Abstract
CIBER (Center for Biomedical Network Research; Centro de Investigación Biomédica En Red) is a public national consortium created in 2006 under the umbrella of the Spanish National Institute of Health Carlos III (ISCIII). This innovative research structure comprises 11 different specific areas dedicated to the main public health priorities in the National Health System. CIBERER, the thematic area of CIBER focused on rare diseases (RDs) currently consists of 75 research groups belonging to universities, research centers, and hospitals of the entire country. CIBERER's mission is to be a center prioritizing and favoring collaboration and cooperation between biomedical and clinical research groups, with special emphasis on the aspects of genetic, molecular, biochemical, and cellular research of RDs. This research is the basis for providing new tools for the diagnosis and therapy of low‐prevalence diseases, in line with the International Rare Diseases Research Consortium (IRDiRC) objectives, thus favoring translational research between the scientific environment of the laboratory and the clinical setting of health centers. In this article, we intend to review CIBERER's 15‐year journey and summarize the main results obtained in terms of internationalization, scientific production, contributions toward the discovery of new therapies and novel genes associated to diseases, cooperation with patients' associations and many other topics related to RD research.
Keywords: genetics, new therapeutic approaches, novel genes, rare diseases, research network

1. INTRODUCTION
In 2006, a public consortium known as “Center for Biomedical Network Research” (CIBER, Centro de Investigación Biomédica En Red) was created under the umbrella of the Spanish National Institute of Health Carlos III (ISCIII). This highly innovative research structure comprises 11 thematic areas dedicated to the main public health priorities of the National Health System (NHS), 1 one of them being devoted to research on rare diseases (CIBERER).
In this article, we review CIBERER's 15‐year journey and summarize the most outstanding results obtained in terms of internationalization, scientific production, gene discovery, contributions toward the discovery of new therapies and novel genes associated to diseases, cooperation with patient associations and many other topics related to RD research.
2. CIBER CONSORTIUM, COLLABORATION, AND NETWORKING IN BIOMEDICAL RESEARCH
The CIBER constitutes a relevant research consortium of excellence in the fields of Biomedicine and Health Sciences within the Spanish NHS and the Science and Technology Research System. CIBER is structured into 11 thematic areas and comprises 427 research groups selected through a competitive process, contributing more than 6000 researchers and a core staff of 804 people (Table 1). 2 CIBER is a national collaborative network consortium belonging to ISCIII, the national body that translates relevant biomedical research results to the NHS. CIBER's thematic areas are: Bioengineering, Biomaterials, and Nanomedicine (CIBERBBN), Diabetes and Metabolic Diseases (CIBERDEM), Physiopathology of Obesity and Nutrition (CIBEROBN), Liver and Digestive Diseases (CIBEREHD), Respiratory Diseases (CIBERES), Epidemiology and Public Health (CIBERESP), Mental Health (CIBERSAM), Frailty and Healthy Aging (CIBERFES), Cardiovascular Diseases (CIBERCV), Cancer (CIBERONC), and Rare Diseases (CIBERER) (www.ciberisciii.es).
TABLE 1.
Characteristics of the CIBER structure and distribution of the 11 thematic areas
| CIBER acronym | CIBER name | Number of research groups (2020) | Total number of researchers in 2020 | Number of publications in 2020 | Mean number of publications per group (2020) | Publications in Q1 (2020) | Percentage of publication in Q1 | Publications in D1 (2020) | Percentage of publication in D1 |
|---|---|---|---|---|---|---|---|---|---|
| BBN | Bioengineering, biomaterials and nanomedicine | 45 | 558 | 739 | 16.4 | 558 | 76 | 211 | 29 |
| CV | Cardiovascular diseases | 40 | 499 | 757 | 18.9 | 441 | 58 | 157 | 21 |
| DEM | Diabetes and associated metabolic diseases | 31 | 250 | 362 | 11.7 | 269 | 74 | 100 | 28 |
| EHD | Liver and digestive diseases | 44 | 402 | 690 | 15.7 | 396 | 57 | 204 | 30 |
| ER | Rare diseases | 57 | 561 | 727 | 12.8 | 454 | 62 | 196 | 27 |
| ES | Respiratory diseases | 33 | 394 | 789 | 23.9 | 391 | 50 | 156 | 20 |
| ESP | Epidemiology and public health | 51 | 557 | 1367 | 26.8 | 764 | 56 | 347 | 25 |
| FES | Frailty and healthy aging | 20 | 145 | 249 | 12.5 | 153 | 61 | 51 | 20 |
| OBN | Physiopathology of obesity and nutrition | 33 | 451 | 747 | 22.6 | 454 | 61 | 114 | 15 |
| ONC | Cancer | 50 | 618 | 711 | 14.2 | 456 | 64 | 238 | 33 |
| SAM | Mental health | 23 | 423 | 852 | 37.0 | 516 | 61 | 197 | 23 |
| Totals (CIBER) | Spanish national biomedical network research (Centro de Investigación Biomédica en Red) | 427 | 4858 | 7990 | 18.7 | 4852 | 61 | 1971 | 25 |
CIBER is integrated by researchers from universities, research centers, hospitals, health administrations and health services, in addition to an important research structure rooted in patient care. There are 104 institutions associated to the CIBER structure. The main aim of the CIBER is to promote collaborative research, favoring synergies from a multidisciplinary approach to science. CIBER relies on a team of expert managers allocated to different technical departments. There are areas supporting the internationalization and project departments managing more than 150 research projects, 30 at European level and 5 in the American continent, besides many collaborations among various research groups. The Technology Transfer Area oversees licensing agreements with industry and manages patent portfolios; the Contracting and Purchasing Department manages most service contracts; and the Institutional Relations Area manages the negotiation of agreements and scientific contracts, providing legal support to the Innovation Unit. Lastly, the Communication Area is dedicated to the dissemination of CIBER's results, both internally (newsletters), and externally.
3. CIBER'S RESOURCES AND INFRASTRUCTURES
CIBER has access to numerous infrastructures distributed nationwide. CIBER is part of and contributes to the National Biobank Network through the CIBERER‐Biobank, a public, non‐profit biorepository centralizing the reception of RD biological samples; the CIBERES‐Pulmonary Biobank Platform, which includes samples and matched clinical information from patients with lung diseases; and FATBANK, whose adipose tissue biobank constitutes a national referent. CIBERES‐Pulmonary Biobank Platform has acted as the coordinator of the National Biobank Network Platform until recently.
Other specialized centralized platforms are: (1) the so‐called Singular Scientific and Technical Infrastructures (ICTS), which are large installations, resources, facilities, and services, unique in its kind, dedicated to cutting edge and high‐quality research and technological development, as well as to promote exchange, transmission and preservation of knowledge, technology transfer, and innovation. CIBER coordinates NANBIOSIS, an important ICTS for the development of nanotechnology or biomaterials‐based products from their design phase to their preclinical validation stage; (2) Bioinformatics platforms: both CIBEREHD and CIBERER areas, have developed platforms performing data quality control services, gene expression analysis, genomic mapping, variant analysis, and data mining; (3) BiblioPRO, a virtual library of documents in Spanish on health‐related quality of life and health outcomes perceived by patients (Patient Reported Outcomes‐PRO); (4) Neuroimaging Platform, a database containing thousands of neuroimages developed by CIBERSAM; (5) Patient registry platforms: the REHEVASC platform collects medical records from patients with rare liver diseases from 17 hospitals nationwide and the National Chronic Hepatitis C Database in collaboration with the Spanish Association for the Study of the Liver (AEEH); and the RENACER Patient Registry is a joint initiative of the CIBERCV and the Spanish Agency of Medicines and Medical Devices that, in collaboration with the Spanish Society of Cardiology and other medical societies, registers cardiovascular disease patients. Additionally, CIBEREHD coordinates the Hepamet cohort and the European NAFLD Registry (non‐alcoholic fatty liver disease), and CIBERER takes part in the National Registry of patients with RDs, being the promoter of GenRaRe, a clinical registry of genetic diseases.
In addition to the aforementioned infrastructure, CIBER participates in the ITEMAS Network, a platform promoted by the ISCIII supporting healthcare innovation. Its objective is to ensure that ideas of health professionals on innovation are delivered to health services.
4. CIBERER: THE CENTER FOR BIOMEDICAL NETWORK RESEARCH ON RARE DISEASES
CIBERER, the thematic area of CIBER devoted to RDs (www.ciberer.es/en) was launched in 2006. At present, it is composed of 75 research groups (57 CIBERER groups plus 18 associated clinical groups) belonging to consolidated research structures from universities, health institutions, research centers and hospitals nationwide. CIBERER is organized into seven different research programs: (a) Translational Genomic Medicine; (b) Inherited Mitochondrial and Metabolic Medicine; (c) Neurological Diseases; (d) Pediatric and Developmental Medicine; (e) Sensorineural Pathology; (f) Endocrine Medicine; and (g) Hereditary Cancer, Hematological and Dermatological Diseases.
