Abstract
Mechanisms that determine the survival of midbrain dopaminergic (mDA) neurons in the adult central nervous system (CNS) are not fully understood. Netrins are a family of secreted proteins that are essential for normal neural development. In the mature CNS, mDA neurons express particularly high levels of netrin‐1 and its receptor Deleted in Colorectal Cancer (DCC). Recent findings indicate that overexpressing netrin‐1 protects mDA neurons in animal models of Parkinson’s disease (PD), with a proposed pro‐apoptotic dependence function for DCC that triggers cell death in the absence of a ligand. Here, we sought to determine if DCC expression influences mDA neuron survival in young adult and ageing mice. To circumvent the perinatal lethality of DCC null mice, we selectively deleted DCC from mDA neurons utilizing DATcre/loxP gene‐targeting and examined neuronal survival in adult and aged animals. Reduced numbers of mDA neurons were detected in the substantia nigra pars compacta (SNc) of young adult DATcre/DCCfl/fl mice, with further reduction in aged DATcre/DCCfl/fl animals. In contrast to young adults, aged mice also exhibited a gene dosage effect, with fewer SNc mDA neurons in DCC heterozygotes (DATcre/DCCfl/wt). Notably, loss of mDA neurons in the SN was not uniform. Neuronal loss in the SN was limited to ventral tier mDA neurons, while mDA neurons in the dorsal tier of the SN, which resist degeneration in PD, were spared from the effect of DCC deletion in both young and aged mice. In the ventral tegmental area (VTA), young adult mice with conditional deletion of DCC had normal mDA neuronal numbers, while significant loss occurred in aged DATcre/DCCfl/fl and DATcre/DCCfl/wt mice compared to age‐matched wild‐type mice. Our results indicate that expression of DCC is required for the survival of subpopulations of mDA neurons and may be relevant to the degenerative processes in PD.
Keywords: unbiased stereology, netrin‐1, calbindin D‐28k, Parkinson’s Disease, substantia nigra, ventral tegmental area, dopamine
Netrin‐1 and its receptor Deleted in Colorectal Cancer (DCC) are highly expressed in midbrain dopaminergic (mDA) neuron subpopulations. To determine how DCC impacts mDA survival, we selectively deleted DCC from mDA neurons utilizing gene‐targeting. DCC loss results in reduced mDA numbers in the substantia nigra pars compacta (SNc) in young adult mice, with further reduction in aged animals. The ventral tier mDA neuron subpopulation that is vulnerable in Parkinson’s disease (PD) is also especially vulnerable to DCC loss. DCC is required for normal survival of mDA subpopulations, and deficits in netrin‐1‐DCC signalling may be relevant to PD.
Abbreviations
- ABC
avidin/biotin complex
- AAV
adeno‐associated virus
- ANOVA
analysis of variance
- BSA
Bovine serum albumin
- CB
calbindin D‐28k
- CNS
central nervous system
- cDCC
conditional deleted in colorectal carcinoma knockout
- DAB
3,3’‐Diaminobenzidine
- DAT
dopamine transporter
- DCC
deleted in colorectal carcinoma
- Het
heterozygote
- HSD
honestly significant difference
- IR
immunoreactivity
- KO
knockout
- mDA
Midbrain dopaminergic
- ml
medial lemniscus
- PB
phosphate buffer
- PD
Parkinson disease
- PFA
paraformaldehyde
- PBS
phosphate‐buffered saline
- RRF
retrorubral field
- RT
room temperature
- SNc
substantia nigra, pars compacta
- SNr
substantia nigra, pars reticulate
- TBS
tris‐buffered saline
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
- WT
wildtype
1. INTRODUCTION
The cellular and molecular mechanisms that underlie the premature loss of ventral mDA neurons in Parkinson’s disease (PD) and related conditions are not well understood. Although a number of factors have been identified that support the survival of mDA neurons, effective neuroprotective therapies for different types of age‐related mDA degeneration remain elusive (Sidorova & Saarma 2020). Netrins are a family of secreted proteins that were initially identified as axon guidance cues (Sun et al. 2011) but have since been demonstrated to also influence neuronal survival (Negulescu & Mehlen 2018). The netrin receptor Deleted in Colorectal Cancer (DCC) is a type‐1 transmembrane Ig superfamily protein that directs cell and axon extension during embryogenesis (Keino‐Masu et al. 1996; Sun et al. 2011; Fazeli et al. 1997). DCC and netrin‐1 are essential for normal mammalian development, and mice devoid of DCC or netrin‐1 (DCC null / netrin‐1 null mice) die during embryogenesis or shortly after birth (Serafini et al. 1994; Bin et al. 2015; Serafini et al. 1996; Fazeli et al. 1997). mDA neurons express particularly high levels of DCC and netrin‐1 during embryogenesis, which persist during post‐natal maturation and in the adult brain (Livesey & Hunt 1997; Volenec et al. 1998; Vosberg et al. 2020; Reyes et al. 2013).
DCC expression by mDA neurons in the mature brain is heterogeneous, with high levels expressed by mDA neurons of the substantia nigra pars compacta (SNc), and comparatively low to non‐detectable expression in the ventral tegmental area (VTA) and retrorubral fields (RRF), respectively (Reyes et al. 2013; Osborne et al. 2005; Livesey & Hunt 1997; Xu et al. 2010). Notably, the DCC‐expressing mDA population in the ventral tier of the SNc is particularly vulnerable to neurodegeneration in PD and in PD models (German et al. 1992; Yamada et al. 1990; Lavoie & Parent 1991; Luk et al. 2013; Hassani et al. 2020; Damier et al. 1999). Patients with mutations in the coding sequences of the human DCC or netrin‐1 genes show mirror movements (Srour et al. 2010; Meneret et al. 2017) that may resemble those seen in adult‐onset PD (Cincotta et al. 2006; Espay et al. 2005). Genetic studies have identified single nucleotide polymorphisms in the human DCC and netrin‐1 genes that are correlated with the age of onset and susceptibility to develop PD (Lesnick et al. 2007; Lin et al. 2009; Sun et al. 2018).
The ventral midbrain expresses amongst the highest levels of DCC and netrin‐1 in the CNS. Previous studies have identified a role for netrin‐1 and DCC in neural development in regulating mDA precursor cell migration, axon guidance, terminal arborization and survival (Xu et al. 2010; Brignani et al. 2020; Flores et al. 2005). A recent study provided evidence that reducing netrin‐1 expression in the substantia nigra (SN) of adult mice results in the loss of mDA neurons, and that netrin‐1 is neuroprotective for mDA neurons in rodent models of PD (Jasmin et al. 2021). DCC is suggested to regulate cell survival, although it is unclear whether it functions as a dependence receptor that promotes mDA neuron death in the absence of its ligand netrin‐1 (Mehlen et al. 1998; Llambi et al. 2001; Negulescu & Mehlen 2018), or whether it mediates a survival role by itself.
The use of conventional DCC knockout mice to study processes relevant to ageing or neurodegenerative diseases is limited since DCC nulls die within a few hours after birth (Fazeli et al. 1997). Furthermore, interpreting findings in conventional DCC nulls is confounded by DCC expression by many neuronal and glial cell types. Here, we used cell‐type‐specific genetic deletion to determine if DCC expression impacts survival of mDA subpopulations during development and normal aging. We report reduced numbers of mDA neurons in the SNc of young adult DATcre/DCCfl/fl mice, with further reduction in aged DATcre/DCCfl/fl animals. In contrast to young adults, aged mice also exhibited a gene dosage effect, with fewer SNc mDA neurons in DCC heterozygotes (DATcre/DCCfl/wt). Loss of mDA neurons in the SN was not uniform, but rather was limited to the ventral tier, with sparing of mDA neurons in the dorsal tier of the SN and in the retrorubral field (RRF). In the ventral tegmental area (VTA), young adult mice with conditional deletion of DCC had normal mDA cell numbers, while significant loss occurred in aged DATcre/DCCfl/fl and DATcre/DCCfl/wt mice compared to age‐matched wild‐types. Our findings indicate that during postnatal maturation and healthy aging, DCC is required for the survival of specific subpopulations of mDA neurons. mDA neuron loss preferentially in the ventral tier of the SN suggests that DCC may be relevant to the degenerative process in PD.
