Abstract
Objective
The ObserVational survey of the Epidemiology, tReatment and Care of MigrainE (OVERCOME; United States) study is a multicohort, longitudinal web survey that assesses symptomatology, consulting, diagnosis, treatment, and impact of migraine in the United States.
Background
Regularly updating population‐based views of migraine in the United States provides a method for assessing the quality of ongoing migraine care and identifying unmet needs.
Methods
The OVERCOME (US) 2018 migraine cohort involved: (I) creating a demographically representative sample of US adults using quota sampling (n = 97,478), (II) identifying people with active migraine in the past year via a validated migraine diagnostic questionnaire and/or self‐reported medical diagnosis of migraine (n = 24,272), and (III) assessing consultation, diagnosis, and treatment of migraine (n = 21,143). The current manuscript evaluated whether those with low frequency episodic migraine (LFEM; 0–3 monthly headache days) differed from other categories on outcomes of interest.
Results
Among the migraine cohort (n = 21,143), 19,888 (94.1%) met our International Classification of Headache Disorders, 3rd edition‐based case definition of migraine and 12,905 (61.0%) self‐reported a medical diagnosis of migraine. Respondents’ mean (SD) age was 42.2 (15.0) years; 15,697 (74.2%) were women. Having at least moderate disability was common (n = 8965; 42.4%) and around half (n = 10,783; 51.0%) had consulted a medical professional for migraine care in the past year. Only 4792 (22.7%) of respondents were currently using a triptan. Overall, 8539 (40.4%) were eligible for migraine preventive medication and 3555 (16.8%) were currently using migraine preventive medication. Those with LFEM differed from moderate and high frequency episodic migraine and chronic migraine on nearly all measures of consulting, diagnosis, and treatment.
Conclusion
The OVERCOME (US) 2018 cohort revealed slow but steady progress in diagnosis and preventive treatment of migraine. However, despite significant impact among the population, many with migraine have unmet needs related to consulting for migraine, migraine diagnosis, and getting potentially beneficial migraine treatment. Moreover, it demonstrated the heterogeneity and varying unmet needs within episodic migraine.
Keywords: diagnosis, episodic migraine, headache, migraine, treatment, unmet need
Abbreviations
- AMPP
American Migraine Prevalence and Prevention Study
- AMS
American Migraine Study
- CGRP
calcitonin gene‐related peptide
- CIs
confidence intervals
- CM
chronic migraine
- EM
episodic migraine
- HCP
healthcare providers
- HFEM
high frequency episodic migraine
- ICHD‐3
International Classification of Headache Disorders, 3rd edition
- IRB
Institutional Review Board
- LFEM
low frequency episodic migraine
- MAST
Migraine in America Symptoms and Treatment
- MFEM
moderate frequency episodic migraine
- MIDAS
Migraine Disability Assessment
- ORs
odds ratios
- OTC
over‐the counter
- OVERCOME
ObserVational survey of the Epidemiology, tReatment and Care of MigrainE
- RRs
rate ratios
- SD
standard deviation
- SR‐MD
self‐reported a medical diagnosis
- US
United States
INTRODUCTION
Migraine is a chronic neurological disease that affects ~15% of individuals in the United States 1 , 2 and causes substantial personal and economic costs. 2 , 3 , 4 , 5 , 6 , 7 The World Health Organization ranks migraine as the second leading cause of years lived with disability and the leading cause of disability in women age 15–49 years. 8 Monitoring patterns of consultation, diagnosis, and treatment provides a method for assessing the quality of ongoing medical care and identifying barriers to better outcomes. The US population‐based studies of migraine over the past 30 years have provided ongoing snapshots of migraine 9 , 10 , 11 , 12 , 13 that reflect evolving consulting, diagnostic, medication, and impact/burden patterns (Table 1). These studies have shown that the percentage of those responding to population‐based surveys whose symptoms identify them as having migraine has gone up over time and the impact of migraine may be increasing. 9 , 10 , 11 , 12 , 13 Emerging developments in migraine treatment may herald a new era of migraine care. 14 In particular, novel monoclonal antibodies that target calcitonin gene‐related peptide (CGRP), small molecule CGRP receptor antagonists, serotonin 5‐HT1F agonists, devices, and biobehavioral approaches offer healthcare providers (HCPs) a broader range of treatment options. 15 , 16
TABLE 1.
US national surveys focused on migraine using ICHD criteria a
| Study name | ||||||
|---|---|---|---|---|---|---|
| Study characteristic | AMS‐I 9 | AMS‐II 10 | AMPP 11 | CaMEO 12 | MAST 13 | OVERCOME (US) |
| Focus of study and the migraine community at time of study initiation | Estimate migraine prevalence/magnitude of disease and identify acute treatment needs | Characterize changes in diagnosis and acute treatment patterns with addition of novel migraine‐specific acute treatments | Reassess the prevalence/impact of migraine and characterize patterns of preventive medication use and unmet prevention needs | Understand the natural history of migraine over the course of 1 year including transitions from EM to CM | Assess the current symptomatology and acute treatment patterns of the population in anticipation of novel migraine‐specific acute treatments | Understand the consultation, diagnosis, treatment, impact, and unmet needs of those with migraine overall and across monthly headache day categories concurrent with addition of novel migraine‐specific preventive and acute treatments |
| Study type | Cross‐sectional, single cohort | Cross‐sectional, single cohort | Longitudinal | Longitudinal | Longitudinal | Longitudinal |
| Annual assessments for 5 years | Quarterly assessments for 1 year | One 6 month follow‐up assessment | Semi‐annual follow‐up assessments for up to 2 years | |||
| Single cohort | Single cohort | Single cohort | Multiple cohorts | |||
| Age range, years | 12–80 | 12 and older | 12 and older | 12 and older | 18 and older | 18 and older |
| Disease‐related inclusion criteria | ICHD‐based diagnostic questionnaire | ICHD‐based diagnostic questionnaire | ICHD‐based diagnostic questionnaire | ICHD‐based diagnostic questionnaire | ICHD‐based diagnostic questionnaire | ICHD‐based diagnostic questionnaire or self‐reported medical diagnosis of migraine |
| Data collection | Mailed survey | Mailed survey | Mailed survey | Web‐based Survey | Web‐based survey | Web‐based survey |
| Sampling type | Stratified random sample | Stratified random sample | Stratified random sample | Quota sampled | Quota sampled | Simple random sample derived from quota sample |
| Year of initial—final assessment | 1989 | 1999 | 2004–2009 | 2012–2013 | 2017–2018 | 2018–2022 (planned) |
| Respondent type | Individuals in a household | Individuals in a household | Individuals in a household | Individual | Individual | Individual |
| Respondents (individuals) | 20,468 | 29,727 | 162,576 | 80,783 | 117,150 | Total TBD |
| Respondents with migraine completing full survey | 2479 | 3738 | 18,968 | 16,789 | 18,353 | Total TBD |
Abbreviations: AMPP, American Migraine Prevalence and Prevention; AMS, American Migraine Study; CaMEO, Chronic Migraine Epidemiology and Outcomes; CM, chronic migraine; EM, episodic migraine; ICHD, International Classification of Headache Disorders; MAST, Migraine in America Symptoms and Treatment; OVERCOME, ObserVational survey of the Epidemiology, tReatment and Care of MigrainE; TBD, to be determined.
The ObserVational survey of the Epidemiology, tReatment and Care of MigrainE (OVERCOME; United States) study is a longitudinal, multicohort, web‐based study annually recruited in a demographically representative sample of the US population. The primary objective of OVERCOME (US) is to monitor changes in patterns of consultation, diagnosis, acute and preventive treatment for migraine, and the impact of migraine over time as novel classes of treatment come into wider use. The current manuscript is the primary analyses of the initial OVERCOME (US) 2018 cohort and establishes the baseline of consulting, treatment, and impact of migraine while also considering potentially relevant covariates, such as sociodemographic characteristics and having a self‐reported medical diagnosis (SR‐MD) of migraine overall and across monthly headache day categories. We hypothesized that those with low frequency episodic migraine (LFEM; 0–3 monthly headache days) would differ from those with moderate frequency EM (MFEM; 4–7 monthly headache days), high frequency EM (HFEM; 8–14 monthly headache days), and chronic migraine (CM; ≥15 monthly headache days) on sociodemographics, consultation, and treatment patterns.