The vision of CIBERER is to be a center where collaboration and cooperation between biomedical and clinical research groups are prioritized, with special emphasis on the aspects of genetic, molecular, biochemical, and cellular research on RDs. This research is the basis for providing new diagnostic and therapeutic tools, favoring the translation of research from the scientific environment of the laboratory to healthcare settings. CIBERER is aligned with the new Horizon European Program and it shares the International Rare Diseases Research Consortium (IRDiRC) vision and goals. 3 IRDiRC is an initiative launched in 2010 by the European Commission and the National Health Institutes of the United States to achieve 1000 new therapies and innovative diagnostic tools, in addition to a global network of national registries, by 2027.
The specific CIBERER's objectives are mainly based on the development of new treatments and the improvement in access to the diagnosis of RDs. In order to achieve this challenge, CIBERER maintains close collaboration with all the stakeholders involved in the RD field, both at a national and international level to (a) Establish and provide access to harmonized and relevant RD data and information; (b) Carry out the molecular and clinical characterization of RD; (c) Promote translational, preclinical, and clinical research (d) Rationalize ethical regulations and procedures. To cover all these aspects, the following lines of action were defined: networking, excellence research, collaborative and cooperative research, translation, and transfer of results, as well as social visibility of RDs. In addition, the internationalization of research, the effective relationship with the productive sector and the institutional visibility are continuously being promoted to complement the aforementioned lines of action.
5. CIBER AND CIBERER'S SCIENTIFIC PRODUCTION
The global scientific productivity of the CIBER Consortium in its 15 years of existence has been very relevant, thus becoming one of the top 10 national institutions according to its scientific production. As shown by the relevant statistics, an indexed publication with a CIBER affiliation is displayed every 66 min in Medline; specifically ~ two publications/day have CIBERER affiliation (727 publications in 2020; Table 1 and Figure 1).
FIGURE 1.
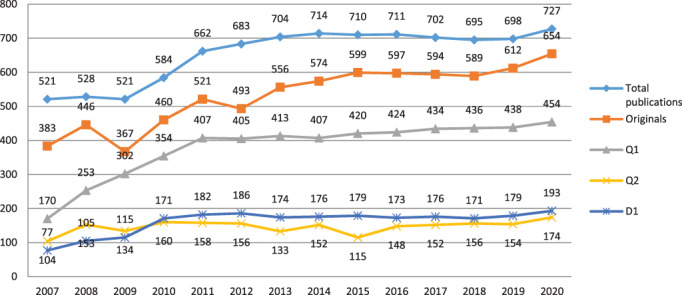
Number of CIBERER publications per year. The total number of original publications in the 2007–2020 period has been 7445 [Colour figure can be viewed at wileyonlinelibrary.com]
Regarding its participation in projects and leadership, currently, CIBERER has more than 30 active grants underway in the frame of different national and international calls. Furthermore, CIBERER has financed 85 intramural projects in the past 10 years, for an overall amount of more than 4.5 million euros. Moreover, CIBERER has recently received close to 8 million euros to deploy two projects focused on the genetic aspects of the COVID‐19 pandemic and the implementation of the IMPaCT project (see below).
6. INTERNATIONAL PARTICIPATION OF CIBERER
CIBERER researchers participate and are beneficiaries of relevant national and international projects within the scope of RDs. Some of the initiatives in which CIBERER is engaged are: (a) Since 2010, CIBERER supports the coordination of Orphanet‐Spain 4 within Orphanet, the portal for RDs and orphan drugs that has become a consortium of 40 countries, within Europe and across the globe. 5 CIBERER is in charge of translation of Orphanet contents into Spanish, which is relevant for more than 470 million people in the world regarding medical, scientific and social aspects of RDs. 5 This resource, along with MAPER, a database of available RD research resources in Spain, makes CIBERER an institution generating and giving access to the largest amount of information about RD related activities in Spain; (b) European Joint Program on Rare Diseases 6 (EJPRD); this specific Joint Action for RD has brought together all those European institutions working in this field with the aim of improving the integration, effectiveness, production and social impact of RD research. The EJPRD brings together more than 130 institutions from 35 countries to create a sustainable ecosystem enabling a virtuous cycle between research, care and medical innovation; (c) The IRDiRC consortium 7 is a global effort to contribute to the development of new therapies and to accelerate and facilitate RD diagnosis. CIBERER played an active role during the genesis of the consortium, being involved in the bilateral meetings held between the European Union (EU) and the National Institutes of Health (NIH), and participating since then and up until recently in the consortium's interdisciplinary working group, in which CIBER members continue performing different responsibilities; (d) The 1 + Million Genomes 8 is one of the most ambitious initiatives in precision medicine worldwide in which CIBER Consortium and CIBERER play a very active role by leading the Spanish action. CIBERER groups are represented in practically all the work programs of the Spanish “mirror group” of this initiative, (e) The RD‐Connect project, 9 which gave rise to the RD‐Connect Community later on, was launched in November 2012 as a 6‐year project funded by the European Commission. CIBERER participated as an associated member of the consortium; (f) Genome‐phenome Archive (EGA) 10 is one of the two largest archives encouraging the distribution and sharing of genomic and phenotypic data, adopting strict protocols for information management, storage, security and dissemination such as those proposed by ELIXIR, an European platform coordinating, integrating and sustaining bioinformatics resources across its member states where many CIBER groups participate very actively; (g) Global Alliance for Genomics and Health (GA4GH) 11 is an international, non‐profit alliance launched in 2013 that brings together more than 600 leading organizations working on the generation of models and standards to enable the responsible, voluntary and secure sharing of omics and health‐related data. CIBERER is one of its members; (h) European Openscreen, 12 an ERIC (European Research Infrastructures Consortium) integrating high‐throughput screening facilities for molecules and drug development comprising more than 140 000 compounds and offering open access to resources for researchers from academic institutions. (i) The European infrastructure EATRIS, 13 the main support structure for translational biomedical research in biomedicine in Europe, is widely represented in the consortium, with 14 health research institutions. Likewise, many of the CIBER's consorted institutions participate in SCReN, the Platform of Clinical Research and Clinical Trial Units, which in turn is integrated into ECRIN, 14 its equivalent at European level; (j) RD‐CODE project 14 promotes the use of the Orphanet nomenclature for implementation into health information systems, thus enabling data sharing at European level in a standardized and consistent manner.
In 2020, The Council of Ministers of the Spanish Government approved “The Spanish Strategy for Personalized Medicine” with a total funding of 77.3 million euros. The first action of the newly adopted strategy, reflected in the Emergency Plan for Science and Innovation, was the Call for the launch of the Infrastructure for Precision Medicine associated with Science and Technology (Infraestructura de Medicina de Precisión Asociada a la Ciencia y Tecnología¸ IMPaCT). With an endowment of 25.8 million euros, it will foster scientific knowledge and increase the capacity to exploit all the information available improving the quality and efficiency of the healthcare system. This initiative comprises three programs: Predictive Medicine, Data Science, and Genomic Medicine, two of which are led by CIBER (Predictive Medicine and Genomic Medicine; CIBERESP and CIBERER, respectively).
CIBERER also coordinates the ENoD initiative (Undiagnosed Rare Diseases Program); this project has achieved a diagnosis for 29% of the patients eligible for the program, thus facilitating appropriate genetic counseling to many families.
7. CIBERER PARTICIPATION IN THE EUROPEAN REFERENCE NETWORKS
European reference networks (ERNs) 15 are virtual networks involving healthcare providers across Europe. They aim to tackle complex or RDs and conditions that require highly specialized treatments and a concentration of knowledge and resources. There are 24 ERNs involving 25 EU countries plus Norway, with over 900 healthcare units located in more than 300 hospitals and covering all major disease groups. CIBERER groups participate in nine ERNs: EURO‐NMD, METAB‐ERN, ERN‐RND, Endo‐ERN, ITHACA, BOND, TRANSPLANTCHILD, ERN GUARD‐HEART, and EuroBloodNet (Table 2).
TABLE 2.
CIBERER representation in the European reference networks
| European reference network (ERN) | Acronym | CIBERER group–Institution |
|---|---|---|
| Rare neuromuscular diseases | EURO‐NMD | Hospital de la Santa Creu i Sant Pau |
| Hospital Universitario La Fe | ||
| Hospital Sant Joan de Déu | ||
| Hospital Universitario Vall d'Hebrón | ||
| Hereditary metabolic disorders | METAB‐ERN | Hospital Universitario 12 de Octubre |
| Hospital Universitario Santiago de Compostela | ||
| Hospital Universitario de Cruces | ||
| Hospital Universitario Vall d'Hebrón | ||
| Hospital Sant Joan de Déu | ||
| Rare neurological diseases | ERN‐RND | Hospital Sant Joan de Déu |
| Hospital Vall d'Hebrón | ||
| Hospital Universitario Clinic de Barcelona | ||
| Rare hematological diseases | EuroBloodNet | Hospital Universitario Vall d'Hebrón |
| Rare endocrine conditions | Endo‐ERN | Hospital Universitario Vall d'Hebrón |
| Hospital Universitario de Cruces | ||
| Hospital de la Santa Creu i Sant Pau | ||
| Rare congenital malformations and rare intellectual disability | ITHACA |
Hospital Universitario La Paz Hospital Sant Joan de Déu Hospital Universitario Vall d'Hebrón |
| Rare bone diseases | BOND |
Hospital Universitario La Paz Hospital Universitario Virgen de la Arrixaca |
| Transplantation in children | TRANSPLANT‐CHILD | Hospital Universitario La Paz |
| Rare and low‐prevalence complex diseases of heart | GUARD‐HEART | Hospital Universitario Virgen de la Arrixaca |
8. MAIN CONTRIBUTION OF GENETIC RESEARCH BY CIBERER
In the past 15 years, CIBERER researchers have contributed to the discovery and description of new entities and/or associated genes. At least 100 new genes associated with human disease have been described by CIBERER groups in the last decade, in many cases (71) leading the publication as first or senior authors (Supplementary Table 1). Similarly, CIBERER researchers have described a number of new disorders. The main contributions occurred in the fields of rare bone diseases, central and peripheral nervous system, metabolics, oncogenetics, or vision and hearing impairments, among others.
