Abstract
Objective
This study was aimed at identifying differences in the prodromal symptoms and their duration, risk factors and markers of vulnerability in patients presenting a first episode mania (FEM) or psychosis (FEP) with onset in late adolescence or adulthood in order to guide tailored treatment strategies.
Methods
Patients with a FEM or FEP underwent a clinical assessment. Prodromes were evaluated with the Bipolar Prodrome Symptom Scale‐Retrospective (BPSS‐R). Chi‐squared tests were conducted to assess specific prodromal symptoms, risk factors or markers of vulnerability between groups. Significant prodromal symptoms were entered in a stepwise forward logistic regression model. The probabilities of a gradual versus rapid onset pattern of the prodromes were computed with logistic regression models.
Results
The total sample included 108 patients (FEM = 72, FEP = 36). Social isolation was associated with the prodromal stage of a FEP whilst Increased energy or goal‐directed activity with the prodrome to a FEM. Physically slowed down presented the most gradual onset whilst Increased energy presented the most rapid. The presence of obstetric complications and difficulties in writing and reading during childhood were risk factors for FEP. As for markers of vulnerability, impairment in premorbid adjustment was characteristic of FEP patients. No specific risk factor or marker of vulnerability was identified for FEM.
Conclusion
Early characteristics differentiating FEP from FEM were identified. These findings might help shape early identification and preventive intervention programmes.
Keywords: first episode mania, first episode psychosis, markers of vulnerability, prodromes, risk factors
Significant outcomes
Increased energy or goal‐directed activity was associated with the prodromal stage of a first episode of mania (FEM) whilst Social isolation was associated with the prodrome of a first episode of psychosis (FEP).
Even though the duration of the FEP prodrome was longer than the FEM prodrome, the prodromal phase of FEM patients was long enough to enable an early intervention.
A higher prevalence of obstetric complications and difficulties in writing and reading during childhood were risk factors for FEP patients while impairment in premorbid adjustment was a marker of vulnerability more characteristic of FEP patients.
Limitations
The FEM and the FEP groups had different sample sizes, which might have influenced the assessment of differences between groups.
The retrospective assessment of prodromes might have caused a possible recall bias.
The lack of a control group should be acknowledged since it might have helped in assessing the specificity of prodromal symptoms and risk factors.
1. INTRODUCTION
In recent years, there has been an increased focus on the early identification of different disorders and on their prompt intervention, with attempts to predict the clinical characteristics of those individuals who will progress to full‐threshold disorder. 1 , 2 Particularly, researchers have concentrated on the prodromal phase given its potential clinical relevance in early intervention.
The prodromal phase of first episode psychosis (FEP) has been more frequently studied than the prodrome of first episode mania (FEM). A major limitation of previous literature is that a majority of studies have failed to distinguish between affective and non‐affective FEP, considering them as a single factor. 3 Due to the overlap in psychotic symptoms between FEPs and FEMs, there is a real possibility to incorrectly classify these patients at their first episode. 4 For example, about 10% of affective psychoses progress to schizophrenia (SCZ) in the long‐term. 4 The identification during the prodromal phase of specific symptoms that allow differentiating patients developing SCZ or bipolar disorder (BD) might help tailor early interventions. 5
Previous studies assessing and directly comparing the manifestations and duration of the prodromal phase in both patients presenting a FEP or a FEM are scant in the literature, with studies investigating FEM 6 , 7 and FEP 8 , 9 , 10 patients separately. In addition, these previous studies generally assessed prodromes to early onset BD or SCZ, in children and adolescents. 11 , 12
In terms of risk factors or markers of vulnerability, BD was more frequently associated with a family history for mood disorders and ADHD 7 , 13 whilst obstetric/perinatal complications, 14 worse premorbid adjustment, 15 , 16 and high rates of cannabis abuse before the first episode of disease 17 were more frequently reported in SCZ.
1.1. Aims of the study
Given the research suggesting the clinical significance of the challenges in early treatment strategies in FEM and FEP, the main aims of this study were to identify symptomatic and temporal differences between the proximal prodromal phases of a first episode of mania compared with first episode of psychosis with onset in late adolescence or adulthood. In addition, the study was aimed at exploring differences in risk factors and markers of vulnerability between these groups.
2. MATERIAL AND METHODS
2.1. Participants
This is a cross‐sectional study with a retrospective assessment of information on early manifestations. Subjects were drawn from the ‘Prodromes and Predictors in First Episode Mania and Psychosis’—ProPreF project, a 2‐year longitudinal, multicentric study investigating prodromes and predictors of clinical and longitudinal outcomes in patients presenting a FEM/FEP. Its ultimate goal was to comprehensively and exhaustively assess the clinical presentation of the patients together with their biomarkers in order to guide early identification and tailored early intervention strategies. The study included the Bipolar and Depressive Disorders Unit of IDIBAPS‐Hospital Clinic in Barcelona, the FIDMAG research foundation and the Institut Pere Mata, under the umbrella of the Spanish Research Network on Mental Health (CIBERSAM). 18
2.2. Procedures
To determine the presence of a full FEP or FEM, the summaries of the patients’ clinical history and the assessment of the clinical presentation at onset of FEM or FEP were reviewed by at least two investigators, and an agreement was reached on the diagnosis. Then, patients were clinically assessed by a trained investigator using a semi‐structured interview based on the Structured Clinical Interview for DSM Disorders (SCID‐I‐II), 19 , 20 and diagnoses were confirmed according to DSM‐5 criteria. 21 If the patient met the DSM‐5 A‐D criteria for a manic episode, they were subsequently categorized as a FEM. If the patient presented at least two of the five symptoms of the criterion A for a DSM‐5 psychotic disorder but had not experienced the DSM‐5 A‐D criteria for a manic episode, they were categorized as a FEP.
Only outpatients with either a FEM or a FEP in late adolescence (≥15 years old) or adulthood (≤45 years old) were enrolled. Patients were included if they (i) were aged between 18 and 45 years at the time of evaluation; (ii) had experienced their FEM/FEP in the previous 4 years and (iii) provided signed informed consent. Exclusion criteria were the presence of (i) a mental intellectual disability (defined as intelligence quotient (IQ) <70); (ii) any severe medical condition (i.e. cancer, HIV); (iii) alcohol/substance dependence or (iv) electroconvulsive therapy (ECT) in the previous 12 months.
The study was carried out following the latest version of the Declaration of Helsinki, and was reviewed by the ethical committee of the recruiting institutions.
2.3. Socio‐demographic information
Socio‐demographic data (i.e. age, educational level and working status) were collected and stored in an electronic data repository. Parental socioeconomic status (SES) was determined using Hollingshead's Two‐Factor Index of Social Position. 22
2.4. Clinical assessment
Clinical information on onset features (i.e. age at onset, age at first hospitalization) was collected. The duration of untreated psychosis (DUP) was calculated as the number of days between the first manifestations of psychotic symptoms and the initiation of psychiatric treatment (for those patients presenting psychotic symptoms at onset).
Clinical symptoms at the time of evaluation were assessed with the Positive and Negative Syndrome Scale (PANSS), 23 the Young Mania Rating Scale (YMRS) 24 and the Montgomery–Asberg Depression Rating Scale (MADRS). 25 Psychosocial functioning was assessed through the Functional Assessment Short Test (FAST). 26
Information was also collected for physical or psychiatric comorbidities as well as for current pharmacological treatment. Current antipsychotic mean doses were measured as risperidone equivalents, 27 , 28 current antidepressant mean doses were measured as fluoxetine equivalents 29 and current benzodiazepine mean doses were measured as diazepam equivalents, 30 based on international consensus.
Patients also underwent an assessment of adherence to treatment, which was categorized as good, irregular and bad; well‐being was assessed through the World Health Organization Well Being Index (WHO‐5); and insight was evaluated with the Insight Scale. 31
2.5. Information on early manifestations
In line with the procedures adopted in a previous study 32 and through a process of adaptation of these procedures to the clinical environment of the recruiting centres, information was collected on three domains: 1. Prodromal phenomenological manifestations; 2. Risk factors and 3. Markers of vulnerability.
