Abstract
The study of secretory protein kinase is an emergent research field in recent years. The secretion phenomenon of type II cGMP‐dependent protein kinase (PKG II) was found in our latest research and our previous study confirmed that PKG II inhibited platelet‐derived growth factor receptor β (PDGFRβ) activation induced by platelet‐derived growth factor BB (PDGF‐BB) within the gastric cancer cells. Thus, this study was designed to investigated effect of secretory PKG II on PDGFRβ. Transwell assay and CCK8 assay indicated that secretory PKG II reversed PDGF‐BB‐induced cell migration, invasion, and proliferation. Immunoprecipitation, GST pull down and Western blotting results showed that secretory PKG II combined with extracellular domains of PDGFRβ and phosphorylated it, and thereby inhibited PDGF‐BB‐induced activation of PDGFRβ, and downstream PI3K/Akt and MAPK/ERK pathways. Mutation at Ser254 of PDGFRβ to alanine abolished the above inhibitory effects of secretory PKG II on PDGFRβ, indicating that Ser254 was the specific site phosphorylated by secretory PKG II. In conclusion, secretory PKG II inhibited PDGFRβ activation via Ser254 site.
Keywords: gastric cancer cells, PDGFRβ, PKG II, secretory protein kinase
1. INTRODUCTION
The phosphorylation of extracellular proteins is a phenomenon that has long been noticed. Recently, Klement et al summarized the phosphorylated extracellular proteins, suggesting that extracellular protein phosphorylation is an important part of the biological activity of protein phosphorylation modification and has potentially important biological significance (Klement & Medzihradszky, 2017). However, the role of which kinases cause the phosphorylation of extracellular proteins has not been clearly reported for a long time. Until recent years, several researchers published articles about the secretion of protein kinases such as Fam20C, VLK and PKM2 to regulate extracellular protein phosphorylation (Bordoli et al., 2014; Hsu et al., 2016; Tagliabracci et al., 2012, 2015), indicating that the secretion of protein kinases has attracted great attention and is becoming a new research field.
Gastric cancer as one of the most common cancers in the world, caused 109 million new cases and 769 000 deaths in 2020, making it the fifth most common malignant tumor and the fourth leading cause of cancer death (Sung et al., 2021). Due to the lack of obvious symptoms to attract enough attention in the early stage and limited screening methods, mostly patients with gastric cancer at diagnosis are in the advanced stage (den Hoed & Kuipers, 2016; Smyth et al., 2020). Although progress has been made in treatment methods and drug development, the prognosis of gastric cancer is still poor (Johnston & Beckman, 2019; Marano et al., 2016; Patel & Cecchini, 2020).
Platelet‐derived growth factor receptor (PDGFR), which consists of PDGFRα and PDGFRβ, are members of the receptor tyrosine kinases (RTKs) (Kazlauskas, 2017). PDGFRs have important functions in body growth, development, angiogenesis, and wound healing. The activation and expression of PDGFRs are tightly controlled, and their over expression, mutation and gene rearrangement may lead to activation of PDGFRs and the enhanced PDGFR signaling, which is associated with various human diseases, including cancers and fibrotic diseases (Ramachandran et al., 2019; Roskoski, 2018). Therefore, they are ideal drug targets (Papadopoulos & Lennartsson, 2018). PDGF‐BB/PDGFRβ, as an angiogenic factor, is closely related to the occurrence and development of tumors including promoting proliferation, differentiation, migration, and invasion abilities, and is a target for the treatment of various tumors and a biomarker of prognosis (Appiah‐Kubi et al., 2016; C. Wang et al., 2019).
cGMP‐dependent protein kinase (PKG II) is a serine/threonine protein kinase in eukaryotic cells, which was originally isolated from small intestinal mucosa. PKG II is involved in the regulation of intestinal mucosal cell secretion, gene expression, renin and aldosterone secretion (Collado‐Alsina et al., 2014; Wincott et al., 2014). Recently, more and more evidence show that PKG II is involved in regulating biological activities such as cell proliferation, migration, apoptosis and differentiation, and is closely related to the occurrence and development of tumors (Cook & Haynes, 2004; Fallahian et al., 2011; Swartling et al., 2009). Previous studies from our lab have found that PKG II blocked the activation of PDGFRβ by PDGF‐BB, thereby inhibiting signal transduction initiated by PDGFRβ and related cell proliferation, migration, and other activities (Y. Wang et al., 2018). In addition, our recent data confirmed that PKG II could be secreted out of the cell and existed in the supernatant of cultured cells and human and mouse serum. This paper was designed to explore whether secretory PKG II could inhibit PDGFRβ activation.
