scholar]
- 25.Lampert W. Inhibitory and toxic effects of blue-green algae on Daphnia. Int Rev Gesamten Hydrobiol. 1981;66:285–298. [Google Scholar]
- 26.Lampert W. Feeding and nutrition in Daphnia. Mem Ist Ital Idrobiol. 1987;45:143–192. [Google Scholar]
- 27.MacKintosh C, Beattie K A, Klump S, Cohen P, Codd G A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatase-1 and 2A from both mammals and higher plants. FEBS Lett. 1990;244:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 28.Martin C, Oberer L, Ino T, König W A, Busch M, Weckesser J. Cyanopeptolins, new depsipeptides derived from the cyanobacterium Microcystis aeruginosa PCC 7806. J Antibiot. 1993;46:1550–1556. doi: 10.7164/antibiotics.46.1550. [DOI] [PubMed] [Google Scholar]
- 29.Matveev V, Matveeva L, Jonez G J. Study of the ability of Daphnia carinata King to control phytoplankton and resist cyanobacterial toxicity: implications for biomanipulation in Australia. Aust J Mar Freshw Res. 1994;45:889–904. [Google Scholar]
- 30.Nicklisch A, Kohl J-G. Growth kinetics of Microcystis aeruginosa Kütz as a basis for modelling its population dynamics. Int Rev Gesamten Hydrobiol. 1983;68:317–326. [Google Scholar]
- 31.Nizan S, Dimentman C, Shilo M. Acute toxic effects of cyanobacterium Microcystis aeruginosa on Daphnia magna. Limnol Oceanogr. 1986;31:497–502. [Google Scholar]
- 32.Oliver C J, Shenolikar S. Physiologic importance of protein phosphatase inhibitors. Front Biosci. 1998;3:961–972. doi: 10.2741/a336. [DOI] [PubMed] [Google Scholar]
- 33.Pflugmacher S, Wiegand C, Oberemm A, Beattie K A, Krause E, Codd G A, Steinberg C E W. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: the first step of detoxication. Biochim Biophys Acta. 1998;1425:527–533. doi: 10.1016/s0304-4165(98)00107-x. [DOI] [PubMed] [Google Scholar]
- 34.Reinikainen M. Acute and sublethal effects of cyanobacteria with different toxic properties on cladocerean zooplankton. PhD thesis. Åbo, Finland: Åbo Akademi University; 1997. [Google Scholar]
- 35.Riemann B, Christoffersen K. Microbial trophodynamics in temperate lakes. Mar Microb Food Webs. 1993;7:69–100. [Google Scholar]
- 36.Rocha O, Duncan A. The relationship between cell carbon and cell volume in freshwater algal species used in zooplanktonic studies. J Plankton Res. 1985;7:279–294. [Google Scholar]
- 37.Rohrlack T, Henning M, Kohl J-G. Mechanisms of the inhibitory effect of the cyanobacterium Microcystis aeruginosa on Daphnia galeata's ingestion rate. J Plankton Res. 1999;21:1489–1500. [Google Scholar]
- 38.Rohrlack T, Henning M, Kohl J-G. Does the toxic effect of Microcystis aeruginosa on Daphnia galeata depend on microcystin ingestion rate? Arch Hydrobiol. 1999;146:385–395. [Google Scholar]
- 39.Rohrlack T, Dittmann E, Henning M, Börner T, Kohl J-G. Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl Environ Microbiol. 1999;65:737–739. doi: 10.1128/aem.65.2.737-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenberg S A, Carlson R E. Direct and indirect effects of zooplankton grazing on phytoplankton in a hypertrophic lake. Oikos. 1984;42:291–302. [Google Scholar]
- 41.Thompson J M, Ferguson A J D, Reynolds C S. Natural filtration rates of zooplankton in a closed system: the derivation of a community grazing index. J Plankton Res. 1982;4:545–560. [Google Scholar]
- 42.Thostrup L, Christoffersen K. Accumulation of microcystin in Daphnia magna feeding on toxic Microcystis. Arch Hydrobiol. 1999;145:447–467. [Google Scholar]
- 43.Tillett D, Dittmann E, Erhard M, von Döhren H, Börner T, Neilan B A. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem Biol. 2000;7:753–764. doi: 10.1016/s1074-5521(00)00021-1. [DOI] [PubMed] [Google Scholar]
- 44.Trampisch H J, Windeler J. Medizinische Statistik. Berlin, Germany: Springer Verlag; 1997. [Google Scholar]
- 45.Wera S, Hemmings B A. Serine/threonine protein phosphatases. Biochem J. 1995;311:17–29. doi: 10.1042/bj3110017. [DOI] [PMC free article] [PubMed] [Google Scholar]