2.5.1. Prodromal symptoms type and duration
Patients (and their caregivers when available) were asked to retrospectively report prodromal symptoms’ type and duration. First, we explained psychosis in clear language. We then provided the patient with the date of their first hospitalization or contact with the mental health service in order to set a time point for diagnosis. Following this, patients were asked when they first experienced changes in behaviour or prodromal symptoms. During the interview, we used a timeline and asked for important dates in the life of the patients in order to help the recalling process. A trained evaluator conducted a semi‐structured interview with the Bipolar Prodrome Symptom Scale‐Retrospective (BPSS‐R), 11 which systematically assesses the onset pattern, duration, severity and frequency of 39 symptoms and signs newly emerging of the proximal prodrome and includes subthreshold manic, depressive, general psychopathology and psychotic symptoms (see Table S1). The prodromal symptoms were categorized into four subgroups, according to the psychopathological index of each symptom: 1. Mania Index Symptoms; 2. Depressive Index; 3. General Psychopathology Index Symptoms and 4. Psychosis Index. Specific symptoms, such as the Lack of concentration can be considered as both manic and depressive. The total score of each Index considers all symptoms within a specific subgroup. On the contrary, the BPSS‐R total score is calculated by adding the main subscores, considering the duplicated items only once.
Trained evaluators conducted a semi‐structured interview with the Bipolar Prodrome Symptom Scale‐Retrospective (BPSS‐R), 11 focusing mainly on prodromic symptoms’ type, frequency and duration. Prodromal symptoms’ severity and frequency were rated on an ordinal scale from 0 (absent) to 4 (static lifetime or character trait). 6 , 11 Any prodromal symptom independent of severity, frequency and any episode occurring before the FEM/FEP in the lifetime of the patient was assessed in the interview. Only those symptoms occurring within 3 years and 1 month before 12 the first full episode was considered as part of the proximal prodrome. Only symptoms of at least moderate severity were considered in the analyses. 6 , 11 In addition, symptoms with a frequency score of 4 = static lifetime or character trait, were not included as they are not considered part of the proximal prodrome. 6 , 11 The onset pattern of the prodromal stage was defined as ‘gradual’ if ≥4 months or ‘rapid’ if <4 months. The deterioration pattern was defined as ‘slow’ if ≥4 weeks and ‘rapid’ if <4 weeks. Three different patterns of presentation were then derived, namely 1 = gradual onset with slow deterioration; 2 = gradual onset with rapid deterioration and 3 = rapid onset with rapid deterioration.
2.5.2. Risk factors
Specific risk factors have been identified as increasing the a risk of developing schizophrenia or bipolar disorder. 1 , 32 Such risk factors are not early manifestations of the disorder per se. The patients and their parents (when available) were interviewed on the presence of the following risk factors: (i) history of illness in first‐degree relatives for psychiatric disorders. At baseline, the participants were asked to report family history of psychiatric disorders. Specifically, information was gathered on the presence of first‐degree family history, namely parents’ history of psychotic, depressive, bipolar, personality and substances related disorders. When possible, the information was double‐checked with relatives or medical records; (ii) obstetric complications, assessed through the Lewis–Murray 33 scale, as a dichotomous variable (yes/no); (iii) life stressors occurring during the 12 months preceding the first episode of mania or psychosis. Life‐time traumatic events were considered when patients reported at least one traumatic event or situation of the list of the Post‐Traumatic Stress Disorder (PTSD) Symptom Scale 34 (PSS); (iv) childhood traumas, assessed through the Childhood Trauma Questionnaire (CTQ‐30) 35 ; (v) delayed language acquisition, when the individual produced sentences with two words only after the 24th month; (vi) delayed psychomotor development, when the individual was able to walk only after the 18th month; (vii) nocturnal enuresis, after the age of five 36 and (viii) difficulties in writing and reading, either self‐reported or when the patient was referred to a speech therapist as a child).
2.5.3. Markers of vulnerability
Some early developmental, behavioural or personality patterns and some biological characteristics are more frequently reported in the life history of patients that later develop bipolar disorder or schizophrenia. 1 , 32 These may be early manifestations of the disorder itself and are considered as ‘markers of vulnerability’. Specific lifetime diagnoses, such as attention deficit hyperactivity disorder (ADHD) or Conduct Disorder and the presence of previous depressive episodes or hypomania can be reported by patients that later develop a first episode of mania or psychosis as well as specific personality features. In the present study, clinical information on prior contact with mental health services during childhood/adolescence as well as on previous diagnoses and pharmacological treatment was collected. The presence of features associated with ADHD was evaluated through The Wender Utah Rating Scale. 37 Life‐time impulsivity was assessed through the Barratt Impulsiveness Scale (BIS‐11). 38 In addition, the assessment of lifetime history of alcohol‐ and drug‐related problems was of interest. We specifically assessed information on the presence of suicidal ideas or attempts and on the use of cannabis and alcohol during the prodromal phase. Conus et al. 32 also assessed the Premorbid Adjustment Scale (PAS) 39 to examine attainment of developmental goals at each of several periods of a participant's life before the onset of the illness. In the present study, only childhood and adolescence life periods were assessed.
2.6. Statistical analysis
Descriptive statistics were used to define sample characteristics, the prevalence of prodromes and the duration of the prodromal stage. Continuous variables were given as mean value ± standard deviation and were compared using Student t‐test. Categorical variables were expressed as total number and percentages and differences among groups were assessed through Chi‐square of Fisher's exact test.
The prodromal symptoms were categorized into subgroups according to their psychopathological index 6 , 12 , 40 (Appendix S1 and Table S1). The BPSS‐R total score was calculated by adding the main subscores, considering the duplicated items only once.
Univariate logistic regression models (FEM versus FEP as the dependent variable) were computed for each of the potential predictors, considering only those prodromal symptoms with at least a minimum number of events, in order to fit the predictive model. Significant prodromal symptoms were entered in a stepwise forward selection algorithm to select variables included in the final multivariate logistic regression model (FEM versus FEP as the dependent variable). Odds ratio and 95% confidence interval (CI) were calculated. Receiver operating characteristic (ROC) analysis was used to evaluate the model performance.
Differences between FEP and FEM patients in the duration of the prodromal symptoms in months were assessed through Student t‐test. We wanted to assess if each specific prodromal symptom (considering only prodromal symptoms that significantly differed in prevalence between FEP or FEM patients) appeared more gradually or rapidly during the proximal prodrome. In order to do so, we computed the predicted probabilities of a gradual versus rapid onset pattern of each prodromal symptom with a logistic regression model, defining prodromal symptoms as the predictive variables. We evaluated the distribution of prodromal symptoms in the whole sample to assess which of them appeared more gradually or rapidly.
Statistical analysis was performed using R for Windows (version 4.0.3, R Project for Statistical Computing, Vienna, Austria). Statistical significance was set at p < 0.05.
3. RESULTS
The total sample included 108 participants, 72 (66.7%) patients with a FEM and 36 (33.3%) patients with a FEP (flow chart in Figure S1). No differences were identified in socio‐demographic variables (Table 1 and Table S2).
TABLE 1.