2. MATERIALS AND METHODS
2.1. Animals
The use of conventional DCC knockout mice to study processes relevant to ageing or neurodegenerative diseases is limited, since DCC nulls die within a few hours after birth (Fazeli et al. 1997). Furthermore, interpretation of findings in conventional DCC nulls is confounded by DCC expression by many neuronal and glial cell types. To overcome the challenges posed by early postnatal death of conventional DCC nulls and the lack of cellular specificity of conventional DCC knockouts, we used a cell‐type specific cre/loxP gene‐targeting strategy (Sauer 1998) to target DCC in mDA neurons of adult and aged animals. Mice with loxP sites flanking exon 24 of the DCC gene (Sauer 1998; Krimpenfort et al. 2012; Horn et al. 2013) were crossed with Slc6a3 tm1.1(cre)Bkmn (DATcre) mice that express Cre‐recombinase (Cre) regulated by expression of the dopamine transporter (DAT) gene (Backman et al. 2006)) (purchased from The Jackson Laboratory, Bar Harbor, ME, USA, catalogue number 006660) to generate mice with conditional deletion of DCC in mDA neurons. In this DATcre line, mDA neurons initiate cre expression at ~E16 and maintain expression throughout adulthood. We generated DATcre/DCCfl/fl, DATcre/DCCfl/wt, and DATcre/DCCwt/wt genotypes, and quantified survival of mDA subpopulations in young adults and healthy aged mice. We also determined if vulnerable subpopulations of mDA neurons correspond to those especially susceptible to degeneration in PD. To validate the specificity of the gene‐targeting strategy, DATcre mice were crossed with ROSA26‐lacZ reporter mice (Soriano 1999) (129S‐Gt(ROSA)26Sortm1Sor/J, The Jackson Laboratory, catalogue number 003310). ROSA reporter expression in mDA neurons was detected using β‐galactosidase histochemistry (Soriano 1999).
All lines were maintained on a C57BL/6 background and housed with a 12 hr light/dark cycle in the Montreal Neurological Institute (MNI) animal care facility (Edge Cage System with Micro Barrier Top; Allentown Inc), with up to five cage companions, with ad libitum access to food and water. All procedures involving animals were performed in accordance with the Canadian Council on Animal Care’s guidelines for the use of animals in research, according to a protocol approved by the McGill University Institutional Review Board (protocol no. AUP4532). To reduce variability related to sex, only male mice were used. Young adult and aged mice were each derived from 5 L (n = 7–9 mice/litter). The litters were genotyped and either young adult DATcre/DCCwt/wt (n = 4), DATcre/DCCfl/wt (n = 4) and DATcre/DCCfl/fl (n = 4) mice, or aged DATcre/DCCwt/wt (n = 5), DATcre/DCCfl/wt (n = 5) and DATcre/DCCfl/fl (n = 5) mice were selected for analysis based on optimal perfusion quality. Young adults weighed 25–30 g, and aged mice 35–40 g. Predetermined inclusion and exclusion criteria, and randomization are not relevant to this study, and are therefore not reported.
2.2. Immunohistochemistry
Mice were transcardially perfused under deep anesthesia (ketamine 100 mg/kg, xylazine 10 mg/kg, acepromazine 3 mg/kg, i.p) with heparinized phosphate buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (PFA, ACROS Organics‐Fisher, cat. no. 41678‐0030) in phosphate buffer (PB, 0.1 M, pH 7.4, 4°C). Brains were removed and immersed in the same fixative for 12 h, and transferred into cold 30% phosphate buffered (PB, pH 7.4) sucrose solution for an additional 48 h. 40‐μm‐thick brain sections were cut in the coronal plane using a freezing microtome and free‐floating sections collected in PBS.
For immunohistochemistry, one set of free‐floating sections out of a series of 6 were initially incubated for 1 h in PBS containing 10% bovine serum albumin (BSA) and 0.3% Triton X‐100. Sections were then washed with PBS (1 × 5 min) and incubated overnight (18 h) at 4°C in PBS solution containing 0.1% Triton X‐100, 2% BSA, and a monoclonal antibody against tyrosine hydroxylase (TH) (1:1000; Immunostar Inc, cat. no. 22941). This antibody does not cross‐react with dopamine‐β‐hydroxylase, phenylethanolamine‐N‐methyltransferase or tryptophan hydroxylase and has been extensively employed to identify catecholaminergic neurons in rodents (van den Munckhof et al. 2003; Hassani et al. 2020; Murphy & Deutch 2018; Luk et al. 2013). The present experiments omitted primary antibody as a control for the specificity of the TH labeling, which revealed absent immunolabelling. Following washes with PBS (3 × 5 min), sections were incubated in PBS solution containing secondary antibody (biotinylated anti‐mouse IgG, 1:200, cat no. BA‐2000; Vector Labs), 0.1% Triton X‐100, and 2% BSA, followed by washes in PBS (3 × 5 min). Sections were then incubated for 1 h at RT in PBS containing peroxidase‐linked avidin‐biotin complex (ABC, 1:100, cat. no: PK‐6100; Vector Labs), 0.1% Triton X‐100, and 2% BSA. Finally, PBS washed sections (3 × 5 min) were developed using Tris buffer (0.05 M, pH 7.6) containing diaminobenzidine (0.25 mg/ml, DAB, cat. no. D5905; Sigma), 1% imidazole (1.0 M stock solution; cat. no. 56750; Sigma), and hydrogen peroxide (0.006%, cat. no. H323; Fisher) for 15 min to yield a light brown immunohistochemical reaction product. Thoroughly washed sections (PBS 6 × 5 min) were mounted out of distilled water onto glass slides (Superfrost/Plus, cat. no. 12‐550‐15; Fisher), air‐dried, dehydrated in a graded series of alcohols, cleared in Xylene substitute (HC700; Fisher), and coverslipped with Permount (cat. no. SP‐15; Fisher).
Sections were also immunolabelled for calbindin D‐28k to identify the dorsal tier mDA neuron subpopulation of the SNc. Free‐floating sections were incubated in Tris buffer saline (TBS) containing 10% methanol and 3% hydrogen peroxide for 10 min at RT to inhibit endogenous peroxidase activity. Sections were then washed 3 times and incubated for one hr in TBS containing 10% horse serum and 0.3% Triton X‐100. Sections were incubated for 16 h at 4°C in a TBS solution (10% horse serum and 0.1% Triton X‐100) containing a monoclonal antibody against CB (1:3000 in PBS, cat. no: 300; Swant Inc), followed by incubation in secondary antibody (biotinylated anti‐mouse IgG, 1:200 in PBS, Vector). Finally, sections were incubated with the peroxidase‐linked avidin‐biotin complex, and the ABC reaction product revealed using DAB as a chromogen, as described above. The specificity of the CB antibody has been well‐validated for immunohistochemistry including demonstrating the absence of immunostaining in CB knockout mice and preadsorption controls (Sequier et al. 1990). The antibody has been widely used for immunohistochemical analysis on sections of rodent brain (Rymar et al. 2004; van den Munckhof et al. 2003; Hassani et al. 2020; Luk et al. 2013) and reveals CB in regions that express CB mRNA (Sequier et al. 1990).
2.3. Stereological quantification of midbrain dopaminergic neurons
All image analysis and quantification of TH‐immunoreactivity (IR) or CB‐IR neurons was conducted by an individual blinded to the experimental conditions. Unbiased estimates of the number of neurons were obtained using the optical dissector method as described in detail in our previous work (van den Munckhof et al. 2003; Luk et al. 2013; Hassani et al. 2020). The rostrocaudal extent of the midbrain was examined in TH‐IR 40‐μm‐thick coronal serial sections of adult and ageing DATcre/DCCfl/fl, DATcre/DCCfl/wt and DATcre/DCwt/wt mice. Delineation of the boundaries of mDA nuclei was performed with reference to a mouse brain stereotactic atlas (Paxinos & Franklin 2003), and in keeping with previous morphological analysis of mDA subpopulations in mouse brain (Nelson et al. 1996). The SNc included mDA neurons identified in the compact portion of the substantia nigra and the neighboring lateral part of the SN (Nelson et al. 1996). mDA neurons in the VTA and RRF were delineated as defined in previous work (Nelson et al. 1996). Estimates of the total number of TH‐IR and CB‐IR neurons in young adults and aged mice were generated using an Olympus BX40 microscope equipped with motorized stage and Stereo Investigator software (Microbrightfield). Parameters for stereological analysis were essentially identical for TH‐IR and CB‐IR, with the mentioned exceptions. Briefly, a systematic random sampling grid (150 × 150 μm for TH‐IR; 125 × 125 μm for CB‐IR) was applied to the ventral midbrain region of interest. An optical brick (50 × 50 × 10 μm for TH‐IR; 50 × 60 × 10 μm for CB‐IR) with exclusion lines and guard zones (1 μm) was applied at each intersection point on the grid. Each optical brick was then analyzed using a 100× objective (oil immersion, numerical aperture 1.3, with a matching condenser).