METHODS
Study design
OVERCOME (US) is a prospective, longitudinal (up to 2 years), multicohort, web‐based survey of adults with and without migraine in the United States. The current analyses focus specifically on the 2018 migraine cohort baseline cross‐sectional survey, fielded from September to November 2018. The study received approval from Sterling Institutional Review Board (IRB ID #6425‐001); all respondents provided electronic informed consent to participate in a general health‐related survey. Participation was voluntary and respondents received a nominal honorarium (survey panel points that accumulate and can be used toward gift cards and other cash equivalent vouchers).
OVERCOME (US) is a closed survey and requires panel members to log‐in to participate. Following consent, cookies were created to identify unique users, however, identifiable data were not collected in the study data. Prior to fielding, the survey underwent qualitative testing. During and following fielding, quality control measures included verifying programmed response ranges, performing consistency checks, evaluating length of interview, and ensuring an answer for each question was entered before moving to the next. Where appropriate, response options of “prefer not to answer,” “don’t know,” “does not apply to me,” and “don't remember” were included to accommodate those unable or unwilling to provide a definitive answer to a specific question. When appropriate, survey response option randomization and adaptive question logic were applied. Table S4 provides full details regarding the programming, recruitment, enrollment, and survey administration of OVERCOME (US).
Establishing the migraine cohort involved three phases: (1) creating a demographically representative sample of US adults, (2) identifying those with active migraine, and (3) characterizing symptomatology, consultation, treatment, and impact of migraine. Figure 1 provides a diagram of respondent flow.
FIGURE 1.
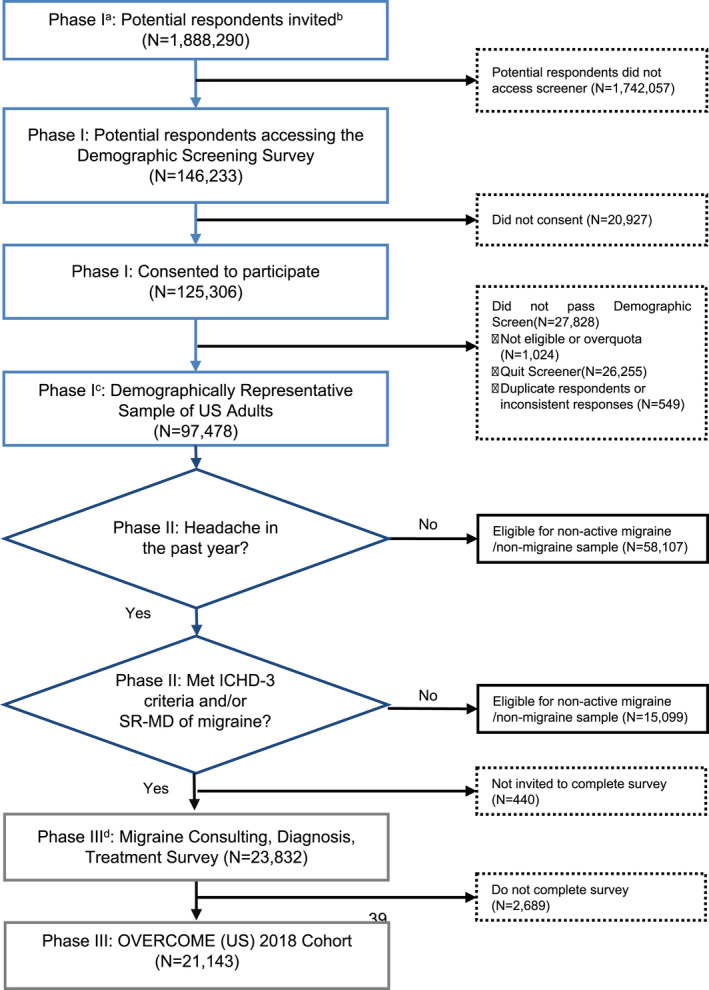
Consort diagram for OVERCOME (US) 2018 migraine cohort wave 1 (N = 21,143). SR‐MD, self‐reported medical diagnosis of migraine. aPhase I = Creating a demographically representative sample of US adults. bTargeted sampling to represent the US adult population in terms of key demographic characteristics (age, sex, race, and geography) were applied. cPhase II = Identifying Respondents with Migraine. dPhase III = Establishing the Migraine Cohort. ICHD‐3, International Classification of Headache Disorders 3rd edition; OVERCOME, Observational survey of the Epidemiology, Treatment, and Care of Migraine; SR‐MD, self‐reported medical diagnosis of migraine [Color figure can be viewed at wileyonlinelibrary.com]
Phase I: Creating a demographically representative sample of US adults
The purpose of phase I was to create, via quota sampling, a respondent population that was demographically representative of US adults reflecting the marginal distribution of geography (Northeast, Midwest, South, and West), age (18–34, 35–44, 45–54, 55–64, and 65+ years) race (American Indian/Alaska native, Asian/Asian American/Native Hawaiian/Asian or Pacific Islander, Black/African American, White/Caucasian, and other), and sex, with age and race nested in sex.
Potential respondents came from five commercial consumer survey panels: Lightspeed Research, Dynata, DISQO, EMI Research Solutions, and Market Cube. These panels recruit members via email, e‐newsletter campaigns, banner placement, partnerships, and direct mail and require double opt‐in (individuals sign up for the panel and then confirm participation via email; see Table S4 for further details).
Inclusion criteria for phase I were: (1) age 18 years or older, (2) US resident, (3) online survey panel member, (4) internet access, (5) ability to read and write English, and (6) ability to provide electronic informed consent. Email invitations, taking into account an individual’s demographic characteristics, were sent in batches to random panel members to participate in a general health survey. Sample performance was monitored daily (using results from a demographic screener within the OVERCOME survey) and as quota targets were reached, the random selection process was refined to target panel members matching demographic characteristics for quotas not yet reached. Industry standards (e.g., using digital fingerprints) were applied to retain confidentiality while preventing multiple entries from the same individual across panels. A total of 97,478 individuals responded to the invitation, consented to participate, were eligible, completed the demographic screener, and made up the demographically representative sample of US adults.
Phase II: Identifying respondents with migraine
The purpose of phase II was to identify respondents with migraine in the demographically representative sample. This phase involved initially asking a series of questions surrounding the respondent’s health and comorbidities, including a question about whether the respondent had at least one headache in the past 12 months not associated with head injury/illness/hangover. These potentially eligible individuals were assessed for migraine in two ways: (1) they completed the validated American Migraine Study (AMS)/American Migraine Prevalence and Prevention Study (AMPP) migraine diagnostic questionnaire to determine if they had migraine (algorithmic details can be found elsewhere) 9 , 10 , 11 , 17 and/or (2) they had an SR‐MD of migraine defined by indicating that a health care provider had told them that they had migraine” and/or “chronic migraine or transformed migraine.” We identified 23,832 individuals with migraine and also sampled a control group of 10,000 individuals without active migraine in the past year and no SR‐MD of migraine. The control group was demographically matched to the US Census data. The sample of respondents (n = 23,832) who met the above criteria for migraine were invited to complete the Migraine Assessment Survey in order to achieve our sample aim of at least 20,000 with migraine (to analyze smaller subgroups and account for potential loss at follow‐up surveys).
Phase III: Establishing the migraine cohort
Those with migraine were invited to complete the phase III survey, which assessed consultation, treatment, and impact of migraine. To be included in the migraine cohort, individuals were required to answer all questions assessing the consultation, treatment, and impact of migraine. Among the 23,832 with migraine, 2689 (11.3%) did not complete the entire assessment and were not included in the migraine cohort. OVERCOME (US) aimed to have at least 20,000 people with migraine in each cohort in order to provide 90% power for detecting statistically significant differences within the cohort longitudinal analyses at 1 year and allow for evaluation of smaller subgroups that would not be feasible with a smaller baseline sample. The 21,143 people who made up the 2018 migraine cohort met the cohort sample size aim.