2. MATERIALS AND METHODS
2.1. Antibodies and reagents
Recombinant Human Protein PKG II with GST Tag (PR7572A), Lipofectamine 3000 Transfection Reagent (L3000015), and Opti‐MEM (31985070) were from Thermo Fisher Scientific. Analogue of the natural signal molecule cyclic GMP 8‐pCPT‐cGMP (C 009) was from BioLog Life Science Institute. Anti‐PKG II (sc‐393126), anti‐β Actin (#sc‐8432) antibodies were from Santa Cruz Biotechnology. Anti‐p‐PDGFRβ (#3166), anti‐PDGFRβ (#3169), anti‐N‐Cadherin (#13116), anti‐E‐Cadherin (#3195), anti‐p‐PI3K (#17366), anti‐PI3K (#4257), anti‐p‐Akt (#4060), anti‐Akt (#4691), anti‐p‐MEK (#9154), anti‐MEK (#9122), anti‐p‐ERK (#4370), anti‐ERK (#4695), anti‐p‐(Ser/Thr) (#9631), anti‐His (#12698), anti‐GST (#2624), Kinase Buffer (#9802) and ATP (9804) were from Cell Signaling Technology. The horseradish peroxidase (HRP) conjugated goat anti‐mouse IgG (115‐035‐003) and goat anti‐rabbit IgG (111‐035‐003) secondary antibodies were from Jackson ImmunolResearch. Cell culture medium RPMI 1640 (350‐006‐CL), F‐12K (312‐250‐CL), DMEM (319‐006‐CL), fetal bovine serum (FBS, 086‐150), and antibiotic‐antimycotic (450‐115‐EL) were from Wisent. Transwell chamber (3422) was from Corning Life Sciences. ECM gel (E6909) and anti‐flag (F1804) were from Sigma‐Aldrich. CCK8 Kit (CK04) was from Dojindo. RIPA Lysis Buffer (P0013B), Cell Lysis Buffer for IP (P0013J), Enhanced BCA Protein Assay Kit (P0010), Protein A + G agarose (P2055), Protease and Phosphatase inhibitor (P1046) and phenylmethanesulfonyl fluoride (PMSF, ST506) were from Beyotime. Glutathione Speharose 4B (17‐0756‐01) was from GE Healthcare. Immobilion ECL Western HRP Substrate (WBKLS0500) and Immobilon PVDF membranes (IPVH00010) were from Merck KGaA. QuikChange Lightning Site‐Directed Mutagenesis Kit (#210519) was from Agilent Technologies. Recombinant the extracellular domain of human PDGFRβ protein (Met 1‐Lys 531) (10514‐H08H) with His Tag and the plasmid pCMV3‐C‐FLAG‐PDGFRβ (HG10514‐CF) were from Sino Biological.
2.2. Cell culture
Human gastric cancer cells HGC‐27 (TCHu22) and AGS (TCHu232), and SV40‐transformed African green monkey kidney cells COS‐7 (SCSP‐508) were kindly provided and maintained based on the protocol published by Stem Cell Bank, Chinese Academy of Sciences. Cells were grown in RPMI 1640, F‐12K or DMEM, with 10% FBS and antibiotic‐antimycotic.
2.3. Transwell assay
For transwell assay, transwell chambers with 8 μm pore were plated into 24‐well plates. Cells were added onto the top chamber coated with (invasion assay) or without (migration assay) ECM gel. After incubation, cells at the bottom side of chamber were visualized and counted after fixed with 4% PFA for 10 min and stained with 0.1% crystal violet for 30 min.
2.4. Proliferation assay
For cell proliferation assay in vitro, CCK8 assay was performed using the CCK8 Kit following the manufacturer's protocol. Briefly, cell suspension (100 μl/well) was inoculated in 96‐well plate. After incubating with CCK8 solution (10 μl/well) at 37℃ for 3 h, the absorbance value at 450 nm was determined.
2.5. Western blot analysis
Cells were lysed in RIPA lysis buffer and protein concentrations were measured using Enhanced BCA Protein Assay Kit. Protein samples were separated on SDS‐PAGE gels and transferred to PVDF membrane. After blocking with 5% BSA and subsequently incubated with respective antibodies, protein bands were visualized using ECL.
2.6. Immunoprecipitation(IP)
Cells were washed with PBS and lysed in cell lysis buffer for IP supplemented with protease and phosphatase inhibitor and PMSF. Cell lysates after centrifugation were incubated with antibodies in a vertical rotator at 4℃ overnight, followed by mixing with protein A + G agarose for 6 h. After washing six times with lysis buffer, beads were boiled in SDS loading buffer at 100℃ for 5 min and detected by western blotting.
2.7. Kinase assay and GST pull down
For kinase assay, GST‐PKG II and His‐PDGFRβ were added to the Kinase Buffer supplemented with ATP. For GST pull down, the mixtures were incubated with glutathione speharose 4B at 4℃ for 1 h and then washed three times with kinase buffer.