2.4. Statistical analysis
Statistical analyses applied one‐way or two‐way ANOVA as appropriate, with Tukey’s HSD posthoc tests. Two‐tailed tests were used in all cases (Graphpad Prism, v9.3.0). All datasets used for comparisons passed the Shapiro–Wilk test for normality, with an absence of outliers (ROUT test; Graphpad Prism, v9.3.0). Sample size estimates were initially based on review of G*Power software (v3.1.9.4, Faul et al. 2007), but no formal sample size calculation was performed in this study. Rather, sample sizes used were based on previous publications using similar analysis of midbrain dopaminergic subpopulations in rodent models (Hassani et al. 2020; Luk et al. 2013; van den Munckhof et al. 2003). The primary endpoint was to determine if mDA neuron numbers in different nuclei were reduced in response to reduction in DCC gene dosage in young adult or ageing mice. As a secondary endpoint, we determined if mDA subpopulations of the dorsal tier of the SN were spared from the effect of reduction of DCC gene dosage. The chosen significance levels to testing hypotheses were: *p < 0.05, **p < 0.01, ***p < 0.001. Randomization of animals was not relevant to this study. This study was not pre‐registered prior to completion of experiments and manuscript submission.
3. RESULTS
3.1. Selective deletion of DCC from dopaminergic neurons
We first assessed the cell‐type specificity of our gene‐targeting strategy by crossing DATcre mice (Slc6a3 tm1.1(cre)Bkmn ) (Backman et al. 2006) with ROSA26‐lacZ reporter mice (Soriano 1999). Cre‐induced recombination results in Lac‐Z transcription and accumulation of cytoplasmic β–galactosidase that we detected histochemically in the ventral midbrain of DATcre/ROSA +ve, with only background staining in sections from DATwt/ROSA +ve mice (Figure 1a,b). These findings confirm that this DATcre strategy targets mDA neurons, in agreement with previous studies that used these mice (Wang et al. 2010).
FIGURE 1.
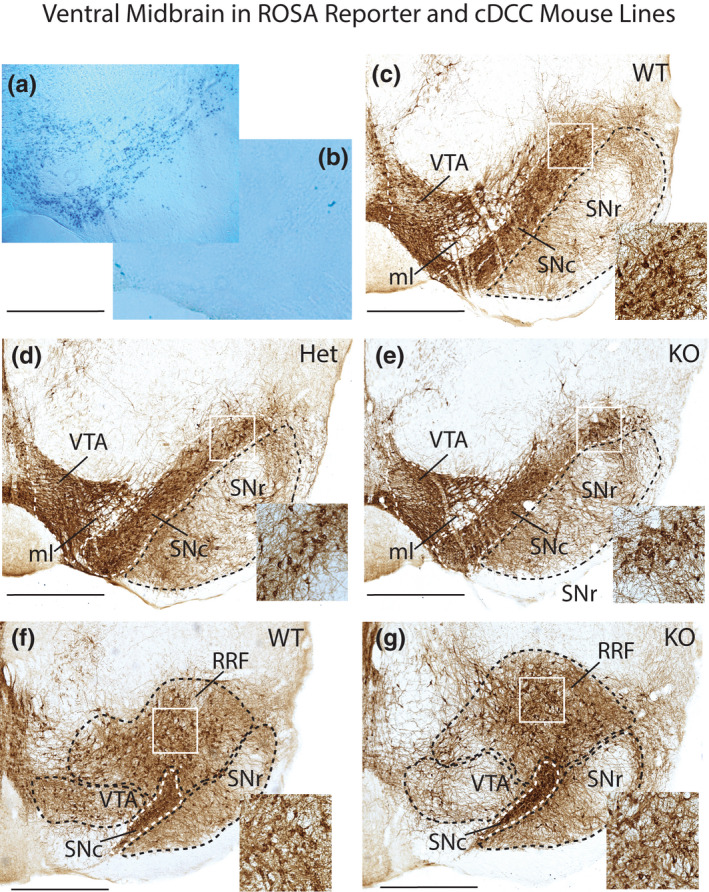
Ventral midbrain in DATcre/ROSA26‐lacZ reporter and cDCC mice. Photomicrographs of midbrain from ROSA26 reporter lines, and cDCC WT (DATCre/DCCwt/wt), cDCC Het (DATCre/DCCwt/fl) and cDCC KO (DATCre/DCCfl/fl) mice. (a, b) To validate the specificity of the gene‐targeting strategy, the progeny of ROSA26‐lacZ reporter mice were crossed either with DATcre positive mice that express cre recombinase in midbrain dopaminergic neurons (mDA) neurons (a), or with DATcre negative mice (b). Sections from these mice were processed for β‐galactosidase histochemistry. The blue β‐galactosidase reaction product reveals DAT expressing mDA neurons in DATcre positive mice and absence of stain in the control. (c–e) Tyrosine hydroxylase immunoreactive (TH‐IR) mDA neurons in coronal sections from cDCC WT (c), cDCC Het (d) or cDCC KO mice (e) at a mid‐level along the rostro‐caudal axis of the substantia nigra‐ventral tegmental area (SN‐VTA). (f, g) More caudal level showing the retrorubral field (RRF) in addition to the SN and VTA, comparing cDCC WT and cDCC KO mice. Inset shows higher magnification images corresponding to the white bounding box in the SNc (c–e) and RRF (f, g). All 3 genotypes exhibit broadly similar patterns of TH‐IR. SNc, Substantia nigra pars compacta; SNr, Substantia nigra pars reticulata; VTA, ventral tegmental area; RRF, retrorubral field; ml, medial lemniscus. Scale bars, 700 μm
3.2. Quantitative analysis of mDA neurons in young adult mice
To determine whether mDA number is altered following cell‐type‐specific conditional DCC knockout, the TH‐immunolabelled sections from 2.5‐month‐old young adult mice were quantified by unbiased stereology using the optical fractionator probe. Two‐way ANOVA with Tukey’s HSD post‐hoc tests was used to compare neuron numbers in different midbrain areas in the conditional DCC knockout genotypes that target mDA neurons: DATcre/DCCwt/wt, DATcre/DCC fl/wt, DATcre/DCC fl/fl, abbreviated henceforth as cDCC WT, cDCC Het and cDCC KO (Figure 1c–g). The total number of TH‐IR neurons within ventral mDA nuclei (SNc, VTA and RRF) was significantly decreased in cDCC KO mice compared with either cDCC WT (WT, 18450 ± 1296, mean ± SD, n = 4 vs KO, 14455 ± 827, n = 4; F (2,9) = 21.90, p = 0.0005) or cDCC Het mice (Het, 17741 ± 353, n = 4 vs KO 14455 ± 827, n = 4; F (2,9) = 21.90, p = 0.0019). However, the total number of TH‐IR neurons was statistically similar in cDCC Hets compared to cDCC WTs in young adult mice (WT, 18450 ± 1296, n = 4, Het, 17741 ± 353; F (2,9) = 21.90, p = 0.6566). There was no significant change in the size of reference spaces containing TH‐IR neurons in SNc, VTA and RRF in the three different genotypes. Furthermore, visual inspection of coronal sections containing mDA nuclei showed similar distribution and intensity of TH‐immunostaining, with differences appreciated on quantitative rather than qualitative analysis (Figures 1c–g and 2b–g).
FIGURE 2.
