Abstract
Sex‐specific physiology is commonly reported in animals, often indicating lower immune indices and higher oxidative stress in males than in females. Sexual selection is argued to explain these differences, but empirical evidence is limited. Here, we explore sex differences in immunity, oxidative physiology and packed cell volume of wild, adult, breeding birds (97 species, 1997 individuals, 14 230 physiological measurements). We show that higher female immune indices are most common across birds (when bias is present), but oxidative physiology shows no general sex‐bias and packed cell volume is generally male‐biased. In contrast with predictions based on sexual selection, male‐biased sexual size dimorphism is associated with male‐biased immune measures. Sexual dichromatism, mating system and parental roles had no effect on sex‐specificity in physiology. Importantly, female‐biased immunity remained after accounting for sexual selection indices. We conclude that cross‐species differences in physiological sex‐bias are largely unrelated to sexual selection and alternative explanations should be explored.
Keywords: antioxidants, dichromatism, immune system, mating system, oxidative damage, parental care, sex‐biased physiology
Sex differences in physiology are often reported in various animal species, but the established patterns are often contradictory. Here, we explore physiological sex differences across wild, adult, breeding birds (97 species) and establish that immune measures are generally female‐biased (when bias is present), and haematocrit is overwhelmingly male‐biased. No general sex bias can be detected in oxidative physiology across birds. Interestingly, measures of the intensity of sexual selection do not explain the cross‐species differences in physiological sex differences.
INTRODUCTION
Sex‐specific physiology represents a set of physiological characteristics that differ considerably between males and females of the same species. Besides the most obvious sex‐differences, such as reproductive roles driven by the sex‐specific endocrine systems, it has been demonstrated that sexes can also differ in their immune and oxidative physiologies (Costantini, 2018; Kelly et al., 2018; Klein & Flanagan, 2016). Given that these physiological systems are both tightly linked to reproduction and survival (Metcalf et al., 2020; Monaghan et al., 2009; Vágási et al., 2019; Zuk, 2009), sexual dimorphism in physiology might covary with sex differences in major life‐history traits, including fitness or demography (Metcalf et al., 2020; Nystrand & Dowling, 2020; Stoehr & Kokko, 2006; Tidière et al., 2020). Recent studies suggest that although sex‐specific physiology might occur anywhere from insects to fish, birds or mammals (Costantini, 2018; Kelly et al., 2018; Nunn et al., 2009), it is not ubiquitous across species (e.g. Kelly et al., 2018). Currently, our understanding of the generality, direction (female‐biased vs. male‐biased), as well as the evolutionary causes of sex‐specificity in physiology is rudimentary even in well‐studied vertebrate groups, such as birds or mammals.
Large‐scale phylogenetic comparative and meta‐analytic studies are needed to advance our understanding of the generality of sex‐specific physiologies. Nonetheless, such studies are scarce to date, and their results are often contradictory. For instance, the weak female‐bias in immunity disappeared after controlling for phylogenetic effects in a meta‐analysis covering 104 species (including both vertebrates and invertebrates; Kelly et al., 2018), indicating no general sex‐bias in physiology across animals. A comparative study on zoo mammals found that females tend to have generally higher white blood cell counts (Nunn et al., 2009), indicating female‐bias in immunity across mammals. In contrast, a recent meta‐analysis on wild birds showed that multiple components of the immune system are in fact more active/higher in males than in females during breeding, although no sex‐difference was detected when the season was not considered (Valdebenito et al., 2021). Contradictory conclusions of these studies could partly be explained by non‐standardised sampling, such as lack of controlling for the effect of age (Jakubas et al., 2015) or breeding status (sex‐related differences in physiology often manifest during the breeding season, but not outside of it; Pap et al., 2010), or the non‐uniform sexual dimorphism across the inspected immune parameters (Metcalf & Graham, 2018) or among taxonomic groups. For instance, it was suggested that among vertebrates, females should have stronger adaptive and non‐inflammatory immune responses, whereas males should mount stronger innate and inflammatory immune responses (Lee, 2006). The argument is that adaptive and non‐inflammatory immune responses are less costly, and have limited influence on reproduction in females. Males, on the contrary, should invest in arms of the immune system that help them cope with wounds or infections resulting from aggressive interactions (Lee, 2006). Sex differences in oxidative physiology across vertebrates were so far only inspected in a single meta‐analysis (Costantini, 2018), indicating that females in reptiles and in vertebrates with no parental care are less resistant to oxidative stress than males. Nonetheless, this meta‐analysis indicated no general sex‐bias across 82 vertebrate species or in the case of fish, birds and mammals (Costantini, 2018). On the contrary, species‐level case studies often indicate clear sex‐differences in oxidative physiology, especially among adult breeding individuals (e.g. Emaresi et al., 2016; Heiss & Schoech, 2012; Rubolini et al., 2012). Consequently, to determine the generality and consistency of physiological sex‐differences or to establish its evolutionary roots, cross‐species studies are needed that control for the confounding effects of methodology, age or seasonality (see Martin et al., 2008; Pap et al., 2010).
Several hypotheses have been formulated to explain differences in physiology between the sexes. Theoretical works have emphasised the importance of evolutionary explanations to why sex‐specific physiologies emerge, irrespective of the proximate (e.g. hormonal) mechanisms driving them (Metcalf et al., 2020; Metcalfe & Alonso‐Alvarez, 2010; Rolff, 2002; Stoehr & Kokko, 2006). One of the key mechanisms could be sexual selection, a powerful driver of phenotypic evolution (Alonso‐Alvarez et al., 2007; Hasselquist, 2007; Monaghan et al., 2009). The sexual selection hypothesis posits that differential allocation into reproduction, including mate acquisition (e.g. sexual display, intrasexual competition) or into different parental roles can alter resource availability for self‐maintenance in a sex‐specific manner (Schantz et al., 1999; Zuk, 2009). The hypothesis implies that the sex with higher variance in reproductive success (generally males in vertebrates) allocates more resources into mate attraction and less into immune function or oxidative homeostasis, thus increasing their fitness through the number of matings at the expense of a shorter life (Zuk, 2009). On the contrary, the sex which maximises its fitness via longevity (generally females, being limited by their fecundity) will allocate more resources into self‐maintenance, increasing thus their survival probabilities and their fitness through the number of breeding attempts (Zuk, 2009). Nonetheless, evidence for the link between sexual selection and sexual dimorphism in physiology is limited. For instance, sex differences in immune function across captive mammals were unrelated to sexual size dimorphism (Nunn et al., 2009). On the contrary, reduction of spleen mass (an indirect measure of immune suppression) in males was more pronounced in bird species with increased extra‐pair paternity, but was unrelated to sexual dichromatism (Møller et al., 1998). Thus, results on how the intensity of sexual selection relates to sexual dimorphism in physiology remain contradictory, questioning the general validity of the sexual selection hypothesis.
