Summary
Background
Interleukin (IL)‐31 affects the inflammatory response, is involved in epidermal barrier disruption in atopic dermatitis (AD) and plays a key role in pruritus. Nemolizumab, a humanized monoclonal antibody against IL‐31 receptor A, reduced pruritus in patients with AD after a 16‐week administration period.
Objectives
To examine the long‐term effectiveness and safety of nemolizumab in patients aged ≥ 13 years with AD and inadequately controlled moderate‐to‐severe pruritus.
Methods
In two long‐term phase III studies, nemolizumab 60 mg every 4 weeks (Q4W) was administered subcutaneously, concomitantly with topical treatments. Study‐JP01 patients received double‐blind nemolizumab or placebo for 16 weeks, and then entered a 52‐week extension period in which all patients received nemolizumab (nemolizumab/nemolizumab and placebo/nemolizumab groups). Study‐JP02 patients received nemolizumab for 52 weeks. Both studies included an 8‐week follow‐up period.
Results
Study‐JP01 nemolizumab/nemolizumab and placebo/nemolizumab, and Study‐JP02 nemolizumab groups comprised 143, 72 and 88 patients, respectively. In the nemolizumab/nemolizumab group, there were clinically meaningful improvements from the start of treatment to week 68 in the pruritus visual analogue scale (66% decrease) and Eczema Area and Severity Index (78% decrease). Quality of life (QoL) indicators improved after the first nemolizumab dose; improvements were maintained during the follow‐up period. The long‐term safety profile was consistent with previous studies, with no unexpected late‐onset adverse events.
Conclusions
Nemolizumab 60 mg Q4W with concomitant topical treatments in patients with AD and inadequately controlled moderate‐to‐severe pruritus produced a continuous improvement in pruritus, signs of AD, and QoL for up to 68 weeks, with a favourable safety profile.
What is already known about this topic?
Pruritus, a characteristic symptom of atopic dermatitis (AD), causes distress to patients, reducing quality of life and affecting sleep and daily activities.
Nemolizumab (plus topical agents) has previously been shown to reduce pruritus associated with AD to a greater extent than placebo over 16 weeks.
As patients with AD suffer from repeated phases of relapse and remission, it is important to extend the periods of relief from pruritus and rash.
What does this study add?
Data from two long‐term (≥ 52 weeks) phase III studies confirmed that nemolizumab plus topical agents increased or maintained effectiveness through the study duration, with continuous improvement after week 16.
Acute itchiness or flare of AD were rarely observed during the 8‐week follow‐up period.
The results support the long‐term use of nemolizumab with concomitant topical agents in patients with AD and inadequately controlled moderate‐to‐severe pruritus.

Linked Comment: S. Barbarot. Br J Dermatol 2022; 186:608.
Plain language summary available online
Pruritus is a characteristic symptom of atopic dermatitis (AD), 1 an inflammatory skin condition which affects up to a quarter of children and 5% of adults worldwide. 2 , 3 The itch–scratch cycle associated with pruritus causes distress to patients, reducing quality of life (QoL) and affecting sleep and daily activities. 4 , 5 , 6 , 7 As AD is a chronic condition in which patients suffer from repeated phases of relapse and remission, 1 , 6 it is important to extend the periods of remission from pruritus and rash, in order to improve the quality of daily life.
The pathogenesis of allergic skin diseases is complex, and the definitive cause of pruritus in AD remains unclear, but cytokines appear to play a key role. 8 In particular, interleukin (IL)‐31 is a key mediator for pruritus in skin conditions including AD and prurigo nodularis, 9 , 10 , 11 , 12 , 13 and appears to have proinflammatory and immunomodulatory functions as well as pruritogenic activity. 14 , 15
The humanized monoclonal antibody nemolizumab targets IL‐31 receptor A, 16 and in a recent 16‐week, double‐blind, phase III study, nemolizumab plus topical agents produced a greater reduction in pruritus associated with AD compared with placebo plus topical agents. 17 The mean percentage change in pruritus visual analogue scale (VAS) score from baseline to week 16 favoured nemolizumab vs. placebo[difference −21·5%; 95% confidence intervals (CI) −30·2% to −12·7%; P < 0·001], and the mean percentage change in secondary endpoints such as the Eczema Area and Severity Index (EASI) confirmed the benefits of nemolizumab treatment (difference −12·6%; 95% CI −24·0% to −1·3%). 17 Current Japanese guidelines for AD recommend the first‐line use of topical agents, and oral antihistamines may be used as adjunctive therapy to reduce pruritus. 18 Thus, by administering nemolizumab alongside current therapies, the design of this pivotal study reflected the management situation of many patients with AD and pruritus. A phase IIb study of nemolizumab administered concomitantly with topical corticosteroids (TCS) also reported sustained pruritus improvements in patients with AD over 24 weeks of treatment. 19
Herein, we report data from two phase III clinical studies examining the effectiveness and safety of long‐term (up to 68 weeks) nemolizumab, administered concomitantly with TCS and/or topical calcineurin inhibitors (TCI), in patients with AD with inadequately controlled moderate‐to‐severe pruritus.
Patients and methods
Study design, treatments and blinding
We conducted two phase III, multicentre, long‐term studies of nemolizumab for the treatment of pruritus associated with AD, which was inadequately controlled by topical agents and oral antihistamines. The study designs are shown in Figure S1 (see Supporting Information). Studies were conducted in compliance with the Declaration of Helsinki, Good Clinical Practice and Japanese regulatory ordinance. Trial documentation was approved by the institutional review boards at each centre. Patients (or their legal guardian) provided written informed consent prior to treatment.
