Abstract
Background
The ratio of early diastolic mitral inflow velocity (E) to early diastolic mitral annular tissue velocity (e’), or E/e’, is an echocardiographic measure of left ventricular filling pressure. Peri‐operative changes in E/e’ and association with outcomes have been demonstrated in adults undergoing surgery for aortic stenosis (AS). We sought to explore changes in E/e’ and other diastolic indices in the setting of congenital AS surgery and to assess for association with post‐operative outcomes among children and young adults.
Methods
A retrospective, single‐center study was performed among patients 6 months to 30 years of age who underwent congenital AS surgery from 2006 to 2018. Tissue Doppler indices were collected from pre‐ and post‐operative echocardiograms. Post‐operative outcomes were reviewed.
Results
Sixty‐six subjects with subvalvar (45%), valvar (47%), and supravalvar (8%) AS underwent surgery at a median age of 9.5 years (IQR: 4.0–14.8). Pre‐operatively, the lateral E/e’ ratio was 8.6 (6.7–11.0); 33% had E/e’≥10. Post‐operatively, the lateral e’ decreased to 9.9 cm/s (8.0–11.4), the E/e’ ratio increased to 10.4 (8.3–13.1); and 53% had E/e’≥10 (p‐values < 0.0001, 0.0072, and < 0.001, respectively). Pre‐operative lateral e’ correlated modestly with duration of intubation (ρ = −0.24, p‐value 0.048) and post‐operative lateral e’ correlated modestly with duration of intubation and length of hospital stay (ρ = −0.28 and −0.26, p‐values = 0.02 and 0.04, respectively).
Conclusions
Children and young adults who underwent congenital AS surgery had echocardiographic evidence of diastolic dysfunction pre‐operatively that worsened post‐operatively. Lateral e’ may be a sensitive indicator of impaired ventricular relaxation in these patients and may impact duration of intubation and hospital stay.
Keywords: aortic valve, aortic valve, congenital heart disease, congenital heart surgery, echocardiography, left, repair, replacement, ventricle
1. INTRODUCTION
Patients with aortic stenosis (AS) are exposed to chronic pressure overload that may result in left ventricular (LV) remodeling, diastolic dysfunction, and elevated LV filling pressure. Echocardiographic indices of diastolic function have proven to have prognostic value in many disease states, including AS. 1 , 2 , 3 One specific tissue Doppler index, the ratio of early diastolic mitral inflow velocity (E) to early diastolic mitral annular tissue velocity (e’), or E/e’, serves as a validated non‐invasive index of LV filling pressure 4 and, importantly, is less impacted by variable loading conditions, including the presence of aortic valve disease. 5 Adult studies have described the changes in pre‐ and post‐operative E/e’ and shown associations between pre‐and post‐operative E/e’ and post‐operative clinical outcomes, including mortality, in those undergoing aortic valve replacement. 6 , 7
Children and young adults undergoing congenital aortic valve surgery have a range of post‐operative clinical outcomes. 8 , 9 While E/e’ and other tissue Doppler indices have been shown to correlate with LV filling pressures in pediatric patients, 10 the peri‐operative changes in these measures have not been well‐described. Moreover, the utility of these measures in assessing operative risk and post‐operative outcomes in the setting of congenital aortic valve surgery has not been evaluated. Therefore, we sought (1) to describe the changes in E/e’ and other diastolic indices by echocardiography among children and young adults undergoing congenital aortic valve surgery; and (2) to assess whether pre‐ or post‐operative E/e’ may serve as a non‐invasive marker for early post‐operative outcomes, including duration of intubation and hospital stay.
2. PATIENTS AND METHODS
We performed a retrospective study of children and young adults between the ages of 6 months and 30 years who underwent surgery for congenital AS at Morgan Stanley Children's Hospital and Milstein Hospital of Columbia University Irving Medical Center from 2006 to 2018. Patients with primary diagnoses of subvalvar, valvar, and/or supravalvar AS were abstracted from our congenital cardiac surgical database. Since it is not uncommon for congenital patients with obstructive aortic lesions to have mixed disease, those with a secondary diagnosis of aortic insufficiency (AI) at the time of surgical intervention were included. Similarly, given that a substantial number of patients with congenital valvar AS undergo balloon aortic valvuloplasty as an initial intervention, these patients were also included if the primary indication for surgical re‐intervention was AS. Patients were excluded if they had (1) concomitant complex congenital heart disease (including hemodynamically significant ventricular septal defect and more than mild mitral stenosis or regurgitation), (2) prior surgical aortic valve intervention, or (3) insufficient tissue Doppler imaging available. This study was approved by the Institutional Review Board with a waiver of informed consent.