CIBERER's groups have contributed with the discovery of many new diseases and genes associated to previously known disorders. For example, one of the many contribution was the finding in 2011 of a new gene (MAX) associated to hereditary pheochromocytoma (PCC) in 8 single patients. 16
9. ORPHAN DRUGS, NEW THERAPIES, AND REPURPOSED DRUGS CONTRIBUTED BY CIBERER
In the past 10 years, CIBERER has contributed with the designation of 25 orphan drugs by either the European Medicines Agency (EMA) or its American counterpart, the Federal Food and Drugs Administration (FDA). CIBERER has sponsored 12 of the 25 orphan drugs. These drugs/compounds with an orphan designation are listed in Table 3.
TABLE 3.
Orphan drug designations with CIBERER as sponsor
| Substance | Indication/RD | Sponsor | Agency | Reference |
|---|---|---|---|---|
| Lentiviral vector carrying the Fanconi anemia‐A (FANCA) gene | Fanconi anemia type A | CIBER |
EMA FDA |
EU/3/10/822 FDA 16–5193 |
| Lentiviral vector containing the human liver and erythroid pyruvate kinase (PKLR) gene | Pyruvate kinase deficiency | CIBER |
EMA FDA |
EU/3/14/1330 FDA 16–5168 |
| Temsirolimus | Adrenoleukodystrophy | CIBER | EMA | EU/3/16/1669 |
| Hematopoietic stem cells modified with a lentiviral vector containing the CD18 gene | Leukocyte adhesion deficiency type I | CIBER |
EMA FDA |
EU/3/16/1753 FDA 16–5453 |
| Ubiquinol | Primary coenzyme Q10 deficiency syndrome | CIBER | EMA | EU/3/16/1765 |
| Metformin | Progressive myoclonic epilepsy type 2 (Lafora disease) | CIBER |
EMA FDA |
EU/3/16/1803 FDA 17–6161 |
| Etamsylate | Hereditary haemorrhagic telangiectasia | CSIC & CIBER | EMA | EU/3/18/2087 |
| Gefitinib | Fanconi anemia | CIBER | EMA | EU/3/18/2075 |
| Afatinib | Fanconi anemia | CIBER | EMA | EU/3/18/2110 |
| Dimethyl fumarate | Adrenoleukodystrophy | CIBER | EMA | EU/3/19/2236 |
| Autologous skin equivalent graft composed of keratinocytes and fibroblasts genetically corrected by CRISPR/Cas9‐mediated excision of mutation‐carrying COL7A1 exon 80 | Epidermolysis bullosa | CIBER | EMA | EU/3/20/2253 |
| L‐Ergothioneine | Cystinuria | CIBER | EMA | EU/3/21/2445 |
In addition, the different research programs developed by CIBERER over the last decade have resulted in 28 patent applications with a therapeutic potential. Seven of them have been licensed, signing long‐term cooperation agreements with leading companies in the pharmaceutical and the biotech sectors. These technologies are being developed to treat different groups of RD including mitochondrial, 17 metabolic, 18 skin, 19 or rare hematological diseases. 20 Thus, it is worth highlighting our ex‐vivo lentiviral vectors‐based gene therapy programs. These are our most advanced programs in clinical phases, three of which are licensed and running in collaboration with Rocket Pharmaceuticals, Inc. Specifically, they are focused on pyruvate kinase deficiency (PKD), 21 leukocyte adhesion deficiency type I (LAD‐1) 22 and Fanconi Anemia (FA), the latest being the most advanced one, running under a globally registered clinical trial. 23 In 2019 CIBERER has reported a successful engraftment of gene‐corrected hematopoietic stem cells in non‐conditioned patients with FA, a DNA repair syndrome generated by mutations in any of the 22 FA genes discovered to date. The authors demonstrated that lentiviral‐mediated hematopoietic gene therapy reproducibly confers engraftment and proliferation advantages of gene‐corrected hematopoietic stem cells supporting that gene therapy should constitute an innovative low‐toxicity therapeutic option for this life‐threatening disorder. 24
10. A HAND‐IN‐HAND COLLABORATION WITH PATIENTS ASSOCIATIONS
CIBERER maintains extensive collaborations with patient associations representing all RDs. Noteworthy is the close collaboration with FEDER (Federación Española de Enfermedades Raras; Spanish Alliance of Rare Diseases), an umbrella organization established in 1999 with the aim of being the voice of ~ three million people living with a low‐prevalence disease in Spain. At present, FEDER has more than 370 affiliated associations and has developed a network of strategic alliances and collaborations with EURORDIS (a non‐governmental patient‐driven alliance of patient organizations representing 949 RD patient organizations in 73 countries.), RDI (Rare Disease International, the global alliance of people living with a RD of all nationalities across all RDs), SWAN (Syndromes Without A Name) and ALIBER (Ibero‐American Alliance for RDs).
In 2017, CIBERER constituted the Patient Advisory Council (PAC) with the aim of fostering patients' participation in CIBERER's activities as a means to know first‐hand their expectations and needs and to achieve their engagement in CIBERER's research policies. The PAC is integrated by various patient associations, nominated from the different CIBERER research programs and renewed every 2 years.
CIBERER and FEDER have signed collaboration agreements focused on FEDER's participation in both the Scientific Advisory Board (SAB) and the PAC with two designated members, and on CIBERER's participation as advisor at the Information and Guidance Service for patients (Servicio de Información y Orientación; SIO) launched and managed by FEDER. Additionally, most Principal Investigators of CIBERER's research groups are involved with patient associations, serving as Scientific Advisors or as members of their Scientific Committees.
11. THE SPECIALTY OF GENETICS OR MEDICAL GENETICS IN SPAIN
Spain is the only country in Europe that does not currently have this specialty. It is essential that the specialty of Clinical Genetics is approved in Spain to facilitate the training of specialists in the diagnosis, management and treatment of genetic diseases. The lack of regulation of the specialty causes a great heterogeneity in the type of genetic services provided in the Hospitals with a serious inequity of patient access in the NHS. This situation delays diagnoses and their corresponding treatments in patients, hinders the prevention of new cases in families, reduces the efficiency of the health system (by requesting unnecessary or irrelevant tests) and does not guarantee that all professionals who provide genetic services are adequately trained for this activity, which is detrimental to the health of citizens and the quality of life of patients. CIBERER as many other stakeholders have been reporting this irregular situation for years.
12. FINAL REMARKS
Although Spain has excellent standards in Public Health Services, comparable to those of the most advanced economies in the world, there are still some hurdles preventing timely diagnosis and treatment of RDs patients and a full deployment of Genomic Medicine in the country. Some of these barriers are: (a) the lack of an official Clinical Genetics specialty;( b) the inequality in access to public and universal high‐quality genetics and genomic medicine services; c) the existence of 17 Spanish Regional Health Systems responsible for implementing health policies; and (d) the partial implementation of Next Generation Sequencing (NGS) technologies in the country, which could reduce the public NHS's spending significantly.
In this context, CIBERER has emerged as a flagship research institution, concentrating interdisciplinary and multi‐institutional research efforts and resources devoted to RDs in Spain, with a preferential dedication of its financial resources to the RDs networks integrated by centers and research groups dependent on different administrations and public and private institutions.
After an experience of 15 years, CIBERER has shown a clear orientation to generate an impact on the health of the RD community and become an exceptional partner in Spain in the path to achieve the IRDiRC objectives.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose. All authors declare that they have no conflicts of interest according to editorial policies and ethical considerations.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/cge.14113.
Supporting information
Supplemental table 1 New genes reported in the last 10 years with CIBERER participation.
ACKNOWLEDGEMNTS
This study has been funded by Instituto de Salud Carlos III (ISCIII) and Spanish Ministry of Science and Innovation
APPENDIX A.