Study‐JP01 (JapicCTI‐173740)
Patients were first enrolled into Part A (16 weeks, randomized, double‐blind, placebo‐controlled) and randomly assigned (2 : 1 ratio) to receive nemolizumab 60 mg or placebo every 4 weeks (Q4W) by subcutaneous injection (both plus TCS/TCI and/or oral antihistamines). Full details of Part A have been published. 17 Patients completing Part A could enter a 52‐week, open‐label, long‐term extension period (Part B); no additional selection criteria were imposed for Part B entry. All patients in Part B received nemolizumab 60 mg Q4W up to week 64, resulting in nemolizumab/nemolizumab and placebo/nemolizumab assessment groups.
Study‐JP02 (JapicCTI‐183894)
All patients received nemolizumab 60 mg Q4W up to week 48. Administration at baseline, week 4 and week 8 was by a medical professional. From week 12, half of the patients switched to self‐injection.
In both studies, at the end of treatment, there was an 8‐week follow‐up period.
Patients
Eligible patients were aged ≥ 13 years, with a bodyweight of ≥ 30·0 kg, and a confirmed diagnosis of AD (as per the criteria of Hanifin and Rajka 20 ) with pruritus. At the time of informed consent, patients were required to have a score of ≥ 3 on a five‐level itch scale, 21 indicating inadequate pruritic response, despite treatment with medium‐potency (or stronger) 6 TCS/TCI administered at a stable dose for ≥ 4 weeks, and oral antihistamines administered at a stable dose for ≥ 2 weeks, or an inability to receive such therapies. A VAS score for pruritus 22 of ≥ 50 was also an inclusion criterion for both studies.
Exclusion criteria were any clinically relevant medical condition that could endanger the patient or render them inappropriate for study participation, abnormal laboratory values for liver enzymes or haematological parameters, or presence of diseases likely to affect the assessment of AD eczema and pruritus.
Prohibited concomitant therapies included antibody drugs (excluding nemolizumab), phototherapy and hyposensitization therapies, and systemic immunosuppressive treatments. Concomitant stable medium‐potency TCS/TCI were used during Part A of Study‐JP01, and TCS of any potency could be used during Part B of Study‐JP01 and throughout Study‐JP02.
Outcomes
The primary efficacy endpoint for Part A of Study‐JP01 (the percent change in the weekly mean pruritus VAS score from baseline to week 16) has been described previously. 17 For Part B of Study‐JP01 and for Study‐JP02, efficacy endpoints included the change over time in the following measures (where higher scores denote more severe symptoms): pruritus VAS score (range 0–100), five‐level itch scale (range 0–4), the pruritus numeric rating scale (NRS, range 0–10), 23 the EASI (range 0–72) score, 24 the static Investigator’s Global Assessment (sIGA, range 0–5) score, 25 and the Insomnia Severity Index (ISI, range 0–28). 26 In addition, the total Dermatology Life Quality Index (DLQI, range 0–30), 27 the Patient‐Oriented Eczema Measure (POEM, range 0–28) score, 28 and the mean daily quantity of topical agents used during the study period were assessed in Study‐JP01. Patients used an electronic diary (daily from baseline to week 16, then weekly) to input pruritus VAS, NRS and five‐level itch scale scores. EASI and sIGA were assessed by the investigator. The ISI, DLQI and POEM were completed by patients at study visits.
Additional efficacy measures were the proportions of patients in both studies who achieved the following: a 50%, 75% or 90% decrease in the pruritus VAS score or the EASI score from baseline, a score on the five‐level itch scale of ≤ 1, a decrease of ≥ 4 points from baseline in the pruritus NRS score, a decrease of two or more levels in the sIGA score (i.e. final score of ≤ 1), a score of ≤ 7 on the ISI, and an improvement of ≥ 6 points on the ISI. In Study‐JP01, the proportions of patients with a decrease of ≥ 4 points from baseline in the total DLQI score [considered to be the minimal clinically important difference (MCID)], 29 and a decrease of ≥ 4 points from baseline (MCID) 30 , 31 , 32 in the POEM total score were calculated.
Safety endpoints included treatment‐emergent adverse events (TEAEs), serious TEAEs, TEAEs requiring discontinuation or interruption of study treatment, and TEAEs of special interest. Injection‐related reactions were defined as adverse reactions which developed within 24 h after nemolizumab administration. Severity of TEAEs was determined by the investigator as mild (discomfort without limiting normal activities of daily living), moderate (discomfort disturbing or affecting activities of daily living) or severe (disturbing work or normal activities of daily living).
Statistical methods
For Study‐JP01, the target sample size for Part A was 204 (nemolizumab 136, placebo 68) as per the POWER procedure (t‐test) in SAS software (SAS Institute Inc., Cary, NC, USA); 17 no additional power calculations were conducted for Part B, which included patients who completed Part A. For Study‐JP02, the target sample size was 80; this was intended to ensure that enough patients completed long‐term treatment (accounting for people who dropped out) to allow for sufficient data to evaluate long‐term efficacy and safety.
The modified intention‐to‐treat population included all patients who met the inclusion/exclusion criteria, who received at least one dose of study treatment, and had data available for evaluation. For Study‐JP01, baseline for this analysis was at the time of randomization to Part A; for Study‐JP02, baseline was at study entry. Summary statistics were recorded at each timepoint. Missing data were not imputed for the continuous endpoints but were imputed as nonresponse for binary endpoints. The safety analysis set included all patients who received at least one dose of the study treatment. Integrated safety results for all nemolizumab‐treated patients in both studies are provided.
Results
Patients
In total, 215 patients were randomly assigned to treatment in Study‐JP01 (nemolizumab/nemolizumab, n = 143; placebo/nemolizumab, n = 72), of whom 206 (n = 139 and n = 67, respectively) proceeded to Part B. In Study‐JP02, 88 patients received nemolizumab treatment (of whom 44 switched to self‐injection at week 12), as shown in Figure S2 (see Supporting Information). Completion rates were high in both studies.
Baseline demographic data are shown in Table 1. Overall, the populations of the two studies were comparable, with the exception that in Study‐JP02, a higher percentage of patients had a sIGA score of 4 or more, compared with Study‐JP01. Due to differing medication usage rules, TCS use varied between Study‐JP01 and Study‐JP02. Overall, around 60% of patients in both studies had an allergic disease at baseline.