Demographic, clinical, and echocardiographic data were collected for each patient. Demographic and clinical variables included age, gender, presence of a genetic syndrome, prior aortic valve interventions, primary surgery type, and cardiopulmonary bypass time. Complete pre‐operative echocardiograms within 3 months prior to the operation and post‐operative echocardiograms within 14 days after the operation and prior to discharge were reviewed. The severity of AS, presence of AI, and aortic valve morphology were noted. LV size, hypertrophy, and function were assessed both qualitatively and quantitatively, measured by M‐mode and shortening fraction (SF). Early (E) and late (A) mitral inflow velocities were measured from the standard apical four‐chamber view. Tissue Doppler imaging was reviewed, and the following measurements were also performed from the standard apical four‐chamber view: septal e’, lateral e’, isovolumic relaxation time (IVRT), mitral valve inflow time (MVIT), and deceleration time. The septal and lateral E/e’ ratios were determined, and a lateral E/e’ ratio ≥10 was characterized as abnormal. Age‐based E, E/A, septal and lateral e’ and E/e’ z‐scores were derived using the Boston Z‐score calculator. 11 , 12
The primary post‐operative outcome measures were duration of intubation and length of hospital stay. Given the known low operative mortality in children and young adults undergoing congenital aortic valve surgery, mortality was noted but not included as an outcome measure.
Data are presented as median (inter‐quartile range) or frequency (%) where appropriate. Non‐parametric analyses were employed. To assess the changes in pre‐ to post‐operative E, E/A, septal and lateral e’ and E/e’, IVRT, MVIT, and deceleration time, the Wilcoxon signed‐rank test was used. Comparisons between these indices and post‐operative outcomes (duration of intubation and length of hospital stay) were performed using Spearman's rho and Wilcoxon rank‐sum. A p‐value < 0.05 was considered statistically significant. Analyses were performed with Stata (College Station, TX, USA).
3. RESULTS
Sixty‐six patients met inclusion criteria at a median age of 9.5 years (4.0–14.8). As demonstrated in Table 1, most were male (n = 47 or 71%). Patients underwent surgery for subvalvar (n = 30, 45%), valvar (n = 31, 47%), or supravalvar (n = 5, 8%) AS; six patients had relief of multi‐level AS. The most common primary surgeries performed included subaortic membrane resection (n = 30, 45%), Ross with or without Konno procedure (n = 20, 31%), supravalvar AS repair (n = 8, 12%), and aortic valve replacement (n = 4, 6%). A small group of patients required secondary interventions including VSD closure (n = 2, 3%), mitral valve intervention (n = 2, 3%), and coarctation repair (n = 1, 2%). Median cardiopulmonary bypass time was 96.0 minutes (51.8–139.0).
TABLE 1.
Patient characteristics (n = 66)
| Median or n | IQR or % a | |
|---|---|---|
| Age (years) | 9.5 | 4.0–14.8 |
| Weight (kg) | 31.9 | 17.0–59.9 |
| Height (cm) | 133 | 100.3–160.4 |
| Gender | ||
| Male | 47 | 71.2 |
| Female | 19 | 28.8 |
| Presence of genetic syndrome | 7 | 10.6 |
| Primary type of aortic stenosis | ||
| Subvalvar | 30 | 45.5 |
| Valvar | 31 | 46.9 |
| Supravalvar | 5 | 7.6 |
| Prior intervention | 27 | 40.9 |
| Balloon aortic valvuloplasty | 21 | 31.8 |
| Coarctation repair or stent | 8 | 13.6 |
| Primary surgery | ||
| Subaortic membrane resection | 30 | 45.5 |
| Aortic valve repair | 1 | 1.5 |
| Aortic valve replacement | 4 | 6.1 |
| Ross +/‐ Konno | 20 | 30.3 |
| Ozaki | 2 | 3.0 |
| Bentall | 1 | 1.5 |
| Supravalvar repair | 8 | 12.1 |
All percentages represent the proportion of the entire study population.