Measures
Table S1 provides a full list of domains measured within Wave 1. Sociodemographic data included in the current analyses were age (truncated at 85 years for data privacy), sex, marital status, employment status, household income (reported in $25K increments where $100K and above was consolidated for modeling purposes), education (where groups were consolidated into less than high school degree, high school degree/less than college degree, college degree or more for modeling purposes), ethnicity, and race. Migraine‐related characteristics included age at migraine diagnosis, years between first attack and diagnosis, average monthly headache days (over the past 3 months). The Migraine Symptom Severity Scale assessed how often specific migraine‐related symptoms (unilateral pain, pulsatile pain, moderate or severe pain, pain made worse by activity, nausea, photophobia, and phonophobia) were experienced (response options included: “never,” “rarely,” “less than half the time,” “half the time or more,” and “all or nearly all the time”). 13 , 18 Symptom presence was defined as a response of “half the time or more” or “all or nearly all the time.” Migraine related‐disability was assessed using the five‐item Migraine Disability Assessment (MIDAS) scale. 19 , 20 MIDAS quantifies the number of days an individual missed/had reduced productivity at work/home/social events over the last 3 months. 20 , 21 The number of days are categorized into the following disability grades: I: 0–5 = little/no disability; II: 6–10 = mild; III: 11–20 = moderate; and IV: ≥21 = severe.
Respondents with migraine were asked about two aspects of health care consulting for headache: (1) lifetime consultation by specialty (primary care, neurology, headache specialist, pain specialist, emergency department, urgent care, retail clinic, and other) for headache/migraine (marking “yes” for all that applied) and then reporting the number of visits by specialty in the last 12 months and (2) reporting the number of HCP visits by specialty for any reason in the last 12 months. Within the survey, the term “specialist” (e.g., “headache specialist”) did not imply a formal designation (e.g., United Council for Neurological Subspecialties certification or National Headache Foundation Certificate of Added Qualification in Headache Medicine).
Respondents identified lifetime and current use of acute and preventive medications for migraine available at the time of the survey. Acute medication use was defined as “currently using or typically keeping on hand”; preventive medication use was defined by having “taken or used in the last 3 months” for migraine prevention. Eligibility for migraine prevention could be met in one of three ways: 3 monthly headache days with severe disability (MIDAS ≥21), 4–5 monthly headache days with at least some disability (MIDAS ≥6), or ≥6 monthly headache days. 10 , 11 , 15 Individuals also identified lifetime and current use of nonsurgical neurostimulation or biobehavioral treatments for migraine prevention. Both brand and generic name(s) for acute and preventive medications were provided in the survey.
Statistical analysis
Mean and standard deviations (SDs) or median and range were reported for continuous variables and frequencies; percentages were reported for categorical variables. Sensitivity and positive predictive values of an SR‐MD of migraine were calculated. Given that those who did not have a headache in the last 12 months not due to injury/illness were not asked any further questions regarding migraine, specificity the negative predictive value was not calculable. Results were calculated for the overall cohort and then stratified by monthly headache day category (LFEM, MFEM, HFEM, and CM) to test our hypothesis that individuals with LFEM would differ from those with MFEM, HFEM, and CM in sociodemographics, consultation, and treatment patterns. To test these hypotheses, we utilized unadjusted independent regression modeling in which we assessed the relationship between respondents’ characteristics with LFEM to MFEM, HFEM, and CM groups. Logistic regression models were used for dichotomized outcomes (odds ratios [ORs]), linear regression models were used for continuous variables with a normal distribution (beta), and Poisson regression models were used for continuous variables with a Poisson distribution (rate ratios [RRs]). The 95% confidence intervals (CIs) were calculated with alpha set to 0.05 to evaluate the difference between LFEM and the group to which it is being compared (MFEM, HFEM, and CM) on the outcome/measure of interest. Results where the 95% CI did not include 1.00 for OR and RR, or 0.00 for beta, indicated a significant difference among the groups. The null hypothesis was that there was no difference between LFEM and other headache categories on these variables. For certain variables, the “prefer not to answer” response was excluded from analysis (see Table 2). Statistical analyses were performed using SAS Software version 9.4 (SAS Institute Inc., Cary, NC).
TABLE 2.
Demographic features, consultation, diagnosis, and treatment use; stratified by monthly headache days OVERCOME (US) 2018 migraine cohort
| Total | Monthly headache days | Comparison between monthly headache day groups OR/RR/beta (95% CI) q | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–3 | 4–7 | 8–14 | ≥15 | ||||||||||
| (N = 21,143) | (N = 12,299) | (N = 4070) | (N = 2291) | (N = 2483) | 4–7 vs. 0–3 | 8–14 vs. 0–3 | ≥15 vs. 0–3 | ||||||
| N/mean | %/SD/range | N/mean | %/SD/range | N/mean | %/SD/range | N/mean | %/SD/range | N/mean | %/SD/range | ||||
| Demographics | |||||||||||||
| Age, years | 42.2 | 15.0 | 42.3 | 15.3 | 41.5 | 14.5 | 41.4 | 14.4 | 43.5 | 14.8 | Beta = −0.83 (−1.36, −0.30) | Beta = −0.93 (−1.60, −0.26) | Beta = 1.20 (0.55, 1.85) |
| Female | 15,697 | 74.2% | 8807 | 71.6% | 3101 | 76.2% | 1786 | 78.0% | 2003 | 80.7% | OR = 1.27 (1.17, 1.38) | OR = 1.40 (1.26, 1.56) | OR = 1.65 (1.49, 1.84) |
| Marital status a | |||||||||||||
| Married | 9288 | 43.9% | 5505 | 44.8% | 1757 | 43.2% | 958 | 41.8% | 1068 | 43.0% | OR = 0.972 (0.91, 1.04) | OR = 1.00 (0.92, 1.10) | OR = 1.04 (0.95, 1.13) |
| Living with partner | 2577 | 12.2% | 1399 | 11.4% | 501 | 12.3% | 332 | 14.5% | 345 | 13.9% | |||
| Single | 5901 | 27.9% | 3545 | 28.8% | 1164 | 28.6% | 611 | 26.7% | 581 | 23.4% | |||
| Divorced | 2234 | 10.6% | 1232 | 10.0% | 437 | 10.7% | 254 | 11.1% | 311 | 12.5% | |||
| Widowed | 619 | 2.9% | 339 | 2.8% | 109 | 2.7% | 70 | 3.1% | 101 | 4.1% | |||
| Separated | 450 | 2.1% | 234 | 1.9% | 90 | 2.2% | 61 | 2.7% | 65 | 2.6% | |||