Here, we use a dataset comprising 14 230 physiological measurements of 1997 wild birds, belonging to 97 species. Sampling was restricted to the breeding season (when the effect of sexual selection should be the most prominent), to the same geographic region, and was performed using identical field protocols (minimising handling stress) and uniform laboratory measurements. We explore sex differences in ten immunological, four oxidative physiology parameters, as well as in packed cell volume, reflecting blood oxygen‐carrying capacity. We adopt an evolutionary framework in our analyses, where we inspect how characters reflecting the strength of sexual selection correlate with patterns of physiological sex‐differences across the avian phylogeny. The intensity of sexual selection can manifest in many forms, including sexually different sizes (dimorphism), colours (dichromatism), in divergent mating systems (e.g. polygyny) or sex‐specific parental roles (e.g. from uniparental to unequal biparental offspring care), or as a combination of these traits (Dunn et al., 2001; Liker et al., 2015). Based on the sexual selection hypothesis of physiological sex‐differences we predict larger sex differences in physiology in species subject to strong sexual selection (larger sexual size dimorphism or dichromatism, in polygamous over monogamous species and in species with sex‐biased parental roles). Similarly, we predict lower immune indices and stronger oxidative stress (lower concentration of antioxidants and higher oxidative damage) in the sex subject to more intense sexual selection (i.e. the larger size, more colourful, non‐caring sex).
METHODS
Sample collection
Blood samples were collected between 2009 and 2013, and between 2016 and 2019. We captured birds with mist‐nets and nest traps at various sites across Romania (see Pap et al., 2015; Vágási et al., 2019). We timed the blood sampling to the breeding season (between April and July) to capture the state of birds during the most demanding life‐history stage when sex differences manifest strongly (Pap et al., 2010) and thus its effect on physiological sex differences is expected to be the largest. Detailed descriptions of the fieldwork can be found in the Supplementary Methods.
Physiological variables
Immune capacity was quantified by counting the number of total and specific white blood cells (WBCs) relative to erythrocyte numbers (heterophils, lymphocytes, monocytes, eosinophils, basophils, heterophil:lymphocyte ratio and total white blood cell count), by quantifying the levels of natural antibodies (NAbs) and complement (agglutination and lysis, respectively), and by measuring the bacterial killing activity of the plasma against Escherichia coli (French & Neuman‐Lee, 2012; Matson et al., 2005). The oxidative state was assessed by measuring the level of three non‐enzymatic antioxidant markers (total antioxidant status, TAS; uric acid, UA; total glutathione, tGSH) and of the level of peroxidative damage to membrane lipids (malondialdehyde, MDA) (Vágási et al., 2019). We also estimated the packed cell volume (hematocrit, Ht%, by measuring the ratio of erythrocytes to total blood volume (Møller et al., 2013). Detailed protocols of laboratory assays are described in the Supplementary Methods).
Higher WBC count can mark a more potent immune system (Blount et al., 2003; Hõrak et al., 1998; Nunn, 2002; Nunn et al., 2003), nonetheless, high WBC count and differential increase in white blood cell types can also signal specific health conditions. For instance, an increase in heterophils and lymphocyte counts marks a change in first‐line defence and upregulated adaptive defences, respectively (Davis et al., 2008). Similarly, decreased levels of leukocytes (e.g. lymphocytes) may signal stress‐induced immunosuppression, or reduced ability to fight parasites (Blount et al., 2003; Davis et al., 2008). The heterophil:lymphocyte ratio can be associated with a state of readiness to cope with infection and is also considered a reliable proxy of physiological stress (Gross & Siegel, 1983; Minias, 2019). Inter‐individual differences in monocyte and eosinophil counts might indicate a difference in phagocytic and antigen‐presenting capacity, reflecting defence potential against parasitic infections. Basophils are indicative of inflammatory reactions (Davison et al., 2008). NAbs and the activity of the complement system are two associated measures of the constitutive innate immune system (Matson et al., 2005). Higher scores mean that the immune constituents of the plasma can agglutinate or lyse foreign erythrocytes at lower concentrations (indicating better immune capacity). Bacterial killing activity is a direct measure of constitutive innate immunity in birds (Millet et al., 2007; Tieleman et al., 2005) and characterises the capacity of the blood to limit bacterial infection. All oxidative physiology markers measured here have previously been shown to be associated with fitness parameters in wild‐living organisms. For instance, decreased non‐enzymatic antioxidant levels are indicative of stress or increased reproductive effort (Alonso‐Alvarez et al., 2004; Vágási et al., 2019; Wiersma et al., 2004). Similarly, oxidative damage to lipids (i.e. MDA) is indicative of higher reproductive effort in both birds and mammals (Blount et al., 2016; Metcalfe & Alonso‐Alvarez, 2010; Monaghan et al., 2009; Pap et al., 2018; Stier et al., 2012; Xu et al., 2014).
Field and molecular sexing of birds
Sex was determined during field sampling whenever it was possible based on morphological characteristics (plumage traits and/or presence of brood patch for species where only females develop brood patch; see Table S1). Molecular sexing was performed in sexually monomorphic species, where sexing in the field was not possible (see Supplementary Methods).
Measures of sexual selection
Data on sex‐specific body mass were extracted from Storchová and Hořák (2018), but were verified using alternative sources and some data were updated using recent primary literature data or our field measurements (for specific data sources see Table S2). Sexual size dimorphism (SSD) was calculated as log male body mass minus log female body mass, where positive values indicate larger size in males (hereafter male‐biased SSD) (Mikula et al., 2021).
To quantify sexual dichromatism, plumage colouration was measured in both sexes following the methodology described in Dale et al. (2015) and Carballo et al. (2020) (see Supplementary Methods for details). The mating system was scored based on information obtained from birdsoftheworld.org, Snow et al. (1998) and other studies (Liker et al., 2015; Olson et al., 2008). Sex‐differences in parental care were extracted from Liker et al. (2015) or, for species without data in this reference, we used other two sources (birdsoftheworld.org; Snow et al., 1998, see Supplementary Methods for details). Data, as well as sources on body mass and measures of sexual selection, are provided in Table S2.
Statistical analyses
Although we aimed to maintain the complete homogeneity in the assessment of our physiological samples, given the long‐time frame of data collection complete homogeneity could not be achieved. A few aspects of our laboratory assays changed over the years, including laboratory personnel, equipment or batches of reagents, which potentially added noise to the data, mainly contingent on and reflected by the year of sample collection. Consequently, to quantify sex‐specificity in physiological parameters whilst controlling for temporal data heterogeneity, we used the following procedure. First, for each physiological parameter (dependent variable) we built a general linear mixed‐effects model (LMM). All models contained species, sex and the interaction between these two variables as fixed factors, as well as the year of data collection as a random factor. Second, from these models, we extracted the critical values (t‐values) of sex‐effect for each species using pairwise differences in estimated marginal means, by utilising R package emmeans (Lenth, 2021). These critical values, along with sex‐specific sample sizes were then used to calculate corrected effect sizes (Hedge's d, also called Hedge's g, which is unbiased in the case of small sample sizes, following Lakens, 2013) express differences between the sexes in physiological parameters. Positive values of Hedge's d mark higher values of the measured parameter in males compared to females (i.e. male‐bias), whilst negative values mark larger values in females (i.e. female‐bias). Hedge's d values were used as response variables in meta‐analytic models exploring the cross‐species diversity of sex‐specific physiology and factors affecting it. The advantage of using effect sizes is that, unlike sex‐differences in average values, it considers the variance within sex and provides a value that reflects both the direction and magnitude of the sexual dimorphism in physiology, whilst being independent of the scale of the original variable. Effect sizes were extracted for all species, where both sexes and a minimum of three individuals per species were sampled. However, we also performed sensitivity analyses restricting the analyses to species with a minimum sample size of two, three or four individuals per sex to assess the sensitivity of the results to within‐species and within‐sex sample sizes. Given that sexes were sometimes captured at different times of the breeding season, we also tested and concluded that controlling for capture date differences do not alter any of our conclusions (for details see Supplementary Methods and Table S3).