The pre‐operative echocardiograms were performed at a median of 7 days (4.4–15.9) prior to surgery. As demonstrated in Table 2, most patients had moderate (n = 31, 47%), moderate to severe (n = 8, 12%), or severe (n = 18, 27%) AS, with median peak and mean gradients of 65.0 mm Hg (50.0—78.4) and 34.5 mm Hg (25.5–44.0), respectively. Most patients had LV hypertrophy (n = 46, 70%) and no or mild LV dilation (n = 49, 74%), consistent with a primary diagnosis of AS. Most had qualitatively normal to hyperdynamic LV systolic function (n = 63, 95%); the median SF was 43.2% (38.4–46.6).
TABLE 2.
Pre‐operative echocardiographic findings (n = 66)
| Median or n | IQR or % | |
|---|---|---|
| Time to echocardiogram (days) | 7.0 | 4.4‐15.9 |
| Aortic stenosis | ||
| None | 0 | 0 |
| Mild | 7 | 10.6 |
| Mild‐moderate | 2 | 3.0 |
| Moderate | 31 | 47.0 |
| Moderate‐severe | 8 | 12.1 |
| Severe | 18 | 27.3 |
| Peak gradient (mm Hg) | 65.0 | 50.0‐78.4 |
| Mean gradient (mm Hg) | 34.5 | 25.5‐44.0 |
| Aortic insufficiency | ||
| None | 20 | 30.3 |
| Mild | 22 | 33.3 |
| Mild‐moderate | 3 | 4.5 |
| Moderate | 5 | 7.6 |
| Moderate‐severe | 9 | 13.6 |
| Severe | 7 | 10.6 |
| LV systolic function | ||
| Normal | 55 | 83.3 |
| Hyperdynamic | 8 | 12.1 |
| Mildly decreased | 1 | 1.5 |
| Moderately decreased | 1 | 1.5 |
| Severely decreased | 1 | 1.5 |
| Shortening fraction (%) | 43.2 | 38.4‐46.6 |
| LV hypertrophy | ||
| None | 20 | 30.3 |
| Mild | 24 | 36.4 |
| Mild‐moderate | 10 | 15.2 |
| Moderate | 9 | 13.6 |
| Moderate‐severe | 1 | 1.5 |
| Severe | 2 | 3.0 |
| LV dilation | ||
| None | 41 | 62.1 |
| Mild | 8 | 12.1 |
| Mild‐Moderate | 2 | 3.0 |
| Moderate | 6 | 9.1 |
| Moderate‐Severe | 2 | 3.0 |
| Severe | 7 | 10.6 |
| Aortic valve morphology | ||
| Tricuspid | 42 | 63.6 |
| Bicuspid | 23 | 34.8 |
| Unicuspid | 1 | 1.5 |
Abbreviations: LV, left ventricle; LVOT, left ventricular outflow tract.
The pre‐ and post‐operative diastolic findings are displayed in Table 3. Pre‐operatively, subjects demonstrated mild diastolic dysfunction. The median medial e’ and lateral e’ z‐scores of −1.5 and −1.8, respectively, were relatively low, and the median medial and lateral E/e’ z‐scores were slightly higher than age‐based norms with z‐scores of +2.3 and +2.0, respectively. One‐third of patients (n = 22) had lateral E/e’ ≥10.
TABLE 3.