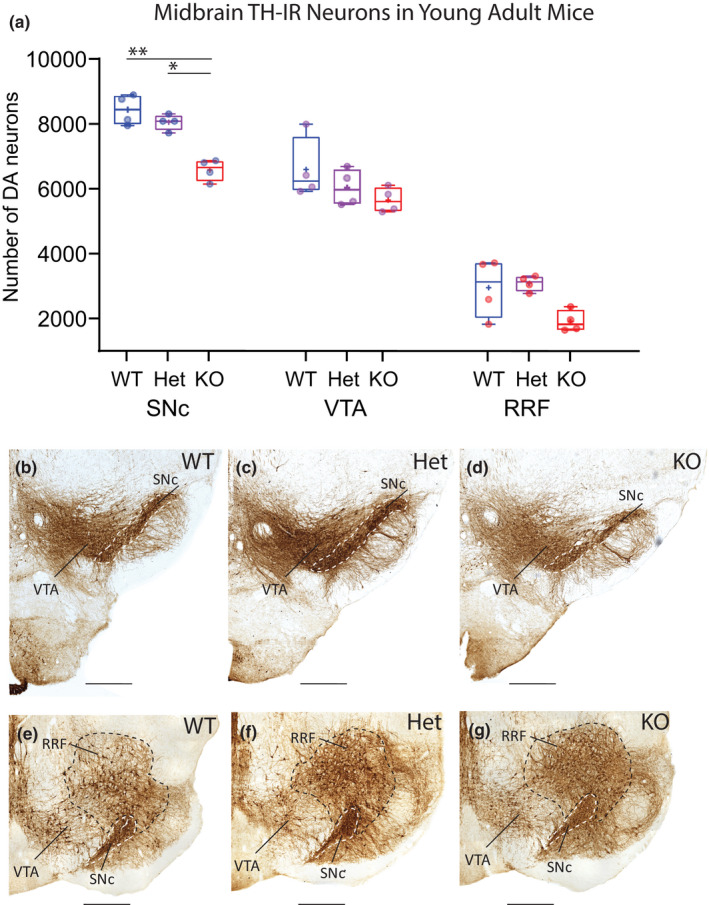
Reduced numbers of mDA neurons in SNc of young adult cDCC KO mice but not in cDCC Hets. (a) Comparison of stereological counts of mDA neuron subpopulations in 2.5‐month‐old young adult cDCC WT (DATCre/DCCwt/wt, n = 4), cDCC Het (DATCre/DCCfl/wt, n = 4) and cDCC KO (DATCre/DCC fl/fl, n = 4) mice. cDCC KO mice have significantly fewer mDA neurons in the SNc compared to either cDCC WTs (**p= 0.0018) or cDCC Hets (*p = 0.0207). The VTA and RRF contain similar numbers of mDA neurons in all 3 genotypes indicating that in young adults these neurons are spared from the impact of DCC loss. Statistical analysis applied is a two‐way ANOVA with Tukey’s HSD posthoc tests. Statistical significance was set as *p < 0.05, **p < 0.01, ***p < 0.001). Graphs are box and whiskers plots including all data points, median, mean (+ sign), 1st and 3rd quartile delimiting box, and minimum and maximum values at whiskers. n = number of animals. (b–g) The distribution and intensity of TH‐IR in the SNc (dashed boundary), VTA and RRF in all 3 genotypes is similar, and differences in mDA counts are discerned by quantitative methods using unbiased stereology. SNc, Substantia nigra pars compacta; VTA, ventral tegmental area; RRF, retrorubral field. Scale bars B‐G, 500 μm
Regional analysis revealed that the SNc of cDCC KO mice contained significantly lower numbers of TH‐IR neurons compared to cDCC WTs (WT 8449 ± 464, mean ± SD, n = 4 vs KO 6600 ± 330, n = 4; F (2,27) = 17.70; p = 0.0018) (Figure 2). In contrast, counts of TH‐IR neurons in the VTA (WT 6615 ± 953, n = 4 vs KO 5671 ± 384, n = 4; F (2,27) = 17.70; p = 0.3182) and RRF (WT 2970 ± 913, n = 4 vs KO 1934 ± 331, n = 4; F (2,27) = 17.70; p = 0.02147) were not significantly different between genotypes. Numbers of TH‐IR neurons in young adult cDCC Hets (SNc 8066 ± 242, VTA 6053 ± 566, RRF 3105 ± 238, n = 4) when compared with young adult cDCC WT mice were also similar in all midbrain regions (F (2,27) = 17.70; p = 0.9850, p = 0.8735, p = 0.9999 respectively) (Figure 2). Comparison of young adult cDCC Hets with cDCC KO mice revealed significantly lower numbers in the SNc of KOs (Het 8066 ± 242, n = 4 vs KO 6600 ± 330, n = 4; F (2,27) = 17.70; p = 0.0207), but not in the VTA (Het 6053 ± 566, n = 4 vs KO 5671 ± 384, n = 4; F (2,27) = 17.70, p = 0.9853) or RRF (Het 3105 ± 238, n = 4 vs KO 1934 ± 331, n = 4; F (2,27) = 17.70, p = 0.1112). In summary, the number of mDA neurons in young adult cDCC KO mice is selectively reduced in the SNc, with sparing of other mDA groups. In contrast, young adult cDCC Hets have numbers of mDA neurons comparable to WT littermates in all midbrain regions.
3.3. Quantitative analysis of mDA neurons in aged adult mice
The reduced number of mDA neurons detected in the SNc of young adult cDCC KO mice, with relative sparing of the VTA and RRF, is reminiscent of the differential vulnerability of mDA cell loss found in PD (Fearnley & Lees 1991; German et al. 1992; Reyes et al. 2013; Yamada et al. 1990). To determine if loss of DCC makes mDA neurons more vulnerable to the degenerative stress of ageing, we quantified the number of mDA neurons in midbrain subnuclei of littermate cohorts aged 16 months.
The total number of TH‐IR cells within mDA nuclei (SNc, VTA and RRF) was significantly decreased in both aged adult cDCC KO mice and cDCC Hets compared with cDCC WT littermates (WT 13968 ± 1623, mean ± SD, n = 5 vs KO 11520 ± 360, n = 5; F (2,12) = 6.374, p = 0.0151); WT 13968 ± 1623, vs Het 11943 ± 1125, n = 5, F (2,12) = 6.374, p = 0.0423). Although the mean number of TH‐IR dorsal tier neurons was also lower in aged cDCC KOs compared with cDCC Hets, this trend was not statistically significant (F (2,12) = 6.374; p = 0.8162) . There was no change in the size of reference spaces containing TH‐IR neurons in SNc, VTA and RRF when comparing either the 3 different aged genotypes, or when comparing similar areas in young adult versus aged mice. Furthermore, visual inspection of coronal sections containing mDA nuclei showed similar distribution and intensity of TH‐immunostaining in the different genotypes, with differences appreciated on quantitative rather than qualitative analysis (Figure 3b–g).
FIGURE 3.
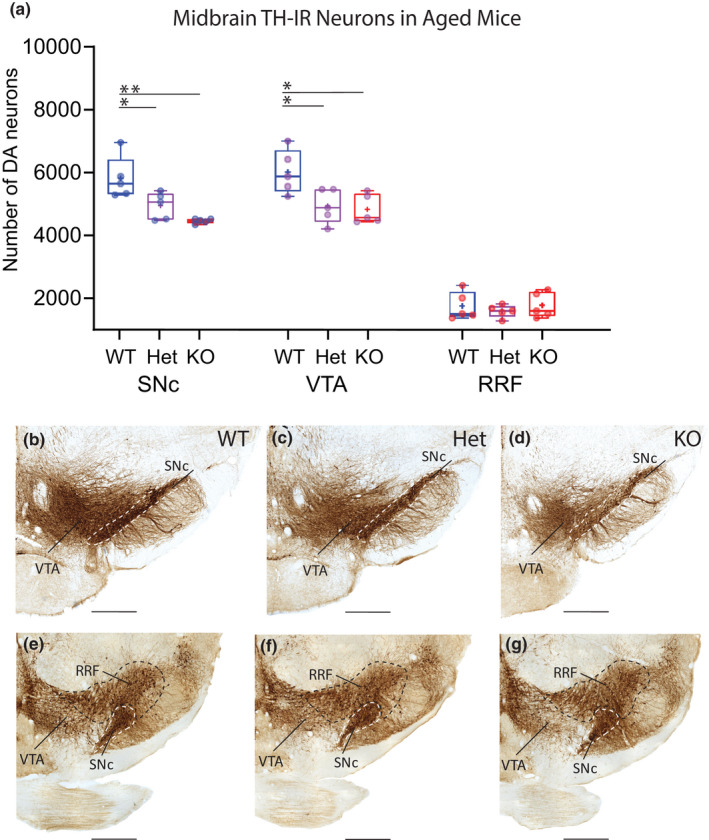
Reduced numbers of mDA neurons in SNc and VTA of aged cDCC Hets and cDCC KO mice. (a) Comparison of stereological counts of mDA neuron subpopulations in aged 16 month old cDCC WT (DATCre/DCCwt/wt, n = 5), cDCC Het (DATCre/DCCfl/wt, n = 5) and cDCC KO (DATCre/DCCfl/fl, n = 5) mice. Significantly fewer mDA neurons were detected in the SNc of aged cDCC Het and cDCC KO mice compared to cDCC WT (*p = 0.0407 and **p = 0.0019, respectively). Unlike young adults, the VTA of cDCC HET and cDCC KO mice also shows reduced mDA neuron numbers compared to cDCC WT (*p = 0.0245 and ** p= 0.0095, respectively). mDA neuron numbers in the RRF remain unaltered between the 3 genotypes. Statistical analysis applied is a two‐way ANOVA with Tukey’s HSD posthoc tests. Statistical significance was set as *p < 0.05, **p < 0.01, ***p < 0.001. Graphs are box and whiskers plots including all data points, median, mean (+ sign), 1st and 3rd quartiles delimit box, and minimum and maximum values at whiskers. n= number of animals. (b–g) The distribution and intensity of TH immunolabelling in the SNc (dashed boundary), VTA and RRF in all 3 genotypes is similar, and differences in mDA counts are discerned using quantitative methods using unbiased stereology. SNc, Substantia nigra pars compacta; VTA, ventral tegmental area; RRF, retrorubral field. Scale bar B‐G, 500 μm
Analysis of specific subregions identified a significant decrease in the number of mDA neurons in the SNc of aged cDCC KOs compared to aged cDCC WT mice (WT 5841 ± 678, mean ± SD, n = 5 vs KO 4482 ± 75, n = 5; F (2,36) = 13.45, p = 0.0019) (Figure 3). In contrast to the young adult animals, analysis of aged mice revealed significant mDA loss in the SNc of cDCC Hets compared to cDCC WT mice (Het 4968 ± 426, n = 5; F (2,36) = 13.45, p = 0.0407), suggesting an effect of DCC gene dosage. Whereas homozygous deletion of DCC resulted in early mDA neuronal loss in the SNc in young adult mice, in cDCC Hets, mDA neuronal loss was only detected in aged mice. Furthermore, while no significant loss of TH‐IR neurons was found in the VTA of young adult cDCC KOs or cDCC Hets, significant loss of TH‐IR neurons was detected in the VTA of aged cDCC KOs and cDCC Hets compared to cDCC WT mice (WT 6039 ± 698, n = 5 vs Het 4959 ± 542, n = 5; F (2,36) = 13.45, p = 0.0245); WT 6039 ± 698, vs KO 4851 ± 467, n = 5; F (2,36) = 13.45, p = 0.0095) (Figure 3). Finally, mDA neurons in the RRF were preserved in the 3 genotypes in aged mice (WT 1773 + 442, n = 5, Hets 1611 + 200, n = 5, KOs 1800 + 401, n = 5; F (2,36) = 13.45; p = 0.9998 (WT vs. Hets), p = 0.9999 (WT vs. KOs), p = 0.9993 (Hets vs. KOs).