Table 1.
Demographics and other baseline characteristics (modified intention‐to‐treat population)
| Study‐JP01 | Study‐JP02 | ||
|---|---|---|---|
| Nem/nem (n = 143) | Plb/nem (n = 72) | Nem (n = 88) | |
| Male sex, n (%) | 93 (65·0) | 48 (66·7) | 56 (63·6) |
| Age (years), median (Q1–Q3) | 39·0 (27·0–47·0) | 40·5 (29·5–48·0) | 40·0 (32·0–46·0) |
| Disease duration (years), median (Q1–Q3) | 30·3 (19·2–38·5) | 28·9 (19·2–38·1) | 31·0 (20·0–38·5) |
| Pruritus VAS score, median (Q1–Q3)a | 75·7 (69·0–82·1) | 75·1 (69·1–82·1) | 78·9 (70·9–87·6) |
| Pruritus NRS score, median (Q1–Q3)a | 7·3 (6·9–8·0) | 7·4 (7·0–8·0) | 7·7 (6·9–8·4) |
| 5‐level itch scale score, median (Q1–Q3)a | 3·0 (3·0–3·1) | 3·0 (3·0–3·0) | 3·0 (3·0–3·2) |
| EASI score, median (Q1–Q3) | 24·2 (16·9–36·1) | 22·7 (15·5–33·8) | 27·0 (18·7–37·4) |
| sIGA score of 4 or more, n (%) | 61 (42·7) | 27 (37·5) | 55 (62·5) |
| ISI score | n = 142 | n = 72 | n = 87 |
| Median (Q1–Q3) | 12·0 (8·0–18·0) | 12·0 (8·0–16·0) | 11·0 (7·0–16·0) |
| DLQI score | n = 136 | n = 69 | – |
| Median (Q1–Q3) | 12·0 (8·0–16·0) | 12·0 (8·0–14·0) | – |
| POEM score | n = 142 | n = 72 | – |
| Median (Q1–Q3) | 22·0 (18·0–26·0) | 20·5 (15·0–25·0) | – |
| Baseline treatment, n (%) | |||
| Topical therapyb | 143 (100·0) | 72 (100·0) | 87 (98·9) |
| Potent/highly potent TCS | 0 | 0 | 85 (96·6) |
| Medium‐potency TCS | 139 (97·2) | 70 (97·2) | 31 (35·2) |
| TCI | 59 (41·3) | 29 (40·3) | 43 (48·9) |
| Oral antihistamines | 128 (89·5) | 63 (87·5) | 82 (93·2) |
| Nonsedating | 127 (88·8) | 61 (84·7) | 70 (79·5) |
| Sedating | 17 (11·9) | 11 (15·3) | 16 (18·2) |
| Use of TCS/TCI (g daily), median (Q1–Q3)c | 2·9 (1·6–5·7) | 2·9 (1·9–4·8) | – |
| Allergic diseases at baseline, n (%) | 94 (65·7) | 45 (62·5) | 52 (59·1) |
| Seasonal allergy | 37 (25·9) | 23 (31·9) | 18 (20·5) |
| Rhinitis allergic | 38 (26·6) | 15 (20·8) | 19 (21·6) |
| Conjunctivitis allergic | 32 (22·4) | 13 (18·1) | 15 (17·0) |
| Food allergy | 21 (14·7) | 11 (15·3) | 16 (18·2) |
| Asthma | 21 (14·7) | 8 (11·1) | 23 (26·1) |
All patients included in the trial (100%) were Japanese.
DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; ISI, Insomnia Severity Index; Nem, nemolizumab; NRS, numeric rating scale; Plb, placebo; POEM, Patient‐Oriented Eczema Measure; Q, quartile; sIGA, static Investigator’s Global Assessment; TCI, topical calcineurin inhibitors; TCS, topical corticosteroids; VAS, visual analogue scale.
aThe pruritus VAS score, pruritus NRS score, and five‐level itch scale score were the average measurement over the previous 24 h. bThe use of multiple agents was allowed. cThe median daily usage was calculated using data collected over a 4‐week period.
Efficacy outcomes
The percentage change in pruritus VAS score is shown in Figure 1a. In Study‐JP01, the shift in the mean value of the pruritus VAS scores demonstrated a continuing trend towards reduced pruritus over time. In the nemolizumab/nemolizumab group, the decrease from baseline in pruritus VAS at week 68 was 65·9%. At the end of the 8‐week follow‐up period (12 weeks after the last administration), the pruritus VAS score showed only minimal increases, indicating that the effectiveness of nemolizumab against pruritus was durable. In Study‐JP02, no differences were observed between the patients who self‐injected or those who continued to receive administration from a medical professional (data not shown); overall, all patients receiving nemolizumab had a decrease in pruritus VAS scores from baseline at each study timepoint. The improvement in pruritus was similar in Study‐JP01 and Study‐JP02. A scatterplot indicating durable improvements in pruritus VAS over time and a graph showing the absolute VAS score are shown in Figure S3 and S4a (see Supporting Information).
Figure 1.