Pre‐ and post‐operative tissue doppler indices (n = 66)
| Pre‐operative | Post‐operative | ||||
|---|---|---|---|---|---|
| Median or n | IQR or % | Median or n | IQR or % | p‐value | |
| E (cm/s) | 108.0 | 92.9 ‐ 121.8 | 99.1 | 85.8 ‐ 118.5 | 0.073 |
| E z‐score | +0.9 | 0.0 ‐ +1.7 | +0.4 | −0.2 ‐ +1.5 | 0.11 |
| E/A | 1.7 | 1.4 ‐ 2.0 | 1.6 | 1.1 ‐ 2.1 | 0.36 |
| E/A z‐score | −0.7 | −0.1 ‐ −1.1 | −0.9 | −0.2 ‐ −1.4 | 0.35 |
| Medial e’ (cm/s) | 9.6 | 8.4 ‐ 11.0 | 8.6 | 7.0 ‐ 9.7 | 0.0003 |
| Medial e’ z‐score | −1.5 | −0.7 ‐ −2.4 | −2.2 | −1.3 ‐ −3.0 | 0.0002 |
| Medial E/e’ | 11.3 | 8.9 ‐ 12.9 | 11.8 | 9.2 ‐ 14.8 | 0.12 |
| Medial E/e’ z‐score | 2.3 | +1.0 ‐ +3.4 | 2.6 | +1.0 ‐ +4.7 | 0.11 |
| Lateral e’ (cm/s) | 12.1 | 10.0 ‐ 14.4 | 9.9 | 8.0 ‐ 11.4 | <0.0001 |
| Lateral e’ z‐score | −1.8 | −0.9 ‐ −2.8 | −2.5 | −1.8 ‐ −3.4 | <0.0001 |
| Lateral E/e’ | 8.6 | 6.7 ‐ 11.0 | 10.4 | 8.3 ‐ 13.1 | 0.0072 |
| Lateral E/e’ z‐score | +2.0 | +0.8 ‐ +3.6 | +3.0 | +1.8 ‐ +4.9 | 0.0074 |
| Lateral E/e’ ≥10 | 22 | 33 | 35 | 53% | <0.001 |
| IVRT (msec) | 70.4 | 63.4 ‐ 88.0 | 71.3 | 56.3 ‐ 84.5 | 0.63 |
| MVIT (msec) | 332.8 | 241.0 ‐ 421.6 | 223.5 | 179.6 ‐ 288.5 | <0.0001 |
| Deceleration time (msec) | 137.3 | 119.0 ‐ 181.6 | 140.4 | 115.1 ‐ 167.2 | 0.10 |
Abbreviations: A, Late diastolic (atrial) mitral inflow velocity; E, early diastolic mitral inflow velocity; e’, early diastolic mitral annular tissue velocity; IVRT, isovolumic relaxation time; MVIT, mitral valve inflow time.
The post‐operative echocardiograms were performed at a median of 3 days (2.6–4.0) after surgery. The medial and lateral e’ and e’ z‐scores decreased significantly, and the lateral E/e’ increased from a median of 8.6 pre‐operatively to 10.4 post‐operatively (p = 0.007); see Table 3 and Figure 1. Similarly, the proportion of patients with lateral E/e’ ≥10 rose from 33% to 53% (p < 0.001). MVIT shortened from a median of 332.8 msec pre‐operatively to 223.5 msec post‐operatively (p‐value < 0.0001). There was no significant pre‐ to post‐operative change in median E, E/A, IVRT, or deceleration time.
FIGURE 1.
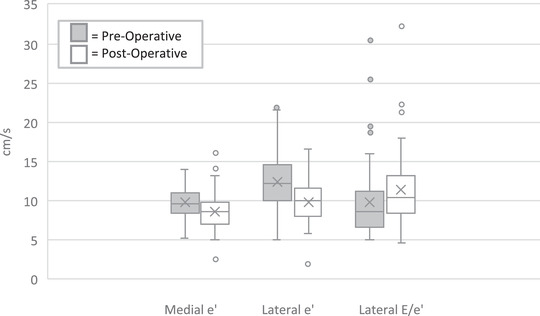
Peri‐operative change in tissue Doppler indices. The box‐whisker plot demonstrates worsening of the medial and lateral e’ velocities (centimeters/sec) and the lateral E/e’ ratio following congenital aortic valve surgery. E = early diastolic mitral inflow velocity, e’ = early diastolic mitral annular tissue velocity
Most (n = 41, 62%) were extubated on post‐operative day 0 (0.0–1.0) and only one‐third required inotropic support for a median duration of 0 days (0.0–1.0). The median length of hospital stay was 4.0 days (3.0‐5.8). There was no early mortality. A lower pre‐operative lateral e’ (ρ = −.24, p‐value 0.048), shorter pre‐operative MVIT (ρ = −.27, p‐value 0.03), and shorter deceleration time (ρ = −0.25, p‐value 0.047) correlated modestly with longer duration of intubation. A lower post‐operative lateral e’ correlated modestly with both longer duration of intubation (ρ = −0.28, p‐value 0.02) and length of hospital stay (ρ = −0.26, p‐value 0.04). A shorter post‐operative deceleration time also correlated modestly with longer duration of intubation (ρ = −0.31, p‐value 0.01) and length of hospital stay (ρ = −0.26, p‐value 0.03).