The CIBERER network
Carmen Aguado1,26; Cristina Aguado27,1; Paula Aguilera149,7,1;Virginia Albiñana28,1; Laura Alías1,3−5; Berta Almoguera29,1; Javier Alonso30,1; Verónica Alonso‐Ferreira31,1; María Isabel Alvarez‐Mora32,7,1; Guillermo Antiñolo34,1; María L. Arbones35,1; Joaquín Arenas36,1; Emi Arjona28,1; Thais Armangue37,38,39,1; Judith Armstrong40,1; María Arnedo41,1; Rafael Artuch1,6; Anna Aulinas Masó17,42,1; Almudena Avila‐Fernandez29,1; Carmen Ayuso29,1; Isabel Badell43,5,1; Celia Badenas32,44,1; María L. Baeza45,46,1; Montserrat Baiget1,3; Susanna Balcells47,48,49,1; María Juliana Ballesta‐Martínez50,51,52,1; María‐José Barahona53,1; Francisco Barros1,14; Paola Chiara Bartoccioni54,1; M. Pilar Bayona‐Bafaluy55,56,57,1; Sara Benito Sanz1,23,24; Carmelo Bernabéu28,1; Sara Bernal1,3−5; Fiona Blanco‐Kelly29,1; Alberto Blázquez58,36,1; Susanna Bodoy54,59,1; Massimo Bogliolo60,61,1; Cristina Borralleras62,1; Salud Borrego34,1; Luisa M. Botella28,1; Francesc Bou de Pieri1,9; Paola Bovolenta63,1; Nereida Bravo‐Gil34,1; Alejandro Brea1,14; Gloria Bueno‐Lozano64,1; Juan Bueren1,2; Ana Bustamante29,1; Teresa Caballero65,24,1; Carlos Camacho‐Macorra63,1; Yolanda Cámara66,1; Núria Camats‐Tarruella67,1; Ángel Campos Barros1,23,24; Victoria Campuzano68,1; Lara Cantarero1,20; Judith Cantó69,7,1; José Antonio Caparrós‐Martín70,1; Francesc Cardellach69,7,1; Rosario Carmona71,72,1; Ángel Carracedo1,13,14; Cristina Carrera149,7,1; Marta Carretero73,74,1; Mercedes Casado75,1; José Antonio Casado1,2; Carlos Casasnovas76,77,1; Alberto Cascón78,1; Patricia Casino79,80,1; Luis Castaño81,82,83,84,1; Laura Castilla‐Vallmanya47,48,49,1; Albert Catala85,86,1; María Luisa Cayuela87,51,1; Rafael Cediel88,70,1; Javier Cervera89,90,1; Marta Codina‐Solà91,1; Julio Contreras92,70,1; Bru Cormand47,48,49,1; Roser Corominas93,1; Javier Corral94,51,1; Virginia Corrochano1; Ana Cortés‐Rodríguez95,96,1; Marta Corton29,1; Mar Costa‐Roger91,1; Mónica Cozar47,48,49,1; Iris Crespo97,1; Fátima Crispi98,7,1; Raquel Cruz1,13; José M. Cuezva63,99,1; Ivon Cuscó91,67,1; Josep Dalmau100,7,1; Sergio de Cima89,1; Susana de la Luna101,102,1; Noemí De Luna103,1; Alfonso de Oyarzabal Sanz104,1; Miguel del Campo105,106,1; Ignacio del Castillo107,1; Lucía Del Pino Molina108,24,1; Ángela Del Pozo1,23,24; Marcela del Río109,73,74,1; Aitor Delmiro58,36,1; Lourdes R. Desviat110,24,1; Mara Dierssen101,111,1; Cristina Domínguez‐González112,36,1; María Domínguez‐Ruiz107,1; Joaquín Dopazo71,72,1; Ekaitz Errasti54,1; María José Escámez113,73,74,1; Maria Cristina Estañ70,1; Jesús Esteban114,1; Raúl Estévez115,1; Begoña Ezquieta1,23,24; Luis Fernández1,23,24; Almudena Fernández1,12; Mónica Fernández‐Cancio67,1; Noèlia Fernàndez‐Castillo47,48,49,1; Patricia Fernández‐San Jose107,1; Cristina Fillat1,7,8; Carmen Fons116,1; Joana Fort54,117,1; Stéphane Fourcade76,1; Mario F. Fraga118,1; Pía Gallano1,3−5; Eduard Gallardo103,1; Marta García113,73,74,1; Elena García‐Arumí66,1; María García‐Bravo1,2; Angels García‐Cazorla116,1; Inés García‐Consuegra36,1; Francesc Josep Garcia‐Garcia69,7,1; Gema García‐García119,1; José Luis García‐Giménez120,121,1; María Adelaida Garcia‐Gimeno122,1; Sixto García‐Miñaur1,23,24; Alberto García‐Redondo114,1; M. Teresa García‐Silva123,36,1; Judit García‐Villoria124,7,1; Fe García Santiago1,23,24; Gloria Garrabou69,7,1; Gema Garrido1,12; Nuria Garrido‐Pérez55,56,57,1; Sonia Gaztambide81,84,82,1; Mercedes Gil‐Campos125,126 127,1; Judith Giroud‐Gerbetant54,1; Guillermo Glover50,51,1; Beatriz Gómez1; Paulino Gómez‐Puertas63,1; Pilar Gonzalez‐Cabo120,121,1; Ingrid Gonzalez‐Casacuberta69,7,1; María González‐del Pozo34,1; Lidia González‐Quereda1,3−5; Adrián González‐Quintana58,36,1; Laura Gort124,7,1; Nadine Gougeard89,1; Eduard Gratacos98,8,7,1; Josep María Grau69,7,1; Daniel Grinberg47,48,49,1; Guillermo Güenechea1,2; Rosa Guerrero70,24,1; Encarna Guillén‐Navarro50,128,51,1; Mariona Guitart‐Mampel69,7,1; Armand Gutiérrez‐Arumí129,1; Karen Heath1,23,24,163; Miguel Heredia89,1; Concepción Hernández‐Chico107,1; Enrique Herreras1; Janet Hoenicka1,20; Aïda Homs1,9; Juan Andrés Jimenez‐Estrada70,1; Cecilia Jimenez‐Mallebrera130,1; Cristina Jou131,1; Diana Luz Juarez‐Flores69,7,1; Pablo Lapunzina23,24,25,1; Fernando Larcher73,113,74,1; Adriana Lasa1,3−5; Luis Lassaletta132,24,1; Ana Latorre‐Pellicer133,1; Daniel Linares1,7; José Luis Llacer89,1; Sara Llames134,135,74,1; Ester Lopez‐Gallardo55,56,1; Eduardo López‐Laso136,126 127,1; Alberto López‐Lera1,24; Daniel Lopez‐Lopez71,72,1; Marcos López‐Sánchez1,9; Miguel López de Heredia1; Eduardo López Granados108,24,1; Isabel Lorda‐Sanchez29,1; María Luisa Lozano94,51,1; Juan Luque1; Irene Madrigal32,7,1; Josep Malvehy149,7,1; Cristina Manguán García70,24,1; Elena Mansilla1,23,24; Clara Marco‐Marín89,1; Gemma Marfany137,48,49,1; Alberto Marina89,1; Ramón Martí66,1; Salvador Martí1,26; Yolanda Martin107,1; Miguel A. Martín58,36,1; Elena Martín‐Hernandez.123,36,1; Inmaculada Martin‐Merida29,1; Rosa Martínez83,84,82,81,1; Francisco Martínez‐Azorín36,1; Beatriz Martinez‐Delgado138,1; Núria Martínez‐Gil47,48,49,1; Víctor M. Martínez‐Glez23,24,25,1; M.A. Martínez‐Momblán139,1; María Carmen Martínez‐Romero50,51,52,1; Pilar Martínez Fernández1,23,24; Lucía Martínez Santamaría113,73,74,1; Loreto Martorell40,1; Patricia Meade55,56,57,1; Álvaro Meana134,135,74,1; Miguel Ángel Medina140,141,1; Andrés Medrano1; Ingrid Mendes1; Cristina Méndez‐Vidal34,1; José M. Millán1,15,16; Pablo Minguez29,1; Jordi Minguillón60,3,61,1; Serena Mirra137,48,49,1; Belén Molla89,1; Eduardo Moltó12,142,1; Raquel Montero75,1; Lluis Montoliu1,12; Julio Montoya55,56,1; María Morán36,1; Constanza Moren69,7,1; Mireia Moreno89,1; José Carlos Moreno1,23,24; Antonio Moreno‐Galdó67,1; Miguel Ángel Moreno‐Pelayo107,1; María A Mori1,23,24; Matías Morin107,1; Beatriz Morte1; Victoriano Mulero51,143,1; Gerard Muñoz‐Pujol124,7,1; Rodolfo Murillas73,74,1; Silvia Murillo‐Cuesta70,24,1; Andrés Nascimento130,1; Susana Navarro1,2; Plácido Navas96,1; Julián Nevado23,24,25,1; Adrià Nicolas54,1; M. Ángela Nieto144,1; Mar O′Callaghan116,1; Leticia Olavarrieta107,1; Aida Ormazabal75,1; Paula Ortiz‐Romero1,9; Ana Osorio145,1; David Páez146,1; Manuel Palacín54,117,1; M. Gabriela Palacios‐Verdú147,1; Francesc Palau1,19−22; Adrián Palencia‐Campos70,1; Federico V. Pallardó120,121,1; María Palomares1,23,24; María Peña‐Chilet71,72,1; Belén Pérez110,24,1; Javier Perez‐Florido71,72,1; Débora Pérez‐García148,1; Eva Perez‐Jimenez89,1; Luis A. Pérez‐Jurado1,9−11; James R. Perkins140,141,1; Rosario Perona70,24,1; Juan Pie133,1; Tomàs Pinós66,1; Sheila Pinto28,1; Miriam Potrony32,7,1; Susana Puig149,7,1; Joan Antón Puig‐Butille150,33,7,1; Beatriz Puisac133,1; Roser Pujol60,3,1; Aurora Pujol76,102,1; Óscar Quintana1,2; Raquel Rabionet47,48,49,1; María José Ramírez de Haro60,3,1; Feliciano J. Ramos151,133,1; Juan A.G. Ranea140,141,1; Judith Reina‐Castillón152,1; Eugenia Resmini1,5,17; Antonia Ribes124,7,1; Itxaso Rica81,83,84,1; Eva Richard110,63,24,1; Pau Riera153,1; Paula Río1,2; Rosa Riveiro‐Alvarez29,1; José Rivera94,51,1; Ana Rivera‐Barahona70,1; Mercedes Robledo78,1; Juan Carlos Rodriguez‐Aguilera95,1; Lourdes Rodríguez‐de la