Socio‐demographic data, clinical characteristics, risk factors and markers of vulnerability
| Groups | Statistics | ||
|---|---|---|---|
| FEM (n = 72) | FEP (n = 36) | t or χ 2, p‐value | |
| Socio‐demographic Variables | |||
| Age (years, mean ± SD) | 26.58 ± 7.03 | 27.78 ± 6.90 | −0.838, 0.404 |
| Female sex (n, %) | 37, 51.4 | 18, 50.0 | 0.000, 1.000 |
| Education Level (n, %) | 4.625, 0.100 | ||
| Primary School | 1, 1.4 | 4, 11.1 | |
| Secondary School | 44, 61.1 | 20, 55.6 | |
| University | 27, 37.5 | 12, 33.3 | |
| Marital status, Married, yes (n, %) | 13, 18.1 | 5, 13.9 | 0.075, 0.784 |
| Employment (n, %) | 0.000,1.000 | ||
| Working | 22, 30.6 | 11, 30.6 | |
| Not Working | 50, 69.4 | 25, 69.4 | |
| Socio‐economic status (n, %) | 4.857, 0.301 | ||
| I | 7, 9.7 | 5, 14.3 | |
| II | 13, 18.1 | 2, 5.7 | |
| III | 10, 13.9 | 7, 20.0 | |
| IV | 16, 22.2 | 11, 31.4 | |
| V | 26, 36.1 | 10, 28.6 | |
| Clinical Variables | |||
| Age at first episode, any type (years, mean ± SD) | 22.90 ± 7.79 | 22.17 ± 7.96 | 0.459, 0.647 |
| Age at FEM or FEP (years, mean ± SD) | 25.78 ± 6.91 | 27.14 ± 7.06 | 0.958, 0.340 |
| Age at first hospitalization (years, mean ± SD) | 25.49 ± 6.97 | 26.48 ± 7.41 | −0.627, 0.532 |
| Duration of Untreated Psychosis (days, mean ± SD) | 29.58 ± 56.01 | 108.67 ± 204.62 | −2.172, 0.037 |
| MADRS Total Score (mean±SD) | 5.47 ± 4.80 | 9.15 ± 5.69 | −3.419, 0.001 |
| PANSS Positive Symptoms Score (mean ± SD) | 7.58 ± 1.67 | 9.52 ± 3.50 | −3.023, 0.004 |
| PANSS Negative Symptoms Score (mean ± SD) | 10.20 ± 4.87 | 15.39 ± 6.87 | −3.916, <0.001 |
| PANSS General Psychopatology Score (mean ± SD) | 22.44 ± 6.34 | 28.27 ± 7.09 | −4.209, <0.001 |
| PANSS Total Score (mean ± SD) | 40.21 ± 11.49 | 53.18 ± 15.09 | −4.836, <0.001 |
| YMRS Total Score (mean ± SD) | 1.64 ± 2.70 | 1.33 ± 1.81 | 0.592, 0.555 |
| FAST Total Score (mean ± SD) | 17.20 ± 13.18 | 25.31 ± 12.69 | −3.019, 0.003 |
| Psychopharmacological Treatment, Current (n, %) | 68, 94.40 | 35, 97.20 | 0.419, 0.663 |
| Length of time between the diagnosis and the study assessment (days, mean ± SD) | 296.76 ± 340.19 | 307.64 ± 333.97 | −0.158, 0.875 |
| Risk factors | |||
| Family History of Psychosis, yes (n, %) | 11, 15.5 | 8, 22.9 | 0.436, 0.509 |
| Family History of Bipolar Disorder, yes (n, %) | 17, 23.9 | 6, 17.1 | 0.301, 0.583 |
| Family History of Major Depressive Episode, yes (n, %) | 28, 39.4 | 15, 42.9 | 0.016, 0.899 |
| Obstetric complications, yes (n, %) | 16, 23.6 | 16, 44.4 | 3.976, 0.046 |
| Abnormal Psychomotor Development, yes (n, %) | |||
| Acquisition of walking ability | 4, 10.3 | 2, 7.4 | 0.157,1.000 |
| Acquisition of language | 3, 7.9 | 4, 18.5 | 1.651, 0.260 |
| Nocturnal enuresis | 5, 13.5 | 7, 25.9 | 0.869, 0.331 |
| Acquisition of reading/writing ability | 1, 2.7 | 6, 25.9 | 7.697, 0.008 |
| Stressors during prodromal phase, yes (n, %) | 49, 70.0 | 26, 72.2 | 0.000, 0.990 |
| Childhood Trauma, yes (n, %) | 16, 43.2 | 8, 61.5 | 0.661, 0.416 |
| Markers of vulnerability | |||
| Previous contact with MHS, yes, (n, %) | 42, 60.0 | 22, 64.7 | 0.061, 0.804 |
| Age at first contact with MHS (mean ± SD) | 20.4 ± 8.97 | 20.04 ± 7.83 | 0.178, 0.859 |
| Psychopharmacological Treatment in childhood/adolescence, (n, %) | 30, 42.30 | 24, 66.70 | 4.761, 0.029 |
| Psychiatric Diagnoses in childhood/adolescence (n, %) | 30, 41.70 | 22, 61.1 | 2.897, 0.089 |
| PAS childhood score (mean ± SD) | 4.56 ± 3.39 | 6.74 ± 4.38 | −2.840, 0.005 |
| PAS adolescence score (mean ± SD) | 6.78 ± 4.31 | 9.94 ± 5.61 | −3.222, 0.002 |
| PAS total score (mean ± SD) | 11.33 ± 6.93 | 16.69 ± 9.01 | −3.388, 0.001 |
| Cannabis use during prodromal phase, yes (n, %) | 41, 59.4 | 17. 48.6 | 0.712, 0.399 |
| Alcohol use during prodromal phase, yes (n, %) | 42, 60.9 | 20, 55.6 | 0.100, 0.752 |
| Suicidal Ideation during prodromal phase, yes (n, %) | 6, 8.3 | 5, 13.9 | 0.810, 0.574 |
| Treatment during prodromal phase, yes (n, %) | 25, 35.2 | 10, 27.8 | 0.310, 0.578 |
| Antidepressants during prodromal phase, yes (n, %) | 16, 22.5 | 3, 8.3 | 2.398. 0.121 |
Abbreviations: FAST, Functional Assessment Staging; FEM, First Episode Mania; FEP, First Episode Psychosis; MADRS, Montgomery‐Asberg Depression Rating Scale; MHS, Mental Health Services; PANSS; PAS, Premorbid Adjustment Scale; Positive and Negative Syndrome Scale; SD, Standard Deviation; WHO, World Health Organization; YMRS, Young Mania Rating Scale. Bold for statistically significant p values.
3.1. Clinical variables among the subgroups
No differences for mean age at onset and age at first hospitalization were found (Table 1). The DUP of FEM was significantly shorter than for FEP patients (Mean Difference (MD) = 79.086, CI = 5.16–153.01).
At the time of evaluation, FEP patients showed higher scores in the PANSS subdomains (positive symptoms: MD = 1.94, CI = 0.64–3.23; negative symptoms: MD = 5.20, CI = 2.53–7.87; general psychopathology: MD = 5.84, CI = 2.94–8.73), PANSS total score (MD = 12.97, CI = 7.03–18.92) and depressive (MADRS total score: MD = 3.68, CI = 1.39–5.97) but not manic symptoms.
The two groups differed in the total FAST score, with worse psychosocial functioning for FEP patients (MD = 12.97, CI = 7.03–18.92).
3.2. Prevalence of prodromal symptoms
The differences in prevalence of prodromal symptoms are displayed in Table 2 and Figure S2. The mean number of total prodromal symptoms was not different between groups, but FEM patients presented more Mania prodromal symptoms than FEP (MD = 2.18, CI = 1.21–3.15) whilst FEP patients reported more psychosis prodromal symptoms than FEM (MD = 0.43, CI = 0.03–0.83). No difference was observed in the mean number of total prodromal symptoms between psychotic and non‐psychotic FEM (6.57 ± 4.59 vs 8 ± 4.76, t = 0.781, p = 0.437).
TABLE 2.