4. DISCUSSION
In this series of children and young adults undergoing congenital aortic valve surgery, the majority had at least mild left ventricular hypertrophy pre‐operatively and one‐third had evidence of diastolic dysfunction by the lateral E/e’ ratio. Peri‐operatively, various indices of diastolic dysfunction worsened, including the absolute and age‐normalized medial and lateral e’ measurements, and over half developed abnormal E/e’ ratios. There were modest but significant correlations of worse pre‐ and post‐operative indices of diastolic function with longer duration of intubation and length of hospital stay, including the lateral e’ measurement, MVIT, and deceleration time.
Patients with congenital AS are at risk of developing diastolic dysfunction secondary to chronic pressure overload on the LV and compensatory development of LV remodeling. LV diastolic dysfunction is routinely assessed by echocardiogram with tissue Doppler imaging, 13 which measures low‐velocity, high‐amplitude velocities of the myocardium. These are typically performed at the medial (septal) and lateral aspects of the mitral valve. The ratio of early diastolic mitral inflow velocity (E) to early diastolic mitral annular tissue velocity (e’), or E/e’, has emerged as a surrogate measure of LV filling pressure. Many studies have correlated E/e’ with invasive measurement of LV filling pressure, including a 2017 European multi‐center study that demonstrated E/e’, among other echo indices, to be independently associated with invasive LV end‐diastolic pressure (LVEDP). 14 , 15 , 16 , 17 , 18 Ommen et al. demonstrated that in adult patients, E/e’ values < 8 and > 15 accurately predicted normal and elevated mean LV diastolic pressure, respectively, with wide variability in those with E/e’ between 8 and 15. 4 However, Friedman et al. went on to show that in children and young adults with AS, lateral E/e’ > 9.5 accurately predicted an LVEDP ≥15 mm Hg. 19
As a reliable surrogate of LV filling pressure, E/e’ has been studied as a clinical and operative prognosticator. In the adult literature, E/e’ has been shown to be predictive of survival and reduced exercise capacity after acute myocardial infarction, decreased ventricular function and mortality after off‐pump coronary artery surgery, morbidity and mortality in patients with congestive heart failure, primary cardiac events in hypertensive patients, and mortality in end‐stage renal disease. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Specific to AS, E/e’ has been used to assess risk in unrepaired adult patients, including the development of symptoms and event‐free survival. 28 , 29 , 30 In those undergoing aortic valve replacement, both pre‐and post‐operative E/e’ have been shown to be associated with post‐operative morbidity, including heart failure and acute kidney injury (AKI), and mortality. 1 , 6 , 7
This study is the first, to our knowledge, in the pediatric literature to describe the changes in tissue Doppler measurements in the setting of congenital aortic valve surgery and to assess the association with early post‐operative outcomes. Eidem et al. previously showed that children with unrepaired AS have significantly increased medial and lateral E/e’ ratios when compared to healthy controls. 31 Our results support this finding, demonstrating baseline elevations in both the absolute pre‐operative lateral E/e’ and the age‐normalized z‐scores. Both the medial and lateral e’ and e’ z‐scores decreased significantly in the peri‐operative period, resulting in a further increase in lateral E/e’ and the associated z‐scores. Interestingly, the E and E/A did not change significantly, suggesting that tissue Doppler imaging may have a greater sensitivity to detect changes in diastolic dysfunction peri‐operatively.
In our series, a lower pre‐operative e’, shorter pre‐operative MVIT, and shorter pre‐operative deceleration time correlated modestly with longer duration of intubation, while a lower post‐operative e’ and shorter post‐operative deceleration time correlated modestly with both longer duration of intubation and length of hospital stay. Although deceleration time was associated with pre‐ and post‐operative outcomes, it did not significantly change peri‐operatively. E/e’ was not associated with short‐term post‐operative outcomes, presumably because E did not change significantly as previously mentioned. This suggests that the absolute e’ value, in particular, may be a more sensitive and clinically relevant indicator of impaired relaxation and recovery following congenital aortic valve surgery in children and young adults.