| Prefer not to answer b | 74 | 0.3% | 45 | 0.4% | 12 | 0.3% | 5 | 0.2% | 12 | 0.5% | |||
| Household income c | |||||||||||||
| Less than $25,000 | 4575 | 21.6% | 2535 | 20.6% | 889 | 21.8% | 514 | 22.4% | 637 | 25.7% | |||
| $25,000 to $49,999 | 5703 | 27.0% | 3199 | 26.0% | 1111 | 27.3% | 667 | 29.1% | 726 | 29.2% | |||
| $50,000 to $74, 999 | 4155 | 19.7% | 2446 | 19.9% | 817 | 20.1% | 433 | 18.9% | 459 | 18.5% | OR = 0.92 (0.86, 0.99) | OR = 0.83 (0.76, 0.91) | OR = 0.72 (0.66, 0.79) |
| $75,000 to $99,999 | 2637 | 12.5% | 1589 | 12.9% | 476 | 11.7% | 276 | 12.0% | 296 | 11.9% | |||
| $100,000 or over | 3351 | 15.8% | 2063 | 16.8% | 663 | 16.3% | 337 | 14.7% | 288 | 11.6% | |||
| Prefer not to answer b | 722 | 3.4% | 467 | 3.8% | 114 | 2.8% | 64 | 2.8% | 77 | 3.1% | |||
| Education d | |||||||||||||
| Less than high school degree | 1304 | 6.2% | 742 | 6.0% | 260 | 6.4% | 141 | 6.2% | 161 | 6.5% | |||
| High school degree and less than college degree | 12,192 | 57.7% | 6868 | 55.8% | 2385 | 58.6% | 1375 | 60.0% | 1564 | 63.0% | |||
| College degree or more | 7599 | 35.9% | 4661 | 37.9% | 1417 | 34.8% | 766 | 33.4% | 755 | 30.4% | OR = 0.88 (0.81, 0.94) | OR = 0.83 (0.75, 0.91) | OR = 0.72 (0.65, 0.78) |
| Prefer not to answer b | 48 | 0.2% | 28 | 0.2% | 8 | 0.2% | 9 | 0.4% | 3 | 0.1% | |||
| Employment status e | |||||||||||||
| Employed full time | 9313 | 44.0% | 5645 | 45.9% | 1847 | 45.4% | 960 | 41.9% | 861 | 34.7% | OR = 0.98 (0.91, 1.05) | OR = 0.85 (0.78, 0.93) | OR = 0.63 (0.57, 0.68) |
| Employed part time | 2744 | 13.0% | 1670 | 13.6% | 515 | 12.7% | 275 | 12.0% | 284 | 11.4% | |||
| Retired | 2489 | 11.8% | 1525 | 12.4% | 383 | 9.4% | 239 | 10.4% | 342 | 13.8% | |||
| Homemaker | 2271 | 10.7% | 1193 | 9.7% | 481 | 11.8% | 276 | 12.0% | 321 | 12.9% | |||
| Long‐term or short‐term disability | 1443 | 6.8% | 614 | 5.0% | 295 | 7.2% | 210 | 9.2% | 324 | 13.0% | |||
| Not employed and looking for work | 1164 | 5.5% | 668 | 5.4% | 212 | 5.2% | 123 | 5.4% | 161 | 6.5% | |||
| Student | 1070 | 5.1% | 642 | 5.2% | 213 | 5.2% | 119 | 5.2% | 96 | 3.9% | |||
| Not employed and not looking for work | 531 | 2.5% | 277 | 2.3% | 104 | 2.6% | 71 | 3.1% | 79 | 3.2% | |||
| Prefer not to answer b | 118 | 0.6% | 65 | 0.5% | 20 | 0.5% | 18 | 0.8% | 15 | 0.6% | |||
| Hispanic ethnicity | |||||||||||||
| Yes | 2152 | 10.2% | 1314 | 10.7% | 420 | 10.3% | 207 | 9.0% | 211 | 8.5% | OR = 0.96 (0.86, 1.08) | OR = 0.83 (0.71, 0.97) | OR = 0.78 (0.67, 0.90) |
| No | 18,385 | 87.0% | 10,640 | 86.5% | 3527 | 86.7% | 2012 | 87.8% | 2206 | 88.8% | |||
| Prefer not to answer b | 606 | 2.9% | 345 | 2.8% | 123 | 3.0% | 72 | 3.1% | 66 | 2.7% | |||
| Race f | |||||||||||||
| White or Caucasian only | 16,758 | 79.3% | 9439 | 76.7% | 3339 | 82.0% | 1905 | 83.2% | 2075 | 83.6% | OR = 1.39 (1.27, 1.52) | OR =1.50 (1.33, 1.68) | OR = 1.55 (1.38, 1.74) |
| Black or African American only | 1824 | 8.6% | 1255 | 10.2% | 288 | 7.1% | 155 | 6.8% | 126 | 5.1% | |||
| Asian or Asian American only | 681 | 3.2% | 502 | 4.1% | 108 | 2.7% | 37 | 1.6% | 34 | 1.4% | |||
| American Indian or Alaska native only | 225 | 1.1% | 125 | 1.0% | 35 | 0.9% | 32 | 1.4% | 33 | 1.3% | |||
| Other only g | 612 | 2.9% | 401 | 3.3% | 106 | 2.6% | 49 | 2.1% | 56 | 2.3% | |||
| Two or more races | 869 | 4.1% | 466 | 3.8% | 163 | 4.0% | 98 | 4.3% | 142 | 5.7% | |||
| Prefer not to answer b | 174 | 0.8% | 111 | 0.9% | 31 | 0.8% | 15 | 0.7% | 17 | 0.7% | |||
| Consultation/diagnosis | |||||||||||||
| Sought care for headache/migraine (LIFETIME) | 16,686 | 78.9% | 9020 | 73.3% | 3442 | 84.6% | 1947 | 85.0% | 2277 | 91.7% | OR = 1.99 (1.81, 2.19) | OR = 2.06 (1.82, 2.32) | OR = 4.02 (3.46, 4.66) |
| No. healthcare visits total in the last 12 months | RR = 1.36 (1.34, 1.38) | RR = 1.69 (1.67, 1.71) | RR = 2.20 (2.17, 2.23) | ||||||||||
| Mean (SD) | 9.2 | (17.6) | 7.2 | (14.3) | 9.7 | (14.9) | 12.1 | (20.5) | 15.7 | (28.2) | |||
| Median (range) | 5 | (0–680) | 4 | (0–533) | 6 | (0–344) | 7 | (0–343) | 9 | (0–680) | |||
| No. healthcare visits for headache/migraine in the last 12 months h | RR = 1.81 (1.77, 1.85) | RR = 2.45 (2.39, 2.51) | RR = 3.62 (3.55, 3.70) | ||||||||||
| Mean (SD) | 2.9 | (7.8) | 1.8 | (5.4) | 3.2 | (7.5) | 4.4 | (10.6) | 6.5 | (12.8) | |||
| Median (range) | 0 | (0–273) | 0 | (0–273) | 1 | (0–184) | 1 | (0–230) | 2 | (0–220) | |||
| Age at migraine diagnosis, mean (SD) i , j | 23.7 | 11.8 | 23.9 | 11.8 | 23.4 | 11.4 | 23.7 | 12.2 | 23.8 | 12.0 | Beta = −0.55 (−1.11, 0.02) | Beta = −0.24 (−0.95, 0.46) | Beta = −0.14 (−0.79, 0.52) |
| Years between migraine onset and migraine diagnosis, mean (SD) j | 3.3 | 6.5 | 3.2 | 6.5 | 3.5 | 6.5 | 3.4 | 6.6 | 3.6 | 6.6 | Beta = 0.31 (−0.03, 0.64) | Beta = 0.27 (−0.15, 0.68) | Beta = 0.40 (0.01, 0.79) |
| Treatment patterns | |||||||||||||
| Migraine medication use (LIFETIME) | |||||||||||||
| Acute treatment, prescription/OTC k , l | 20,520 | 97.1% | 11,814 | 96.1% | 4002 | 98.3% | 2258 | 98.6% | 2446 | 98.5% | OR = 2.42 (1.87, 3.12) | OR = 2.81 (1.97, 4.01) | OR = 2.71 (1.94, 3.80) |
| Acute prescription | 16,239 | 76.8% | 8885 | 72.2% | 3305 | 81.2% | 1898 | 82.9% | 2151 | 86.6% | OR = 1.66 (1.52, 1.81) | OR = 1.86 (1.65, 2.08) | OR = 2.49 (2.20, 2.81) |
| Triptan | 7392 | 35.0% | 3658 | 29.7% | 1606 | 39.5% | 939 | 41.0% | 1189 | 47.9% | OR = 1.54 (1.43, 1.66) | OR = 1.64 (1.50, 1.80) | OR = 2.17 (1.99, 2.37) |
| Opioid | 10,090 | 47.7% | 5138 | 41.8% | 2137 | 52.5% | 1277 | 55.7% | 1538 | 61.9% | OR = 1.54 (1.44, 1.66) | OR = 1.76 (1.60, 1.92) | OR = 2.27 (2.08, 2.48) |
| Preventive use m | 5525 | 26.1% | 2481 | 20.2% | 1166 | 28.7% | 760 | 33.2% | 1118 | 45.0% | OR = 1.59 (1047,1.72) | OR = 1.96 (1.78,2.17) | OR = 3.24 (2.96,3.55) |
| Neurostimulation n | 488 | 2.3% | 187 | 1.5% | 102 | 2.5% | 70 | 3.1% | 129 | 5.2% | OR = 1.67 (1.31, 2.13) | OR = 2.04 (1.55, 2.70) | OR = 3.55 (2.82, 4.46) |
| Biobehavioral o | 3879 | 18.4% | 1726 | 14.0% | 901 | 22.1% | 558 | 24.4% | 694 | 28.0% | OR = 1.74 (1.59, 1.91) | OR = 1.97 (1.77, 2.20) | OR = 2.38 (2.15, 2.63) |
| Migraine medication use (CURRENT) p | |||||||||||||
| Acute treatment, prescription/OTC k | 19,915 | 94.2% | 11,384 | 92.6% | 3927 | 96.5% | 2212 | 96.6% | 2392 | 96.3% | OR = 2.21 (1.84, 2.64) | OR = 2.25 (1.78, 2.85) | OR = 2.11 (1.70, 2.63) |
| OTC | 17,304 | 81.8% | 9921 | 80.7% | 3399 | 83.5% | 1941 | 84.7% | 2043 | 82.3% | OR = 1.21 (1.11, 1.33) | OR = 1.33 (1.18, 1.50) | OR = 1.11 (1.00, 1.25) |
| Acute prescription | 8457 | 40.0% | 4140 | 33.7% | 1841 | 45.2% | 1106 | 48.3% | 1370 | 55.2% | OR = 1.63 (1.51, 1.75) | OR = 1.84 (1.68, 2.01) | OR = 2.43 (2.22, 2.65) |