Reported prevalence and duration of prodromal symptoms prior to First Episode Mania (FEM) or Prior to First Episode Psychosis (FEP)
| Prodromal symptom characteristics | Prevalence (Yes/No) | Duration (Months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FEM (n = 72, 66.7%) | FEP (n = 36, 33.3%) | X 2 or F, p value | FEM (n = 72, 66.7%) | FEP (n = 36, 33.3%) | t, p value | |||||
| N | % | N | % | Mean | SD | Mean | SD | |||
| Mania Index Symptoms | ||||||||||
| Increased energy or goal‐directed activity | 38 | 52.8 | 3 | 8.3 | 18.827, <0.001 | 2.42 | 2.11 | 3.33 | 0.58 | −0.737, 0.465 |
| Overly cheerful, happy, on top of the world | 27 | 37.5 | 4 | 11.1 | 6.928, 0.008 | 2.13 | 1.46 | 3.50 | 1.91 | −1.685, 0.103 |
| Racing thoughts | 24 | 33.3 | 5 | 13.9 | 3.683, 0.055 | 2.39 | 2.38 | 3.20 | 1.92 | −0.705, 0.487 |
| Overly talkative | 27 | 37.5 | 1 | 2.8 | 13.313, <0.001 | 2.09 | 1.26 | 2.00 | ‐ | 0.072, 0.943 |
| Decreased need for sleep | 25 | 34.7 | 2 | 5.6 | 9.389, 0.002 | 2.88 | 6.95 | 1.50 | 0.71 | 0.276, 0.785 |
| Irritability or anger | 29 | 40.3 | 11 | 30.6 | 0.601, 0.438 | 2.93 | 2.67 | 11.36 | 10.54 | −2.622, 0.025 |
| Overly self‐confidence | 22 | 30.6 | 2 | 5.6 | 7.292, 0.007 | 2.20 | 1.31 | 2.50 | 0.70 | −0.309, 0.760 |
| Increased sexual interest | 12 | 16.7 | 2 | 5.6 | 2.626, 0.135 | 2.20 | 1.23 | 4.00 | 1.41 | −1.878, 0.085 |
| Reckless or dangerous behaviours | 4 | 5.6 | 2 | 5.6 | 0.000, 1.000 | 1.25 | 0.50 | 2.00 | 0.00 | −2.000, 0.116 |
| Risky sexual behaviour | 4 | 5.6 | 1 | 2.8 | 0.419, 0.663 | 1.50 | 1.00 | 2.00 | ‐ | −0.447, 0.685 |
| Mania or Depression Index Symptoms | ||||||||||
| Lack of concentration | 21 | 29.2 | 10 | 27.8 | 0.000, 1.000 | 2.81 | 2.05 | 9.70 | 8.18 | −2.625, 0.026 |
| Physical agitation | 26 | 36.1 | 4 | 11.1 | 6.283, 0.012 | 1.96 | 1.39 | 6.50 | 7.68 | −1.179, 0.323 |
| Depression Index Symptoms | ||||||||||
| Depressed mood | 1 | 1.4 | 5 | 13.9 | 7.147, 0.015 | 4.00 | ‐ | 12.80 | 8.41 | −0.955, 0.393 |
| Tiredness or lack of energy | 2 | 2.8 | 7 | 19.4 | 8.573, 0.006 | 9.00 | 7.07 | 13.57 | 12.49 | −0.480, 0.646 |
| Anhedonia | 6 | 8.3 | 6 | 16.7 | 1.688, 0.209 | 6.00 | 3.74 | 10.17 | 8.95 | −1.052, 0.329 |
| Insomnia | 28 | 38.9 | 14 | 38.9 | 0.000, 1.000 | 3.28 | 6.57 | 9.54 | 7.73 | −2.680, 0.011 |
| Feeling of worthlessness or guilt | 2 | 2.8 | 2 | 5.6 | 0.519, 0.599 | 3.50 | 0.71 | 9.50 | 12.02 | −0.705, 0.554 |
| Weight loss or decrease in appetite | 9 | 12.5 | 6 | 16.7 | 0.087, 0.768 | 4.05 | 3.50 | 9.17 | 7.91 | −1.725, 0.108 |
| Physically slowed down | 3 | 4.2 | 6 | 16.7 | 4.909, 0.057 | 6.33 | 6.80 | 19.00 | 11.30 | −1.753. 0.123 |
| Thinking about suicide | 2 | 2.8 | 2 | 5.6 | 0.519, 0.599 | 13.50 | 14.85 | 9.50 | 12.02 | 0.296, 0.795 |
| Hypersomnia | 0 | ‐ | 2 | 5.6 | 4.075, 0.109 | ‐ | ‐ | 10.50 | 9.19 | ‐ |
| Attempting suicide | 0 | ‐ | 1 | 2.8 | 2.019, 0.333 | ‐ | ‐ | 14.00 | ‐ | ‐ |
| Weight gain or increasing in appetite | 3 | 4.2 | 2 | 5.6 | 0.105, 1.000 | 3.67 | 2.31 | 9.50 | 2.12 | −2.842, 0.066 |
| General Index Symptoms | ||||||||||
| Educational and occupational dysfunction | 17 | 23.6 | 13 | 36.1 | 1.298, 0.255 | 5.22 | 6.18 | 11.54 | 11.16 | −1.827, 0.085 |
| Anxiety or nervousness | 28 | 38.9 | 18 | 50.0 | 0.800, 0.371 | 3.96 | 3.91 | 7.71 | 8.19 | −1.758, 0.093 |
| Social Isolation | 7 | 9.7 | 12 | 33.3 | 7.672, 0.006 | 2.93 | 1.88 | 11.33 | 10.07 | −2.809, 0.015 |
| Mood lability | 33 | 45.8 | 7 | 19.4 | 6.080, 0.014 | 3.26 | 6.10 | 8.14 | 9.06 | −1.362, 0.214 |
| Ambivalence/difficulty making decisions | 3 | 4.2 | 8 | 22.2 | 8.553, 0.006 | 3.17 | 1.44 | 5.50 | 4.28 | −0.899, 0.392 |
| Obsessions and compulsions | 10 | 13.9 | 6 | 16.7 | 0.009, 0.924 | 3.35 | 1.83 | 5.17 | 2.86 | −1.564, 0.140 |
| Losing temper frequently or trouble controlling anger | 11 | 78.6 | 3 | 8.3 | 1.077, 0.375 | 1.64 | 1.21 | 9.33 | 12.74 | −1.045, 0.405 |
| Day/Night Sleep Reversal | 2 | 2.8 | 0 | ‐ | 1.019, 0.551 | 14.00 | 16.97 | ‐ | ‐ | ‐ |
| Self‐harming behaviour (no intent to kill self) | 0 | ‐ | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Oppositionality | 2 | 2.8 | 1 | 2.8 | 0.000, 1.000 | 2.50 | 0.71 | 2.00 | ‐ | 0.577, 0.667 |
| Increased creativity | 23 | 31.9 | 1 | 2.8 | 10.185, 0.001 | 2.65 | 2.66 | 3.00 | ‐ | −0.128, 0.899 |
| Giddy, clownish | 2 | 2.8 | 1 | 2.8 | 0.000, 1.000 | 1.50 | 0.71 | 3.00 | ‐ | −1.732, 0.333 |
| Psychosis, Mania and Depression Index Symptoms | ||||||||||
| Difficulty thinking or communicating clearly | 4 | 5.6 | 6 | 16.7 | 3.527, 0.081 | 2.37 | 1.38 | 15.83 | 13.06 | −2.503, 0.053 |
| Psychosis Index Symptoms | ||||||||||
| Suspiciousness/persecutory ideas | 11 | 15.3 | 13 | 36.1 | 4.882, 0.027 | 2.91 | 3.33 | 5.15 | 3.21 | −1.678, 0.108 |
| Strange or unusual ideas | 17 | 23.6 | 12 | 33.3 | 0.713, 0.398 | 2.82 | 3.02 | 4.12 | 2.31 | 0.026, 0.222 |
| Hallucinations | 1 | 1.4 | 1 | 2.8 | 0.255, 1.000 | 2.00 | ‐ | 3.00 | ‐ | ‐ |
| FEM (n = 92) | FEP (n = 28) | t, p value | FEM (n = 92) | FEP (n = 28) | t, p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Mania Index | 3.65 | 2.74 | 1.47 | 2.18 | 4.482, <0.001 | 2.84 | 3.84 | 11.48 | 10.94 | 3.377, 0.003 |
| Depression Index | 1.46 | 1.52 | 2.03 | 1.92 | −1.533, 0.131 | 3.63 | 5.58 | 10.52 | 8.93 | 3.489, 0.001 |
| General Psychopathology Index | 1.90 | 1.56 | 1.94 | 1.76 | −0.129, 0.897 | 3.56 | 3.61 | 9.18 | 7.78 | 3.379, 0.002 |
| Psychosis Index | 0.46 | 0.75 | 0.89 | 1.06 | −2.175, 0.034 | 2.63 | 2.70 | 7.97 | 8.51 | 2.500, 0.022 |
| BPSS‐R Total Score | 6.70 | 4.62 | 5.61 | 4.73 | 1.146, 0.254 | 3.15 | 3.22 | 8.8 | 7.42 | 3.884, <0.001 |
Abbreviations: BPSS‐R, Bipolar Prodrome Symptom Scale‐Retrospective. Bold for statistically significant p values; FEM, First Episode Mania; FEP, First Episode Psychosis; SD, Standard Deviation.