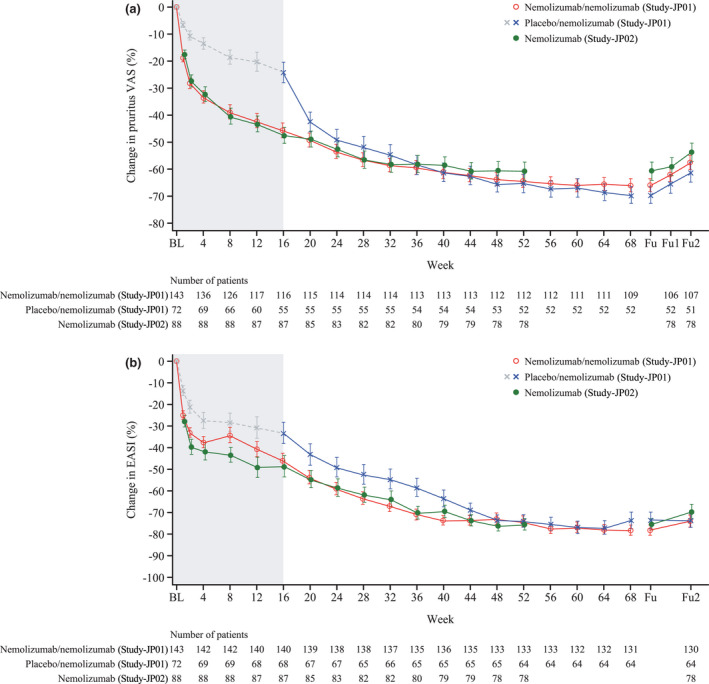
Percentage change in (a) pruritus VAS scores and (b) EASI scores (modified intention‐to‐treat population). Fu1 and Fu2 denote 4 and 8 weeks after the end of the treatment period, respectively. Error bars denote standard error of the mean. Study‐JP01 was double‐blind until week 16 (denoted by shaded area).
BL, baseline; EASI, Eczema Area and Severity Index; Fu, follow‐up; VAS, visual analogue scale.
Similar trends were observed in the change in EASI scores from baseline (Figure 1b); patients in the nemolizumab/nemolizumab group in Study‐JP01 demonstrated a continued decrease in EASI during the long‐term treatment period, and a decrease from baseline at week 68 of 78·2%. The reductions in EASI score were maintained after the end of treatment, with minimal changes during the follow‐up period. The changes in EASI scores were comparable in Study‐JP02. Absolute EASI scores are shown in Figure S4b; EASI scores were decreased to 5·6 (where 1·1–7·0 is defined as mild severity 33 ) at week 68.
As shown in Figure 2, improvements in sleep (ISI) and DLQI were observed by week 16, and these improvements were maintained until the end of treatment. No rapid exacerbations were observed after the final administration of nemolizumab. The proportion of patients with a DLQI score ≤ 4 between baseline and the end of the follow‐up period is shown in Figure S5 (see Supporting Information).
Figure 2.
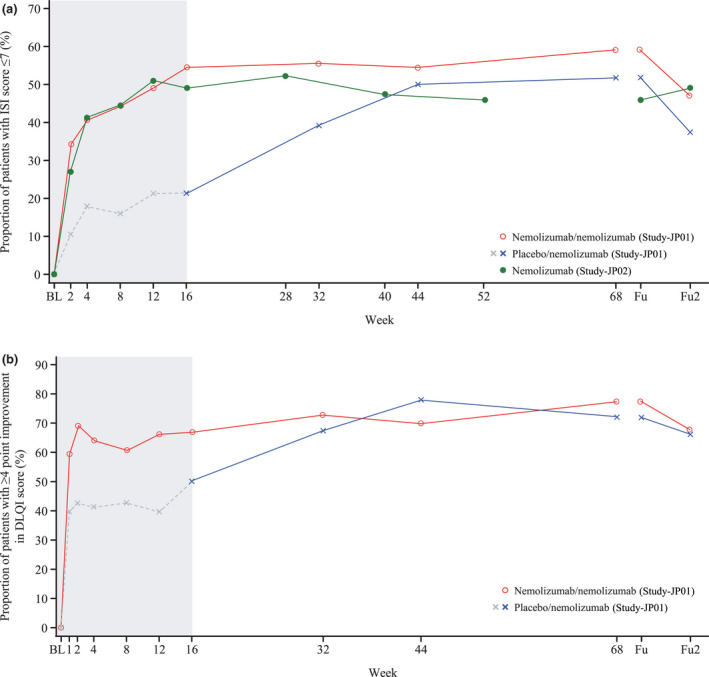
Proportion of patients with (a) ISI score of ≤ 7 and (b) a decrease of ≥ 4 points in DLQI score from baseline to the end of the follow‐up period (modified intention‐to‐treat population). Fu2 denotes 8 weeks after the end of the treatment period. Study‐JP01 was double‐blind until week 16 (denoted by shaded area).
BL, baseline; DLQI, Dermatology Life Quality Index; Fu, follow‐up; ISI, Insomnia Severity Iindex.
A continued decrease in TCS/TCI usage was observed in Study‐JP01 (both groups) during the long‐term administration period (Figure 3). Usage of TCS/TCI did not increase during the 8‐week follow‐up period (12 weeks after the last administration). Outcomes for all other efficacy endpoints are summarized in Table 2; in general, outcome measures showed a tendency towards improvement between weeks 16 and 68 (Study‐JP01) and between weeks 16 and 52 (Study‐JP02). A high proportion of patients (around 80%) achieved an improvement of ≥ 4 points (MCID) in POEM.
Figure 3.
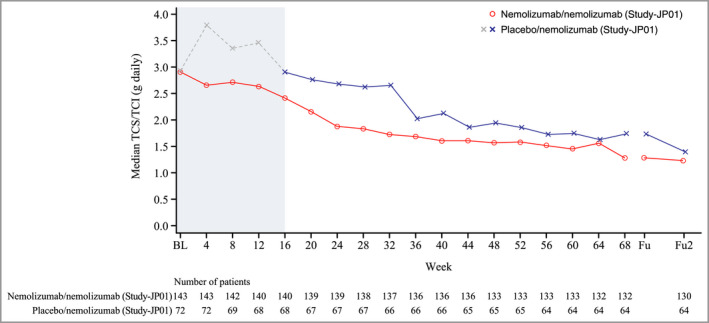
Daily usage of TCS and/or TCI from baseline to the end of the follow‐up period (modified intention‐to‐treat population). The median daily usage was calculated using data collected over a 4‐week period. Fu2 denotes 8 weeks after the end of the treatment period. Study‐JP01 was double‐blind until week 16 (denoted by shaded area).