Rosa70,24,1; Agustí Rodríguez‐Palmero76,154,1; Pilar Rodriguez‐Pombo110,63,24,1; Laia Rodriguez‐Revenga32,7,1; Benjamín Rodríguez‐Santiago1,3; Víctor Rodríguez‐Sureda155,156,1; Marta Rodríguez de Alba29,1; Santiago Rodríguez de Cordoba28,1; Carlos Romá‐Mateo120,121,1; Vicente Rubio89,1; Ángela Ruiz28,1; Montserrat Ruiz76,1; Carlos Ruiz‐Arenas1,9; Victor Luis Ruiz‐Perez70,23,1; Eduardo Ruiz‐Pesini55,56,1; Clara Ruiz‐Ponte1,14; Josep Rullo54,1; Lidia Sabater100,7,1; Juliana Salazar146,1; Eduardo Salido157,1; Carolina Sanchez‐Jimeno29,1; Ana María Sánchez Cuesta96,1; María José Sánchez Soler50,51,1; Fulvio Santacatterina63,1; Marta Santamarina1,14; Alicia Santos1,17; Carlos Santos‐Ocaña96,1; Fernando Santos Simarro1,23,24; Pascual Sanz89,1; Leandro Sastre70,24,1; Agatha Schlüter76,1; José Carlos Segovia1,2; María Segura‐Puimedon158,1; Pedro Seoane140,141,1; Clara Serra‐Juhe1,3−5; Mercedes Serrano116,1; José M. Serratosa159,1; Teresa Sevilla160,161,1; Jordi Surrallés60,3,61,1; Saoud Tahsin‐Swafiri29,1; Gemma Tell‐Martí149,7,1; Jair Antonio Tenorio‐Castaño23,24,25,1; Eduardo Tizzano91,67,1; Ester Tobias69,7,1; Frederic Tort124,7,1; Laura Trujillano162,1; María José Trujillo‐Tiebas29,1; Cristina Ugalde36,1; Olatz Ugarteburu124,7,1; Roser Urreizti75,1; Inés Urrutia83,84,82,81,1; María Valencia70,1; Patricia Vallcorba1,23,24; Elena Vallespín1,23,24; Isabel Varela‐Nieto70,24,1; Ana Vega1,14; Valentina Vélez‐Santamaria76,77,1; Juan J. Vílchez160,1; Olaya Villa158,1; Manuela Villamar107,1; Susan M. Webb1,17,18; José Manuel Zubeldia45,46,1; Olga Zurita29,1.
1, Centre for Biomedical Network Research on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid, Spain.
2, Hematopoietic Innovative Therapies Division, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT), Instituto de Investigaciones Sanitarias Fundación Jiménez Díaz (IIS‐FJD), Madrid, Spain.
3, Genetics Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
4, Institut de Recerca Hospital de la Santa Creu i Sant Pau (IIB Sant Pau), Barcelona, Spain.
5, Universitat Autònoma de Barcelona, Barcelona, Spain.
6, Clinical Biochemistry Department, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
7, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
8, Universitat de Barcelona, Barcelona, Spain.
9, Department of Experimental and Health Sciences, Universitat Pompeu Fabra (UPF), Barcelona, Spain.
10, Genetics Service, Hospital del Mar, Barcelona, Spain.
11, Hospital del Mar Research Institute (IMIM), Barcelona, Spain.
12, Department of Molecular and Cellular Biology, National Centre for Biotechnology (CNB‐CSIC), Madrid, Spain.
13, Grupo de Medicina Xenómica, Centro Singular de Investigación en Medicina Molecular y Enfermedades Crónicas (CIMUS), Universidade de Santiago de Compostela, Santiago de Compostela, Spain.
14, Fundación Pública Galega de Medicina Xenómica (SERGAS), IDIS, Santiago de Compostela, Spain.
15, Unidad de Genética, Hospital Universitario y Politécnico La Fe, Valencia, Spain.
16, Biomedicina Molecular Celular y Genómica, Instituto Investigación Sanitaria La Fe, Valencia, Spain.
17, Hospital S Pau, Dept Medicine/Endocrinology, IIB‐Sant Pau, Research Center for Pituitary Diseases, Barcelona, Spain.
18, Departamento de Medicina, Universitat Autònoma de Barcelona, Barcelona, Spain.
19, Department of Genetic and Molecular Medicine, Hospital Sant Joan de Deu, Barcelona, Spain.
20, Laboratory of Neurogenetics and Molecular Medicine ‐ IPER, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
21, Institute of Medicine & Dermatology, Hospital Clínic, Barcelona, Spain.
22, Division of Pediatrics, University of Barcelona School of Medicine, Barcelona, Spain.
23, INGEMM‐Instituto de Genética Médica y Molecular,Hospital Universitario La Paz, Madrid, Spain.
24, Instituto de Investigación Sanitaria Hospital La Paz (IdiPAZ), Madrid, Spain.
25, ERN‐ITHACA.
26, CIBERER Biobank, Valencia, Spain.
27, Laboratorio de Oncología, Pangaea Oncology, Hospital Quirón Dexeus, Barcelona, Spain.
28, Centro de Investigaciones Biologicas‐Margarita Salas (CSIC), Madrid, Spain.
29, Department of Genetics, Health Research Institute‐Fundación Jiménez Díaz University Hospital, Universidad Autónoma de Madrid (IIS‐FJD, UAM), Madrid, Spain.
30, Unidad de Tumores Sólidos Infantiles, Instituto de Investigación de Enfermedades Raras, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain.
31, Instituto de Investigación de Enfermedades Raras, Instituto de Salud Carlos III, Madrid, Spain.
32, Biochemistry and Molecular Genetics Department, Hospital Clínic de Barcelona, Universitat de Barcelona, Barcelona, Spain.
33, Biochemistry and Molecular Genetics Department, Hospital Clínic de Barcelona, University of Barcelona, Spain.
34, Department of Maternofetal Medicine, Genetics and Reproduction, Institute of Biomedicine of Seville, University Hospital Virgen del Rocío/CSIC/University of Seville, Seville, Spain.
35, Instituto de Biología Molecular de Barcelona (IBMB), CSIC, Barcelona, Spain.
36, Grupo de Enfermedades Mitocondriales y Neuromusculares, Instituto de Investigación Hospital 12 de Octubre (imas12), Madrid, Spain.
37, Neuroimmunology Program, Institut d'Investigació Biomèdica August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
38, Hospital Clínic, University of Barcelona, Barcelona, Spain.
39, Pediatric Neuroinmunology Unit, Sant Joan de Deu Children's Hospital, University of Barcelona, Barcelona, Spain.
40, Genetics Department, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
41, Unidad de Genética Clínica y Genética Funcional, Facultad de Medicina, Universidad de Zaragoza, Spain.
42, Departamento de Medicina, Universitat de Vic–Universitat Central de Catalunya, Vic, Barcelona, Spain.
43, Unidad de Hematología Pediátrica, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
44, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
45, Allergy Service, Hospital General Universitario Gregorio Marañón, Madrid, Spain.
46, Gregorio Marañón Health Research Institute (IiGSM), Madrid, Spain.
47, Departamento Genética, Microbiologia y Estadística, Facultad Biología, Universitat de Barcelona, Barcelona, Spain.
48, Institut de Biomedicina Universitat de Barcelona (IBUB), Barcelona, Spain.
49, Institut de Recerca Sant Joan de Déu (IRSJD), Barcelona, Spain.
50, Sección de Genética Médica, Servicio de Pediatría, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain.
51, Instituto Murciano de Investigación Biosanitaria (IMIB), Murcia, Spain.
52, Universidad Católica San Antonio, Murcia, Spain.
53, Endocrinology Department, Hospital Universitari Mútua de Terrassa, Barcelona, Spain.
54, Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technolo‐gy (BIST), Laboratory of Amino acid Transporters and Disease, Barcelona, Spain.
55, Departamento de Bioquímica, Biología Molecular y Celular, Universidad de Zaragoza, Zaragoza, Spain.
56, Instituto de Investigación Sanitaria (IIS) de Aragón, Zaragoza, Spain.
57, Instituto de Biocomputación y Física de Sistemas Complejos (BIFI), Universidad de Zaragoza, Zaragoza, Spain.
58, Servicio de Bioquímica, Hospital Universitario 12 de Octubre, Madrid, Spain.
59, Universitat de Vic‐Central de Catalunya, Vic, Spain.