Evidence of diastolic dysfunction on echocardiogram may be partly attributable to the surgery itself, as several studies have shown associations between cardiopulmonary bypass and aortic cross‐clamp times with reductions in tissue Doppler velocities. 32 , 33 , 34 Cardiopulmonary bypass and cross‐clamping are known to induce myocardial injury. In addition to direct injury, there is a well‐described systemic inflammatory response associated with exposure to exogenous materials, which exacerbates endothelial dysfunction. Cellular ischemia also occurs, which results in the depletion of high‐energy phosphates, increases of intra‐cellular calcium stores, and build‐up of toxic metabolites. 35 , 36 Reperfusion injury adds to this insult. Patients with pre‐operative diastolic dysfunction may be both more susceptible to this injury and/or slower to recover.
Vassalos et al. demonstrated recovery of pre‐operative LV peak systolic velocities by tissue Doppler by 15 hours after cardiac surgery in most children, suggesting that abnormal measurements beyond this time more likely represent true abnormalities. In this same study, reduced pre‐ and post‐operative LV systolic velocities were associated with longer duration of intubation, 3 Similarly, Nam et al. showed that among adults undergoing cardiac surgery, post‐operative lateral E/e’ > 15 was independently associated with post‐operative AKI and mortality at 1 and 5 years. The authors proposed that, in addition to underlying diastolic dysfunction, elevated peri‐ and post‐operative LV filling pressure may be indicative of more significant hemodynamic instability, resulting in an increased risk of post‐operative complications. They went on to show that 92.4% of patients with elevated E/e’ measured within 10 post‐operative days continued to have abnormal values at 1 year, suggesting prolonged disturbances in diastolic dysfunction 7 Together, these studies and ours suggest that LV tissue Doppler indices may have prognostic value in determining both short‐term and long‐term post‐operative outcomes.
There were several limitations to our study. Although we included patients with a primary diagnosis of AS, there was heterogeneity in the level of AS and the degree of AI. The LV, however, is likely exposed to similar conditions regardless of the type of outflow obstruction. Moreover, the appearance of the LV pre‐operatively in our cohort—predominantly hypertrophied and not significantly dilated—supports our inclusion of patients with primary AS. Given the retrospective nature of the study, a minority of subjects could not be included due to lack of tissue Doppler acquisition. We chose to focus on indices of diastolic function measured by tissue Doppler and, therefore, did not examine other parameters, such as left atrial volume. Most importantly, our single‐center study was performed at a large academic medical center in which our subjects had very favorable post‐operative outcomes. Because of the short duration of intubation and length of hospital stay, there was limited ability to detect marked associations between diastolic indices and outcomes. That being said, there were significant peri‐operative changes and associations noted that suggest that tissue Doppler indices may be useful prognosticators in children, as they are in adults. Long‐term follow‐up to assess for serial changes in diastolic indices and their relationship to symptoms and outcomes in a multi‐center cohort of children and young adults may prove to be quite informative.
Ultimately, the goal of aortic valve surgery is to prevent irreversible LV changes and long‐term diastolic dysfunction. 37 The early presence of diastolic dysfunction in children and young adults may impact the decision regarding timing of intervention or impact risk‐stratification with regard to post‐operative care. In our study, we found that the lateral e’ measurement may be the most sensitive indicator of not only peri‐operative changes in diastolic function but also impaired relaxation and recovery following congenital aortic valve surgery. Moving forward, a multi‐center, collaborative approach would be worthwhile to elucidate the utility of this measurement and its relationship to both short‐ and long‐term outcomes.