| Triptan | 4792 | 22.7% | 2261 | 18.4% | 1096 | 26.9% | 657 | 28.7% | 778 | 31.3% | OR = 1.64 (1.51, 1.78) | OR = 1.79 (1.61, 1.98) | OR = 2.03 (1.84, 2.23) |
| Opioid | 4033 | 19.1% | 1888 | 15.4% | 879 | 21.6% | 557 | 24.3% | 709 | 28.6% | OR = 1.52 (1.39, 1.66) | OR = 1.77 (1.59, 1.97) | OR = 2.20 (1.99, 2.44) |
| Preventive m | 3555 | 16.8% | 1624 | 13.2% | 747 | 18.4% | 467 | 20.4% | 717 | 28.9% | OR = 1.48 (1.34, 1.63) | OR = 1.68 (1.50, 1.89) | OR = 2.67 (2.41, 2.95) |
| Neurostimulation n | 250 | 1.2% | 103 | 0.8% | 50 | 1.2% | 36 | 1.6% | 61 | 2.5% | OR = 1.47 (1.05, 2.07) | OR = 1.89 (1.29, 2.77) | OR = 2.98 (2.17, 4.11) |
| Biobehavioral o | 2994 | 10.8% | 1292 | 10.5% | 712 | 17.5% | 441 | 19.2% | 549 | 22.1% | OR = 1.81 (1.64, 2.00) | OR = 2.03 (1.80, 2.29) | OR = 2.42 (2.16, 2.70) |
Abbreviations: CI, confidence interval; OR, odds ratio; OTC, over‐the‐counter; OVERCOME, ObserVational survey of the Epidemiology, tReatment and Care of MigrainE; RR, rate ratio; SD, standard deviation.
Marital status was dichotomized as married or living with a partner versus single, divorced, widowed, and separated.
Survey questions with responses “Prefer not to answer” were excluded from this analysis.
Household income was dichotomized as greater or equal to $50,000 versus less than $50,000.
Education was dichotomized as having a college degree versus no college degree.
Employment status was dichotomized as employed full time versus employed part time, retired, homemaker, long‐term or short‐term disability, not employed and looking for work, student, and not employed and not looking for work.
Race was dichotomized as White or Caucasian only versus Black or African American only, Asian or Asian American only, American Indian or Alaska native only, Other only, and two or more races.
Includes native Hawaiian/Pacific Islander only or other race only.
N = 106 (1%) respondents answered “don’t know” on headache/migraine healthcare visits.
Maximum age of respondents was 95 years; however due to study design and data privacy‐related issues, all n = 21 respondents 85 years and older were included in the age group 85 years.
Among those with a migraine diagnosis.
Acute medications, prescription and OTC, used for the acute treatment of migraine included specific names (brand/generic) of triptans, opioids, barbiturates, ergot alkaloids, nonsteroidal anti‐inflammatory drugs (NSAIDs), and simple/combination analgesics.
OTC reflects current use.
Preventive medications used for the preventive treatment of migraine included specific names (brand/generic) of antidepressants, antiseizure drugs, antihypertensive drugs, botulinum neurotoxins, and calcitonin gene‐related peptide (CGRP) monoclonal antibodies available at the time of the survey.
Neurostimulation involved non‐surgical external nerve stimulator devices.
Biobehavioral treatments involved those treatments used specifically for migraine prevention.
“Current” defined as “currently using or typically keep on hand” for acute medications and “last 3 months” for preventive medications.
Models assessed the effect of each monthly headache day group 4–7, 8–14, and ≥15 versus 0–3 monthly headache days on each dichotomized variable (predicted event italicized). Logistic regression models were used to assess odds ratios (ORs) for binary outcomes, linear regression models were used and the beta was reported for continuous outcomes with a normal distribution, and Poisson regression models were used and rate ratios (RRs) were approximated and presented for continuous variables with a Poisson distribution. All models were unadjusted, and 95% CIs reported. Parameter estimates (OR, beta, or RR) were bolded to indicate statistical significance.
RESULTS
Respondent flow
Email invitations were sent to 1,888,290 individuals (Figure 1), 146,233 responded, and 125,306 consented to participate. Among those, 97,478 were eligible for and completed the demographic screener; these comprised a demographically representative sample of US adults, constructed to match the 2018 US Census data (see Table S2 for comparisons). Among the 97,478 participants, 39,371 had greater than or equal to one headache in the past year and were potentially eligible for the migraine cohort. Among those, 24,272 met criteria for migraine (24.9% of those eligible); however, 440 were not invited to complete the assessment of consulting, diagnosis, and treatment of migraine as they responded after the migraine cohort was closed. Among the 23,832 invited to complete the full migraine assessment, 21,143 did so and constituted the migraine cohort. Among those, monthly headache day frequency distribution was: LFEM = 12,299 (58.2%); MFEM = 4070 (19.2%); HFEM = 2291 (10.8%); and CM = 2483 (11.7%).
Sociodemographics and migraine‐related characteristics
As shown in Table 2, respondents had a mean (SD) age of 42.2 years (15.0), 74.2% identified as female at birth, 56.1% were married/living with a partner, 35.9% had a college degree, 44% were employed full time, 49.6% had annual household income greater than or equal to $50,000, 10.2% identified as Hispanic, and 79.3% identified as White.
The logistic regression models revealed significant differences of note between those with LFEM relative to MFEM/HFEM/CM regarding sex, race, income, education, and employment. Relative to LFEM (71.6%), respondents in the MFEM (76.2%), HFEM (78.0%), and CM (80.7%) categories were more likely to be female (OR [95% CI] range: MFEM 1.27 [1.17, 1.38]; HFEM 1.40 [1.26, 1.56]; and CM 1.65 [1.49, 1.84]). Similarly, relative to LFEM (76.7%), respondents in the MFEM (82.0%), HFEM (83.2%), and CM (83.6%) categories were more likely to identify as White (OR range: MFEM 1.39 [1.27, 1.52]; HFEM 1.50 [1.33, 1.68]; and CM 1.55 [1.38, 1.74]). For income, education, and employment, relative to LFEM (53.4% income >$50,000 annually, 37.9% college degree, and 45.9% employed full time) those in the HFEM and CM categories were less likely to have annual income greater than $50,000 annually (HFEM 48.5% and CM 45.1%), less likely to have a college degree (HFEM 33.4% and CM 30.4%) and less likely to be employed full time (HFEM 41.9% and CM 34.7%) and ORs across those three outcomes ranged from 0.63 to 0.85.
Among migraine‐related symptoms, the five most commonly reported were moderate or severe pain intensity (73.2%); pounding/pulsating/throbbing pain (71.2%); phonophobia (62.9%); photophobia (61.2%); and unilateral pain (53.0%). The average MIDAS score was 19.3 (SD = 31.9). Overall, 42.4% of respondents reported at least moderate disability (i.e., MIDAS ≥11) and increased with headache day frequency from MFEM (57.5%), to HFEM (68.8%), and CM (79.7%) categories (Figure 2).
FIGURE 2.