60, Genomic Instability and DNA Repair Syndromes Group and Joint Research Unit on Genomic Medicine UAB‐Sant Pau Biomedical Research Institute (IIB Sant Pau), Institut de Recerca Hospital de la Santa Creu i Sant Pau‐IIB Sant Pau, Barcelona, Spain.
61, Department of Genetics and Microbiology, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain.
62, Gebro Pharma Lab, Barcelona, Spain.
63, Centro de Biología Molecular Severo Ochoa, Consejo Superior de Investigaciones Científicas‐Universidad Autónoma de Madrid, Madrid, Spain.
64, Unidad Endocrinología, Servicio Pediatría, Hospital Clínico Universitario ‘Lozano Blesa’, Zaragoza, Spain.
65, Allergy Department, Hospital Universitario La Paz, Madrid, Spain.
66, Research Group on Neuromuscular and Mitochondrial Diseases, Vall d'Hebron Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
67, Vall d'Hebron Institut de Recerca (VHIR), Vall d'Hebron Hospital Universitari, Barcelona, Spain.
68, Departament de Biomedicina, Facultat de Medicina i Ciències de la Salut, Universitat de Barcelona, Barcelona, Spain.
69, Muscle Research and Mitochondrial Function Laboratory, Faculty of Medicine and Health Sciences‐University of Barcelona, Internal Medicine Department‐Hospital Clinic of Barcelona, Barcelona, Spain.
70, Instituto de Investigaciones Biomédicas “Alberto Sols”, CSIC‐UAM, Madrid, Spain.
71, Clinical Bioinformatics Area, Fundación Progreso y Salud (FPS), CDCA, Hospital Virgen del Rocio, Sevilla, Spain.
72, Computational Systems Medicine, Institute of Biomedicine of Seville (IBIS), Hospital Virgen del Rocio, Sevilla, Spain.
73, División de Biomedicina Epitelial, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT), Madrid, Spain.
74, IIS‐FJD, Madrid, Spain.
75, Clinical Biochemistry Department, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
76, Neurometabolic Diseases Laboratory, Bellvitge Biomedical Research Institute (IDIBELL), L'Hospitalet de Llobregat, Spain.
77, Neuromuscular Unit, Neurology Department, Hospital Universitari de Bellvitge, Universitat de Barcelona, L'Hospitalet de Llobregat, Spain.
78, Grupo de Cáncer Endocrino, Programa de Genética del Cáncer Humano, Centro Nacional de Investigaciones Oncológicas, CNIO Spain.
79, Departament de Bioquímica i Biología Molecular, Universitat de València, Burjassot, Spain.
80, Estructura de Recerca Interdisciplinar en Biotecnologia i Biomedicina (ERI BIOTECMED), Universitat de València, Burjassot, Spain.
81, Cruces University Hospital, UPV/EHU, Barakaldo, Spain.
82, Endo‐ERN.
83, Biocruces Bizkaia Health Research Institute, Barakaldo, Spain.
84, Centre for Biomedical Network Research Diabetes and Associated Metabolic Disorders (CIBERDEM), Instituto de Salud Carlos III, Madrid, Spain.
85, Pediatric Hematology and Oncology departments, Hospital Sant Joan de Deú, Barcelona, Spain.
86, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
87, Grupo de Telomerasa, Cáncer y Envejecimiento, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain.
88, Department of Animal Medicine and Surgery, Complutense University of Madrid, Madrid, Spain.
89, Instituto de Biomedicina de Valencia, Consejo Superior de Investigaciones Científicas (IBV‐CSIC), Valencia, Spain.
90, Laboratorio de Reconocimiento Molecular, Centro de Investigación Príncipe Felipe, Valencia, Spain.
91, Department of Clinical and Molecular Genetics, Hospital Vall d'Hebron, Barcelona, Spain.
92, Anatomy and Embriology Department, Faculty of Veterinary, Universidad Complutense de Madrid, Madrid, Spain.
93, Department of Genetics, Microbiology and Statistics, Universitat de Barcelona, Barcelona, Spain.
94, Servicio de Hematología y Oncología Médica, Hospital Universitario Morales Meseguer, Centro Regional de Hemodonación, Universidad de Murcia, Murcia, Spain.
95, Servicio de Fisiopatologia Celular y Bioenergética, Universidad Pablo de Olavide, Sevilla, Spain.
96, Centro Andaluz de Biología del Desarrollo, Universidad Pablo de Olavide‐CSIC‐JA, Sevilla, Spain.
97, School of Medicine and Health Sciences, Universitat Internacional de Catalunya, Barcelona, Spain.
98, BCNatal‐Fetal Medicine Research Center (Hospital Clínic and Hospital Sant Joan de Déu, University of Barcelona), Barcelona, Spain.
99, Instituto de Investigación Hospital 12 de Octubre (i + 12), Madrid, Spain.
100, Service of Neurology, Hospital Clínic, Barcelona, Spain.
101, Centre for Genomic Regulation (CRG), The Barcelona Institute of Science and Technology (BIST), Barcelona, Spain.
102, Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain.
103, Lab Neuromuscular diseases, Institut de Recerca Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain.
104, Synaptic metabolism laboratory, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
105, Division of Dysmorphology and Teratology, Department of Pediatrics, University of California San Diego (UCSD), La Jolla, CA, USA.
106, Division of Genetics, Rady Children's Hospital San Diego, San Diego, CA, USA.
107, Servicio de Genética, Hospital Universitario Ramón y Cajal, IRYCIS, Madrid, Spain.
108, Clinical Immunology Department, La Paz University Hospital and Lymphocyte Pathophysiology in Immunodeficiencies Group, Madrid, Spain.
109, Universidad Carlos III de Madrid, Departamento de Bioingeniería e Ingeniería Aeroespacial, Madrid, Spain.
110, Centro de Diagnóstico de Enfermedades Moleculares, Centro de Biología Molecular, Universidad Autónoma de Madrid, Madrid, Spain.
111, Universitat Pompeu Fabra (UPF), Barcelona, Spain.
112, Servicio de Neurología, Unidad de Enfermedades Neuromusculares, Hospital Universitario 12 de Octubre, Madrid, Spain.
113, Universidad Carlos III de Madrid, Departamento de Bioingeniería e Ingeniería Aeroespacial, Madrid, Spain.
114, Unidad de Eclerosis Lateral Amiotrófica (ELA‐ALS), Instituto de Investigación Hospital 12 de Octubre (imas12), Madrid, Spain.
115, Unitat de Fisiologia, Department de Ciències Fisiològiques, IDIBELL‐Institut de Neurociències, Universitat de Barcelona, L'Hospitalet, Spain.
116, Neuropediatrics Department, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
117, Department of Biochemistry and Molecular Biomedicine, Universitat de Barcelona, Barcelona, Spain.
118, Cancer Epigenetics and Nanomedicine Laboratory, Department of Organisms and Systems Biology, Nanomaterials and Nanotechnology Research Center (CINN‐CSIC), Health Research Institute of Asturias (ISPA), Institute of Oncology of Asturias (IUOPA), University of Oviedo, Oviedo, Spain.
119, Instituto de Investigación Sanitaria La Fe, Valencia, Spain.
120, Department of Physiology, Faculty of Medicine and Dentistry, Universitat de València, Valencia, Spain.
121, Biomedical Research Institute INCLIVA, Valencia, Spain.
122, Department of Biotechnology, Polytechnic University of Valencia, Valencia, Spain.
123, Servicio de Pediatría, CSUR Errores Congénitos del Metabolismo, Hospital Universitario 12 de Octubre, Madrid, Spain.
124, Secció d'Errors Congènits del Metabolisme–IBC, Servei de Bioquímica i Genètica Molecular, Hospital Clínic de Barcelona, Barcelona, Spain.
125, Pediatric Research and Metabolism Unit, Reina Sofia University Hospital, Córdoba, Spain.
126, Maimónides Institute for Biomedical Research of Córdoba (IMIBIC), Córdoba, Spain.
127, University of Córdoba, Córdoba, Spain.
128, Departamento de Pediatría, Universidad de Murcia (UMU), Murcia, Spain.
129, Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona, Spain.
130, Neuromuscular Unit, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
131, Pathology Department, Institut de Recerca Sant Joan de Déu, Barcelona, Spain.
132, Department of Otorhinolaryngology, La Paz University Hospital, Madrid, Spain.
133, Unidad de Genética Clínica y Genómica Funcional, Facultad de Medicina, Universidad de Zaragoza, Zaragoza, Spain.
134, Centro Comunitario de Sangre y Tejidos del Principado de Asturias, Oviedo, Spain.
135, División de Biomedicina Epitelial, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (UNIDAD MIXTA CCST‐PA CIEMAT), Madrid, Spain.
136, Pediatric Neurology Unit, Department of Pediatrics, University Hospital Reina Sofía, Córdoba, Spain.
137, Dept, de Genètica, Microbiologia i Estadística, Universitat de Barcelona, Barcelona, Spain.
138, Molecular Genetics, Instituto de Investigación de Enfermedades Raras (IIER), Instituto de Salud Carlos III (ISCIII), Madrid, Spain.
139, Department of Fundamental and Medical‐Surgical Nursing, School of Nursing, Faculty of Medicine and Health Sciences, Universitat de Barcelona, Barcelona, Spain.