To assess whether sex‐differences in the measured physiological parameters are species‐specific, as required for phylogenetic comparative analyses, we tested their repeatabilities. To do this, we performed bootstrap sampling without replacement on the existing measurements. We extracted n random measurements for both males and females of a particular physiological parameter. Based on these samples, we extracted the effect sizes of the sex‐difference in each species following the LMM procedure described above. We repeated this bootstrap sampling and the extraction of effect sizes 100 times and then calculated cross‐species repeatability of the effect sizes using R package irr (Gamer et al., 2019). The above repeatability test was performed for each physiological parameter and with a within‐species, within‐sex sample size (n) of two, three, four and five individuals. Sex‐differences in all physiological parameters were highly repeatable across species at each within‐species, within‐sex sample size (n) (see Table S4).
Given that species represent non‐independent data points, phylogenetic effects can arise. Therefore, we explored phylogenetic effects in physiological sex‐biases and controlled for this effect in each meta‐analytic model. To do this, we obtained 1000 equally parsimonious phylogenies from http://birdtree.org (Jetz et al., 2012) using the Hackett backbone tree (Hackett et al., 2008), and we assembled a rooted, ultrametric consensus tree using the SumTrees Python library (Sukumaran & Holder, 2010). This consensus tree was then used to estimate the phylogenetic effects in physiological sex‐biases (R function ‘phylosig’; Revell, 2012) and to control for these in meta‐analytic models.
In order to explore the generality of sex‐differences in physiology across several species, we used intercept‐only meta‐regression models with phylogenetic correlation incorporated to assess the overall effect size using restricted maximum likelihood approximation. We then performed moderator analyses in order to explore which sexual selection variables influence the magnitude and direction of sex‐differences in various physiological parameters. All meta‐analytic models were performed using the ‘rma.mv’ function in the R package metafor (v. 2.4‐0; Viechtbauer, 2010). All analyses were carried out using R v. 4.0.4 (R Development Core Team, 2021).
RESULTS
Phylogenetic effect
The phylogenetic effect of sex‐differences in physiological parameters was not detected except in the case of basophil counts (Table S5, Figure 1). Phylogenetic signals (or lack thereof) remained consistent for each parameter after the exclusion of species with small sex‐specific sample sizes (Table S5).
FIGURE 1.
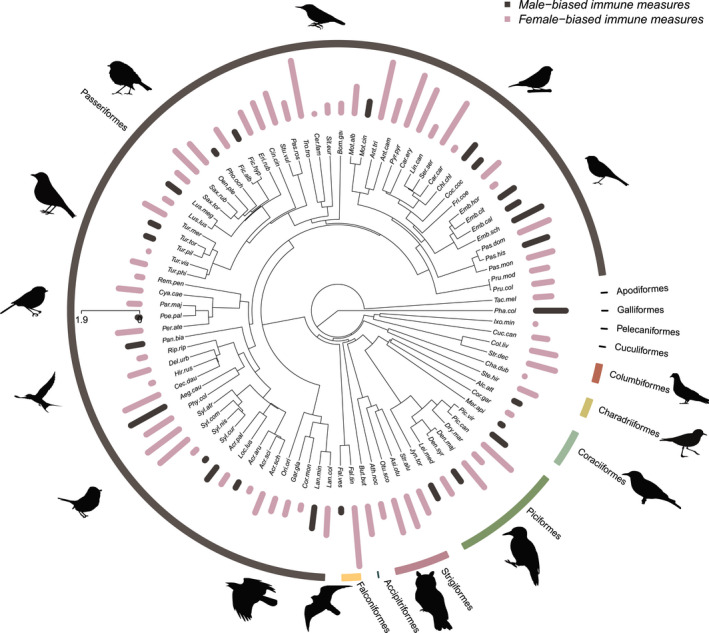
Phylogenetic distribution of sex‐differences in total white blood cell counts, illustrating the phylogenetic coverage of the data and the lack of phylogenetic signal in physiological sex‐specificity in our sample. Female‐bias (marked in pink) in total white blood cell number is more prominent than male‐bias (marked in grey) in our sample. The height of the bars reflects the magnitude of sex difference (i.e. the absolute value of the effect sizes)
Sex‐differences in physiology
Effect sizes obtained from intercept‐only meta‐analytic models indicated overwhelmingly female‐biased immune measures, although the magnitude and direction of sexual dimorphism varied considerably among immune parameters (Table S6, Figure 2). Females had higher agglutination scores as well as higher total WBC, heterophil and lymphocyte counts than males. No overall sex‐bias could be detected in the case of rarer WBC types (monocytes, eosinophils and basophils) or heterophil:lymphocyte ratios. Lysis, as well as bacterial killing activity, appeared to be slightly but not detectably higher in females. No overall sex‐bias could be detected in any of the oxidative physiology parameters (i.e. TAS, UA, tGSH and MDA concentrations; Table S6, Figure 2). The overall effect size of Ht% indicated a higher packed cell volume in males compared to females. The general pattern of sexual difference in physiology was only negligibly sensitive to the sex‐specific sample sizes, as the results remained generally consistent when species with lower sex‐specific sample sizes were excluded, even though these sensitivity analyses reduced the species pool of the models (Table S6). This indicates that sexual dimorphism in certain physiological parameters is robust to within‐species, within‐sex sample sizes.
FIGURE 2.
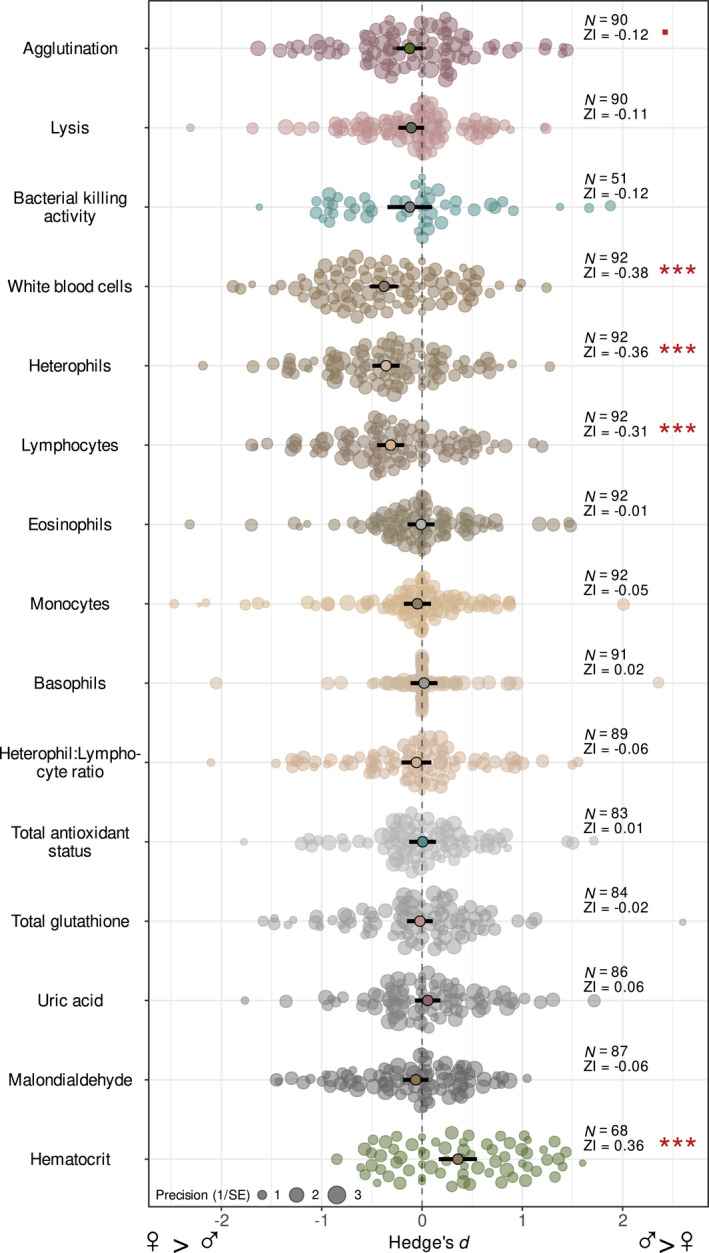
Orchard plot for the intercept only meta‐analytic models showing the overall difference in physiological measures between males and females. Mean effect sizes with confidence intervals (bold lines) are shown in the middle of the dot charts, where individual dots are individual (i.e. species‐specific) effect sizes. The size of dots shows the precision (inverse of standard error) of the effect size estimate of different physiological parameters. Number of species (N), overall effect size (ZI) and p‐value indicating the difference of ZI from zero are given. Negative effect sizes indicate female‐bias (larger values in females), whilst positive values mark male‐bias in the respective parameter. Significance levels are denoted with red as follows: ■ p < 0.1, ***p < 0.0001
The role of sexual selection
The agglutination and lysis scores were increasingly female‐biased in species with more male‐biased SSD (Table 1, Figure 3), though the association was statistically non‐significant. The overall higher agglutination and lysis scores in females compared to males (i.e. negative intercept) remained unchanged after controlling for SSD (Table 1). Total WBC, heterophil, lymphocyte and monocyte counts were more female‐biased in species with female‐biased SSD, whilst the sexes became similar in these immunological traits with more male‐biased SSD (Table 1, Figure 3). Again, controlling for SSD did not influence the overall female‐bias in these leukocyte counts (Table 1). SSD was unrelated to sexual dimorphism in eosinophils, basophils, heterophil:lymphocyte ratio and in bacterial killing activity, and SSD did not influence the overall sex‐specificity of these parameters (Table 1).