2.8. Plasmid mutation and transfection
The protein kinase phosphorylation site prediction tool GPS3.0 software was used to predict the possible sites of secretory PKG II acting on PDGFRβ. Construction of mutant plasmid Ser254Ala (S254A), Thr335Ala (T335A), and Ser373Ala (S373A) of PDGFRβ were performed by using QuikChange Site‐Directed Mutagenesis Kit. Primer sequences are the following: S254A, ATCATACGCGGCAGGCCCAAGCAACATGGTCAG (forward primer) and CTGACCATGTTGCTTGGGCCTGCCGCGTAT GAT (reverse primer); T335A, ATCATACGCGGCAGGAACAAGCAACATGGTCAG (forward primer) and CTGACCATGTTGCTTGTTCCTG CCGCGTATGAT (reverse primer); S373A, AAGGAATTAAGAGAAGCACCATCTCCGAAAGCCAAC (forward primer) and GTTGGCTTTCGGAGATGGTGCTTCTCTTAATTCCTT (reverse primer). COS‐7 cells were transfected with plasmids using lipo3000 and Opti‐MEM.
2.9. Statistical analysis
Data were expressed as mean ± standard deviation (SD) and analyzed by two‐way ANOVA performed with GraphPad Prism software. P < 0.05 was considered statistically significant (*p < 0.05).
3. RESULTS
3.1. Secretory PKG II inhibits PDGFRβ‐mediated migration, invasion, and proliferation of HGC‐27 and AGS cells
In this study, we used recombinant protein GST‐PKG II to simulate the effect of secretory PKG II on cells. To clarify the effect of secretory PKG II on PDGFRβ, Transwell assay and CCK‐8 assay were used to determine the effects of GST‐PKG II on PDGFRβ mediated cell migration, invasion, and proliferation. The results of the transwell assay showed that GST‐PKG II effectively inhibited PDGF‐BB induced migration and invasion of gastric cancer cells under the activation of cGMP (Figure 1a, Supplemental Figure 1). Similarly, proliferation capacity that enhanced by PDGF‐BB was effectively prevented by activated GST‐PKG II (Figure 1b).
Figure 1.
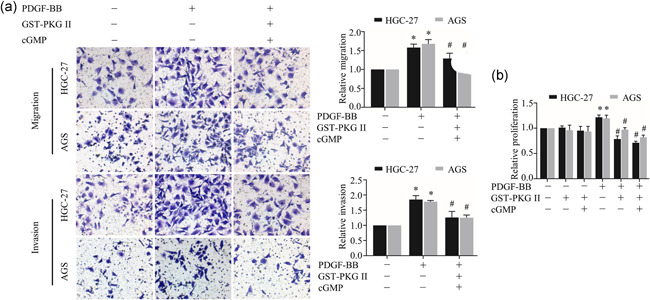
Effects of secretory PKG II on PDGF‐BB‐induced migration, invasion, and proliferation activities. (a) The detection of migration and invasion by Transwell assay. (b) The detection of proliferation by CCK8 assay. The gastric cancer cell lines HGC‐27 and AGS were treated with GST‐PKG II (100 ng/ml) and cGMP (250 μΜ) for 1 h, and then treated with PDGF‐BB (100 ng/ml) for 12 h (migration assay) or 24 h (invasion assay and CCK8 assay). (*p < 0.05, compared to control group; # p < 0.05, compared to PDGF‐BB group). Abbreviations: cGMP, cyclic guanosine monophosphate; PDGF, platelet‐derived growth factor; PDGFR, platelet‐derived growth factor receptor; PKG II, type II cGMP‐dependent protein kinase
3.2. Secretory PKG II inhibits PDGFRβ‐mediated protein expression related to migration, invasion, and proliferation
To further define the impact of the GST‐PKG II, we performed western blotting to detect the level of related proteins. The results showed that compared to the corresponding PDGF‐BB treated group, activated GST‐PKG II significantly decreased the expression of N‐Cadherin, MMP9, and PCNA, whereas expression of E‐Cadherin was upregulated (Figure 2). The above results demonstrated that GST‐PKG II had profound inhibitory activities on PDGF‐BB‐induced metastasis and proliferation.
Figure 2.