Five out of 72 FEM patients (6.9%) and eight out of 36 FEP patients (22.2%) reported no prodromal symptoms (OR = 0.26, CI = 0.08–0.87, p = 0.030).
Univariate and multivariate logistic regression findings are reported in Table 3. In the multivariate logistic regression (Chi‐square = 30.532, df = 2, p < 0.001), the prodromal General Psychopathology symptom Social isolation was associated with the prodromal stage of a FEP (OR = 4.95, CI = 1.47–16.66, p = 0.010), whilst the mania symptom Increased energy or goal‐directed activity with the prodromal stage of a FEM (OR = 0.08, CI = 0.02–0.29, p < 0.001). The ROC analysis supported the utility of the model and its variables because it performed significantly better than chance in predicting the association between prodromal symptoms and presenting a FEP versus a FEM, with an area under the curve (AUC) = 0.78 (CI = 0.70–0.86) (SF 3).
TABLE 3.
Associations of prodromal symptoms to a First Episode Mania (FEM) or Prior to First Episode Psychosis (FEP)
| Type of symptoms | Univariate analyses | Multivariate forward stepwise analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | Beta coefficient | |
| Mania Index Symptoms | |||||
| Racing thoughts | 0.32 (0.11–0.94) | 0.037 | |||
| Overly cheerful or happy | 0.21 (0.07–0.65) | 0.007 | |||
| Increased energy or goal‐directed activity | 0.08 (0.02–0.29) | <0.001 | 0.08 (0.02–0.29) | <0.001 | −2.544 |
| Mania or Depression Index Symptoms | |||||
| Physically agitated | 0.22 (0.07–0.70) | 0.010 | |||
| Depression Index Symptoms | |||||
| Physically slowed down | 4.60 (1.08–19.62) | 0.039 | |||
| General Index Symptoms | |||||
| Social isolation | 4.64 (1.63–13.18) | 0.004 | 4.95 (1.47–16.66) | 0.010 | 1.599 |
| Ambivalence/difficulty making decisions | 6.57 (1.62– 25.59) | 0.008 | |||
| Mood lability | 0.29 (0.11–0.74) | 0.009 | |||
| Psychosis Index Symptoms | |||||
| Suspiciousness | 3.13 (1.23–7.99) | 0.017 | |||
| Constant | −0.336 | ||||
Abbreviations: FEM, First Episode Mania; FEP, First Episode Psychosis; IC, Intervals of confidence; OR, Odds ratio. Bold for statistically significant p values.
3.3. Duration of prodromal symptoms
More FEP patients reported a pattern of presentation with gradual onset and slow deterioration than FEM patients (52.8% versus 25%, OR = 3.35, CI = 1.44–7.78).
The duration of prodromal symptoms prior to a FEP or a FEM is shown in Table 2. The mean duration of the BPSS‐R prodromal stage was longer for FEP patients than for FEM patients (MD = 5.66, CI = 2.69–8.63). Moreover, the mean duration of the Manic, Depression, General Psychopathology and Psychotic prodromes was statistically longer in FEP than FEM patients. The mean duration of the prodromal stage did not significantly differ between psychotic FEM (3.35 ± 3.31 months) and non‐psychotic FEM (1.13 ± 0.24 months) (t = −1.629, p = 0.108).
As for the predicted probabilities of a gradual versus rapid onset pattern (Figure 1), the Depression symptom Physically slowed down was the symptom that most probably presented a gradual onset pattern, followed by two General Psychopathology Symptoms, Social isolation and Ambivalence/difficulty making decisions. The Manic Symptom Increased energy or goal−directed activity was the symptom that most probably presented a rapid onset pattern, followed by the mania or depression symptom Physical agitation.
FIGURE 1.
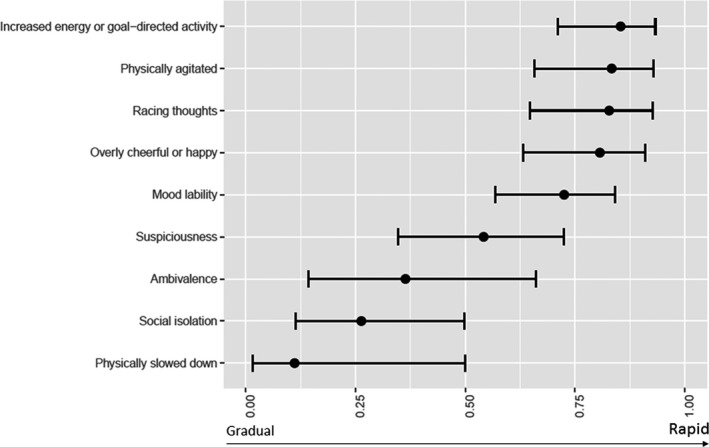
Probabilities of a gradual versus rapid onset pattern of prodromal symptoms
3.4. Risk factors
No difference was reported in family history of psychiatric disorders (Table 1).
FEP patients reported higher rates of OC (OR = 2.59, CI = 1.10–6.08) and difficulties in writing and reading (OR = 12.6, CI = 1.45–109.85).
Both FEM and FEP patients frequently experienced stressors in the prodromal phase. Similarly, they reported high rates of lifetime traumatic experiences and childhood abuse, with no difference between groups.
3.5. Markers of vulnerability
A similarly high percentage of FEM and FEP patients reported previous contact with mental health services (60% versus 64.7%) with a similar mean age at first contact (around 20 years old) (Table 1). Patients in the FEP group more frequently received psychopharmacological treatment in the past than FEM patients (OR = 2.73, CI = 1.18–6.32).
As for premorbid adjustment, FEP patients reported worse performance than FEM (PAS childhood: 6.74 ± 4.38 versus 4.56 ± 3.39, MD = 2.187, CI = 0.66–3.71; PAS adolescence: 9.94 ± 5.61 versus 6.78 ± 4.31, MD = 3.17, CI = 1.01–5.32; PAS total score: MD = 5.35, CI = 2.22–8.4). The PAS scale revealed a progressive decline in both FEP (Mean = 3.2, CI = 1.66–4.74, t = −4.234, p < 0.001) and FEM (Mean = 2.22, CI = 1.41–3.04, t = −5.442, p < 0.001) patients.
No differences were observed in cannabis and alcohol use in the prodromal phase. Notably, both FEM and FEP patients reported high rates of cannabis (59.4% versus 48.6%) and alcohol (60.9% versus 55.6%) consumption.