Fu, follow‐up; TCI, topical calcineurin inhibitors; TCS, topical corticosteroids.
Table 2.
Summary of other efficacy endpoints (modified intention‐to‐treat population)
| Week 16 | Week 68 (Study‐JP01) or Week 52 (Study‐JP02) | |||||
|---|---|---|---|---|---|---|
| Study‐JP01 | Study‐JP02 | Study‐JP01 | Study‐JP02 | |||
|
Nem/nem n = 143 |
Plb/nem n = 72 |
Nem n = 88 |
Nem/nem n = 143 |
Plb/nem n = 72 |
Nem n = 88 |
|
| Improvement in pruritus VAS, % | ||||||
| 50% | 34·3 | 13·9 | 45·5 | 57·3 | 58·3 | 55·7 |
| 75% | 14·7 | 4·2 | 14·8 | 32·2 | 34·7 | 34·1 |
| 90% | 6·3 | 2·8 | 4·5 | 14·0 | 13·9 | 20·5 |
| 5‐level itch scale score ≤ 1, % | 16·8 | 5·6 | 20·5 | 38·5 | 40·3 | 36·4 |
| Improvement in pruritus NRS, % | ||||||
| ≥ 4 points | 32·2 | 12·5 | 44·3 | 49·7 | 56·9 | 55·7 |
| Improvement in EASI, % | ||||||
| 50% | 51·7 | 40·3 | 56·8 | 79·7 | 75·0 | 80·7 |
| 75% | 25·9 | 18·1 | 33·0 | 66·4 | 59·7 | 52·3 |
| 90% | 7·0 | 4·2 | 12·5 | 40·6 | 33·3 | 27·3 |
| Improvement in sIGA, % | ||||||
| ≥ 2 points and a score of 0 or 1 | 5·6 | 5·6 | 8·0 | 28·7 | 16·7 | 12·5 |
| Improvement in ISI, % | n = 118 | n = 61 | n = 87 | n = 118 | n = 61 | n = 87 |
| ≥ 6 points | 55·1 | 26·2 | 39·1 | 62·7 | 50·8 | 29·9 |
| Improvement in POEM, %a | n = 142 | n = 72 | – | n = 142 | n = 72 | – |
| ≥ 4 points | 73·2 | 44·4 | – | 79·6 | 77·8 | – |
| Usage of TCS and TCI (g daily), median (Q1–Q3)a,b | 2·42 (1·29–4·00) | 2·91 (1·87–4·48) | – | 1·29 (0·55–2·64) | 1·73 (0·82–2·96) | – |
EASI, Eczema Area and Severity Index; ISI, Insomnia Severity Index; Nem, nemolizumab; NRS, numeric rating scale; Plb, placebo; POEM, Patient‐Oriented Eczema Measure; Q, quartile; sIGA, static Investigator’s lobal Assessment; TCI, topical calcineurin inhibitors; TCS, topical corticosteroids; VAS, visual analogue scale.
aStudy‐JP01 only. bThe median daily usage was calculated using data collected over a 4‐week period.
Safety outcomes
Overall, TEAEs occurred in more than 90% of patients who received nemolizumab in the two studies (Table 3), but the majority were mild in severity; severe TEAEs occurred in < 5% of patients. The most common TEAEs were nasopharyngitis (33·9%) and AD (25·2%). The frequency of injection‐related reactions decreased over time, to < 1% during long‐term administration, with no occurrence of unexpected late‐onset TEAEs (Table S1; see Supporting Information).
Table 3.
Treatment‐emergent adverse events (TEAEs) occurring after the first dose of nemolizumab (safety analysis set)
| Pooled nemolizumab (n = 298)a | ||
|---|---|---|
| n (%) | n per 100 PY | |
| Patients with ≥ 1 TEAE | 281 (94·3) | 75·7 |
| Severe TEAEs | 14 (4·7) | 3·8 |
| Moderate TEAEs | 108 (36·2) | 29·1 |
| Mild TEAEs | 266 (89·3) | 71·6 |
| Patients with ≥ 1 serious TEAE | 28 (9·4) | 7·5 |
| Treatment modification due to TEAEs | ||
| Discontinuation | 14 (4·7) | 3·8 |
| Dose interruption | 20 (6·7) | 5·4 |
| Injection‐related reaction | 22 (7·4) | 5·9 |
| Most frequently reported TEAEs (≥ 5% of patients in the pooled nemolizumab treatment group) by preferred term | ||
| Nasopharyngitis | 101 (33·9) | 27·2 |
| Atopic dermatitis | 75 (25·2) | 20·2 |
| Blood creatine phosphokinase increased | 27 (9·1) | 7·3 |
| Contact dermatitis | 26 (8·7) | 7·0 |
| Influenza | 26 (8·7) | 7·0 |
| Urticaria | 24 (8·1) | 6·5 |
| Acne | 22 (7·4) | 5·9 |
| Cellulitis | 21 (7·0) | 5·7 |
| Headache | 21 (7·0) | 5·7 |
| Dental caries | 19 (6·4) | 5·1 |
| Upper respiratory tract inflammation | 19 (6·4) | 5·1 |
| Gastroenteritis | 17 (5·7) | 4·6 |
PY, person‐years. aIncludes all patients in the nemolizumab/nemolizumab group in Parts A and B of Study‐JP01 (n = 143), all patients in the placebo/nemolizumab group who received nemolizumab during Part B of Study‐JP01 (n = 67), and all of the patients who received nemolizumab in Study‐JP02 (n = 88).
Cytokine abnormalities [increased level of thymus and activation‐regulated chemokine (TARC)] were observed in 4·7% of patients (Figure S6; see Supporting Information). However, by 32 weeks after the start of treatment, TARC levels had returned to baseline, and were reduced still further after 56 weeks.