In summary, our findings indicate that mDA neuronal survival in aged mice is decreased with reduction or loss of DCC gene expression. In contrast to young adults where SNc mDA neuronal loss is evident only in cDCC KOs, aged mice show additional mDA loss in cDCC Hets. Furthermore, aged mice exhibit loss of mDA neurons in VTA in both cDCC Hets and cDCC KOs, in contrast to the young adults where the VTA is spared after DCC reduction or loss. Unlike the SNc and VTA, the survival of mDA neurons in the RRF is not dependent on DCC expression in young adults or in ageing mice.
Our findings reveal a reduction in the total number of TH‐IR neurons in aged cDCC WT mice compared to young adult cDCC WT mice (young adult cDCC WT 18450 ± 1296, mean ± SD, n = 4 vs aged cDCC WT 13968 ± 1623, n = 5, F (1,28) = 46,97, p < 0.0001) (Figure 3). Regional analysis revealed significant reduction of mDA neurons in the SNc (cDCC WT, young adult vs aged, 8449 ± 464, n = 4 vs 5841 ± 678, n = 5, F (1,28) = 46,97, p = 0.0080). In contrast, there was no significant difference in TH‐IR counts in the VTA of aged cDCC WT mice compared to young adults (cDCC WT, young adult vs aged, 6615 ± 953, n = 4 vs 6039 ± 698, n = 5, F (1,28) = 46,97, p = 0.9845). Thus, ageing in these mice is associated with a preferential loss of TH‐IR neurons in the SN, with relative resistance of mDA neurons in the VTA and RRF (young adults vs. aged, F (1,28) = 46,97, p = 0.5929). In cDCC KOs and cDCC Hets, a general effect of ageing could contribute to the observed mDA loss in the SNc but does not account for cell loss in the VTA. Furthermore, despite the reduction in mDA neurons with ageing in the SNc in all genotypes, an impact of DCC gene dosage is evident, since Hets show mDA loss compared to WT counterparts in aged mice, but not in young adults. These findings collectively suggest that reduced levels of DCC expression by mDA neurons increases the vulnerability of SNc and VTA neurons to the degenerative stress of ageing.
3.4. Quantitative analysis of dorsal tier SNc neurons identified using CB‐IR
The mDA subpopulation that occupies the dorsal tier of the SNc is identified by expression of the calcium binding protein CB, and is notably resistant to several toxic insults and in PD (German et al. 1992; Yamada et al. 1990; Lavoie & Parent 1991; Luk et al. 2013; Duda et al. 2016; Hassani et al. 2020; Gerfen et al. 1987; Damier et al. 1999; Dopeso‐Reyes et al. 2014). To determine whether mDA neurons of the dorsal tier of the SN specifically resist loss with reduced DCC gene dosage, we quantified CB‐IR neurons in the SNc of young adult and aged mice (Figure 4a–f). In young adults, there was no significant loss of CB‐IR neurons in the SNc of cDCC KOs or cDCC Hets compared to cDCC WT mice (cDCC WT 1616 ± 35, mean ± SD, n = 4 vs KO 1595 ± 47, n = 4, cDCC Hets 1604 ± 85, n = 5; F (2,10) = 0,1192, p = 0.9529 (WT vs. Hets); p = 0.8795 (WT vs. KOs); p = 0.9748 (Hets vs. KOs)) (Figure 4a). These results indicate that the loss of SNc mDA neurons observed in young adults after loss of DCC expression is limited to the ventral tier, without significant loss in the dorsal tier.
FIGURE 4.
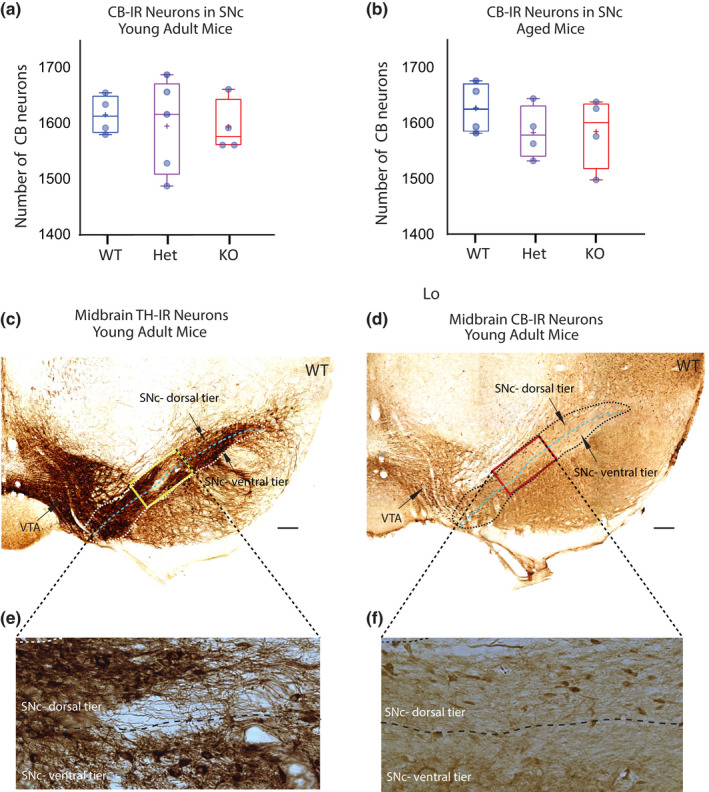
DCC expression does not impact mDA neuron survival in the SNc dorsal tier identified using calbindin D‐28k. (a, b) Stereological counts of mDA neurons in the dorsal tier of the SNc in young adult (a) and aged mice (b). Calbindin D‐28k (CB)‐IR identifies the dorsal tier subpopulation of SNc neurons that resists degenerative stress in Parkinson’s disease. Comparison of cDCC WT (DATCre/DCCwt/wt), cDCC Het (DATCre/DCCfl/wt) and cDCC KO (DATCre/DCCfl/fl) mice indicates that mDA neurons of the SNc dorsal tier are not dependent on DCC for survival in either young adult or aged mice (n= 4 for all groups, except aged Het group where n = 5). Statistical analysis applied is a one‐way ANOVA with Tukey’s HSD posthoc tests. Graphs are box and whiskers plots including all data points, median, mean (+ sign), 1st and 3rd quartile delimiting box, and minimum and maximum values at whiskers. n = number of animals. (c, d) Photomicrographs of adjacent ventral midbrain sections from young adult WT control mice immunolabelled for either tyrosine hydroxylase (TH) (c) or CB (d). The dorsal and ventral tier of the SNc are identified using adjacent CB‐IR sections. (e, f) Higher magnification images of TH‐IR or CB‐IR neurons within SN area identified by the bounding box. The dorsal tier of the SNc is populated by CB‐IR mDA neurons with very few CB‐IR neurons detected in the SNc ventral tier. SNc, Substantia nigra pars compacta; VTA, ventral tegmental area. Scale Bars (c), (d) 200 μm
Comparison of CB‐IR neuron counts in the SNc of aged cDCC WT mice with cDCC KOs or cDCC Hets also revealed no significant differences between genotypes (WT 1626 ± 46, mean ± SD, n = 4 vs either KO 1584 ± 64, n = 4, or Hets 1582 ± 48, n = 4; F (2,9) = 0.8895, p = 0.4970 (WT vs. Hets); p = 0.5163 (WT vs. KOs); p = 0.9994 (Hets vs. KOs) (Figure 4b). CB‐IR mDA neurons in the dorsal tier of the SNc are therefore not dependent on DCC for survival in aged mice, in contrast to mDA neurons in the SNc ventral tier.