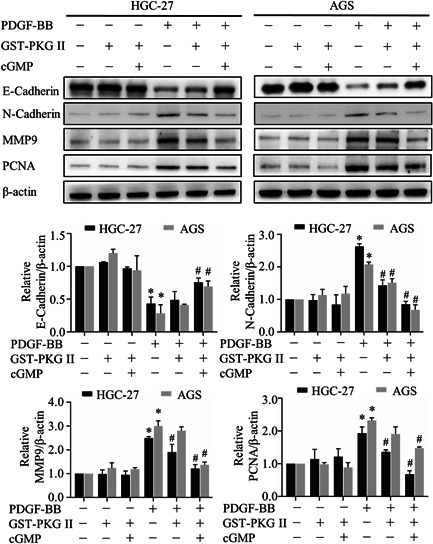
Effects of secretory PKG II on PDGF‐BB‐induced protein expression correlated with migration, invasion, and proliferation. The cells were treated with the same conditions as in Figure 1 and detected by Western blotting with antibodies against E‐cadherin, N‐cadherin, MMP9, and PCNA. (*p < 0.05, compared to control group; # p < 0.05, compared to PDGF‐BB group). Abbreviations: PDGF, platelet‐derived growth factor; PKG II, type II cGMP‐dependent protein kinase
3.3. Secretory PKG II blocks activation of PDGFRβ and signal transductions of PI3K/Akt and MAPK/ERK pathways
PI3K/Akt and MAPK/ERK pathways regulated by PDGF‐BB were related to the metastasis and proliferation of cancer cells, and thereby the effects of PKG II on the above two signal transduction pathways were detected. PDGF‐BB treatment increased levels of phosphorylated PDGFRβ, PI3K, Akt, MEK, and ERK, but activated GST‐PKG Ⅱ remarkably reduced the phosphorylation levels of above‐mentioned proteins (Figure 3), suggesting GST‐PKG II could block activation of PDGFRβ and downstream MAPK/ERK and PI3K/Akt signal pathways.
Figure 3.
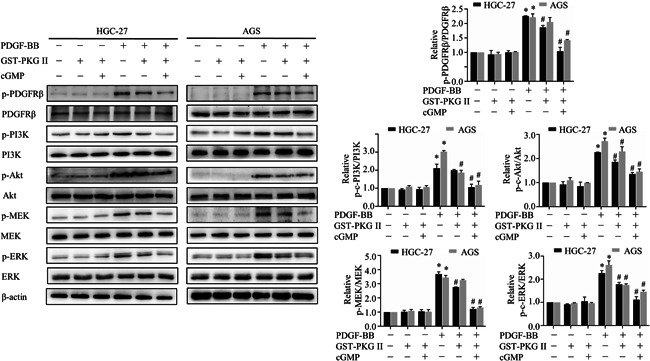
Effects of secretory PKG II on PDGF‐BB‐induced activation of PDGFRβ and downstream PI3K/Akt and MAPK/ERK pathway. The AGS and HGC‐27 cells were pretreated with GST‐PKG II (100 ng/ml) and cGMP (250 μΜ) for 1 h, and then treated with PDGF‐BB (100 ng/ml) for 10 min followed by detection performed by Western blotting with antibodies against p‐ PDGFRβ, PDGFRβ, p‐PI3K, PI3K, p‐Akt, Akt, p‐MEK, MEK, p‐ERK, ERK. (*p < 0.05, compared to control group; # p < 0.05, compared to PDGF‐BB group). Abbreviations: cGMP, cyclic guanosine monophosphate; PDGF, platelet‐derived growth factor; PDGFR, platelet‐derived growth factor receptor; PKG II, type II cGMP‐dependent protein kinase
3.4. Secretory PKG II interacts with PDGFRβ
Based on the above results, we set out to explore the molecular mechanism of blocking PDGFRβ activation by GST‐PKGII. As the interaction with the target substrate protein is the prerequisite for PKG II function, thus Co‐IP was performed to analyze GST‐PKG II‐PDGFRβ interactions in two gastric cancer cells. The results showed the binding of GST‐PKG II with PDGFRβ (Figure 4a). Furthermore, we performed kinase assay in vitro with GST‐PKG II and His‐PDGFRβ (Met1‐Lys531). After the reaction of His‐PDGFRβ (Met1‐Lys531), GST‐PKG II, and cGMP for 1 h, GST‐PKG II was pulled down by GST pull down assay and His‐PDGFRβ (Met1‐Lys531) in the precipitate was detected. The results showed GST‐PKG II could pull down His‐PDGFRβ (Met1‐Lys531), further confirming the interaction between GST‐PKG II and PDGFRβ (Figure 4c).
Figure 4.