Discussion
In this analysis of data from two long‐term (≥ 52 weeks) phase III studies of nemolizumab administered concomitantly with TCS/TCI, all of the measured efficacy outcomes were improved following initiation of nemolizumab, with effectiveness maintained or increased through the duration of the studies. Moreover, acute itchiness or flare of AD (e.g. relapse of pruritus, or worsening of the signs or extent of AD) were rarely observed during the 8‐week follow‐up period.
In patients enrolled in Parts A and B of Study‐JP01, and who received nemolizumab for the entire 68‐week treatment period, pruritus VAS decreased by 66% from the start of treatment. This compares with an improvement of 42·8%, which was previously reported at 16 weeks in Study‐JP01. 17 Although the absolute pruritus VAS score at baseline (74·9–78·4) indicated severe pruritus, 22 , 34 scores up to week 68 had decreased to a level (23·1–31·0) indicative of mild pruritus, suggesting a clinically meaningful improvement for patients. Although a higher percentage of patients in Study‐JP02 had a sIGA score of 4 or more compared with Study‐JP01, the degree of itchiness reached after long‐term nemolizumab administration was the same in both studies.
Several immunotherapies have either recently been approved or are currently being developed for the treatment of AD. 35 However, nemolizumab was specifically developed to inhibit the IL‐31 signalling pathway; IL‐31 is known to be a key factor in pruritogenic activity, with additional effects on proinflammatory and immunomodulatory responses. 14 , 15 It is known that an itch–scratch cycle is promulgated in patients with AD and pruritus, resulting in a worsening of rash associated with increased itching. By targeting IL‐31, nemolizumab is able to decrease pruritus and suppress itch, thereby reducing scratching behaviours and blocking the itch–scratch cycle, which, in addition to its anti‐inflammatory effects subsequently reduces the severity of skin inflammation and eczema. Thus, patients with pruritus associated with AD that was inadequately controlled by topical anti‐inflammatory drugs and oral antihistamines were chosen as the study population for both long‐term studies. Study‐JP01 included the additional eligibility criterion of patients with a baseline EASI score ≥ 10 on the day of randomization. 17 We found that the EASI score decreased by 78% from the start of treatment to week 68 in the nemolizumab/nemolizumab group, from 27·6 at baseline to 5·6 at week 68. In comparison, in Part A of Study‐JP01, the EASI score improved to 45·9% at week 16. 17 The MCID has been reported to be 6·6 points; 30 thus, our data indicate a clinically meaningful improvement for patients.
Both DLQI and ISI, indicators of QoL, were maintained even after the end of treatment administration. This is an important and clinically relevant point, as QoL in patients with AD and pruritus is known to be negatively affected both economically and psychosocially. 4 , 36 , 37 The consideration that the amount of concomitant TCS/TCI could be reduced by around half during nemolizumab administration may also help to reduce the burden on patients, primarily via a decrease in the application time required for topical treatments and also, potentially, by reducing some of the side‐effects (e.g. thinning of the skin) associated with steroid use.
TEAEs occurred in approximately 90% of patients across the two studies; however, most events were mild in severity. Few TEAEs resulted in treatment interruption or discontinuation, and no unexpected delayed‐onset TEAEs occurred during the later study periods. Overall, the safety profile was consistent with previously reported study results. 19 , 38 , 39 The rate of injection‐related reactions between the first dose of nemolizumab and week 12 was 5·0%, but this reduced over time, and there was no increased risk associated with long‐term administration for up to 68 weeks. The TEAEs of infection which occurred during the studies were mostly associated with seasonal diseases.
Although a previous publication has also reported positive long‐term (64‐week) data from a phase II study of nemolizumab, 38 our analysis both confirms and expands the clinical knowledge base for nemolizumab. Whereas the prior phase II study permitted the use of only low‐potency TCS, 38 our phase III studies did not place the same limitations on TCS usage, making the setting of these studies more comparable with actual clinical practice. Furthermore, the phase II study was unable to provide data regarding the duration of effectiveness and safety after treatment completion, whereas our Study‐JP01 and Study‐JP02 included data obtained during a post‐treatment follow‐up period. These data demonstrated that the beneficial effects of nemolizumab on pruritus, rash and QoL continued for 8 weeks after treatment cessation (12 weeks after the last administration) and, overall, nemolizumab was well tolerated for up to 68 weeks. The observation that nemolizumab efficacy against pruritus increases over time and can be maintained even after treatment cessation is an important therapeutic attribute when considering the optimal management of this chronic disease. Exacerbation of AD reported as a TEAE mostly appeared during the first 12 weeks of nemolizumab administration but decreased thereafter. By 12 weeks after the last administration, only five out of 295 patients reported exacerbations, and the risk of rapid relapse of AD following treatment cessation was considered low.
Limitations associated with this analysis include the lack of a control arm during the long‐term administration period in both studies, which may have given rise to an evaluation bias. 40 The lack of imputation of missing data for patients who discontinued prior to week 52 may have been another source of potential bias. The follow‐up period was 12 weeks, which may have been too short to observe exacerbations after treatment ended. In addition, the generalizability of the data may be restricted by the inclusion of only Japanese patients and those aged ≥ 13 years of age. However, a clinical study in paediatric patients (JapicCTI‐205385) and a long‐term study in US patients (NCT03989206) are currently ongoing.
In conclusion, long‐term use of nemolizumab 60 mg Q4W with concomitant TCS/TCI, in patients with AD and moderate‐to‐severe pruritus inadequately controlled by topical agents and oral antihistamines, resulted in a continuous improvement in pruritus, signs of AD and QoL for patients, with a favourable long‐term safety profile. These beneficial and durable effects were likely due to interruption of the itch–scratch cycle, and were maintained for 12 weeks after the last administration.