4. DISCUSSION
The following key findings emerged from the present study. First, Social isolation was associated with the prodromal stage of a FEP whilst Increased energy or goal‐directed activity was associated with the prodrome to a FEM. Second, the duration of the FEP prodrome was longer than the FEM prodrome, although in FEM patients, the prodrome was long enough to enable an early intervention. Third, the risk factors that clearly differentiate FEM from FEP patients were the higher prevalence of OC and difficulties in writing and reading during childhood in FEP patients. Finally, FEM and FEP patients shared a number of vulnerability factors, but a clear impairment in premorbid adjustment was more characteristic of FEP patients.
In terms of psychopathology, Mania prodromal symptoms, namely Increased energy or goal‐directed activity, Physical agitation, Racing thoughts, Overly cheerful, happy, and on top of the world, were more frequently reported in FEM patients whilst Psychosis prodromal symptoms, such as Suspiciousness/persecutory ideas, or the depression symptoms Physically slowed down, were more frequently included in the FEP prodrome. The manic prodrome was characterized by at least three subthreshold manic symptoms in more than half of the youth assessed by Correll and colleagues. 6 In a systematic review, subthreshold manic symptoms such as Overly talkative, Racing thoughts and Physical agitation were among the most frequently reported symptoms in the initial prodrome to mania in BD type I. 41 On the contrary, Reduced drive and motivation, Anergia, Depressed mood and subsyndromal Delusional ideas or Suspiciousness were among the prodromal features most commonly described in FEP studies. 3 , 42 In the comparison between the psychotic and the manic prodromes, attenuated psychotic symptoms 12 and unusual subsyndromal ideas 11 were the symptoms more frequently associated with the prodromal phase to a FEP, whilst subsyndromal manic symptoms were more frequently associated with the manic prodrome. 11 , 12
Even though prodromal symptoms were differentiated in the two groups depending on their nature, it was not rare to find that a small percentage of FEP patients reported subsyndromal manic symptoms. This could due to the heterogeneity that characterizes FEMs and FEPs at their onset and to the dimensional nature of their clinical presentation that sometimes does not match perfectly with dichotomous diagnostic entities. Furthermore, the most frequent symptoms reported by FEP patients that felt within the Mania Index were generally symptoms that were shared by other Indexes, such as lack of concentration (Mania or Depression Index) or difficulty thinking or communicating clearly (Psychosis, Mania and Depressive Index Symptoms). Nonetheless, only a minority of FEP patients reported symptoms included in the Mania Index.
When we assessed the prodromal symptoms in a predictive model, Social isolation was the symptom most associated with the prodromal stage of a FEP, whilst Increased energy or goal‐directed activity was more associated with the FEM prodrome. Social isolation is one of the most prevalent prodromal symptoms of a FEP found in literature. 8 , 43 , 44 It was considered an essential component of the initial prodrome in SCZ and preceded the onset of psychosis by 2–4 years. 45 However, Jackson and colleagues argued that it could be a sensitive but poorly specific prodrome for SCZ. 8 Nevertheless, Social Isolation was considered, together with cognitive deficits, affective disturbances and school failure, one of the four domains of the vulnerability core for SCZ, regardless of the severity of emerging positive symptoms. 43 Moreover, Social isolation in adolescence was reported in one out of three patients that later developed a FEP and was found to differentiate patients hospitalized for psychosis from those hospitalized for other reasons. 44 Furthermore, it presented a predictive role in the following social development, since the level of social abilities reached during the prodromal phase set the upper limit on the social development reached along the course of illness. 43 , 45 Consequently, Social isolation might be addressed in early intervention. 43
As for Increased energy, it was one of the most frequently described prodromal symptoms in FEM. 7 , 11 Specifically, half of the patients reported having experienced Increased energy in a study evaluating the manic prodrome, 6 suggesting that this symptom, and other subthreshold manic symptoms, might serve as specific markers of emerging bipolarity. 6 Furthermore, it was significantly more common in patients with later psychotic mania than in patients not presenting psychotic symptoms during their FEM, thus representing an early predictor of severity. 6 Finally, Increased energy, and other prodromal subthreshold manic symptoms, showed a more rapid onset than other prodromal symptoms although their duration was long enough to allow preventive strategies, such as clinical high‐risk programs for individuals with a first‐degree relative suffering from BD. 11
OC and difficulties in writing and reading during childhood were the only risk factors that differentiated FEP from FEM patients. OC have been identified as a risk factor for psychosis, representing a marker of increased severity of the clinical presentation 16 , 46 and further outcomes. 47 Similarly, low performance in spelling, reading skills and comprehension in childhood was associated with psychotic experiences in early adolescence. 16 , 48 As a result, our findings support the neurodevelopmental hypothesis of SCZ, in which pre/perinatal brain damage underlies the later emergence of psychosis. 49 , 50 Similarly, a clear marker of vulnerability was the impairment in premorbid adjustment. FEM patients showed better premorbid adjustment than patients developing a FEP in previous studies, 51 , 52 with important implications in longitudinal outcomes. 53 , 54 Consistently, while FEM patients presented an average minimal functional impairment, FEP patients reported worse psychosocial functioning as well as higher clinical severity at the moment of evaluation. 55
Of note, no differences emerged in terms of both lifetime traumatic experiences and childhood abuse as well as cannabis and alcohol consumption since high rates were reported in both groups. Trauma and drug abuse have been previously reported as intertwined risk factors and early markers of the vulnerability of affective and non‐affective psychoses. 56 , 57 In consideration of the high rates reported in the present study, they represent universal or transdiagnostic targets of primary preventive strategies. In addition, specific prodromal symptoms such as Social isolation or Increased energy or goal‐directed activity should be firstly recognized by psychiatrists but also by general practitioners, families and teachers as warning signs that signal a need to provide primary preventive strategies. In terms of secondary prevention health, the findings of the present study suggest clinical potential to shape early interventions addressing specific differential characteristics of FEP and FEM patients. Since FEP patients presented worse premorbid adjustment and current psychosocial functioning, functional remediation could be a recommended treatment approach. Conversely, FEM patients, who reported a slighter clinical and functional impairment than FEP, might benefit from potentiation strategies. Both groups of patients should receive at least psychoeducation. Finally, family intervention aimed at providing psychoeducational advice and information on prodromic symptoms, risk factors and markers of vulnerabilities in families with a higher genetic load should be guaranteed.
The present study has limitations. First, the group of FEP patients was smaller, which might have influenced the assessment of differences between groups. As a result, the present study should be considered as exploratory and needs replication in further studies with bigger sample sizes. Second, to assess prodromal symptoms, we used the BPSS‐R scale, which was developed to assess prodromal symptoms in BD and major depressive disorder. 11 Nonetheless, the development of the BPSS‐R was guided by a review of existing literature on BD, but also on the basis of interviews for the assessment of the psychotic prodrome and inputs from experts in the areas of the SCZ prodrome. Moreover, Correll and colleagues 11 stated that a wide variation in symptomatology was present in their study due to the different methodologies used to ascertain pre‐illness symptoms. Consequently, we used the BPSS‐R to assess both FEM and FEP patients to limit this bias. In addition, the retrospective assessment of prodromes with possible recall bias should be acknowledged. Even though previous studies assessed individuals at risk for developing a FEP or a FEM to evaluate their prodromes prospectively, only individuals with higher genetic risk, that is those with family history for BD and SCZ were included, thus not assessing prodromal symptoms of those patients without a genetic liability. 13 , 42 Finally, the presence of a control group might have allowed an evaluation of the specificity of the prodromal symptoms and other risk factors. However, in comparing FEM and FEP patients, we identified which prodromal symptoms were more specific for each group. Despite these limitations, the present study contains a quite large sample size. Moreover, it is the first study of its kind to assess the prodromes of late adolescence and adult‐onset FEP or FEM patients. A core strength of the project is the use of a comprehensive and exhaustive assessment that goes beyond the standard clinical practice, allowing a complete phenotypic characterization of the patients recruited in the study. As the ProPreF project is longitudinal by nature, assessing the predictive role of prodromes on the mid‐term clinical outcomes is possible once the programmed follow‐up study is complete.