TABLE 1.
Result of single‐predictor meta‐analytic models performed separately for each physiological parameter and each predictor (sexual size dimorphism, sexual dichromatism, male polygyny score and parental care)
| Response variable | N | Sexual size dimorphism | Sexual dichromatism | Male polygyny score | Parental care |
|---|---|---|---|---|---|
|
Intercept ZI (p‐value) Predictor ZI (p‐value) | |||||
| Immunological measuress | |||||
| Agglutination | 90 |
−0.11 (0.0903) −1.49 (0.0525) |
−0.14 (0.1341) 0.00 (0.8264) |
−0.08 (0.3242) −0.04 (0.4698) |
−0.11 (0.2929) 0.04 (0.8768) |
| Lysis | 90 |
−0.10 (0.1401) −1.21 (0.1151) |
−0.15 (0.0923) 0.00 (0.4665) |
−0.07 (0.4175) −0.04 (0.4764) |
−0.04 (0.7296) 0.20 (0.3773) |
| Bacterial killing activity | 51 |
−0.13 (0.2686) 0.82 (0.5347) |
−0.10 (0.5246) 0.00 (0.8645) |
−0.26 (0.0771) 0.14 (0.1336) |
−0.10 (0.5887) 0.08 (0.8455) |
| White blood cells | 92 |
−0.40 (< 0.0001) 1.75 (0.0393) |
−0.38 (0.0001) 0.00 (0.9392) |
−0.35 (0.0001) −0.03 (0.5650) |
−0.40 (0.0003) −0.05 (0.8389) |
| Heterophils | 92 |
−0.38 (< 0.0001) 1.85 (0.0287) |
−0.42 (< 0.0001) 0.01 (0.3742) |
−0.33 (0.0002) −0.03 (0.6498) |
−0.34 (0.0019) 0.07 (0.7725) |
| Lymphocytes | 92 |
−0.33 (< 0.0001) 1.70 (0.0431) |
−0.22 (0.0218) −0.01 (0.3742) |
−0.23 (0.0088) −0.08 (0.1678) |
−0.40 (0.0002) −0.27 (0.2782) |
| Eosinophils | 92 |
−0.02 (0.8005) 0.72 (0.3823) |
−0.01 (0.8971) 0.00 (0.9684) |
0.03 (0.7767) −0.04 (0.5396) |
−0.01 (0.9004) −0.01 (0.9658) |
| Monocytes | 92 |
−0.07 (0.3438) 1.75 (0.0365) |
0.04 (0.7169) −0.01 (0.2399) |
0.01 (0.8771) −0.06 (0.3002) |
−0.05 (0.6106) −0.03 (0.9136) |
| Basophils | 91 |
0.01 (0.8908) 0.81 (0.3441) |
0.04 (0.6814) 0.00 (0.7737) |
0.00 (0.9760) 0.02 (0.6864) |
0.07 (0.5197) 0.14 (0.5518) |
| Heterophil:Lymphocyte ratio | 89 |
−0.06 (0.4213) 0.71 (0.4081) |
−0.11 (0.3008) 0.01 (0.4654) |
−0.11 (0.2521) 0.06 (0.3619) |
0.01 (0.9108) 0.19 (0.4645) |
| Oxidative physiology | |||||
| Total antioxidant status | 83 |
0.01 (0.9232) −0.19 (0.8212) |
−0.06 (0.5054) 0.01 (0.3077) |
−0.01 (0.8883) 0.02 (0.7509) |
−0.05 (0.6361) −0.17 (0.5025) |
| Total glutathione | 84 |
−0.02 (0.7189) 0.56 (0.5226) |
0.02 (0.7963) 0.00 (0.5027) |
−0.07 (0.4274) 0.05 (0.3992) |
−0.08 (0.4191) −0.19 (0.4331) |
| Uric acid | 86 |
0.06 (0.3690) −0.17 (0.8378) |
0.18 (0.0452) −0.01 (0.0522) |
−0.04 (0.6024) 0.10 (0.0728) |
0.09 (0.3629) 0.11 (0.6497) |
| Malondialdehyde | 87 |
−0.05 (0.4052) −1.43 (0.0816) |
−0.05 (0.5574) 0.00 (0.8854) |
−0.03 (0.7294) −0.03 (0.5355) |
−0.03 (0.8013) 0.10 (0.6554) |
| Oxygen carrying capacity of the blood | |||||
| Hematocrit | 73 |
0.42 (< 0.0001) −0.39 (0.7320) |
0.37 (0.0057) 0.00 (0.8878) |
0.36 (0.0053) 0.00 (0.7772) |
0.38 (0.0120) 0.06 (0.8617) |
For each model, the coefficients for the intercept (first row; Intercept ZI) and the slope of the predictor (second row; Predictor ZI) are given along with corresponding p‐values in parentheses. The number of species the models of each physiological parameter are based on are also shown (N). Intercepts mark overall sex‐biases (negative values indicating female‐bias and are marked in red, positive values indicate male‐bias and are marked in blue). Significant effects (p‐value ≤ 0.05) are marked in bold.
FIGURE 3.