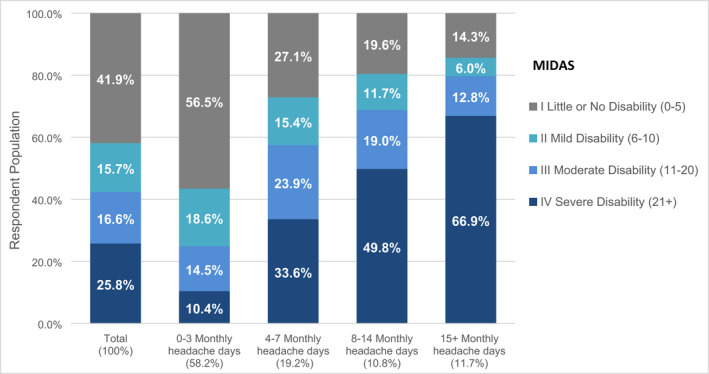
MIDAS by monthly headache days stratified by monthly headache days (N = 21,143)
Monthly headache day category consultation and diagnosis
The proportion with at least one lifetime medical consultation for headache/migraine was high (78.9%; Table 2). Consultation in primary care was most common (70.3%), followed by consultation in neurology (28.1%) or headache specialist (15.6%; Figure 3). A total of 31.0% had consulted at an emergency department or urgent care center at least once. Lifetime consulting for headache/migraine at a community/pharmacy walk‐in/convenient care center was relatively uncommon (12.3%). Among the 51.0% of the population who had consulted for headache/migraine in the past 12 months, they had, on average, 2.9 (SD = 7.8) visits. However, the median number of headache/migraine visits was 0 for the population, 1 for those with MFEM/HFEM, and 2 for those with CM (Table 2).
FIGURE 3.
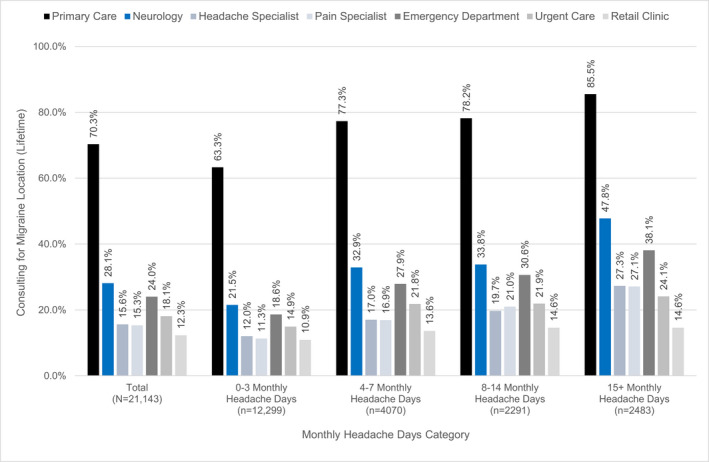
Lifetime consultation for migraine or headache by specialty, stratified by monthly headache days (N = 21,143). Primary care = primary care, family medicine, internal medicine office/clinic; Neurology = general neurologist office/clinic (not a headache specialist); Headache Specialist = headache specialist office/clinic; Pain Specialist = pain specialist office/clinic; Emergency Department = emergency department at a hospital; Urgent Care = urgent care center; Retail Clinic = community/pharmacy walk‐in/convenient care center
The logistic and Poisson regressions revealed significant differences of note between those with LFEM relative to MFEM/HFEM/CM regarding lifetime consultation and number of visits in the last 12 months. Regarding lifetime consultation, those in the MFEM (84.6%), HFEM (85.0%), and CM (91.7%) categories were at least 1.99 times more likely to have consulted than LFEM (73.3%; OR range: MFEM 1.99 [1.81, 2.19]; HFEM 2.06 [1.82, 2.32]; and CM 4.02 [3.46, 4.66]). A similar pattern was observed for the number of times consulting for any health care or for headache/migraine in the last 12 months with the RRs being significantly higher for MFEM/HFEM/CM and the absolute ratio rising with increasing monthly headache day frequency.
Almost all respondents (94.1%) screened positive for migraine using the AMS/AMPP migraine diagnostic questionnaire. In total, 61.0% of respondents had an SR‐MD (Table 3). Treating migraine as defined by the diagnostic questionnaire as the gold standard, the overall sensitivity of SR‐MD for migraine was 58.6% and increased with higher monthly headache day frequency, ranging from 51.9% (LFEM) to 73.9% (CM). The positive predictive value of SR‐MD for people screening positive for migraine via the migraine diagnostic questionnaire was 90.3% and was relatively stable across monthly headache day categories, ranging from 87.0% (LFEM) to 94.3% (CM). Among the 61.0% with an SR‐MD of migraine, the average age at migraine diagnosis was 23.7 years (SD = 11.8), and the average number of years between migraine onset and migraine diagnosis was 3.3 years (SD = 6.5; Table 2), and the averages for both age at diagnosis and years between onset and diagnosis varied by half a year or less across monthly headache day categories.
TABLE 3.
Screen positive for migraine via the AMS/AMPP diagnostic migraine screening module and/or self‐reported medical diagnosis of migraine, stratified by monthly headache days (OVERCOME (US) 2018 migraine cohort; N = 21,143)
| Total | Monthly headache days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–3 | 4–7 | 8–14 | ≥15 | |||||||
| (N = 21,143) | (N = 12,299) | (N = 4070) | (N = 2291) | (N = 2483) | ||||||
| Migraine | N | % | N | % | N | % | N | % | N | % |
| AMS/AMPP module positive for migraine, yes | 19,888 | 94.1% | 11,414 | 92.8% | 3897 | 95.7% | 2201 | 96.1% | 2376 | 95.7% |
| SR‐MD of migraine, yes | 12,905 | 61.0% | 6812 | 55.4% | 2675 | 65.7% | 1556 | 67.9% | 1862 | 75.0% |
| SR‐MD, yes among AMS/AMPP positive, yes | 11,650 | 58.6% | 5927 | 51.9% | 2502 | 64.2% | 1466 | 66.6% | 1755 | 73.9% |
| AMS/AMPP positive, yes and SR‐MD, yes | 11,650 | 55.1% | 5927 | 48.2% | 2502 | 61.5% | 1466 | 64.0% | 1755 | 70.7% |
| AMS/AMPP positive, yes and SR‐MD, no | 8238 | 39.0% | 5487 | 44.6% | 1395 | 34.3% | 735 | 32.1% | 621 | 25.0% |
| AMS/AMPP positive, no and SR‐MD, yes | 1255 | 5.9% | 885 | 7.2% | 173 | 4.3% | 90 | 3.9% | 107 | 4.3% |
Abbreviations: AMPP, American Migraine Prevalence and Prevention; AMS, American Migraine Study; OVERCOME, ObserVational survey of the Epidemiology, tReatment and Care of MigrainE; SR‐MD, self‐reported medical diagnosis of migraine.
Treatment patterns
Lifetime (97.1%) and current (94.2) use of acute (prescription or over‐the‐counter [OTC]) treatment for migraine was high (97.1%). Although 76.8% reported having used a prescription medication in their lifetime, only 40.0% currently used prescription medication. In total, 47.7% reported lifetime use of an opioid for migraine and 19.1% reported currently using an opioid for migraine. Lifetime use of triptans was reported by 35.0% whereas 22.7% reported current use. Lifetime use of migraine preventive medication was 26.1% (Table 2). Figure 4 shows that 40.4% of respondents met eligibility criteria for migraine prevention; and 16.8% were currently using a migraine preventive medication and this increased with higher monthly headache day frequency (LFEM = 13.2%; MFEM = 18.4%; HFEM = 20.4%; and CM = 28.9%; Figure 4). Table 2 shows that lifetime use of nonsurgical neurostimulation was 2.3% and current use (last 3 months) was 1.2%. Lifetime use of biobehavioral treatments was 18.4% and current use (last 3 months) was 14.2%.
FIGURE 4.