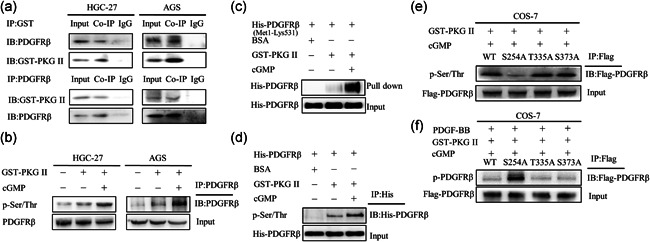
Secretory PKG II inhibited PDGF‐BB ‐induced PDGFRβ activation via phosphorylating its Ser254. (a) Secretory PKG II interacted with PDGFRβ. The HGC‐27 and AGS cells were incubated with GST‐PKG II (100 ng/ml) and cGMP (250 μΜ) for 1 h. The cells were lysed and the lysates were subjected to Co‐IP performed with antibody against GST or PDGFRβ, and then detected by Western blotting with antibodies against PDGFRβ or GST. (b) Secretory PKG II induced PDGFRβ serine/threonine phosphorylation. The cells were incubated with GST‐PKG II (100 ng/ml) and cGMP (250 μΜ) for 1 h. The cells were lysed and the lysates were subjected to IP performed with antibody against PDGFRβ, and then detected by Western blotting with antibodies against p‐Ser/Thr and PDGFRβ. (c) Secretory PKG II interacted with extracellular domain of PDGFRβ. GST‐PKG II (100 ng/ml), cGMP (250 μΜ), and His‐PDGFRβ (Met1‐Lys531) were incubated in kinase buffer for 1 h and the mixtures were subjected to GST pull down, and then detected by Western blotting with antibody against His. (d) Secretory PKG II induced extracellular domain of PDGFRβ serine/threonine phosphorylation. GST‐PKG II (100 ng/ml), cGMP (250 μΜ) and His‐ PDGFRβ (Met1‐ Lys531) were incubated in kinase buffer for 1 h and the mixtures were subjected to IP performed with antibody against PDGFRβ, and then detected by Western blotting with antibodies p‐Ser/Thr and PDGFRβ. (e,f) Ser254 was a secretory PKG II‐specific phosphorylation site in PDGFRβ. The COS‐7 cells were transfected with flag‐tagged plasmids of WT‐PDGFRβ, S254A‐PDGFRβ, T335A‐PDGFRβ, and S373A‐PDGFRβ for 3 days. The cells were incubated with GST‐PKG II (100 ng/ml) and cGMP (250 μΜ) for 1 h, and then treated with PDGF‐BB (100 ng/ml) for 10 min. The cells were lysed and the lysates were subjected to IP performed with antibody against flag and then detected by Western blotting with antibodies against p‐Ser/Thr (in panel e) and p‐PDGFRβ (in panel f). Abbreviations: cGMP, cyclic guanosine monophosphate; PDGF, platelet‐derived growth factor; PDGFR, platelet‐derived growth factor receptor; PKG II, type II cGMP‐dependent protein kinase
3.5. Secretory PKG II induces serine/threonine phosphorylation of PDGFRβ
We next examined the serine/threonine phosphorylation effect of GST‐PKG II on PDGFRβ. After treatment with GST‐PKG II and cGMP in two gastric cancer cells, there was a noticeable increase in serine/threonine phosphorylation of PDGFRβ (Figure 4b). Likewise, a significant increase of pan serine/threonine phosphorylation in His‐PDGFRβ (Met1‐Lys531) under activated GST‐PKG Ⅱ treatment was observed (Figure 4d). These results proved that GST‐PKG Ⅱ could induce serine/threonine phosphorylation in the extracellular domain of PDGFRβ.
3.6. Secretory PKG II inhibits PDGF‐BB‐induced activation of PDGFRβ via phosphorylating its Ser254
Finally, we used the protein kinase phosphorylation site prediction tool GPS3.0 software to predict the possible sites of secretory PKG II acting on PDGFRβ, including Ser254, Thr335, and Ser373. These residues were mutated to nonphosphorylable alanine (S254A, T335A, and S373A) in the plasmid pCMV3‐C‐FLAG‐PDGFRβ, and then wild type(WT) and mutant plasmids were transfected into COS‐7 cells to detect whether mutant plasmids would change the serine/threonine phosphorylation of PDGFRβ by GST‐PKG. After treated with GST‐PKG II and cGMP, the serine/threonine phosphorylation significantly decreased in S254A‐PDGFRβ but not T335A‐PDGFRβ and S373A‐PDGFRβ, compared to WT PDGFRβ, indicating that Ser254 of PDGFRβ was the specific action site for PKG II (Figure 4e). Furthermore, we also investigated whether mutant plasmids would affect the inhibitory effect of PKG II on PDGFRβ activation. The COS‐7 cells were transfected with above mutant plasmids and treated with GST‐PKG II and cGMP before stimulation with PDGF‐BB. The results showed that the mutation at Ser254 of PDGFRβ blocked the inhibitory effect of activated GST‐PKGII on phosphorylation of PDGFRβ (Figure 4f), indicating that PKG II played an inhibitory role in PDGFRβ activation through this site.
4. DISCUSSION
In recent years, series studies have found the existence of secretory protein kinase PKA and PKC that belong to the AGC protein family just like PKG II in tumor patients. Szkudlarek et al. found that the level of PKA in the serum of tumor patients increased, and the purified PKA from the culture supernatant of tumor cells could effectively inhibit angiogenesis (Szkudlarek et al., 2009; H. Wang et al., 2007). Kang et al found that the activity of activated PKC in the plasma of tumor‐bearing mice increased significantly (Kang et al., 2009). However, these reports only regard secretory protein kinases as markers for tumor diagnosis and treatment, and do not conduct in‐depth study on the targets of their extracellular effect.