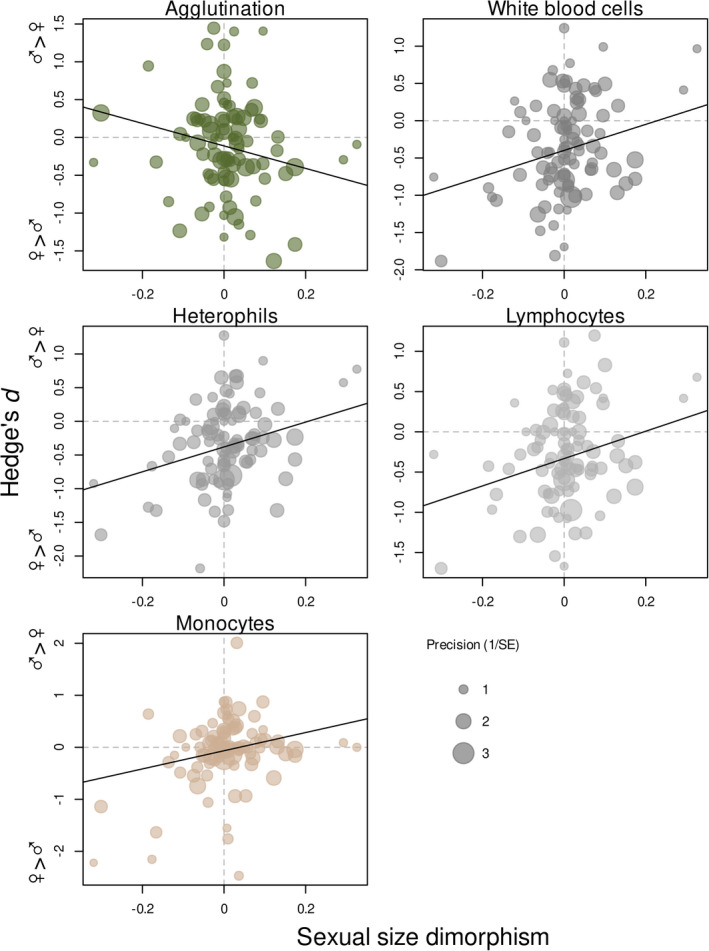
Association between immunological sex‐differences (Hedge's d) and sexual size dimorphism across species. Each point represents a species. The size of points shows the precision (inverse of standard error) of the effect size estimate of different physiological parameters. Positive values mark higher values in males in both parameters. The fitted line originated from corresponding models is presented in Table 1
Despite the lack of overall sex‐specificity in MDA concentration, MDA levels tended to be more female‐biased in species with increasingly male‐biased SSD, but the association was not statistically significant (Table 1). SSD did not explain variation in sexual dimorphism in any non‐enzymatic antioxidant levels (TAS, UA or tGSH) or Ht%, and did not influence the overall sex‐specificity of these parameters (Table 1).
Sexual dimorphism in physiological measures was not associated with sexual dichromatism, mating system (i.e. polygyny score of males) and sex‐differences in parental care (Table 1). None of the overall effect sizes of sexual dimorphism in physiological measures and their association with SSD changed when all possible variables describing the intensity of sexual selection were introduced in multivariate models (Table S7). This indicates that the sex‐differences in physiological parameters and their relationship with SSD are robust.
DISCUSSION
Based on analyses of a unique physiological database comprising 97 European breeding bird species, we make four important conclusions regarding cross‐species variances of physiological sexual dimorphism. First, we provide unambiguous evidence that immunological sex‐bias in birds (when present) is skewed towards females, similarly to what has been observed in mammals (Nunn et al., 2009), and this sex difference remains even after controlling for proxies of sexual selection. Second, oxidative physiological markers do not show consistent sex‐differences across birds even if sampling is homogeneous to age, breeding status, and handling stress. Third, Ht% is consistently male‐biased across breeding birds. Fourth, sex‐biases in all physiological parameters inspected here are phylogenetically flexible and are not explained by measures of the intensity of sexual selection.
Female‐bias in immunity is commonly reported across mammals, including humans (Klein & Flanagan, 2016; Metcalf et al., 2020; Nunn et al., 2009), but the overall pattern in birds appeared more equivocal so far. Higher immune indices in males than in females, and opposite differences (that disappeared after phylogenetic control) were both reported (Kelly et al., 2018; Valdebenito et al., 2021). In contrast, our study indicates a consistent female‐bias in multiple immune parameters and a limited contribution of phylogenetic history to shaping these sex‐differences. The contrasting results likely emerged due to the high sampling heterogeneity in earlier datasets (Kelly et al., 2018), such as lack of consideration for breeding status (see the importance of this in e.g. Pap et al., 2010), or limited sample sizes (Valdebenito et al., 2021, where parameter‐specific sample sizes are often limited to only three species). Compared to these, our study has considerably higher power, due to more homogeneous sampling (e.g. to age, breeding status and handling stress) and a larger species pool. Nonetheless, our results also indicate large inconsistencies in sex‐bias across multiple immune parameters, even though we used a largely identical sample of individuals to measure these. These findings support the conclusion of previous studies, indicating that sexual dimorphism in immune function differs between immune system constituents (Kelly et al., 2018). Differences in sex bias among physiological parameters likely reflect the outcome of differential adaptive up or downregulation of immune effectors between the sexes (Kelly et al., 2018; Metcalf & Graham, 2018). Adaptive and inducible components of the immune system were predicted to be female‐biased, whilst innate and constitutive components to be male‐biased (Lee, 2006). Following Lee's (2006) categorisation of immune measures, our findings are generally inconsistent with these predictions, however, we remain cautious in our conclusions, as some of our immune parameters cannot be unambiguously classified as innate or adaptive, constitutive or inducible (i.e. bacteria‐killing activity, NAbs, lymphocyte count; Demas et al., 2011). Finally, immune parameter‐specific sexual dimorphism can also be explained by the immunological costs of complementary sex roles (e.g. mate attraction in males, parental care in females), which can be similar overall for particular measures, ultimately resulting in comparable physiological traits in males and females.
Immune defence is one of the best predictors of survival and recruitment in wild animals (Møller & Saino, 2004). Consequently, sex‐bias in immunity might contribute to sex‐differences in survival. This theory is supported in mammals, where females have both higher immune indices and higher survival than males (Lemaître et al., 2020; Nunn et al., 2009). In birds, however, immunity appears to be female‐biased (present study), which is inconsistent with the generally male‐biased survival across the avian phylogeny (Székely et al., 2014). The discrepancy in survival and physiological sex‐biases in birds vs. mammals represents a logical challenge, which can be solved by future studies testing the associations between sex‐specific physiology and sex‐specific survival in animals of various taxonomic positions. Finally, sex‐specific survival is also contingent on other immune or physiological parameters that were not considered in our study. Such a factor could be parasite burden, which directly affects immune function and survival. Indeed, sex‐differences in parasitism are commonly reported, often indicating higher parasite load in males than in females (Zuk, 2009), which also manifests in sex‐biased immune function (Moore & Wilson, 2002). However, studies addressing the association between sex‐specific parasitism and sex‐bias in immunity so far yielded conflicting results (McCurdy et al., 1998; Valdebenito et al., 2020). Moreover, the link between immune function and survival might not be linear, with the low and high immune responses being equally sub‐optimal, potentially confounding survival‐immunity associations between the sexes. Therefore, to establish whether physiology plays a role in shaping survival patterns and if so identifying which physiological characters are most important in this respect requires further explorations.