4. DISCUSSION
DCC and netrin‐1 are highly expressed by midbrain dopaminergic neurons (mDA) during neural development and in the mature brain (Livesey & Hunt 1997; Volenec et al. 1998; Osborne et al. 2005; Reyes et al. 2013). DCC null mice die within a few hours after birth (Fazeli et al. 1997), limiting the utility of the conventional knockout allele for the study of DCC loss of function in mDA neurons. Initial studies that examined perinatal DCC null mice revealed cell loss and reduced numbers of mDA neurons (Flores et al. 2005; Xu et al. 2010). However, these studies did not determine if specific expression of DCC by mDA neurons contributes to their survival in adult brain, and if certain mDA subpopulations are especially vulnerable. Here we assessed the function of DCC expressed by mDA neurons using unbiased stereological analysis to quantify the survival of ventral midbrain TH‐IR neurons in young adult and aged cDCC WT (DATcre/DCCwt/wt), cDCC Het (DATcre/DCCfl/wt) and cDCC KO (DATcre/DCC fl/fl) mice.
4.1. DCC and mDA Survival in young adult and aged mice
Our findings reveal a significant decrease in the total number of mDA neurons in the ventral midbrain of young adult mDA cDCC KO mice compared to cDCC Hets or cDCC WT littermates. Analysis of specific subpopulations revealed significantly reduced numbers of mDA neurons in the SNc in cDCC KOs compared to cDCC Hets or cDCC WT mice. In contrast, the number of mDA neurons in the SNc of young adult cDCC Hets was unchanged compared to cDCC WTs. In the VTA and RRF, mDA neuron numbers were similar across all 3 genotypes, indicating these subpopulations are not dependent on DCC for survival in young adult mice. Several developmental abnormalities could contribute to cell loss in the SNc of young adult mice, including neurogenesis, migration, or postnatal cell loss. Since Cre is expressed at about E16 in these mice (Backman et al. 2006), and since most mDA neurogenesis in SN occurs prior to E13 (Bayer et al. 1995), reduced neurogenesis is unlikely as an explanation. Aberrant migration may contribute, but significantly misplaced neurons were not detected in this mutant strain. Thus, the cause of SNc mDA loss in young adult DCC KO mice is likely postnatal, either during or following the perinatal apoptosis period.
To determine how DCC expression by mDA neurons might influence neuronal survival during aging, we examined 16‐month‐old animals. We detected a further reduction in the number of mDA neurons in the SNc of aged cDCC KO mice compared to cDCC WTs. Unlike young adult mice, aged cDCC Hets exhibited a significant loss of mDA neurons in the SNc compared to cDCC WTs. Furthermore, in contrast to young adults, the number of mDA neurons was significantly reduced in the VTA of aged cDCC KOs and cDCC Hets compared with aged WTs. mDA neuron numbers in the RRF remained unaltered in the 3 genotypes in aged mice.
Notably, we detected reduced mDA neuron numbers in the SNc of ageing cDCC WTs, without a change in mDA neuron numbers in the VTA or RRF. The age‐related mDA neuron loss in SNc of cDCC WTs raises the possibility that ageing alone may have driven the neuronal loss detected in cDCC Hets and KOs, rather than reduced DCC gene dosage. Arguing against this possibility, while we found that SNc mDA neuron numbers were unaffected in young cDCC Hets, aged cDCC Hets exhibited significant neuronal loss that approached the reduced number of SNc mDA neurons in cDCC KOs. The critical impact of DCC gene dosage on ageing mDA neurons is further emphasized by our findings in the VTA, which notably did not exhibit neuronal loss with age in cDCC WTs. In contrast, significant loss of mDA neurons was found in the VTA of aged cDCC Hets and cDCC KOs. In summary, our findings demonstrate that DCC expression by SNc mDA neurons contributes to their survival during development and that specific subpopulations of mDA neurons with reduced DCC expression exhibit enhanced vulnerability to degenerative stress associated with ageing.
4.2. Possible Functional Association between DCC and Parkinson’s disease
Recent findings provide evidence for loss of mDA neurons when expression of the DCC ligand netrin‐1 is reduced via stereotaxic injection of an adenovirus associated (AAV)‐Cre into the SNc of mice carrying a floxed allele of netrin‐1 (Jasmin et al. 2021). Analysis of SN homogenates from these mice suggests that acute reduction of netrin‐1 is associated with the loss of mDA neurons. This study also provided evidence that increased levels of netrin‐1 are neuroprotective in rodent models of PD (Jasmin et al. 2021), and that netrin‐1 protein levels are reduced in the ventral midbrain in neuropathological samples from PD patients. A dependence model for netrin‐1 and DCC is proposed, with reduced levels of ligand activating a pro‐apoptotic DCC function (Jasmin et al. 2021). Our findings, in contrast, highlight an additional pro‐survival function for DCC in mDA neurons. Thus, in contrast to a pro‐apoptotic dependence receptor function where reduction in DCC would predict increased mDA survival, our findings suggest that netrin‐1 signaling via DCC activates pro‐survival mechanisms. The dependence receptor hypothesis may be reconciled with our findings by noting that netrin‐1 expression was not manipulated in our study and the absence of ligand is required for activation of a pro‐apoptotic dependence mechanism.
Notably, the young adult cDCC KOs and cDCC Hets recapitulate the preferential loss of mDA subpopulations within the SNc, with relative VTA and RRF sparing characteristic of many PD patients (German et al. 1992; Fearnley & Lees 1991). Aged cDCC Hets, but not young adult Hets, showed mDA neuron loss in the SNc, indicating that reduced DCC gene dosage increases vulnerability to age‐related degenerative stress. DCC loss also results in additional mDA neuron loss in the VTA in aged mice, a pattern reminiscent of advanced PD (Fearnley & Lees 1991; German et al. 1992). PD patients show a relative sparing of the subpopulation of mDA neurons localized in the dorsal tier of the SN identified by expression of the calcium binding protein CB (Gerfen et al. 1985; Luk et al. 2013; Hassani et al. 2020). Our findings indicate that this dorsal tier SN mDA population was also spared from degeneration in young adult and aged mice following conditional deletion of DCC. Thus, dorsal tier SN mDA neurons that resist degeneration in PD and in neurotoxin‐based models (Yamada et al. 1990; Lavoie & Parent 1991; German et al. 1992; Hassani et al. 2020; Luk et al. 2013) are also resistant to mDA loss induced by reduced DCC gene dosage. In keeping with major genetic mouse models related to familial PD (Airavaara et al. 2020), our initial analysis using open field assays did not reveal a significant locomotor phenotype in cDCC KOs. This is not unexpected, since early developmental loss of mDA neurons is often compensated, with mild or absent locomotor abnormalities (Golden et al. 2013). Furthermore, since PD patients only show motor symptoms following chronic loss of over 30% of mDA neurons (Fearnley and Lees 1991), relevant mouse models with modest mDA loss may also not show overt changes in spontaneous locomotor behavior.
Interestingly, mDA neurons of the SN ventral tier express relatively high levels of DCC compared to the dorsal tier (Osborne et al. 2005). Our findings therefore suggest that one mechanism contributing to degeneration of mDA neurons in PD may be altered function of a DCC‐dependent pathway in ventral tier neurons that express DCC. Indeed, dorsal tier SNc neurons, identified by CB‐IR, do not express DCC (Osborne et al. 2005; Reyes et al. 2013) and are spared when DCC gene dosage is reduced. This suggests that DCC‐dependent survival functions are not required in dorsal tier subpopulations but are necessary for ventral tier populations that are also vulnerable in PD. Neurons in the VTA express lower levels of DCC compared to the ventral tier of the SN (Osborne et al. 2005; Reyes et al. 2013) and these populations may therefore be less dependent on DCC for survival, with mDA neuron loss seen in ageing but not in young DCC mutants. The RRF does not express readily detectable levels of DCC (Osborne et al. 2005; Reyes et al. 2013). As expected, the mDA neurons in the RRF are spared from effects of DCC loss, similar to dorsal tier mDA neurons. Of interest, impaired mitochondrial function is implicated in the vulnerability of SN mDA neurons in PD (Pissadaki & Bolam 2013; Pacelli et al. 2015). The precise role of DCC in the face of neurodegenerative stress requires further investigation. Possibilities include reduced levels of netrin‐1 and/or dysfunction of DCC receptors or a cellular pathway downstream of DCC. Recent findings indicate that local netrin‐1 signaling through DCC triggers subcellular recruitment of mitochondria and enhances mitochondrial function (Nakamura et al. 2021) suggesting one possible mechanism by which netrin‐1 and DCC may maintain mDA survival. It will be important in future studies to determine how DCC function is altered by different age‐related cellular stresses to modulate survival of specific neuronal subpopulations of the CNS.
6. CONFLICT OF INTEREST
The authors have no conflicts to report.