As a serine/threonine protein kinase localized on the inner side of the cell membrane by myristoylation of the second glycine at the N‐terminal of the peptide chain, our lab found that PKG II inhibited the activation of several RTKs by phosphorylating specific sites in the intracellular domain of RTKs (Lan et al., 2019; Y. Wang et al., 2018; Wu et al., 2016). Based on the discovery of the secretion of PKG II, whether secretory PKG II inhibits the activation of RTKs by phosphorylating the extracellular segment of RTKs is worthy of further discussion. Here, we chose PDGFRβ as the extracellular target of secretory PKG Ⅱ to prove that PKG Ⅱ could play a biological regulatory role as secretory protein kinase.
In this study, we first investigated the effect of secretory PKG II on PDGFRβ‐mediated migration, invasion and proliferation of gastric cancer cells, and related protein expression levels including E‐cadherin, N‐cadherin, MMP9, and PCNA. The effects of secretory PKG II on PDGFRβ and signaling molecules in downstream PI3K/Akt and MAPK/ERK pathways were also detected. The results provided that secretory PKG II blocked the activation of PDGFRβ by PDGF‐BB, thereby inhibiting signal transduction and related cell proliferation, migration, and invasion activities initiated by PDGFRβ.
In previous study, we found that PKG II blocked PDGFRβ activation by phosphorylating its specific serine/threonine site Ser712 within the gastric cancer cells (Wu et al., 2016), thus we speculated whether secretory PKG II acted on PDGFRβ in a similar mechanism. IP and western blotting results showed that secretory PKG II could interact with PDGFRβ and induce PDGFRβ serine/threonine phosphorylation. Moreover, protein kinase reaction system consisted of recombinant protein PKG II and recombinant protein of PDGFRβ extracellular domain in vitro was established to further verify the above results. The results were the same as before that secretory PKG II bound with extracellular domain of PDGFRβ and phosphorylated it on serine/threonine residues.
RTKs are a kind of growth factor receptors that exist in cell membrane and has high homology. Their structures are similar, including extracellular region (including ligand binding site), transmembrane region and intracellular region (tyrosine kinase activity) (Ségaliny et al., 2015). When the ligand binds to the extracellular domain of RTKs, it can increase the activity of tyrosine kinase in its intracellular domain, which leads to tyrosine phosphorylation at specific sites and initiates downstream signal transduction. The phosphorylation of RTKs intracellular domain has been deeply studied: on the one hand, many tyrosine sites and their related signal transduction and biological activity changes have been clearly described (Glück et al., 2015, Ségaliny et al., 2015); on the other hand, some serine/threonine sites and the relationship between their phosphorylation and RTKs activity regulation have also been reported (Zhou et al., 2013). In contrast, little is known about the phosphorylation of the extracellular domain of RTKs and its role, and further research is needed.
In this study, we finally focused on determining the potential site of secretory PKG II act on the extracellular domains of PDGFRβ through GPS3.0 software that is used for computational prediction of phosphorylation sites with their cognate protein kinases (Xue et al., 2010). The results showed that when the Ser254 site of PDGFRβ was mutated to alanine, the serine/threonine phosphorylation of PDGFRβ decreased significantly, and the inhibitory effect of PKG II on PDGFRβ tyrosine phosphorylation induced by PDGF‐BB disappeared, indicating that Ser254 site is the extracellular site of PKG II on PDGFRβ. These results confirmed that Ser254 was the specific site of PDGFRβ phosphorylated by secretory PKG II.
The extracellular domain of PDGFRβ includes five Ig‐like domains, and the second and third Ig‐like domains play key roles in binding PDGF‐BB (Shim et al., 2010). Site Ser254 is located in the third Ig‐like domain, indicating that secretory PKGII may affect the binding of PDGF‐BB to PDGFRβ by phosphorylation of site Ser254, thus inhibiting the activation of PDGFR β by PDGF‐BB. This mechanism needs to be further studied.
5. CONCLUSION
Secretory PKG II can bind with PDGFRβ to phosphorylate PDGFRβ serine/threonine, block the activation of PDGFRβ and then inhibit the cell migration, invasion, and proliferation mediated by PDGFRβ. It is suggested that PKG II, as a secretory protein kinase, can inhibit tumor development by acting on the surface of the membrane, revealing a new mode of action of serine/threonine protein kinase, and is a potential tumor suppressor.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (No. 31771564); the Natural Science Foundation Project of Jiangsu Province (No. 17KJB310001); Funding from Health and Health Commission of Jiangsu Province (LGY2018025); 333 project funding plan (BRA2019172).
Pang, J. , Li, G. , Qian, H. , Wu, Y. , & Chen, Y. (2022). Secretory type II cGMP‐dependent protein kinase blocks activation of PDGFRβ via Ser254 in gastric cancer cells. Cell Biology International, 46, 747–754. 10.1002/cbin.11766
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, Ji Pang, upon reasonable request.