In conclusion, both the psychotic and manic prodrome may contribute in the identification of patients with a higher risk of developing their first episode of disease. The specific prodromal symptoms together with risk factors and markers of vulnerability identified in the present study might help develop targeted early treatment strategies on the basis of the individual characteristics of the patients and of the prodromes they experienced.
CONFLICT OF INTEREST
NV has received financial support for CME activities and travel funds from the following entities (unrelated to the present work): Angelini, Janssen, Lundbeck, Otsuka. MG has received grants and served as consultant or advisor for Ferrer, Lundbeck, and Janssen‐Cilag. MSV has received financial support for CME activities and travel funds from Janssen‐Cilag and Lundbeck, and reports no financial or other relationship relevant to the subject of this article. JARQ was on the speakers’ bureau and/or acted as consultant for Eli‐Lilly, Janssen‐Cilag, Novartis, Shire, Takeda, Bial, Shionogui, Lundbeck, Almirall, Braingaze, Sincrolab, Medice and Rubió, Raffo in the last 5 years. He also received travel awards (air tickets + hotel) for taking part in psychiatric meetings from Janssen‐Cilag, Rubió, Shire, Takeda, Shionogui, Bial, Medice and Eli‐Lilly. The Department of Psychiatry chaired by him received unrestricted educational and research support from the following companies in the last 5 years: Eli‐Lilly, Lundbeck, Janssen‐ Cilag, Actelion, Shire, Ferrer, Oryzon, Roche, Psious and Rubió. AMA has received funding for research projects and/or honoraria as a consultant or speaker for the following companies and institutions (work unrelated to the topic of this manuscript): Otsuka, Pfizer, AstraZeneca, Bristol‐Myers Squibb, Lundbeck, the Spanish Ministry of Economy and Competitiveness and Instituto de Salud Carlos III. MB has been a consultant for, received grant/ research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Angelini, Casen Recordati, Janssen‐Cilag, Menarini, Rovi and Takeda. EV has received grants and served as consultant, advisor or CME speaker for the following entities (unrelated to the present work): AB‐Biotics, Abbott, Allergan, Angelini, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, Janssen, Lundbeck, Otsuka, Sage, Sanofi‐Aventis and Takeda. IP has received CME‐related honoraria or consulting fees from ADAMED, Janssen‐Cilag and Lundbeck. The rest of authors report no biomedical financial interests or potential conflicts of interest related to the present article.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/acps.13415.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by The Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2017 SGR 1365; 2017‐SGR‐1271); the Centro de Investigación Biomédica en Red de Salud Mental‐CIBERSAM; the Centres de Recerca de Catalunya‐CERCA Programme; the Spanish Ministry of Science, Innovation and Universities (CPII16/00018 to EPC, CM17/00102 to MG, CM19/00123 to ES, CD20/00177 to SA, PI12/0091 to EV and JU, PI15/00283 to EV, PI18/00805 to EV, PI18/01001 to IP); the ‘Pla estrategic de Recerca i Innovacio en Salut 2016–2020’ (SLT006/17/00357 to EV, SLT006/17/00345 to MB); the Biomedicine international training research programme for excellent clinician‐scientists‐BITRECS project (Marie‐Curie grant No 754550 and ‘La Caixa’ Foundation LCF/PR/GN18/50310006 to NV); the ANID‐PIA‐ACT192064, ANID‐FONDECYT 1180358, 120060 to JU and the Agency for Management of University and Research Grants‐AGAUR (Beatriu de Pinós grant to MR). We thank Dr. Derek Clougher for the English revision.
Verdolini N, Borràs R, Sparacino G, et al. Prodromal phase: Differences in prodromal symptoms, risk factors and markers of vulnerability in first episode mania versus first episode psychosis with onset in late adolescence or adulthood. Acta Psychiatr Scand. 2022;146:36–50. doi: 10.1111/acps.13415
Isabella Pacchiarotti and Silvia Amoretti equally contributed to this work.
Funding information
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding authors.
REFERENCES
- 1. Arango C, Díaz‐Caneja CM, McGorry PD, et al. Preventive strategies for mental health. Lancet Psychiatry. 2018;5:591‐604. [DOI] [PubMed] [Google Scholar]
- 2. Vieta E, Salagre E, Grande I, et al. Early intervention in bipolar disorder. Am J Psychiatry. 2018;175:411‐426. [DOI] [PubMed] [Google Scholar]
- 3. Barajas A, Pelaez T, González O, et al. Predictive capacity of prodromal symptoms in first‐episode psychosis of recent onset. Early Interv Psychiatry. 2019;13(3):414‐424. 10.1111/eip.12498 [DOI] [PubMed] [Google Scholar]
- 4. Fusar‐Poli P, Cappucciati M, Rutigliano G, et al. Diagnostic stability of ICD/DSM first episode psychosis diagnoses: meta‐analysis. Schizophr Bull. 2016;42:1395‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joa I, Gisselgård J, Brønnick K, McGlashan T, Johannessen JO. Primary prevention of psychosis through interventions in the symptomatic prodromal phase, a pragmatic Norwegian ultra high risk study. BMC Psychiatry. 2015;15(1):1‐9. doi: 10.1186/s12888-015-0470-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Correll CU, Hauser M, Penzner JB, et al. Type and duration of subsyndromal symptoms in youth with bipolar I disorder prior to their first manic episode. Bipolar Disord. 2014;16:478‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conus P, Ward J, Lucas N, et al. Characterisation of the prodrome to a first episode of psychotic mania: Results of a retrospective study. J Affect Disord. 2010;124:341‐345. [DOI] [PubMed] [Google Scholar]
- 8. Jackson HJ, McGorry PD, Dudgeon P. Prodromal symptoms of schizophrenia in first‐episode psychosis: Prevalence and specificity. Compr Psychiatry. 1995;36:241‐250. [DOI] [PubMed] [Google Scholar]
- 9. Addington J, Liu LU, Buchy L, et al. North American Prodrome Longitudinal Study (NAPLS 2): the prodromal symptoms. J Nerv Ment Dis. 2015;203:328‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang TH, Xu LH, Tang YY, et al. Prediction of psychosis in prodrome: development and validation of a simple, personalized risk calculator. Psychol Med. 2019;49:1990‐1998. [DOI] [PubMed] [Google Scholar]
- 11. Correll CU, Penzner JB, Frederickson AM, et al. Differentiation in the preonset phases of schizophrenia and mood disorders: evidence in support of a bipolar mania prodrome. Schizophr Bull. 2007;33:703‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kafali HY, Bildik T, Bora E, Yuncu Z, Erermis HS. Distinguishing prodromal stage of bipolar disorder and early onset schizophrenia spectrum disorders during adolescence. Psychiatry Res. 2019;275:315‐325. [DOI] [PubMed] [Google Scholar]
- 13. Faedda GL, Baldessarini RJ, Marangoni C, et al. An International Society of Bipolar Disorders task force report: precursors and prodromes of bipolar disorder. Bipolar Disord. 2019;21:720‐740. [DOI] [PubMed] [Google Scholar]
- 14. Cannon TD, Mednick SA, Parnas J. Genetic and perinatal determinants of structural brain deficits in schizophrenia. Arch Gen Psychiatry. 1989;46:883‐889. [DOI] [PubMed] [Google Scholar]
- 15. Cuesta MJ, Sánchez‐Torres AM, Cabrera B, et al. Premorbid adjustment and clinical correlates of cognitive impairment in first‐episode psychosis: the PEPsCog Study. Schizophr Res. 2015;164:65‐73. [DOI] [PubMed] [Google Scholar]
- 16. Arango C, Fraguas D, Parellada M. Differential neurodevelopmental trajectories in patients with early‐onset bipolar and schizophrenia disorders. Schizophr Bull. 2014;40(Suppl 2):S138‐S146. doi: 10.1093/schbul/sbt198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bioque M, Mas S, Costanzo MC, et al. Gene‐environment interaction between an endocannabinoid system genetic polymorphism and cannabis use in first episode of psychosis. Eur Neuropsychopharmacol. 2019;29:786‐794. [DOI] [PubMed] [Google Scholar]