7. AUTHOR CONTRIBUTIONS
Pik‐Shan Lo and Vladimir V. Rymar contributed equally to this work and are co‐first authors. Pik‐Shan Lo: Performed experiments and analysis, produced the initial figures and graphs, and wrote the initial draft of the paper. Vladimir V. Rymar: Performed experiments and analysis, produced the final figures and graphs, and contributed to the final drafts of paper. Tim E. Kennedy: Conceived experiments, obtained research funding, supervised project, helped write final versions of the paper. Abbas F. Sadikot: Conceived experiments, obtained research funding, supervised project, helped write final versions of the paper.
8.
5. ACKNOWLEDGEMENTS
The research was supported by grants to AFS and TEK from the Canadian Institutes of Health Research (CIHR, Grant No. MOP‐142324, MOP‐148907), the Parkinson Society of Canada and the Natural Sciences and Engineering Research Council of Canada (NSERC, RPIN‐2021‐03853).
All experiments were conducted in compliance with the ARRIVE guidelines.
Lo, P. ‐S. , Rymar, V. V ., Kennedy, T. E. , Sadikot, A. F. (2022) The netrin‐1 receptor DCC promotes the survival of a subpopulation of midbrain dopaminergic neurons: Relevance for ageing and Parkinson’s disease. Journal of Neurochemistry, 161,254–265. 10.1111/jnc.15579
Pik‐Shan Lo and Vladimir V. Rymar contributed equally to this work.
Timothy E. Kennedy and Abbas F. Sadikot are co‐corresponding authors.
This article is highlighted in an Editorial on page 217.
Contributor Information
Timothy E. Kennedy, Email: timothy.kennedy@mcgill.ca.
Abbas F. Sadikot, Email: abbas.sadikot@mcgill.ca.
8.1. DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Airavaara, M. , Parkkinen, I. , Konovalova, J. , Albert, K. , Chmielarz, P. , & Domanskyi, A. (2020). Back and to the Future: From Neurotoxin‐Induced to Human Parkinson's Disease Models. Current Protocols in Neuroscience, 91, e88. [DOI] [PubMed] [Google Scholar]
- Backman, C. M. , Malik, N. , Zhang, Y. , Shan, L. , Grinberg, A. , Hoffer, B. J. , Westphal, H. , & Tomac, A. C. (2006). Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus. Genesis, 44, 383–390. [DOI] [PubMed] [Google Scholar]
- Bayer, S. A. , Wills, K. V. , Triarhou, L. C. , & Ghetti, B. (1995). Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Experimental Brain Research, 105, 191–199. [DOI] [PubMed] [Google Scholar]
- Bin, J. M. , Han, D. , Sun, K. L. W. , Croteau, L. P. , Dumontier, E. , Cloutier, J. F. , Kania, A. , & Kennedy, T. E. (2015). Complete Loss of Netrin‐1 Results in Embryonic Lethality and Severe Axon Guidance Defects without Increased Neural Cell Death. Cell Reports, 12, 1099–1106. [DOI] [PubMed] [Google Scholar]
- Brignani, S. , Raj, D. D. A. , Schmidt, E. R. E. , Düdükcü, Ö. , Adolfs, Y. , De Ruiter, A. A. , Rybiczka‐Tesulov, M. , Verhagen, M. G. , van der Meer, C. , Broekhoven, M. H. , & Moreno‐Bravo, J. A. (2020). Remotely Produced and Axon‐Derived Netrin‐1 Instructs GABAergic Neuron Migration and Dopaminergic Substantia Nigra Development. Neuron, 107(684‐702), e689. [DOI] [PubMed] [Google Scholar]
- Cincotta, M. , Borgheresi, A. , Balestrieri, F. , Giovannelli, F. , Ragazzoni, A. , Vanni, P. , Benvenuti, F. , Zaccara, G. , & Ziemann, U. (2006). Mechanisms underlying mirror movements in Parkinson's disease: a transcranial magnetic stimulation study. Movement Disorders: Official Journal of the Movement Disorder Society, 21, 1019–1025. [DOI] [PubMed] [Google Scholar]
- Damier, P. , Hirsch, E. C. , Agid, Y. , & Graybiel, A. M. (1999). The substantia nigra of the human brain. II. Patterns of loss of dopamine‐containing neurons in Parkinson's disease. Brain, 122(Pt 8), 1437–1448. [DOI] [PubMed] [Google Scholar]
- Dopeso‐Reyes, I. G. , Rico, A. J. , Roda, E. , Sierra, S. , Pignataro, D. , Lanz, M. , Sucunza, D. , Chang‐Azancot, L. , & Lanciego, J. L. (2014). Calbindin content and differential vulnerability of midbrain efferent dopaminergic neurons in macaques. Frontiers in Neuroanatomy, 8, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda, J. , Potschke, C. , & Liss, B. (2016). Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease. Journal of Neurochemistry, 139(Suppl 1), 156–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay, A. J. , Li, J. Y. , Johnston, L. , Chen, R. , & Lang, A. E. (2005). Mirror movements in parkinsonism: evaluation of a new clinical sign. Journal of Neurology, Neurosurgery, and Psychiatry, 76, 1355–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Lang, A‐G. , & Buchner, A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Fazeli, A. , Dickinson, S. L. , Hermiston, M. L. , Tighe, R. V. , Steen, R. G. , Small, C. G. , Stoeckli, E. T. , Keino‐Masu, K. , Masu, M. , Rayburn, H. , & Simons, J. (1997). Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature, 386, 796–804. [DOI] [PubMed] [Google Scholar]
- Fearnley, J. M. , & Lees, A. J. (1991). Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain, 114(Pt 5), 2283–2301. [DOI] [PubMed] [Google Scholar]
- Flores, C. , Manitt, C. , Rodaros, D. , Thompson, K. M. , Rajabi, H. , Luk, K. C. , Tritsch, N. X. , Sadikot, A. F. , Stewart, J. , & Kennedy, T. E. (2005). Netrin receptor deficient mice exhibit functional reorganization of dopaminergic systems and do not sensitize to amphetamine. Molecular Psychiatry, 10, 606–612. [DOI] [PubMed] [Google Scholar]
- Gerfen, C. R. , Baimbridge, K. G. , & Miller, J. J. (1985). The neostriatal mosaic: compartmental distribution of calcium‐binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proceedings of the National Academy of Sciences of the United States of America, 82, 8780–8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen, C. R. , Baimbridge, K. G. , & Thibault, J. (1987). The neostriatal mosaic: III. Biochemical and developmental dissociation of patch‐matrix mesostriatal systems. The Journal of Neuroscience, 7, 3935–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German, D. C. , Manaye, K. F. , Sonsalla, P. K. , & Brooks, B. A. (1992). Midbrain dopaminergic cell loss in Parkinson's disease and MPTP‐induced parkinsonism: sparing of calbindin‐D28k‐containing cells. Annals of the New York Academy of Sciences, 648, 42–62. [DOI] [PubMed] [Google Scholar]
- Golden, J. P. , Demaro, J. A., 3rd , Knoten, A. , Hoshi, M. , Pehek, E. , Johnson, E. M., Jr. , Gereau, R. W. , & Jain, S. (2013). Dopamine‐dependent compensation maintains motor behavior in mice with developmental ablation of dopaminergic neurons. The Journal of Neuroscience, 33, 17095–17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani, O. K. , Rymar, V. V. , Nguyen, K. Q. , Huo, L. , Cloutier, J. F. , Miller, F. D. , & Sadikot, A. F. (2020). The noradrenergic system is necessary for survival of vulnerable midbrain dopaminergic neurons: implications for development and Parkinson's disease. Neurobiology of Aging, 85, 22–37. [DOI] [PubMed] [Google Scholar]
- Horn, K. E. , Glasgow, S. D. , Gobert, D. , Bull, S. J. , Luk, T. , Girgis, J. , Tremblay, M. E. , McEachern, D. , Bouchard, J. F. , Haber, M. , & Hamel, E. (2013). DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell Reports, 3, 173–185. [DOI] [PubMed] [Google Scholar]
- Jasmin, M. , Ahn, E. H. , Voutilainen, M. H. , Fombonne, J. , Guix, C. , Viljakainen, T. , Kang, S. S. , Yu, L. Y. , Saarma, M. , Mehlen, P. , & Ye, K. (2021). Netrin‐1 and its receptor DCC modulate survival and death of dopamine neurons and Parkinson's disease features. The EMBO Journal, 40, e105537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino‐Masu, K. , Masu, M. , Hinck, L. , Leonardo, E. D. , Chan, S. S. , Culotti, J. G. , & Tessier‐Lavigne, M. (1996). Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell, 87, 175–185. [DOI] [PubMed] [Google Scholar]