Pesce M, LaPar D, Kalfa D, Bacha E, Freud L. Peri‐operative changes in diastolic function and outcomes in congenital aortic valve surgery. Echocardiography. 2022;39:178–184. 10.1111/echo.15274
REFERENCES
- 1. Giorgi D, Di Bello V, Talini E, et al. Myocardial function in severe aortic stenosis before and after aortic valve replacement: a Doppler tissue imaging study. J Am Soc Echocardiogr. 2005;18:8‐14. [DOI] [PubMed] [Google Scholar]
- 2. Mcmahon CJ. Echocardiographic predictors of adverse clinical events in children with dilated cardiomyopathy: a prospective clinical study. Heart. 2004;90:908‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vassalos A, Lilley S, Young D, et al. Tissue Doppler imaging following paediatric cardiac surgery: early patterns of change and relationship to outcome. Interactive CardioVasc Thoracic Surg. 2009;9:173‐177. [DOI] [PubMed] [Google Scholar]
- 4. Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures. Circulation. 2000;102(15):1788‐1794. [DOI] [PubMed] [Google Scholar]
- 5. Park J‐H, Marwick TH. Use and limitations of E/e' to assess left ventricular filling pressure by echocardiography. J Cardiovasc Ultrasound. 2011;19:169‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang S‐A, Park P‐W, Sung K, et al. Noninvasive estimate of left ventricular filling pressure correlated with early and midterm postoperative cardiovascular events after isolated aortic valve replacement in patients with severe aortic stenosis. J Thoracic Cardiovasc Surg. 2010;140:1361‐1366. [DOI] [PubMed] [Google Scholar]
- 7. Nam K, Park YS, Kim WH. Perioperative echocardiographic index of left ventricular filling pressure in cardiac surgery. Ann Thoracic Surg. 2019;107:84‐91. [DOI] [PubMed] [Google Scholar]
- 8. Etnel JRG, Elmont LC, Ertekin E, et al. Outcome after aortic valve replacement in children: a systematic review and meta‐analysis. J Thoracic Cardiovasc Surg. 2016;151:143‐152. [DOI] [PubMed] [Google Scholar]
- 9. Pasquali SK, Shera D, Wernovsky G, et al. Midterm outcomes and predictors of reintervention after the Ross procedure in infants, children, and young adults. J Thoracic Cardiovasc Surg. 2007;133:893‐899. [DOI] [PubMed] [Google Scholar]
- 10. Border WL, Michelfelder EC, Glascock BJ, et al. Color M‐mode and Doppler tissue evaluation of diastolic function in children: simultaneous correlation with invasive indices. J Am Soc Echocardiogr. 2003;16:988‐994. [DOI] [PubMed] [Google Scholar]
- 11. Colan SD. Normal echocardiographic values for cardiovascular structures. In: Lai WW, Cohen MS, Geva T, Mertens L, eds. Echocardiography in Pediatric and Congenital Heart Disease. Wiley‐Blackwell, West Sussex, UK; 2009:765‐785, Appendix 1. [Google Scholar]
- 12. Sluysmans T, Colan SD. Structural measurements and adjustment for growth. In: Lai WW, Cohen MS, Geva T, Mertens L, eds. Echocardiography in Pediatric and Congenital Heart Disease. Wiley‐Blackwell, West Sussex, UK; 2009, Chapter 5. [Google Scholar]
- 13. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J ‐ Cardiovasc Imag. 2016;17:1321‐1360. [DOI] [PubMed] [Google Scholar]
- 14. Lancellotti P, Galderisi M, Edvardsen T, et al. Echo‐Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro‐Filling study. Eur Heart J ‐ Cardiovasc Imag. 2017;18:961‐968. [DOI] [PubMed] [Google Scholar]
- 15. Andersen OS, Smiseth OA, Dokainish H, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol. 2017;69:1937‐1948. [DOI] [PubMed] [Google Scholar]
- 16. Bruch C, Stypmann J, Grude M, Gradaus R, Breithardt G, Wichter T. Tissue Doppler imaging in patients with moderate to severe aortic valve stenosis: clinical usefulness and diagnostic accuracy. Am Heart J. 2004;148:696‐702. [DOI] [PubMed] [Google Scholar]
- 17. Kasner M, Westermann D, Steendijk P, et al. Utility of doppler echocardiography and tissue doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction. Circulation. 2007;116:637‐647. [DOI] [PubMed] [Google Scholar]
- 18. Rivas‐Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2003;91:780‐784. [DOI] [PubMed] [Google Scholar]