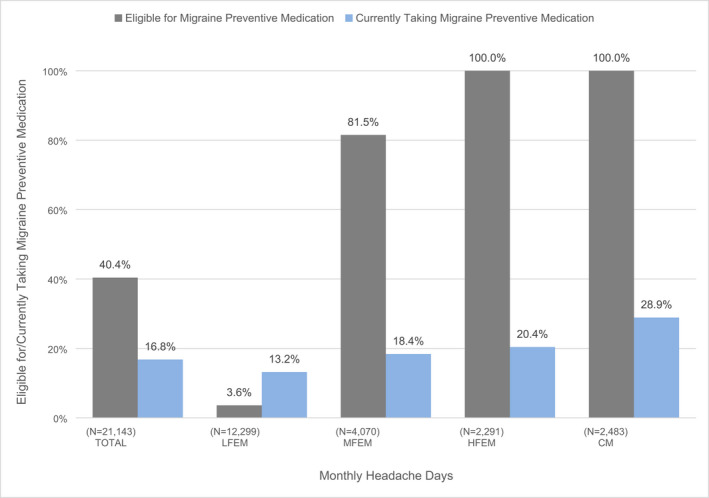
Migraine preventive medication eligibility and currently taking migraine preventive medication, stratified by monthly headache day frequency (N = 21,143). CM, chronic migraine (≥15 monthly headache days); HFEM, high frequency episodic migraine (8–14 monthly headache days); LFEM, low frequency episodic migraine (0–3 monthly headache days); MFEM, moderate frequency episodic migraine (4–7 monthly headache days). aPreventive eligibility considered monthly headache days and Migraine Disability Assessment (MIDAS) disability grade. 10 , 11 , 15 Eligibility was defined three ways: ≥6 monthly headache days, 4–5 monthly headache days with at least some disability (MIDAS ≥6), or 3 monthly headache days with severe disability (MIDAS ≥21). bCurrently taking was defined as “taken or used in the last 3 months.” Migraine preventive medication eligibility considered disability and monthly headache day frequency as specified by the American Headache Society. 15 Currently taking migraine preventive medication use was defined as use within the last 3 months for migraine and is reflective of the percentage among the overall total population within that monthly headache day frequency (regardless of current eligibility for migraine preventive medication)
The logistic regression revealed that those with MFEM/HFEM/CM were all significantly more likely to report lifetime and current use of all acute or preventive medications modeled relative to LFEM and nearly all showed incremental increases in ORs with higher monthly headache day frequency. Of note, those in the MFEM (21.6%), HFEM (24.3%), and CM (28.6%) categories were at least 1.52 times more likely to currently be using an opioid for migraine than LFEM (15.4%; OR range: MFEM 1.52 [1.39, 1.66]; HFEM 1.77 [1.59, 1.97]; and CM 2.20 [1.99, 2.44]). Similarly, those in the MFEM (18.4%), HFEM (20.4%), and CM (28.9%) categories were at least 1.48 times more likely to currently be using a migraine preventive medication than LFEM (13.2%; OR range: MFEM 1.48 [1.34, 1.63]; HFEM 1.68 [1.50, 1.89]; and CM 2.67 [2.41, 2.95]).
DISCUSSION
The OVERCOME (US) study was designed to longitudinally monitor and characterize changes in healthcare consulting for migraine, acute and preventive migraine medication use, and impact on people with migraine in a large representative sample of people in the United States. The current manuscript focuses on cross‐sectional data for the first cohort in this multicohort longitudinal study. This is the most recent in a series of US studies conducted over the past 30 years (Table 1) 9 , 10 , 11 , 12 , 13 and provides a snapshot of migraine care as an unprecedented number of new therapies for migraine became available. Providing regular updated population‐based views of migraine in the United States sheds light on if and how improvements in consulting, diagnosis, and treatment of migraine are progressing at a population level. This allows those committed to addressing clinical, scientific, and/or policy needs for migraine to make informed decisions and identify areas of addressable unmet need.
The median number of consultations for migraine over the previous year were low (0 for LFEM, 1 for MFEM/HFEM; and 2 for CM). Given that the majority of individuals with MFEM/HFEM/CM experienced moderate or severe migraine‐related disability (MFEM = 57.5%; HFEM = 68.8%; and CM = 79.7%), these consulting numbers are concerning. The hope is that more frequent consultation might lead to more effective treatment and reduced disease burden. Interestingly, the median number of total healthcare visits per year (including reasons other than headache) ranges from 6 in the MFEM category to 9 among those with CM. The high rates of consultation for reasons other than headache implies that most respondents have access to health care and yet are not regularly consulting for headache/migraine care. We have shown that the comorbidities of migraine increase with headache frequency; the fact that consultation rates for reasons other than headache also increase with headache frequency suggests that the comorbidities of migraine may drive utilization. This potential driver of health care costs merits additional exploration. Respondents may not recognize migraine as consultation‐worthy or may not communicate effectively with their HCPs about migraine. Communication is beneficial for effective care 22 , 23 , 24 , 25 , 26 , 27 , 28 yet high‐quality interaction regarding migraine and its impact is uncommon. 29 , 30 , 31 This may reduce the effectiveness of a consultation or the likelihood of ongoing consultation.
Lifetime consultation for migraine in primary care was common (70.3%) and consistent with other population‐based survey findings, including AMS‐I and AMS‐II, showing primary care is the predominant site for migraine care. 9 , 10 , 32 Lifetime neurologic consultations occurred in 28.1% of the samples and 15.6% had seen a headache specialist. The number of neurologists and headache specialists in the United States is modest relative to the size of the migraine population and geographic distribution is uneven. 30 Many areas (entire states or certain areas within a large state) have little, if any, close access to a headache specialist and some also have limited access to a neurology office/clinic. When possible, migraine needs to be managed outside a neurologist’s or headache specialist’s office. Primary care providers are well‐positioned to manage migraine and recommendations encourage that primary care clinicians manage migraine, 31 , 33 especially EM. However, primary care providers face time limitations and competing demands across diseases during a visit and this makes it challenging to prioritize migraine management. Programs and initiatives aimed at fitting migraine care into a primary care provider’s practice and/or knowing when to refer could potentially increase ongoing consultation and treatment of migraine in primary care.
Lifetime consultation for migraine at an emergency department was 24.0%; this is higher than the 5%–6% in AMS‐I and AMPP. 34 , 35 Overall, 31.0% had consulted at an emergency department or urgent care center and 12.3% had consulted at a retail clinic. The high rate of utilization outside primary and specialty care may be reflective of broader consulting trends in the United States related to the growth of ambulatory clinics. 36 However, the emergency department/urgent care setting is not ideal for delivering ongoing migraine care due to its environment, the prioritization migraine presentation may receive, the increased likelihood of unnecessary neuroimaging, and the use of opioids as a first‐line treatment for migraine. 37 , 38 , 39 , 40
SR‐MD for migraine (58.6% among those screening positive for migraine and 61.0% overall) is numerically higher than previous population reports. Over the past 30 years, population‐based survey studies of migraine show that SR‐MD rates, among those who screened positive for migraine, have risen from 38% in 1989 (AMS 34 ) to 48% in 1999 (AMS‐II 20 ) to 56% in 2004 (AMPP 41 ), and now 58.6% in 2018. Despite methodological differences among studies, these data suggest progress in the rates of SR‐MD for migraine. Gains from 1989 to 2004 may have come from greater awareness of migraine that occurred concurrent with continuing medical education campaigns and promotional campaigns surrounding the introduction and integration of triptans for the acute treatment of migraine. Between that time and the introduction of biologics for migraine prevention in 2018, treatments introduced were new indications for medicines developed initially for other diseases or novel formulations of migraine therapies. With the approval of CGRP targeted monoclonal antibodies and small molecules, a ditan, and multiple devices, we hope that educational and promotional campaigns result in additional progress in migraine diagnosis. At the same time, 41% did not have an SR‐MD of migraine. This may reflect failure to consult, failure to diagnose migraine among consulters, failure to effectively communicate diagnosis, or a failure to retain and report an HCP diagnosis. 34 Regardless, diagnosing migraine improves the likelihood of getting potentially effective medications 42 for the acute and/or preventive treatment of migraine.