- Krimpenfort, P. , Song, J. Y. , Proost, N. , Zevenhoven, J. , Jonkers, J. , & Berns, A. (2012). Deleted in colorectal carcinoma suppresses metastasis in p53‐deficient mammary tumours. Nature, 482, 538–541. [DOI] [PubMed] [Google Scholar]
- Sun, K. L. W. , Correia, J. P. , & Kennedy, T. E. (2011). Netrins: versatile extracellular cues with diverse functions. Development (Cambridge, England), 138, 2153–2169. [DOI] [PubMed] [Google Scholar]
- Lavoie, B. , & Parent, A. (1991). Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. Neuroreport, 2, 601–604. [DOI] [PubMed] [Google Scholar]
- Lesnick, T. G. , Papapetropoulos, S. , Mash, D. C. , Ffrench‐Mullen, J. , Shehadeh, L. , De Andrade, M. , Henley, J. R. , Rocca, W. A. , Ahlskog, J. E. , & Maraganore, D. M. (2007). A genomic pathway approach to a complex disease: axon guidance and Parkinson disease. PLoS Genetics, 3, e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L. , Lesnick, T. G. , Maraganore, D. M. , & Isacson, O. (2009). Axon guidance and synaptic maintenance: preclinical markers for neurodegenerative disease and therapeutics. Trends in Neurosciences, 32, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey, F. J. , & Hunt, S. P. (1997). Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Molecular and Cellular Neurosciences, 8, 417–429. [DOI] [PubMed] [Google Scholar]
- Llambi, F. , Causeret, F. , Bloch‐Gallego, E. , & Mehlen, P. (2001). Netrin‐1 acts as a survival factor via its receptors UNC5H and DCC. The EMBO Journal, 20, 2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk, K. C. , Rymar, V. V. , van den Munckhof, P. , Nicolau, S. , Steriade, C. , Bifsha, P. , Drouin, J. , & Sadikot, A. F. (2013). The transcription factor Pitx3 is expressed selectively in midbrain dopaminergic neurons susceptible to neurodegenerative stress. Journal of Neurochemistry, 125, 932–943. [DOI] [PubMed] [Google Scholar]
- Mehlen, P. , Rabizadeh, S. , Snipas, S. J. , Assa‐Munt, N. , Salvesen, G. S. , & Bredesen, D. E. (1998). The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature, 395, 801–804. [DOI] [PubMed] [Google Scholar]
- Meneret, A. , Franz, E. A. , Trouillard, O. , Oliver, T. C. , Zagar, Y. , Robertson, S. P. , Welniarz, Q. , Gardner, R. M. , Gallea, C. , Srour, M. , & Depienne, C. (2017). Mutations in the netrin‐1 gene cause congenital mirror movements. Journal of Clinical Investigation, 127, 3923–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, M. J. M. , & Deutch, A. Y. (2018). Organization of afferents to the orbitofrontal cortex in the rat. The Journal of Comparative Neurology, 526, 1498–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, D. S. , Lin, Y. H. , Khan, D. , Gothie, J. M. , de Faria, O., Jr. , Dixon, J. A. , McBride, H. M. , Antel, J. P. , & Kennedy, T. E. (2021). Mitochondrial dynamics and bioenergetics regulated by netrin‐1 in oligodendrocytes. Glia, 69, 392–412. [DOI] [PubMed] [Google Scholar]
- Negulescu, A. M. , & Mehlen, P. (2018). Dependence receptors ‐ the dark side awakens. The FEBS Journal, 285, 3909–3924. [DOI] [PubMed] [Google Scholar]
- Nelson, E. L. , Liang, C. L. , Sinton, C. M. , & German, D. C. (1996). Midbrain dopaminergic neurons in the mouse: computer‐assisted mapping. The Journal of Comparative Neurology, 369, 361–371. [DOI] [PubMed] [Google Scholar]
- Osborne, P. B. , Halliday, G. M. , Cooper, H. M. , & Keast, J. R. (2005). Localization of immunoreactivity for deleted in colorectal cancer (DCC), the receptor for the guidance factor netrin‐1, in ventral tier dopamine projection pathways in adult rodents. Neuroscience, 131, 671–681. [DOI] [PubMed] [Google Scholar]
- Pacelli, C. , Giguere, N. , Bourque, M. J. , Levesque, M. , Slack, R. S. , & Trudeau, L. E. (2015). Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Current Biology, 25, 2349–2360. [DOI] [PubMed] [Google Scholar]
- Paxinos, G. and Franklin, K. (2003) The Mouse Brain In Stereotaxic Coordinates.
- Pissadaki, E. K. , & Bolam, J. P. (2013). The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson's disease. Frontiers in Computational Neuroscience, 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes, S. , Fu, Y. , Double, K. L. , Cottam, V. , Thompson, L. H. , Kirik, D. , Paxinos, G. , Watson, C. , Cooper, H. M. , & Halliday, G. M. (2013). Trophic factors differentiate dopamine neurons vulnerable to Parkinson's disease. Neurobiology of Aging, 34, 873–886. [DOI] [PubMed] [Google Scholar]
- Rymar, V. V. , Sasseville, R. , Luk, K. C. , & Sadikot, A. F. (2004). Neurogenesis and stereological morphometry of calretinin‐immunoreactive GABAergic interneurons of the neostriatum. The Journal of Comparative Neurology, 469, 325–339. [DOI] [PubMed] [Google Scholar]
- Sauer, B. (1998). Inducible gene targeting in mice using the Cre/lox system. Methods, 14, 381–392. [DOI] [PubMed] [Google Scholar]
- Sequier, J. M. , Hunziker, W. , Andressen, C. , & Celio, M. R. (1990). Calbindin D‐28k Protein and mRNA Localization in the Rat Brain. European Journal of Neuroscience, 2, 1118–1126. [DOI] [PubMed] [Google Scholar]
- Serafini, T. , Colamarino, S. A. , Leonardo, E. D. , Wang, H. , Beddington, R. , Skarnes, W. C. , & Tessier‐Lavigne, M. (1996). Netrin‐1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell, 87, 1001–1014. [DOI] [PubMed] [Google Scholar]
- Serafini, T. , Kennedy, T. E. , Galko, M. J. , Mirzayan, C. , Jessell, T. M. , & Tessier‐Lavigne, M. (1994). The netrins define a family of axon outgrowth‐promoting proteins homologous to C. elegans UNC‐6. Cell, 78, 409–424. [DOI] [PubMed] [Google Scholar]
- Sidorova, Y. A. , & Saarma, M. (2020). Can Growth Factors Cure Parkinson's Disease? Trends in Pharmacological Sciences, 41, 909–922. [DOI] [PubMed] [Google Scholar]
- Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics, 21, 70–71. [DOI] [PubMed] [Google Scholar]
- Srour, M. , Riviere, J. B. , Pham, J. M. , Dubé, M. P. , Girard, S. , Morin, S. , Dion, P. A. , Asselin, G. , Rochefort, D. , Hince, P. , & Diab, S. (2010). Mutations in DCC cause congenital mirror movements. Science, 328, 592. [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Ye, L. , Zheng, Y. , & Yang, Z. (2018). Identification of crucial genes associated with Parkinson's disease using microarray data. Molecular Medicine Reports, 17, 3775–3782. [DOI] [PubMed] [Google Scholar]
- van den Munckhof, P. , Luk, K. C. , Ste‐Marie, L. , Montgomery, J. , Blanchet, P. J. , Sadikot, A. F. , & Drouin, J. (2003). Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development (Cambridge, England), 130, 2535–2542. [DOI] [PubMed] [Google Scholar]
- Volenec, A. , Zetterstrom, T. S. , & Flanigan, T. P. (1998). 6‐OHDA denervation substantially decreases DCC mRNA levels in rat substantia nigra compacta. Neuroreport, 9, 3553–3556. [DOI] [PubMed] [Google Scholar]
- Vosberg, D. E. , Leyton, M. , & Flores, C. (2020). The Netrin‐1/DCC guidance system: dopamine pathway maturation and psychiatric disorders emerging in adolescence. Molecular Psychiatry, 25, 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. P. , Li, F. , Shen, X. , & Tsien, J. Z. (2010). Conditional knockout of NMDA receptors in dopamine neurons prevents nicotine‐conditioned place preference. PLoS One, 5, e8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, B. , Goldman, J. S. , Rymar, V. V. , Forget, C. , Lo, P. S. , Bull, S. J. , Vereker, E. , Barker, P. A. , Trudeau, L. E. , Sadikot, A. F. , & Kennedy, T. E. (2010). Critical roles for the netrin receptor deleted in colorectal cancer in dopaminergic neuronal precursor migration, axon guidance, and axon arborization. Neuroscience, 169, 932–949. [DOI] [PubMed] [Google Scholar]
- Yamada, T. , McGeer, P. L. , Baimbridge, K. G. , & McGeer, E. G. (1990). Relative sparing in Parkinson's disease of substantia nigra dopamine neurons containing calbindin‐D28K. Brain Research, 526, 303–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