Author Contribution
Kenji Kabashima: Conceptualization (lead); Methodology (equal); Supervision (lead); Validation (equal); Writing‐review & editing (equal). Takayo Matsumura: Conceptualization (supporting); Data curation (equal); Methodology (equal); Project administration (lead); Validation (equal); Writing‐review & editing (equal). Hiroshi Komazaki: Conceptualization (supporting); Data curation (equal); Formal analysis (lead); Methodology (equal); Validation (equal); Visualization (lead); Writing‐review & editing (equal). Makoto Kawashima: Conceptualization (supporting); Methodology (equal); Supervision (supporting); Validation (equal); Writing‐review & editing (equal).
Supporting information
Table S1 Treatment‐emergent adverse events occurring after the first dose of nemolizumab, by study period (safety analysis set).
Table S2 Study investigators.
Figure S1 Design of (a) Study‐JP01 and (b) Study‐JP02.
Figure S2 Disposition in (a) Study‐JP01 and (b) Study‐JP02.
Figure S3 Scatterplot of data from Study‐JP01 and Study‐JP02 indicating durable improvements in pruritus visual analogue scale scores over time (modified intention‐to‐treat population).
Figure S4 Absolute values of (a) pruritus visual analogue scale scores and (b) Eczema Area and Severity Index scores over time (modified intention‐to‐treat population).
Figure S5 Proportion of patients with a Dermatology Life Quality Index score ≤ 4 between baseline and the end of the follow‐up period (modified intention‐to‐treat population).
Figure S6 Median change in thymus and activation‐regulated chemokine from baseline to the end of the follow‐up period (safety analysis set).
Video S1 Author video.
Acknowledgments
The authors wish to thank the patients who took part in the studies, and their families; the study investigators; and the project team members at Maruho, especially Chieko Tanaka, Yoshiteru Hayakawa, Chie Fujii and Rumiko Kato. They also acknowledge editorial assistance provided by Sally‐Anne Mitchell PhD and publication management provided by Hisanori Yoshida (McCann Health CMC, Japan), funded by Maruho Co. Ltd, Osaka, Japan. A full list of study investigators is provided in Table S2 in the Supporting Information.
Funding sources This research was funded by Maruho Co. Ltd, Osaka, Japan. The trial sponsor was involved in the study design, the collection, analysis, interpretation of data, and the decision to submit the article for publication. Maruho also paid for professional writing assistance. Nemolizumab and placebo were provided by the product manufacturer, Chugai Pharmaceutical Co. Ltd, Tokyo, Japan.
Conflicts of interest K.K. has received grants from Japan Tobacco Inc., Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Pola Pharma, The Procter & Gamble Company, Taiho Pharma and Torii Pharmaceutical, and has received personal fees from Maruho. M.K. has received personal fees from Maruho, Nippon Zoki Pharmaceutical Co. Ltd, Sanofi K.K., Sato Pharmaceutical Co. Ltd and Takeda Pharmaceutical Co. Ltd. T.M. and H.K. are employees of Maruho.
Data availability statement Due to the laws governing use of patient data, and the wording of the informed consent form used in this study, it is not possible to provide even de‐identified patient data from this study to other researchers for analysis. Any other reasonable requests for information will be considered, and may be made by anyone.
Plain language summary available online
References
- 1. Weidinger S, Beck LA, Bieber T et al. Atopic dermatitis. Nat Rev Dis Primers 2018; 4:1. [DOI] [PubMed] [Google Scholar]
- 2. Barbarot S, Auziere S, Gadkari A et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 2018; 73:1284–93. [DOI] [PubMed] [Google Scholar]
- 3. Odhiambo JA, Williams HC, Clayton TO et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009; 124:1251–8. [DOI] [PubMed] [Google Scholar]
- 4. Bridgman AC, Block JK, Drucker AM. The multidimensional burden of atopic dermatitis: an update. Ann Allergy Asthma Immunol 2018; 120:603–6. [DOI] [PubMed] [Google Scholar]
- 5. Ramirez FD, Chen S, Langan SM et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr 2019; 173:e190025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katoh N, Ohya Y, Ikeda M et al. Japanese guidelines for atopic dermatitis 2020. Allergol Int 2020; 69:356–69. [DOI] [PubMed] [Google Scholar]
- 7. Xerfan EMS, Tomimori J, Andersen ML et al. Sleep disturbance and atopic dermatitis: a bidirectional relationship? Med Hypotheses 2020; 140:109637. [DOI] [PubMed] [Google Scholar]
- 8. Topal FA, Zuberbier T, Makris MP et al. The role of IL‐17, IL‐23 and IL‐31, IL‐33 in allergic skin diseases. Curr Opin Allergy Clin Immunol 2020; 20:367–73. [DOI] [PubMed] [Google Scholar]
- 9. Bodoor K, Al‐Qarqaz F, Heis LA et al. IL‐33/13 axis and IL‐4/31 axis play distinct roles in inflammatory process and itch in psoriasis and atopic dermatitis. Clin Cosmet Investig Dermatol 2020; 13:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bagci IS, Ruzicka T. IL‐31: a new key player in dermatology and beyond. J Allergy Clin Immunol 2018; 141:858–66. [DOI] [PubMed] [Google Scholar]
- 11. Singh B, Jegga AG, Shanmukhappa KS et al. IL‐31‐driven skin remodeling involves epidermal cell proliferation and thickening that lead to impaired skin‐barrier function. PLOS ONE 2016; 11:e0161877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeidler C, Yosipovitch G, Stander S. Prurigo nodularis and its management. Dermatol Clin 2018; 36:189–97. [DOI] [PubMed] [Google Scholar]
- 13. Otsuka A, Tanioka M, Nakagawa Y et al. Effects of cyclosporine on pruritus and serum IL‐31 levels in patients with atopic dermatitis. Eur J Dermatol 2011; 21:816–17. [DOI] [PubMed] [Google Scholar]
- 14. Furue M, Yamamura K, Kido‐Nakahara M et al. Emerging role of interleukin‐31 and interleukin‐31 receptor in pruritus in atopic dermatitis. Allergy 2018; 73:29–36. [DOI] [PubMed] [Google Scholar]