- 19. Friedman KG, Mcelhinney DB, Rhodes J, et al. Left ventricular diastolic function in children and young adults with congenital aortic valve disease. Am J Cardiol. 2013;111:243‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fontes‐Carvalho R, Sampaio F, Teixeira M, et al. Left ventricular diastolic dysfunction and E/E′ ratio as the strongest echocardiographic predictors of reduced exercise capacity after acute myocardial infarction. Clin Cardiol. 2015;38:222‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hillis GS, Møller JE, Pellikka PA, et al. Noninvasive estimation of left ventricular filling pressure by e/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol. 2004;43(3):360‐367. [DOI] [PubMed] [Google Scholar]
- 22. Lee E‐H, Yun S‐C, Chin J‐H, et al. Prognostic implications of preoperative E/e′ ratio in patients with off‐pump coronary artery surgery. Anesthesiol: J Am Soc Anesthesiol. 2012;116:362‐371. [DOI] [PubMed] [Google Scholar]
- 23. Sharma R, Pellerin D, Gaze DC, et al. Mitral peak doppler E‐wave to peak mitral annulus velocity ratio is an accurate estimate of left ventricular filling pressure and predicts mortality in end‐stage renal disease. J Am Soc Echocardiogr. 2006;19:266‐273. [DOI] [PubMed] [Google Scholar]
- 24. Sharp ASP, Tapp RJ, Thom SAM, et al. Tissue Doppler E/E' ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J. 2010;31(6):747‐752. [DOI] [PubMed] [Google Scholar]
- 25. Shim JK, Choi YS, Chun DH, Hong SW, Kim DH, Kwak YL. Relationship between echocardiographic index of ventricular filling pressure and intraoperative haemodynamic changes during off‐pump coronary bypass surgery. BJA: Brit J Anaesthesia. 2009;102:316‐321. [DOI] [PubMed] [Google Scholar]
- 26. Hirata K, Hyodo E, Hozumi T, et al. Usefulness of a combination of systolic function by left ventricular ejection fraction and diastolic function by E/E′ to predict prognosis in patients with heart failure. Am J Cardiol. 2009;103:1275‐1279. [DOI] [PubMed] [Google Scholar]
- 27. Okura H, Kubo T, Asawa K, et al. Elevated E/E' predicts prognosis in congestive heart failure patients with preserved systolic function. Circul J. 2009;73:86‐91. [DOI] [PubMed] [Google Scholar]
- 28. Biner S, Rafique AM, Goykhman P, Morrissey RP, Naghi J, Siegel RJ. Prognostic value of E/E′ ratio in patients with unoperated severe aortic stenosis. JACC: Cardiovasc Imag. 2010;3:899‐907. [DOI] [PubMed] [Google Scholar]
- 29. Jassal DS, Tam JW, Dumesnil JG, et al. Clinical usefulness of tissue doppler imaging in patients with mild to moderate aortic stenosis: a substudy of the aortic stenosis progression observation measuring effects of rosuvastatin study. J Ame Soc Echocardiog. 2008;21:1023‐1027. [DOI] [PubMed] [Google Scholar]
- 30. Poh K‐K, Chan MY‐Y, Yang H, Yong Q‐W, Chan Y‐H, Ling LH. Prognostication of valvular aortic stenosis using tissue doppler echocardiography: underappreciated importance of late diastolic mitral annular velocity. J Am Soc Echocardiogr. 2008;21:475‐481. [DOI] [PubMed] [Google Scholar]
- 31. Eidem B. Impact of chronic left ventricular preload and afterload on doppler tissue imaging velocities: a study in congenital heart disease. J Am Soc Echocardiogr. 2005;18:830‐838. [DOI] [PubMed] [Google Scholar]
- 32. Klitsie LM, Hazekamp MG, Roest AAW, et al. Tissue doppler imaging detects impaired biventricular performance shortly after congenital heart defect surgery. Pediatric Cardiol. 2013;34:630‐638. [DOI] [PubMed] [Google Scholar]
- 33. Klitsie LM, Roest AAW, Blom NA, Ten Harkel ADJ. Ventricular performance after surgery for a congenital heart defect as assessed using advanced echocardiography: from doppler flow to 3d echocardiography and speckle‐tracking strain imaging. Pediatric Cardiol. 2014;35:3‐15. [DOI] [PubMed] [Google Scholar]
- 34. Pauliks LB, Ündar A, Clark JB, Myers JL. Segmental differences of impaired diastolic relaxation following cardiopulmonary bypass surgery in children: a tissue doppler study. Artif Organs. 2009;33:904‐908. [DOI] [PubMed] [Google Scholar]
- 35. De Hert S, Moerman A. Myocardial injury and protection related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. 2015;29:137‐149. [DOI] [PubMed] [Google Scholar]
- 36. Hammer S, Loeff M, Reichenspurner H, et al. Effect of cardiopulmonary bypass on myocardial function, damage and inflammation after cardiac surgery in newborns and children. Thorac Cardiovasc Surg. 2001;49:349‐354. [DOI] [PubMed] [Google Scholar]
- 37. Singh AK, Ungerleider RM, Law YM. The impact of aortic valve replacement on left ventricular remodeling in children. Pediatric Cardiol. 2016;37:1022‐1027. [DOI] [PubMed] [Google Scholar]