140, Department of Molecular Biology and Biochemistry, University of Málaga, Málaga, Spain.
141, Institute of Biomedical Research in Málaga (IBIMA), Málaga, Spain.
142, Biochemistry Section, Faculty of Biochemistry and Environmental Sciences and Regional Centre for Biomedical Research, Universidad de Castilla‐La Mancha, Toledo, Spain.
143, Departamento de Biología Celular e Histología, Facultad de Biología, Universidad de Murcia, Murcia, Spain.
144, Instituto de Neurociencias (CSIC‐UMH), Sant Joan d'Alacant, Spain.
145, Unidad de Cáncer Familiar, Programa de Genética del Cáncer Humano, Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, Spain.
146, Oncology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
147, Fundació Dexeus Salut de la Dona, Barcelona, Spain.
148, Departament de Psicología Clínica i de la Salut, Universitat Autònoma de Barcelona, Bellaterra, Barcelona, Spain.
149, Dermatology Department, Melanoma Unit, Hospital Clínic Barcelona, Barcelona, Spain.
150, Molecular Biology CORE, Biomedical Diagnostic Center (CDB), Hospital Clínic de Barcelona, University of Barcelona, Barcelona, Spain.
151, Unidad de Genética Clínica, Servicio de Pediatría, Hospital Clínico Universitario “Lozano Blesa”, Zaragoza, Spain.
152, Reproductive Genetics Unit, IVI, Madrid, Spain.
153, Pharmacy Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
154, Pediatric Neurology Unit, Department of Pediatrics, Hospital Universitari Germans Trias i Pujol, Universitat Autònoma de Barcelona, Catalonia, Spain.
155, Molecular Biology and Biochemistry Research Centre for Nanomedicine (CIBBIM‐Nanomedicine), Vall d'Hebron Institut de Recerca, Universitat Autònoma de Barcelona, Barcelona, Spain.
156, BCNatal ‐ Fetal Medicine Research Center (Hospital Clínic and Hospital Sant Joan de Déu, University of Barcelona), Barcelona, Spain.
157, Hospital Universitario de Canarias, Universidad de La Laguna, Santa Cruz de Tenerife, Spain.
158, Quantitative Genomic Medicine Laboratories (qGenomics), Esplugues del Llobregat, Spain.
159, Epilepsy Unit, Neurology Service, Hospital Universitario and IIS Fundación Jiménez Díaz, Madrid, Spain.
160, Neuromuscular & Ataxias Research group, Instituto de Investigación Sanitaria La Fe, Valencia, Spain.
161, University of Valencia School of Medicine, Valencia, Spain.
162, Servicio de Genética Clínica y Molecular, Hospital Val d'Hebron, Barcelona, Spain.
163, Skeletal dysplasia multidisciplinary Unit (UMDE), ERN‐BOND, Hospital Universitario la Paz, Madrid, Spain.
Luque J, Mendes I, Gómez B, et al. CIBERER: Spanish national network for research on rare diseases: A highly productive collaborative initiative. Clinical Genetics. 2022;101(5‐6):481-493. doi: 10.1111/cge.14113
The CIBERER Network group affiliations are mentioned in Appendix A.
Funding information Instituto de Salud Carlos III; Ministerio de Ciencia e Innovación
Contributor Information
Pablo Lapunzina, Email: pablo.lapunzina@salud.madrid.org, Email: plapunzina@ciberer.es.
The CIBERER Network:
Carmen Aguado, Cristina Aguado, Paula Aguilera, Virginia Albiñana, Laura Alías, Berta Almoguera, Javier Alonso, Verónica Alonso‐Ferreira, María Isabel Alvarez‐Mora, Guillermo Antiñolo, María L. Arbones, Joaquín Arenas, Emi Arjona, Thais Armangue, Judith Armstrong, María Arnedo, Rafael Artuch, Anna Aulinas Masó, Almudena Avila‐Fernandez, Carmen Ayuso, Isabel Badell, Celia Badenas, María L. Baeza, Montserrat Baiget, Susanna Balcells, María Juliana Ballesta‐Martínez, María‐José Barahona, Francisco Barros, Paola Chiara Bartoccioni, M. Pilar Bayona‐Bafaluy, Sara Benito Sanz, Carmelo Bernabéu, Sara Bernal, Fiona Blanco‐Kelly, Alberto Blázquez, Susanna Bodoy, Massimo Bogliolo, Cristina Borralleras, Salud Borrego, Luisa M. Botella, Francesc Bou de Pieri, Paola Bovolenta, Nereida Bravo‐Gil, Alejandro Brea, Gloria Bueno‐Lozano, Juan Bueren, Ana Bustamante, Teresa Caballero, Carlos Camacho‐Macorra, Yolanda Cámara, Núria Camats‐Tarruella, Ángel Campos Barros, Victoria Campuzano, Lara Cantarero, Judith Cantó, JoséAntonio Caparrós‐Martín, Francesc Cardellach, Rosario Carmona, Ángel Carracedo, Cristina Carrera, Marta Carretero, Mercedes Casado, JoséAntonio Casado, Carlos Casasnovas, Alberto Cascón, Patricia Casino, Luis Castaño, Laura Castilla‐Vallmanya, Albert Catala, María Luisa Cayuela, Rafael Cediel, Javier Cervera, Marta Codina‐Solà, Julio Contreras, Bru Cormand, Roser Corominas, Javier Corral, Virginia Corrochano, Ana Cortés‐Rodríguez, Marta Corton, Mar Costa‐Roger, Mónica Cozar, Iris Crespo, Fátima Crispi, Raquel Cruz, JoséM. Cuezva, Ivon Cuscó, Josep Dalmau, Sergio de Cima, Susana de la Luna, NoemíDe Luna, Alfonso de Oyarzabal Sanz, Miguel del Campo, Ignacio del Castillo, Lucía Del Pino Molina, Ángela Del Pozo, Marcela del Río, Aitor Delmiro, Lourdes R. Desviat, Mara Dierssen, Cristina Domínguez‐González, María Domínguez‐Ruiz, Joaquín Dopazo, Ekaitz Errasti, María José Escámez, Maria Cristina Estañ, Jesús Esteban, Raúl Estévez, Begoña Ezquieta, Luis Fernández, Almudena Fernández, Mónica Fernández‐Cancio, Noèlia Fernàndez‐Castillo, Patricia Fernández‐San Jose, Cristina Fillat, Carmen Fons, Joana Fort, Stéphane Fourcade, Mario F. Fraga, Pía Gallano, Eduard Gallardo, Marta García, Elena García‐Arumí, María García‐Bravo, Angels García‐Cazorla, Inés García‐Consuegra, Francesc Josep Garcia‐Garcia, Gema García‐García, JoséLuis García‐Giménez, María Adelaida Garcia‐Gimeno, Sixto García‐Miñaur, Alberto García‐Redondo, M. Teresa García‐Silva, Judit García‐Villoria, Fe García Santiago, Gloria Garrabou, Gema Garrido, Nuria Garrido‐Pérez, Sonia Gaztambide, Mercedes Gil‐Campos, Judith Giroud‐Gerbetant, Guillermo Glover, Beatriz Gómez, Paulino Gómez‐Puertas, Pilar Gonzalez‐Cabo, Ingrid Gonzalez‐Casacuberta, María González‐del Pozo, Lidia González‐Quereda, Adrián González‐Quintana, Laura Gort, Nadine Gougeard, Eduard Gratacos, Josep María Grau, Daniel Grinberg, Guillermo Güenechea, Rosa Guerrero, Encarna Guillén‐Navarro, Mariona Guitart‐Mampel, Armand Gutiérrez‐Arumí, Karen Heath, Miguel Heredia, Concepción Hernández‐Chico, Enrique Herreras, Janet Hoenicka, Aïda Homs, Juan Andrés Jimenez‐Estrada, Cecilia Jimenez‐Mallebrera, Cristina Jou, Diana Luz Juarez‐Flores, Pablo Lapunzina, Fernando Larcher, Adriana Lasa, Luis Lassaletta, Ana Latorre‐Pellicer, Daniel Linares, JoséLuis Llacer, Sara Llames, Ester Lopez‐Gallardo, Eduardo López‐Laso, Alberto López‐Lera, Daniel Lopez‐Lopez, Marcos López‐Sánchez, Miguel López de Heredia, Eduardo López Granados, Isabel Lorda‐Sanchez, María Luisa Lozano, Juan Luque, Irene Madrigal, Josep Malvehy, Cristina Manguán García, Elena Mansilla, Clara Marco‐Marín, Gemma Marfany, Alberto Marina, Ramón Martí, Salvador Martí, Yolanda Martin, Miguel A. Martín, Elena Martín‐Hernandez., Inmaculada Martin‐Merida, Rosa Martínez, Francisco Martínez‐Azorín, Beatriz Martinez‐Delgado, Núria Martínez‐Gil, Víctor M. Martínez‐Glez, M.A. Martínez‐Momblán, María Carmen Martínez‐Romero, Pilar Martínez Fernández, Lucía Martínez Santamaría, Loreto Martorell, Patricia Meade, Álvaro Meana, Miguel Ángel Medina, Andrés Medrano, Ingrid Mendes, Cristina Méndez‐Vidal, JoséM. Millán, Pablo Minguez, Jordi Minguillón, Serena Mirra, Belén Molla, Eduardo Moltó, Raquel Montero, Lluis Montoliu, Julio Montoya, María Morán, Constanza Moren, Mireia Moreno, JoséCarlos Moreno, Antonio Moreno‐Galdó, Miguel