REFERENCES
- Appiah‐Kubi, K. , Wang, Y. , Qian, H. , Wu, M. , Yao, X. , Wu, Y. , & Chen, Y. (2016). Platelet‐derived growth factor receptor/platelet‐derived growth factor (PDGFR/PDGF) system is a prognostic and treatment response biomarker with multifarious therapeutic targets in cancers. Tumour Biology, 37, 10053–10066. [DOI] [PubMed] [Google Scholar]
- Bordoli, M. R. , Yum, J. , Breitkopf, S. B. , Thon, J. N. , Italiano JE, Jr. , Xiao, J. , Worby, C. , Wong, S. K. , Lin, G. , Edenius, M. , Keller, T. L. , Asara, J. M. , Dixon, J. E. , Yeo, C. Y. , & Whitman, M. (2014). A secreted tyrosine kinase acts in the extracellular environment. Cell, 158, 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado‐Alsina, A. , Ramírez‐Franco, J. , Sánchez‐Prieto, J. , & Torres, M. (2014). The regulation of synaptic vesicle recycling by cGMP‐dependent protein kinase type II in cerebellar granule cells under strong and sustained stimulation. Journal of Neuroscience, 34, 8788–8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, A. L. , & Haynes, J. M. (2004). Protein kinase G II‐mediated proliferative effects in human cultured prostatic stromal cells. Cellular Signalling, 16, 253–261. [DOI] [PubMed] [Google Scholar]
- den Hoed, C. M. , & Kuipers, E. J. (2016). Gastric cancer: How can we reduce the incidence of this disease? Current Gastroenterology Reports, 18, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallahian, F. , Karami‐Tehrani, F. , Salami, S. , & Aghaei, M. (2011). Cyclic GMP induced apoptosis via protein kinase G in oestrogen receptor‐positive and ‐negative breast cancer cell lines. FEBS Journal, 278, 3360–3369. [DOI] [PubMed] [Google Scholar]
- Glück, A. A. , Aebersold, D. M. , Zimmer, Y. , & Medová, M. (2015). Interplay between receptor tyrosine kinases and hypoxia signaling in cancer. International Journal of Biochemistry and Cell Biology, 62, 101–114. [DOI] [PubMed] [Google Scholar]
- Hsu, M. C. , Hung, W. C. , Yamaguchi, H. , Lim, S. O. , Liao, H. W. , Tsai, C. H. , & Hung, M. C. (2016). Extracellular PKM2 induces cancer proliferation by activating the EGFR signaling pathway. American Journal of Cancer Research, 6, 628–638. [PMC free article] [PubMed] [Google Scholar]
- Johnston, F. M. , & Beckman, M. (2019). Updates on Management of Gastric Cancer. Current Oncology Reports, 21, 67. [DOI] [PubMed] [Google Scholar]
- Kang, J. H. , Asai, D. , Toita, R. , Kitazaki, H. , & Katayama, Y. (2009). Plasma protein kinase C (PKC)alpha as a biomarker for the diagnosis of cancers. Carcinogenesis, 30, 1927–1931. [DOI] [PubMed] [Google Scholar]
- Kazlauskas, A. (2017). PDGFs and their receptors. Gene, 614, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement, E. , & Medzihradszky, K. F. (2017). Extracellular protein phosphorylation, the neglected side of the modification. Molecular & Cellular Proteomics, 16, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, T. , Pang, J. , Wang, Z. , Wang, Y. , Qian, H. , Chen, Y. , & Wu, Y. (2019). Type II cGMP‐dependent protein kinase phosphorylates EGFR at threonine 669 and thereby inhibits its activation. Biochemical and Biophysical Research Communications, 518, 14–18. [DOI] [PubMed] [Google Scholar]
- Marano, L. , Polom, K. , Patriti, A. , Roviello, G. , Falco, G. , Stracqualursi, A. , De Luca, R. , Petrioli, R. , Martinotti, M. , Generali, D. , Marrelli, D. , Di Martino, N. , & Roviello, F. (2016). Surgical management of advanced gastric cancer: An evolving issue. European Journal of Surgical Oncology, 42, 18–27. [DOI] [PubMed] [Google Scholar]
- Papadopoulos, N. , & Lennartsson, J. (2018). The PDGF/PDGFR pathway as a drug target. Molecular Aspects of Medicine, 62, 75–88. [DOI] [PubMed] [Google Scholar]
- Patel, T. H. , & Cecchini, M. (2020). Targeted therapies in advanced gastric cancer. Current Treatment Options in Oncology, 21, 70. [DOI] [PubMed] [Google Scholar]
- Ramachandran, P. , Dobie, R. , Wilson‐Kanamori, J. R. , Dora, E. F. , Henderson, B. E. P. , Luu, N. T. , Portman, J. R. , Matchett, K. P. , Brice, M. , Marwick, J. A. , Taylor, R. S. , Efremova, M. , Vento‐Tormo, R. , Carragher, N. O. , Kendall, T. J. , Fallowfield, J. A. , Harrison, E. M. , Mole, D. J. , Wigmore, S. J. , … Henderson, N. C. (2019). Resolving the fibrotic niche of human liver cirrhosis at single‐cell level. Nature, 575, 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski, R., Jr. (2018). The role of small molecule platelet‐derived growth factor receptor (PDGFR) inhibitors in the treatment of neoplastic disorders. Pharmacological Research, 129, 65–83. [DOI] [PubMed] [Google Scholar]