- 15. Nakashima C, Otsuka A, Kabashima K. Interleukin‐31 and interleukin‐31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol 2018; 27:327–31. [DOI] [PubMed] [Google Scholar]
- 16. Nemoto O, Furue M, Nakagawa H et al. The first trial of CIM331, a humanized antihuman interleukin‐31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double‐blind, placebo‐controlled study. Br J Dermatol 2016; 174:296–304. [DOI] [PubMed] [Google Scholar]
- 17. Kabashima K, Matsumura T, Komazaki H et al. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med 2020; 383:141–50. [DOI] [PubMed] [Google Scholar]
- 18. Katoh N, Ohya Y, Ikeda M et al. Clinical practice guidelines for the management of atopic dermatitis 2018. J Dermatol 2019; 46:1053–101. [DOI] [PubMed] [Google Scholar]
- 19. Silverberg JI, Pinter A, Pulka G et al. Phase 2b randomized study of nemolizumab in adults with moderate‐severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol 2020; 145:173–82. [DOI] [PubMed] [Google Scholar]
- 20. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatol Venereol (Stockholm) 1980; 92 (Suppl.):44–7. [Google Scholar]
- 21. Kawashima M, Nakagawa H. Olopatadine hydrochloride in children: evidenced efficacy and safety for atopic dermatitis treatment in a randomized, multicentre, double‐blind, parallel group comparative study. Nishinihon J Dermatol 2011; 73:278–89. [Google Scholar]
- 22. Reich A, Heisig M, Phan NQ et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012; 92:497–501. [DOI] [PubMed] [Google Scholar]
- 23. Yosipovitch G, Reaney M, Mastey V et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate‐to‐severe atopic dermatitis. Br J Dermatol 2019; 181:761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barbier N, Paul C, Luger T et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol 2004; 150:96–102. [DOI] [PubMed] [Google Scholar]
- 25. Schmitt J, Langan S, Williams HC et al. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 2007; 120:1389–98. [DOI] [PubMed] [Google Scholar]
- 26. Morin CM, Belleville G, Belanger L et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011; 34:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19:210–16. [DOI] [PubMed] [Google Scholar]
- 28. Charman CR, Venn AJ, Williams HC. The Patient‐Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140:1513–19. [DOI] [PubMed] [Google Scholar]
- 29. Basra MK, Salek MS, Camilleri L et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230:27–33. [DOI] [PubMed] [Google Scholar]
- 30. Schram ME, Spuls PI, Leeflang MM et al. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012; 67:99–106. [DOI] [PubMed] [Google Scholar]
- 31. Gaunt DM, Metcalfe C, Ridd M. The Patient‐Oriented Eczema Measure in young children: responsiveness and minimal clinically important difference. Allergy 2016; 71:1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Howells L, Ratib S, Chalmers JR et al. How should minimally important change scores for the Patient‐Oriented Eczema Measure be interpreted? A validation using varied methods. Br J Dermatol 2018; 178:1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leshem YA, Hajar T, Hanifin JM et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 2015; 172:1353–7. [DOI] [PubMed] [Google Scholar]
- 34. Ständer S, Augustin M, Reich A et al. Pruritus assessment in clinical trials: consensus recommendations from the International Forum for the Study of Itch (IFSI) Special Interest Group Scoring Itch in Clinical Trials. Acta Derm Venereol 2013; 93:509–14. [DOI] [PubMed] [Google Scholar]
- 35. Baylet A, Laclaverie M, Marchand L et al. Immunotherapies in cutaneous pathologies: an overview. Drug Discov Today 2021; 26:248–55. [DOI] [PubMed] [Google Scholar]
- 36. Marron SE, Cebrian‐Rodriguez J, Alcalde‐Herrero VM et al. Psychosocial impact of atopic dermatitis in adults: a qualitative study. Actas Dermosifiliogr 2020; 111:513–17. [DOI] [PubMed] [Google Scholar]
- 37. Silverberg JI, Gelfand JM, Margolis DJ et al. Patient burden and quality of life in atopic dermatitis in US adults: a population‐based cross‐sectional study. Ann Allergy Asthma Immunol 2018; 121:340–7. [DOI] [PubMed] [Google Scholar]
- 38. Kabashima K, Furue M, Hanifin JM et al. Nemolizumab in patients with moderate‐to‐severe atopic dermatitis: randomized, phase II, long‐term extension study. J Allergy Clin Immunol 2018; 142:1121–30. [DOI] [PubMed] [Google Scholar]
- 39. Ruzicka T, Hanifin JM, Furue M et al. Anti‐interleukin‐31 receptor A antibody for atopic dermatitis. N Engl J Med 2017; 376:826–35. [DOI] [PubMed] [Google Scholar]
- 40. Sterne JAC, Hernán MA, McAleenan A et al. Chapter 25: Assessing risk of bias in a non‐randomized study. In: Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (updated February 2021) (Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T et al., eds); 2021. Available at: https://training.cochrane.org/handbook (last accessed 12 April 2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Treatment‐emergent adverse events occurring after the first dose of nemolizumab, by study period (safety analysis set).
Table S2 Study investigators.
Figure S1 Design of (a) Study‐JP01 and (b) Study‐JP02.
Figure S2 Disposition in (a) Study‐JP01 and (b) Study‐JP02.
Figure S3 Scatterplot of data from Study‐JP01 and Study‐JP02 indicating durable improvements in pruritus visual analogue scale scores over time (modified intention‐to‐treat population).
Figure S4 Absolute values of (a) pruritus visual analogue scale scores and (b) Eczema Area and Severity Index scores over time (modified intention‐to‐treat population).
Figure S5 Proportion of patients with a Dermatology Life Quality Index score ≤ 4 between baseline and the end of the follow‐up period (modified intention‐to‐treat population).
Figure S6 Median change in thymus and activation‐regulated chemokine from baseline to the end of the follow‐up period (safety analysis set).
Video S1 Author video.