Ángel Moreno‐Pelayo, María A Mori, Matías Morin, Beatriz Morte, Victoriano Mulero, Gerard Muñoz‐Pujol, Rodolfo Murillas, Silvia Murillo‐Cuesta, Andrés Nascimento, Susana Navarro, Plácido Navas, Julián Nevado, Adrià Nicolas, M. Ángela Nieto, Mar O′Callaghan, Leticia Olavarrieta, Aida Ormazabal, Paula Ortiz‐Romero, Ana Osorio, David Páez, Manuel Palacín, M. Gabriela Palacios‐Verdú, Francesc Palau, Adrián Palencia‐Campos, Federico V. Pallardó, María Palomares, María Peña‐Chilet, Belén Pérez, Javier Perez‐Florido, Débora Pérez‐García, Eva Perez‐Jimenez, Luis A. Pérez‐Jurado, James R. Perkins, Rosario Perona, Juan Pie, Tomàs Pinós, Sheila Pinto, Miriam Potrony, Susana Puig, Joan Antón Puig‐Butille, Beatriz Puisac, Roser Pujol, Aurora Pujol, Óscar Quintana, Raquel Rabionet, María JoséRamírez de Haro, Feliciano J. Ramos, Juan A.G. Ranea, Judith Reina‐Castillón, Eugenia Resmini, Antonia Ribes, Itxaso Rica, Eva Richard, Pau Riera, Paula Río, Rosa Riveiro‐Alvarez, José Rivera, Ana Rivera‐Barahona, Mercedes Robledo, Juan Carlos Rodriguez‐Aguilera, Lourdes Rodríguez‐de la Rosa, Agustí Rodríguez‐Palmero, Pilar Rodriguez‐Pombo, Laia Rodriguez‐Revenga, Benjamín Rodríguez‐Santiago, Víctor Rodríguez‐Sureda, Marta Rodríguez de Alba, Santiago Rodríguez de Cordoba, Carlos Romá‐Mateo, Vicente Rubio, Ángela Ruiz, Montserrat Ruiz, Carlos Ruiz‐Arenas, Victor Luis Ruiz‐Perez, Eduardo Ruiz‐Pesini, Clara Ruiz‐Ponte, Josep Rullo, Lidia Sabater, Juliana Salazar, Eduardo Salido, Carolina Sanchez‐Jimeno, Ana María Sánchez Cuesta, María JoséSánchez Soler, Fulvio Santacatterina, Marta Santamarina, Alicia Santos, Carlos Santos‐Ocaña, Fernando Santos Simarro, Pascual Sanz, Leandro Sastre, Agatha Schlüter, JoséCarlos Segovia, María Segura‐Puimedon, Pedro Seoane, Clara Serra‐Juhe, Mercedes Serrano, JoséM. Serratosa, Teresa Sevilla, Jordi Surrallés, Saoud Tahsin‐Swafiri, Gemma Tell‐Martí, Jair Antonio Tenorio‐Castaño, Eduardo Tizzano, Ester Tobias, Frederic Tort, Laura Trujillano, María JoséTrujillo‐Tiebas, Cristina Ugalde, Olatz Ugarteburu, Roser Urreizti, Inés Urrutia, María Valencia, Patricia Vallcorba, Elena Vallespín, Isabel Varela‐Nieto, Ana Vega, Valentina Vélez‐Santamaria, Juan J. Vílchez, Olaya Villa, Manuela Villamar, Susan M. Webb, JoséManuel Zubeldia, and Olga Zurita
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Pàmpols T, Ramos FJ, Lapunzina P, Gozalo‐Salellas I, Pérez‐Jurado LA, Pujol A. A view on clinical genetics and genomics in Spain: of challenges and opportunities. Mol Genet Genomic Med. 2016;4(4):376‐391. doi: 10.1002/mgg3.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CIBER Activity Annual Report .; 2020. https://www.ciberisciii.es/Memorias/2019/ENG/mobile/index.html#p=1
- 3. IRDiRC vision and goals. 2021. https://irdirc.org/about-us/vision-goals/
- 4. Punto de acceso al sitio web de Orphanet‐España. 2021. http://www.orphanet-espana.es/national/ES-ES/index/inicio/
- 5. 2019 Activity Report, Orphanet Report Series, Report Collection; 2020. https://www.orpha.net/orphacom/cahiers/docs/GB/ActivityReport2019.pdf
- 6. Project structure. 2021. https://www.ejprarediseases.org/what-is-ejprd/project-structure/
- 7. Cutillo CM, Austin CP, Groft SC. A global approach to rare diseases research and orphan products development: the international rare diseases research consortium (IRDiRC). Adv Exp Med Biol. 2017;1031:349‐369. doi: 10.1007/978-3-319-67144-4_20 [DOI] [PubMed] [Google Scholar]
- 8. 1+Million Genomes Roadmap 2020–2022; 2020. https://digitalhealtheurope.eu/results-and-publications/2020-2022-roadmap-of-the-1million-genomes-initiative/
- 9. Thompson R, Johnston L, Taruscio D, et al. RD‐connect: an integrated platform connecting databases, registries, biobanks and clinical bioinformatics for rare disease research. J Gen Intern Med. 2014;29(suppl 3):s780–787. doi: 10.1007/s11606-014-2908-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lappalainen I, Almeida‐King J, Kumanduri V, et al. The European genome‐phenome archive of human data consented for biomedical research. Nat Genet. 2015;47(7):692‐695. doi: 10.1038/ng.3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Framework for responsible sharing of genomic and health‐related data. 2021. https://www.ga4gh.org/genomic-data-toolkit/regulatory-ethics-toolkit/framework-for-responsible-sharing-of-genomic-and-health-related-data/ [DOI] [PMC free article] [PubMed]
- 12. Brennecke P, Rasina D, Aubi O, et al. EU‐OPENSCREEN: a novel collaborative approach to facilitate chemical biology. SLAS Discov. 2019;24(3):398‐413. doi: 10.1177/2472555218816276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eatris–strategic plan 2019–2022; 2018. https://eatris.eu/wp-content/uploads/2018/12/Strategic_Plan_2019-2022_Final_Version.pdf
- 14. RD‐CODE leaflet summary 2019. 2021. http://www.rd-code.eu/wp-content/uploads/2019/06/RDCODE_KOM_Leaflet_V2.pdf
- 15. Héon‐Klin V. European reference networks for rare diseases: what is the conceptual framework? Orphanet J Rare Dis. 2017;12(1):137. doi: 10.1186/s13023-017-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Comino‐Méndez I, Gracia‐Aznárez FJ, Schiavi F, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43(7):663‐667. doi: 10.1038/NG.861 [DOI] [PubMed] [Google Scholar]
- 17. Cámara Y, González‐Vioque E, Scarpelli M, et al. Administration of deoxyribonucleosides or inhibition of their catabolism as a pharmacological approach for mitochondrial DNA depletion syndrome. Hum Mol Genet. 2014;23(9):2459‐2467. doi: 10.1093/HMG/DDT641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pujol A. Novel therapeutic targets and drug candidates for modifying disease progression in adrenoleukodystrophy. Endocr Dev. 2016;30:147‐160. doi: 10.1159/000439340 [DOI] [PubMed] [Google Scholar]
- 19. Bonafont J, Mencía Á, García M, et al. Clinically relevant correction of recessive dystrophic epidermolysis bullosa by dual sgRNA CRISPR/Cas9‐mediated gene editing. Mol Ther. 2019;27(5):986‐998. doi: 10.1016/J.YMTHE.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bueren JA, Quintana‐Bustamante O, Almarza E, et al. Advances in the gene therapy of monogenic blood cell diseases. Clin Genet. 2020;97(1):89‐102. doi: 10.1111/CGE.13593 [DOI] [PubMed] [Google Scholar]
- 21. Navarro S, Quintana‐Bustamante O, Sanchez‐Dominguez R, et al. Preclinical studies of efficacy thresholds and tolerability of a clinically ready lentiviral vector for pyruvate kinase deficiency treatment. Mol Ther Methods Clin Dev. 2021;22:350‐359. doi: 10.1016/J.OMTM.2021.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di L‐R, Aldea M, Sanchez‐Baltasar R, et al. Lentiviral vector‐mediated correction of a mouse model of leukocyte adhesion deficiency type I. Hum Gene Ther. 2016;27(9):668‐678. doi: 10.1089/HUM.2016.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lentiviral‐mediated gene therapy for pediatric patients with fanconi anemia subtype A. 2021. https://clinicaltrials.gov/ct2/show/NCT04069533
- 24. Río P, Navarro S, Wang W, et al. Successful engraftment of gene‐corrected hematopoietic stem cells in non‐conditioned patients with Fanconi anemia. Nat Med. 2019;25(9):1396‐1401. doi: 10.1038/S41591-019-0550-Z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table 1 New genes reported in the last 10 years with CIBERER participation.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