- Ségaliny, A. I. , Tellez‐Gabriel, M. , Heymann, M. F. , & Heymann, D. (2015). Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers. Journal of Bone Oncology, 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, A. H. , Liu, H. , Focia, P. J. , Chen, X. , Lin, P. C. , & He, X. (2010). Structures of a platelet‐derived growth factor/propeptide complex and a platelet‐derived growth factor/receptor complex. Proceedings of the National Academy of Sciences of the United States of America, 107, 11307–11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, E. C. , Nilsson, M. , Grabsch, H. I. , van Grieken, N. C. , & Lordick, F. (2020). Gastric cancer. Lancet, 396, 635–648. [DOI] [PubMed] [Google Scholar]
- Sung, H. , Ferlay, J. , Siegel, R. L. , Laversanne, M. , Soerjomataram, I. , Jemal, A. , & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249. [DOI] [PubMed] [Google Scholar]
- Swartling, F. J. , Ferletta, M. , Kastemar, M. , Weiss, W. A. , & Westermark, B. (2009). Cyclic GMP‐dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines. Oncogene, 28, 3121–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudlarek, M. , Bosio, R. M. , Wu, Q. , & Chin, K. V. (2009). Inhibition of angiogenesis by extracellular protein kinase A. Cancer Letters, 283, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabracci, V. S. , Engel, J. L. , Wen, J. , Wiley, S. E. , Worby, C. A. , Kinch, L. N. , Xiao, J. , Grishin, N. V. , & Dixon, J. E. (2012). Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science, 336, 1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabracci, V. S. , Wiley, S. E. , Guo, X. , Kinch, L. N. , Durrant, E. , Wen, J. , Xiao, J. , Cui, J. , Nguyen, K. B. , Engel, J. L. , Coon, J. J. , Grishin, N. , Pinna, L. A. , Pagliarini, D. J. , & Dixon, J. E. (2015). A single kinase generates the majority of the secreted phosphoproteome. Cell, 161, 1619–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Liu, Y. , & He, D. (2019). Diverse effects of platelet‐derived growth factor‐BB on cell signaling pathways. Cytokine, 113, 13–20. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Li, M. , Lin, W. , Wang, W. , Zhang, Z. , Rayburn, E. R. , Lu, J. , Chen, D. , Yue, X. , Shen, F. , Jiang, F. , He, J. , Wei, W. , Zeng, X. , & Zhang, R. (2007). Extracellular activity of cyclic AMP‐dependent protein kinase as a biomarker for human cancer detection: Distribution characteristics in a normal population and cancer patients. Cancer Epidemiology, Biomarkers and Prevention, 16, 789–795. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Appiah‐Kubi, K. , Lan, T. , Wu, M. , Pang, J. , Qian, H. , Tao, Y. , Jiang, L. , Wu, Y. , & Chen, Y. (2018). PKG II inhibits PDGF‐BB triggered biological activities by phosphorylating PDGFRβ in gastric cancer cells. Cell Biology International, 42, 1358–1369. [DOI] [PubMed] [Google Scholar]
- Wincott, C. M. , Abera, S. , Vunck, S. A. , Tirko, N. , Choi, Y. , Titcombe, R. F. , Antoine, S. O. , Tukey, D. S. , DeVito, L. M. , Hofmann, F. , Hoeffer, C. A. , & Ziff, E. B. (2014). cGMP‐dependent protein kinase type II knockout mice exhibit working memory impairments, decreased repetitive behavior, and increased anxiety‐like traits. Neurobiology of Learning and Memory, 114, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Yao, X. , Zhu, M. , Qian, H. , Jiang, L. , Lan, T. , Wu, M. , Pang, J. , & Chen, Y. (2016). PKG II reverses HGF‐triggered cellular activities by phosphorylating serine 985 of c‐Met in gastric cancer cells. Oncotarget, 7, 34190–34200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Y. , Liu, Z. , Gao, X. , Jin, C. , Wen, L. , Yao, X. , Ren, J. , & Bajic, V. B. (2010). GPS‐SNO: computational prediction of protein S‐nitrosylation sites with a modified GPS algorithm. PLoS One, 5, e11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Tanaka, T. , & Sakurai, H. (2013). Regulation of receptor tyrosine kinases by Ser/Thr phosphorylation. Seikagaku. The Journal of Japanese Biochemical Society, 85, 462–468. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Ji Pang, upon reasonable request.