- 18. Salagre E, Arango C, Artigas F, et al. CIBERSAM: ten years of collaborative translational research in mental disorders. Rev Psiquiatr Salud Ment. 2019;12:1‐8. [DOI] [PubMed] [Google Scholar]
- 19. First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin EJ. Structured Clinical Interview for DSM‐IV Axis II personality disorders (SCID‐II). American Psychiatric Association; 1997. [Google Scholar]
- 20. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM‐IV Axis I Disorders, Clinician Version (SCID‐CV). American Psychiatric Press Inc; 1996. [Google Scholar]
- 21. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 22. Hollingshead AB, Redlich FC. Social class and mental illness: a community study. Am J Public Health. 2007;97:1756‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261‐276. [DOI] [PubMed] [Google Scholar]
- 24. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429‐435. [DOI] [PubMed] [Google Scholar]
- 25. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382‐389. [DOI] [PubMed] [Google Scholar]
- 26. Rosa AR, Sánchez‐Moreno J, Martínez‐Aran A, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM. Dose‐response meta‐analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry. 2020;177:342‐353. [DOI] [PubMed] [Google Scholar]
- 28. Leucht S, Samara M, Heres S, Davis JM. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42:S90‐S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayasaka YU, Purgato M, Magni LR, et al. Dose equivalents of antidepressants: evidence‐based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179‐184. [DOI] [PubMed] [Google Scholar]
- 30. https://clincalc.com/Benzodiazepine/
- 31. Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M. A self‐report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89:62‐67. [DOI] [PubMed] [Google Scholar]
- 32. Conus P, Ward J, Hallam KT, et al. The proximal prodrome to first episode mania a new target for early intervention. Bipolar Disord. 2008;10:555‐565. [DOI] [PubMed] [Google Scholar]
- 33. Lewis SW, Owen MJMR. Obstetric complications and schizophrenia: methodology and mechanisms. In: Schultz SCTC, ed. Schizophrenia. Oxford University Press; 1989:56‐68. [Google Scholar]
- 34. Foa EB, McLean CP, Zang Y, et al. Psychometric properties of the posttraumatic stress disorder symptom scale interview for DSM‐5 (PSSI‐5). Psychol Assess. 2016;28:1159‐1165. [DOI] [PubMed] [Google Scholar]
- 35. Hernandez A, Gallardo‐Pujol D, Pereda N, et al. Initial validation of the spanish childhood trauma questionnaire‐short form: factor structure, reliability and association with parenting. J Interpers Violence. 2013;28:1498‐1518. [DOI] [PubMed] [Google Scholar]
- 36. Salehi B, Chiegan PY, Rafeei M, Mostajeran M. The relationship between child anxiety related disorders and primary nocturnal enuresis. Iran J Psychiatry Behav Sci. 2016;10:1‐4. doi: 10.17795/ijpbs-4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ward MF, Wender PH, Reimherr FW. The Wender Utah rating scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:885‐890. [DOI] [PubMed] [Google Scholar]
- 38. Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impulsiveness scale. J Clin Psychol. 1995;51:768‐774. [DOI] [PubMed] [Google Scholar]
- 39. Cannon‐Spoor HE, Potkin SG, Jed WR. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull. 1982;8:470‐480. [DOI] [PubMed] [Google Scholar]
- 40. Zeschel E, Bingmann T, Bechdolf A, et al. Temperament and prodromal symptoms prior to first manic/hypomanic episodes: results from a pilot study. J Affect Disord. 2015;173:39‐44. [DOI] [PubMed] [Google Scholar]
- 41. Andrade‐González N, Álvarez‐Cadenas L, Saiz‐Ruiz J, Lahera G. Initial and relapse prodromes in adult patients with episodes of bipolar disorder: a systematic review. Eur Psychiatry. 2020;63(1). doi: 10.1192/j.eurpsy.2019.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yung AR, McGorry PO. The prodromal phase of first‐episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353‐370. [DOI] [PubMed] [Google Scholar]
- 43. Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. In: Schizophrenia Bulletin. DHHS Public Health Service. 2003;633‐651. [DOI] [PubMed]
- 44. Mäki P, Koskela S, Murray GK, et al. Difficulty in making contact with others and social withdrawal as early signs of psychosis in adolescents‐the Northern Finland Birth Cohort 1986. Eur Psychiatry. 2014;29:345‐351. [DOI] [PubMed] [Google Scholar]
- 45. Häfner H, Löffler W, Maurer K, Hambrecht M, An Der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100:105‐118. [DOI] [PubMed] [Google Scholar]
- 46. Mezquida G, Fernandez‐Egea E, Treen D, et al. Obstetric phenotypes in the heterogeneity of schizophrenia. J Nerv Ment Dis. 2018;206:882‐886. [DOI] [PubMed] [Google Scholar]
- 47. Garcia‐Rizo C, Bitanihirwe BKY. Implications of early life stress on fetal metabolic programming of schizophrenia: A focus on epiphenomena underlying morbidity and early mortality. Prog Neuro‐psychopharmacology Biol Psychiatry. 2020;101:109910. doi: 10.1016/j.pnpbp.2020.109910 [DOI] [PubMed] [Google Scholar]
- 48. Hameed MA, Lewis AJ, Sullivan S, Zammit S. Child literacy and psychotic experiences in early adolescence: findings from the ALSPAC study. Schizophr Res. 2013;145:88‐94. [DOI] [PubMed] [Google Scholar]
- 49. Davies C, Segre G, Estradé A, et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta‐analysis. Lancet Psychiatry. 2020;7:399‐410. [DOI] [PubMed] [Google Scholar]
- 50. Murray RM, Lewis SW, Lecturer L. Is schizophrenia a neurodevelopmental disorder? Br Med J. 1987;295:681‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Salagre E, Grande I, Vieta E, et al. Predictors of bipolar disorder versus schizophrenia diagnosis in a multicenter first psychotic episode cohort: baseline characterization and a 12‐month follow‐up analysis. J Clin Psychiatry. 2020;81. doi: 10.4088/JCP.19m12996 [DOI] [PubMed] [Google Scholar]
- 52. McClellan J, Breiger D, McCurry C, Hlastala SA. Premorbid functioning in early‐onset psychotic disorders. J Am Acad Child Adolesc Psychiatry. 2003;42:666‐672. [DOI] [PubMed] [Google Scholar]
- 53. Amoretti S, Verdolini N, Mezquida G, et al. Identifying clinical clusters with distinct trajectories in first‐episode psychosis through an unsupervised machine learning technique. Eur Neuropsychopharmacol. 2021;47:112‐129. doi: 10.1016/j.euroneuro.2021.01.095 [DOI] [PubMed] [Google Scholar]
- 54. Salagre E, Grande I, Solé B, et al. Exploring risk and resilient profiles for functional impairment and baseline predictors in a 2‐year follow‐up first‐episode psychosis cohort using latent class growth analysis. J Clin Med. 2020;10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Amoretti S, Mezquida G, Rosa AR, et al. The functioning assessment short test (FAST) applied to first‐episode psychosis: psychometric properties and severity thresholds. Eur Neuropsychopharmacol. 2021;47:98‐111. doi: 10.1016/j.euroneuro.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 56. Langlois S, Zern A, Kelley ME, Compton MT. Adversity in childhood/adolescence and premorbid tobacco, alcohol, and cannabis use among first‐episode psychosis patients. Early Interv Psychiatry. 2020;15(5):1335‐1342. doi: 10.1111/eip.13086 [DOI] [PubMed] [Google Scholar]
- 57. Tomassi S, Tosato S, Mondelli V, et al. Influence of childhood trauma on diagnosis and substance use in first‐episode psychosis. Br J Psychiatry. 2017;211:151‐156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding authors.