Oxidative physiology is highly sensitive to sex‐ and stress hormones, and to intensified physical activity, which is frequently elevated during the reproductive period (Costantini, 2014; Monaghan et al., 2009). Sex differences in parental roles, territory or mate guarding, and participation in aggressive interactions were thus suggested to precipitate in sex differences in oxidative stress (Alonso‐Alvarez et al., 2004, 2007; Schantz et al., 1999). Therefore, we expected detectable sex‐differences in oxidative physiology. Interestingly, however, we did not find general sex‐bias in measures of antioxidants or oxidative damage. This finding brings support for the single multi‐species meta‐analysis, showing no general sex‐differences in markers of oxidative state in birds (Costantini, 2018). It might be possible that the sexes differ in the oxidative state in particular stages of breeding (territory acquisition, incubation or chick provisioning) with the oxidative toll being paid by either males or females in a breeding stage‐specific manner, but overall sex differences remain undetectable.
We found a consistently male‐biased Ht% across breeding birds, contradicting findings of a meta‐analysis based on 36 studies, which concluded no sex‐difference in Ht% in birds (Fair et al., 2007). Ht% reflects blood haemoglobin content and therefore the oxygen‐carrying capacity of the blood. Larger Ht% in males thus likely indicates a generally higher aerobic activity in males compared with females, at least during breeding. Such effect might be the result of more intense locomotor activity due to sexual competition and territory acquisition/defence in males. A mutually non‐exclusive explanation is ‘anaemia’ associated with egg production in females, which could contribute to the sex‐differences in Ht% during the breeding season (Williams et al., 2004).
One of our key findings is that sexual size dimorphism is a significant predictor of immunological sex‐bias for multiple measures of the immune system. It is widely acknowledged that male‐biased sexual size dimorphism is the result of intense mating competition (Fairbairn et al., 2007). Based on the theory on the role of sexual selection in driving physiological adaptations, we predicted weaker immunity and higher levels of oxidative stress in the larger sex, which has a larger variance in fitness. Indeed, sexual size dimorphism was correlated with the sex‐bias in several immune parameters, with dimorphic species being more likely to exhibit sex‐bias in physiology. Surprisingly, however, our prediction was only supported in the case of agglutination, and the opposite pattern was observed in the case of WBC counts (heterophils, lymphocyte, monocytes, total WBCs), with the larger sex exhibiting higher cell counts (not lower as predicted). One possible explanation for the higher WBC count of males in species with larger males is that these cells are important in protection during injuries, and because males of species under strong sexual selection are commonly injured during contests, their number is upregulated to help wound healing (Blount et al., 2003; Nunn, 2002; Nunn et al., 2003). None of the other proxy measures of sexual selection intensity (dichromatism, mating system and parental roles) explained variance in sexual dimorphism of physiological variables. Importantly, female‐biased immunity remained even after accounting for sexual dimorphism, dichromatism, mating system or sex‐differences in parental care. Therefore, we conclude that sexual selection has only limited contribution to shaping immunological sex‐differences and additional evolutionary mechanisms should be explored. Finally, contrary to our predictions based on previous works (Alonso‐Alvarez et al., 2004, 2007; Monaghan et al., 2009; Schantz et al., 1999), none of the measures of sexual selection explained variance in sex‐differences in oxidative physiology. We also show a limited role of parental division of labour in shaping sex‐differences in oxidative stress markers, opposing the results by Costantini (2018). We thus conclude that sexual selection plays little role in shaping sex‐specificity in oxidative physiology across species. Failure to demonstrate associations between sex‐specific oxidative physiology and the intensity of sexual selection, however, does not preclude the existence of such associations. In fact, oxidative physiology changes quickly in response to e.g. locomotion or stress, and changes within the reproductive season as a function of the actual reproductive stage, such as mating, incubation or food‐provisioning is possible (Pap et al., 2018). Exploring such an effect, however, requires the close monitoring of breeding populations and remains to be accomplished by future studies.
CONCLUSION
Our results indicate that physiological sex‐differences are prominent among birds, but the direction and intensity of sex‐specificity are inconsistent among physiological parameters. We conclude that sex‐bias in immunity in birds (when present) is most likely skewed toward females, corresponding to patterns found in mammals. This indicates that female‐bias in immunity might be the general rule in warm‐blooded vertebrates. We find no support for the sexual selection hypothesis explaining sex‐differences in physiology, and we call for exploration of an alternative hypothesis for the origin of physiological sex‐biases. Factors like sex‐specific parasitism, energy expenditure or proximate, hormonal processes could be considered here. Importantly, we also highlight the apparent inconsistency between physiological and survival sex‐biases between mammals and birds. This inconsistency suggests that sex‐differences in survival might be unrelated to physiological sex‐differences.
AUTHOR CONTRIBUTIONS
PLP, CIV and OV conceived the project; PLP, CIV, OV and JP collected the blood samples; PLP, CIV and OV collected literature data; JP, GÁC and CIV carried out the physiological measurements; OV analysed the data; KS and NMM performed the molecular sexing; PLP and OV wrote the manuscript with significant input from CIV. All authors gave final approval for publication and agree to be accountable for the aspects of work that they conducted.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ele.13973.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to many students and colleagues who faithfully helped during fieldwork, to Laura Pătraș and Katja Pohle for their valuable help in physiological measurements, and Mihai Vâlcu for his help in sexual dichromatism measurement. We are grateful to three anonymous reviewers whose comments and suggestions improved the manuscript considerably. This work was carried out with the permission of the Romanian Academy of Sciences and adhered to recommended practices for ringing, measuring and sampling of wild birds for research purposes. The research of PLP, CIV, JP and OV was supported by an Exploratory Research Grant of the Romanian Ministry of Research and Innovation (no. PN‐III‐P4‐ID‐PCE‐2016‐0404). OV and PLP were financed by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (HAS). OV was supported by the New National Excellence Programme of the Hungarian Ministry of Innovation and Technology. GÁC was supported by funds from the Leibniz Institute for Zoo and Wildlife Research, Berlin, Germany.
Vincze, O. , Vágási, C.I. , Pénzes, J. , Szabó, K. , Magonyi, N.M. , Czirják, G.Á. & et al. (2022) Sexual dimorphism in immune function and oxidative physiology across birds: The role of sexual selection. Ecology Letters, 25, 958–970. Available from: 10.1111/ele.13973
Contributor Information
Orsolya Vincze, Email: vincze.orsolya@ecolres.hu.
Péter L. Pap, Email: peterlpap@gmail.com.
DATA AVAILABILITY STATEMENT
All data used in this article are available in the Supporting Information. Sex‐specific body masses, male polygyny score, sexual dichromatism and parental role division are given in Table S2. Species‐ and sex‐specific sample sizes of each physiological parameter, as well as effect size (Hedge's d) indicating the degree of sexual dimorphism in physiological parameters, are given in Table S8. Data used in this manuscript are deposited in Figshare: https://doi.org/10.6084/m9.figshare.18095873.v1.
REFERENCES
- Alonso‐Alvarez, C. , Bertrand, S. , Devevey, G. , Prost, J. , Faivre, B. & Sorci, G. (2004) Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecology Letters, 7, 363–368. [Google Scholar]
- Alonso‐Alvarez, C. , Bertrand, S. , Faivre, B. , Chastel, O. & Sorci, G. (2007) Testosterone and oxidative stress: the oxidation handicap hypothesis. Proceedings of the Royal Society B: Biological Sciences, 274, 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount, J.D. , Houston, D.C. , Møller, A.P. & Wright, J. (2003) Do individual branches of immune defence correlate? A comparative case study of scavenging and non‐scavenging birds. Oikos, 102, 340–350. [Google Scholar]
- Blount, J.D. , Vitikainen, E.I.K. , Stott, I. & Cant, M.A. (2016) Oxidative shielding and the cost of reproduction. Biological Reviews, 91, 483–497. [DOI] [PubMed] [Google Scholar]
- Carballo, L. , Delhey, K. , Vâlcu, M. & Kempenaers, B. (2020) Body size and climate as predictors of plumage colouration and sexual dichromatism in parrots. Journal of Evolutionary Biology, 33, 1543–1557. [DOI] [PubMed] [Google Scholar]
- Costantini, D. (2014) Oxidative stress and hormesis in evolutionary ecology and physiology. Berlin Heidelberg, Berlin, Heidelberg: Springer. [Google Scholar]
- Costantini, D. (2018) Meta‐analysis reveals that reproductive strategies are associated with sexual differences in oxidative balance across vertebrates. Current Zoology, 64, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, J. , Dey, C.J. , Delhey, K. , Kempenaers, B. & Valcu, M. (2015) The effects of life history and sexual selection on male and female plumage colouration. Nature, 527, 367–370. [DOI] [PubMed] [Google Scholar]
- Davis, A.K. , Maney, D.L. & Maerz, J.C. (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Functional Ecology, 22, 760–772. [Google Scholar]
- Davison, F. , Kaspers, B. & Schat, K.A. (2008) Avian immunology. Amsterdam: Elsevier. [Google Scholar]
- Demas, G.E. , Zysling, D.A. , Beechler, B.R. , Muehlenbein, M.P. & French, S.S. (2011) Beyond phytohaemagglutinin: assessing vertebrate immune function across ecological contexts. Journal of Animal Ecology, 80, 710–730. [DOI] [PubMed] [Google Scholar]
- Dunn, P.O. , Whittingham, L.A. & Pitcher, T.E. (2001) Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution, 55, 161–175. [DOI] [PubMed] [Google Scholar]
- Emaresi, G. , Henry, I. , Gonzalez, E. , Roulin, A. & Bize, P. (2016) Sex‐ and melanic‐specific variations in the oxidative status of adult tawny owls in response to manipulated reproductive effort. Journal of Experimental Biology, 219, 73–79. [DOI] [PubMed] [Google Scholar]
- Fair, J. , Whitaker, S. & Pearson, B. (2007) Sources of variation in haematocrit in birds. Ibis, 149, 535–552. [Google Scholar]
- Fairbairn, D.J. , Blanckenhorn, W.U. & Székely, T. (2007) Sex, size and gender roles: evolutionary studies of sexual size dimorphism. New York: Oxford University Press. [Google Scholar]
- French, S.S. & Neuman‐Lee, L.A. (2012) Improved ex vivo method for microbiocidal activity across vertebrate species. Biology Open, 1, 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer, M. , Lemon, J. , Fellows, I. & Sing, P. (2019) irr: Various coefficients of interrater reliability and agreement. R package version 0.84.1. https://CRAN.R‐project.org/package=irr.
- Gross, W.B. & Siegel, H.S. (1983) Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Diseases, 27, 972–979. [PubMed] [Google Scholar]
- Hackett, S.J. , Kimball, R.T. , Reddy, S. , Bowie, R.C.K. , Braun, E.L. , Braun, M.J. et al. (2008) A phylogenomic study of birds reveals their evolutionary history. Science, 320, 1763–1768. [DOI] [PubMed] [Google Scholar]
- Hasselquist, D. (2007) Comparative immunoecology in birds: hypotheses and tests. Journal of Ornithology, 148, 571–582. [Google Scholar]
- Heiss, R.S. & Schoech, S.J. (2012) Oxidative cost of reproduction is sex specific and correlated with reproductive effort in a cooperatively breeding bird, the Florida Scrub Jay. Physiological and Biochemical Zoology, 85, 499–503. [DOI] [PubMed] [Google Scholar]
- Hõrak, P. , Ots, I. & Murumägi, A. (1998) Haematological health state indices of reproducing Great Tits: a response to brood size manipulation. Functional Ecology, 12, 750–756. [Google Scholar]
- Jakubas, D. , Wojczulanis‐Jakubas, K. & Kośmicka, A. (2015) Factors affecting leucocyte profiles in the Little Auk, a small Arctic seabird. Journal of Ornithology, 156, 101–111. [Google Scholar]
- Jetz, W. , Thomas, G.H. , Joy, J.B. , Hartmann, K. & Mooers, A.O. (2012) The global diversity of birds in space and time. Nature, 491, 444–448. [DOI] [PubMed] [Google Scholar]
- Kelly, C.D. , Stoehr, A.M. , Nunn, C. , Smyth, K.N. & Prokop, Z.M. (2018) Sexual dimorphism in immunity across animals: a meta‐analysis. Ecology Letters, 21, 1885–1894. [DOI] [PubMed] [Google Scholar]
- Klein, S.L. & Flanagan, K.L. (2016) Sex differences in immune responses. Nature Reviews Immunology, 16, 626–638. [DOI] [PubMed] [Google Scholar]
- Lakens, D. (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t‐tests and ANOVAs. Frontiers in Psychology, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. (2006) Linking immune defenses and life history at the levels of the individual and the species. Integrative and Comparative Biology, 46, 1000–1015. [DOI] [PubMed] [Google Scholar]
- Lemaître, J.‐F. , Ronget, V. , Tidière, M. , Allainé, D. , Berger, V. , Cohas, A. et al. (2020) Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proceedings of the National Academy of Sciences of the United States of America, 117, 8546–8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth, R. (2021) emmeans: estimated marginal means, aka least‐squares means. Available at: https://CRAN.R‐project.org/package=emmeans. Last accessed 19 November 2018
- Liker, A. , Freckleton, R.P. , Remeš, V. & Székely, T. (2015) Sex differences in parental care: gametic investment, sexual selection, and social environment. Evolution, 69, 2862–2875. [DOI] [PubMed] [Google Scholar]
- Martin, L.B. , Weil, Z.M. & Nelson, R.J. (2008) Seasonal changes in vertebrate immune activity: mediation by physiological trade‐offs. Philosophical Transactions of the Royal Society B: Biological Sciences, 363, 321–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson, K.D. , Ricklefs, R.E. & Klasing, K.C. (2005) A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Developmental & Comparative Immunology, 29, 275–286. [DOI] [PubMed] [Google Scholar]
- McCurdy, D.G. , Shutler, D. , Mullie, A. & Forbes, M.R. (1998) Sex‐biased parasitism of avian hosts: relations to blood parasite taxon and mating system. Oikos, 82, 303–312. [Google Scholar]
- Metcalf, C.J.E. & Graham, A.L. (2018) Schedule and magnitude of reproductive investment under immune trade‐offs explains sex differences in immunity. Nature Communications, 9, 4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf, C.J.E. , Roth, O. & Graham, A.L. (2020) Why leveraging sex differences in immune trade‐offs may illuminate the evolution of senescence. Functional Ecology, 34, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe, N.B. & Alonso‐Alvarez, C. (2010) Oxidative stress as a life‐history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Functional Ecology, 24, 984–996. [Google Scholar]
- Mikula, P. , Valcu, M. , Brumm, H. , Bulla, M. , Forstmeier, W. , Petrusková, T. et al. (2021) A global analysis of song frequency in passerines provides no support for the acoustic adaptation hypothesis but suggests a role for sexual selection. Ecology Letters, 24, 477–486. [DOI] [PubMed] [Google Scholar]
- Millet, S. , Bennett, J. , Lee, K. , Hau, M. & Klasing, K.C. (2007) Quantifying and comparing constitutive immunity across avian species. Developmental and Comparative Immunology, 31, 188–201. [DOI] [PubMed] [Google Scholar]
- Minias, P. (2019) Evolution of heterophil/lymphocyte ratios in response to ecological and life‐history traits: a comparative analysis across the avian tree of life. Journal of Animal Ecology, 88, 554–565. [DOI] [PubMed] [Google Scholar]
- Møller, A.P. & Saino, N. (2004) Immune response and survival. Oikos, 104, 299–304. [Google Scholar]
- Møller, A.P. , Sorci, G. & Erritzøe, J. (1998) Sexual dimorphism in immune defense. The American Naturalist, 152, 605–619. [DOI] [PubMed] [Google Scholar]
- Møller, A.P. , Vágási, C.I. & Pap, P.L. (2013) Risk‐taking and the evolution of mechanisms for rapid escape from predators. Journal of Evolutionary Biology, 26, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Monaghan, P. , Metcalfe, N.B. & Torres, R. (2009) Oxidative stress as a mediator of life history trade‐offs: mechanisms, measurements and interpretation. Ecology Letters, 12, 75–92. [DOI] [PubMed] [Google Scholar]
- Moore, S.L. & Wilson, K. (2002) Parasites as a viability cost of sexual selection in natural populations of mammals. Science, 297, 2015–2018. [DOI] [PubMed] [Google Scholar]
- Nunn, C.L. (2002) A comparative study of leukocyte counts and disease risk in primates. Evolution, 56, 177–190. [DOI] [PubMed] [Google Scholar]
- Nunn, C.L. , Gittleman, J.L. & Antonovics, J. (2003) A comparative study of white blood cell counts and disease risk in carnivores. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn, C.L. , Lindenfors, P. , Pursall, E.R. & Rolff, J. (2009) On sexual dimorphism in immune function. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrand, M. & Dowling, D.K. (2020) Effects of immune challenge on expression of life‐history and immune trait expression in sexually reproducing metazoans—a meta‐analysis. BMC Biology, 18, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, V.A. , Liker, A. , Freckleton, R. & Székely, T. (2008) Parental conflict in birds: comparative analyses of offspring development, ecology and mating opportunities. Proceedings of the Royal Society B: Biological Sciences, 275, 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap, P.L. , Czirják, G.Á. , Vágási, C.I. , Barta, Z. & Hasselquist, D. (2010) Sexual dimorphism in immune function changes during the annual cycle in House Sparrows. Naturwissenschaften, 97, 891–901. [DOI] [PubMed] [Google Scholar]
- Pap, P.L. , Vágási, C.I. , Vincze, O. , Osváth, G. , Veres‐Szászka, J. & Czirják, G.Á. (2015) Physiological pace of life: the link between constitutive immunity, developmental period, and metabolic rate in European birds. Oecologia, 177, 147–158. [DOI] [PubMed] [Google Scholar]
- Pap, P.L. , Vincze, O. , Fülöp, A. , Székely‐Béres, O. , Pătraș, L. , Pénzes, J. et al. (2018) Oxidative physiology of reproduction in a passerine bird: a field experiment. Behavioral Ecology and Sociobiology, 72, 18. [Google Scholar]
- R Development Core Team (2021) R: A Language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. URL http://www.R‐project.org/.
- Revell, L.J. (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. [Google Scholar]
- Rolff, J. (2002) Bateman’s principle and immunity. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269, 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubolini, D. , Colombo, G. , Ambrosini, R. , Caprioli, M. , Clerici, M. , Colombo, R. et al. (2012) Sex‐related effects of reproduction on biomarkers of oxidative damage in free‐living Barn Swallows (Hirundo rustica). PLoS One, 7, e48955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow, D. , Perrins, C.M. & Cramp, S. (1998). The Complete Birds of the Western Palearctic on CD‐ROM.
- Stier, A. , Reichert, S. , Massemin, S. , Bize, P. & Criscuolo, F. (2012) Constraint and cost of oxidative stress on reproduction: correlative evidence in laboratory mice and review of the literature. Frontiers in Zoology, 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr, A.M. & Kokko, H. (2006) Sexual dimorphism in immunocompetence: what does life‐history theory predict? Behavioral Ecology, 17, 751–756. [Google Scholar]
- Storchová, L. & Hořák, D. (2018) Life‐history characteristics of European birds. Global Ecology and Biogeography, 27, 400–406. [Google Scholar]
- Sukumaran, J. & Holder, M.T. (2010) DendroPy: a Python library for phylogenetic computing. Bioinformatics, 26, 1569–1571. [DOI] [PubMed] [Google Scholar]
- Székely, T. , Liker, A. , Freckleton, R.P. , Fichtel, C. & Kappeler, P.M. (2014) Sex‐biased survival predicts adult sex ratio variation in wild birds. Proceedings of the Royal Society B: Biological Sciences, 281, 20140342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidière, M. , Badruna, A. , Fouchet, D. , Gaillard, J.‐M. , Lemaître, J.‐F. & Pontier, D. (2020) Pathogens shape sex differences in mammalian aging. Trends in Parasitology, 36, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman, B.I. , Williams, J.B. , Ricklefs, R.E. & Klasing, K.C. (2005) Constitutive innate immunity is a component of the pace‐of‐life syndrome in tropical birds. Proceedings of the Royal Society B: Biological Sciences, 272, 1715–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vágási, C.I. , Vincze, O. , Pătraș, L. , Osváth, G. , Pénzes, J. , Haussmann, M.F. et al. (2019) Longevity and life history coevolve with oxidative stress in birds. Functional Ecology, 33, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdebenito, J.O. , Halimubieke, N. , Lendvai, Á.Z. , Figuerola, J. , Eichhorn, G. & Székely, T. (2021) Seasonal variation in sex‐specific immunity in wild birds. Scientific Reports, 11, 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdebenito, J.O. , Liker, A. , Halimubieke, N. , Figuerola, J. & Székely, T. (2020) Mortality cost of sex‐specific parasitism in wild bird populations. Scientific Reports, 10, 20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010) Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
- von Schantz, T. , Bensch, S. , Grahn, M. , Hasselquist, D. & Wittzell, H. (1999) Good genes, oxidative stress and condition–dependent sexual signals. Proceedings of the Royal Society B: Biological Sciences, 266, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma, P. , Selman, C. , Speakman, J.R. & Verhulst, S. (2004) Birds sacrifice oxidative protection for reproduction. Proceedings of the Royal Society B: Biological Sciences, 271, S360–S363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, T.D. , Challanger, W.O. , Christians, J.K. , Evanson, M. , Love, O. & Vezina, F. (2004) What causes the decrease in haematocrit during egg production? Functional Ecology, 18, 330–336. [Google Scholar]
- Xu, Y.‐C. , Yang, D.‐B. , Speakman, J.R. & Wang, D.‐H. (2014) Oxidative stress in response to natural and experimentally elevated reproductive effort is tissue dependent. Functional Ecology, 28, 402–410. [Google Scholar]
- Zuk, M. (2009) The sicker sex. PLoS Path, 5, e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Supplementary Material
Data Availability Statement
All data used in this article are available in the Supporting Information. Sex‐specific body masses, male polygyny score, sexual dichromatism and parental role division are given in Table S2. Species‐ and sex‐specific sample sizes of each physiological parameter, as well as effect size (Hedge's d) indicating the degree of sexual dimorphism in physiological parameters, are given in Table S8. Data used in this manuscript are deposited in Figshare: https://doi.org/10.6084/m9.figshare.18095873.v1.