Patterns of acute treatment vary among studies. Current triptan use (22.7%) in OVERCOME (US) was similar to rates in AMPP 43 (20%) but higher than in Migraine in America Symptoms and Treatment (MAST) 13 (16%). Triptans are still not used regularly despite the strong evidence for efficacy. 44 , 45 Current triptan use is below 20% for those with LFEM, which likely reflects both those who do not need triptans as well as failures to deliver guideline‐based care. Rates of current triptan use are modest among those with MFEM (18.4%) and HFEM (28.7%). Although triptans are contraindicated for certain comorbid conditions, this alone cannot fully account for their lack of use. 46 , 47 Current opioid use for migraine (19.1%) was lower than lifetime use (47.7%) and may reflect favorable shifts in treatment away from opioids and toward triptans. 42 , 48 , 49 Regular use of opioids is well documented to increase risk of headache worsening and the onset of CM. 50 , 51
Overall, 40.4% of respondents were deemed prevention eligible and 16.8% were currently using a migraine prevention medication. The proportion of use among those with at least one headache day a week is higher, yet only 18.4% of those with MFEM, 20.4% with HFEM, and 28.9% with CM were currently taking migraine preventive medication. Although low, the overall rate of 16.8% is higher than the 12%–13% rate reported in AMPP 11 and suggests potentially modest improvement in preventive medication use. The proportion of people with migraine is similar to the findings from the AMPP study but the overall rates of use have increased by 25%.
The reported current use of nonsurgical neurostimulation (1.2%) and biobehavioral treatments (14.2%) is novel. Biobehavioral use was higher than expected given how infrequently healthcare professionals specializing in biobehavioral treatments (e.g., psychologists and licensed clinical social workers) are utilized. However, individuals can access biobehavioral treatments (e.g., mindfulness, meditation, relaxation, biofeedback, and cognitive‐behavioral therapy) through written/auditory/visual online materials, books, and mobile apps. This may account for the reported utilization rate.
Overall, the percentage of current preventive treatment use is concerning given the high rates of at least moderate disability (57.5% within LFEM, 68.8% within HFEM, and 79.7% within CM). These increasing rates of disability as monthly headache day frequency increases is consistent with other population‐based findings. 52 , 53 , 54 , 55 EM is not a homogenous group. Studies looking at those with EM would do well to consider the unique needs of those with LFEM (where most are effectively managing migraine with potential need for improved acute treatment for some) relative to those with MFEM/HFEM (where most are experiencing significant disability from migraine and could potentially benefit from migraine preventive treatment). It is possible that the introduction of preventive medications specifically designed for migraine prevention may accelerate growth in the use of preventive treatments.
This current study demonstrates that, at the time of the survey in 2018, progress is being made related to diagnosis and the preventive treatment of migraine. This is encouraging as it shows that slow but steady progress has continued over time and suggests that efforts to inform persons living with migraine and healthcare professionals caring for/about migraine regarding the value in recognizing and treating migraine are worth continuing. In particular, reports of an increase in preventive medication use (16.8%) relative to what was reported in AMPP (12%–13%) is encouraging. OVERCOME (US) will continue to monitor this over time and see whether the addition of new preventive medications (CGRP monoclonal antibodies and small molecule antagonists) designed specifically for migraine with promising efficacy and accompanying efforts to raise awareness of the value of migraine prevention will reveal further gains in migraine preventive use. These gains are encouraging and yet opportunities to recognize and treat migraine abound. Most respondents report not regularly consulting for migraine, around 40% do not report a diagnosis of migraine, and 75% are not currently using a triptan or preventive medication for migraine. Further efforts are needed to increase the likelihood individuals consult regularly and that HCPs recognize and treat migraine as a disease.
This study had several strengths. The OVERCOME (US) 2018 cohort drew from a representative US sample that allowed invitees an equal opportunity to participate in the phase I screening and included respondents with varying levels of monthly headache day frequency and burden. Data were collected at a time (2018) when novel preventive and acute treatments designed specifically for migraine were becoming available and prior to the introduction of novel medications for the acute treatment of migraine. Respondents were identified as having migraine using the validated AMS/AMPP migraine diagnostic questionnaire and/or SR‐MD. The latter, although not typical of other studies in this area, allows for a more complete picture of the spectrum of people with migraine. The study captured respondents (not consulting, not diagnosed, and not treated with prescription medication for migraine) and patient‐reported outcomes not commonly found in other large‐scale real‐world evidence studies that use claims or electronic health records data. Validated measures were used wherever possible (e.g., AMS/AMPP migraine diagnostic questionnaire, MIDAS). The inclusion of migraine frequency spanning from LFEM to CM and including those not consulting for migraine aimed to reduce selection bias. Moreover, evaluating LFEM, MFEM, and HFEM separately provides a more nuanced understanding of those with EM so that the needs of those with EM garner appropriate attention.
Along with these strengths, this study had several important limitations. The participation rate of 7% created the potential for participation bias. The eligible sample approximated US Census through quota sampling rather than traditional random sampling methods. Relative to US Census data, women, individuals over the age of 55 years, and people who were married were over‐represented whereas people of Hispanic origin or with annual household incomes greater than or equal to $100,000 were under‐represented (see Table S2). Requiring internet access may underestimate the needs of some of the most vulnerable and the requirement to read/write in English may account for the under‐representation of those of Hispanic origin. However, these patterns are not unusual in internet‐based population survey research. Requiring individuals to complete the entire survey to be included in the cohort and the analyses may have introduced another form of participation bias and loss of potentially relevant information/non‐random missingness of data. Relative to another population survey (AMPP), the OVERCOME (US) 2018 migraine cohort includes individuals with higher monthly headache day frequency and higher reports of severe migraine‐related disability (see Table S3). This may have been a result of different sampling methods, participation bias, evolving recognition of migraine, and its impact among the population, or increased impact from migraine. Finally, survey data were self‐reported and were not validated by a medical professional, healthcare claims, or electronic health records. As such, they may be susceptible to recall bias.
The current findings spur ideas about other questions that OVERCOME (US) or other population‐based studies may address, including better understanding how sociodemographics, geography, clinical characteristics, individual’s beliefs about migraine and migraine care, and migraine burden influence the likelihood of consulting; how many patients only use the emergency department, urgent care, or retail clinics for migraine; how prescribing patterns may differ across care settings; and the influence of including an SR‐MD only group. OVERCOME (US) will be able to build on the current findings as new data emerges longitudinally from this cohort and baseline/longitudinal findings from other cohorts. Finally, there is a need for manuscripts that provide a more detailed analysis of the trajectory of migraine care as viewed from population‐based surveys over the last three plus decades.
The OVERCOME (US) 2018 cohort provides the latest serial “snapshot” of the migraine landscape. It is timely as the snapshot is of a time just as newly available therapies were becoming available. The results show that, relatively to previous population‐based findings, consultation may now be more likely to include ambulatory clinics, diagnosis rates have shown slow and consistent improvement over time, and use of preventive medication may be slowly improving. The current study also provides evidence that the needs of those with EM vary and should not be considered homogenous when it comes to treatment or research. Several opportunities for optimizing migraine care, including patients seeking care in primary care, more people getting diagnosed with migraine and prescribed potentially beneficial acute and preventive medication, were identified. This baseline OVERCOME (US) 2018 cohort study will be followed by other unique cohorts followed longitudinally to assess changes in the migraine care landscape concurrent with the introduction of novel therapeutic classes for preventing or treating migraine.
CONFLICT OF INTEREST
Richard B. Lipton, MD, has received research support from the National Institutes of Health, the FDA and the National Headache Foundation. He serves as consultant, advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, Dr. Reddy’s Laboratories (Promius), electroCore, Eli Lilly, GlaxoSmithKline, Lilly, Lundbeck, Merck, Novartis, Teva, Vector, and Vedanta Research. He receives royalties from Wolff’s Headache, 8th edition (Oxford University Press, 2009), and Informa. He holds stock/options in Biohaven and CntrlM. Robert A. Nicholson, PhD, is an employee and minor stockholder of Eli Lilly and Company. Michael L. Reed, PhD, has received research support from the National Headache Foundation. He serves as consultant, advisory board member, or has received honoraria or research support from Abbvie/Allergan, Amgen, Dr. Reddy’s Laboratories (Promius), and Eli Lilly. Andre B. Araujo, PhD, reports prior employment and is a minor stockholder of Eli Lilly and Company. Dena H. Jaffe, PhD, is an employee of Kantar Health which receives support from Eli Lilly and Company. Douglas E. Faries, PhD, is an employee and minor stockholder of Eli Lilly and Company. Dawn C. Buse, PhD, has received research support from the FDA and the National Headache Foundation. She serves as consultant, advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, Dr. Reddy’s Laboratories (Promius), Eli Lilly, Lundbeck, Novartis, and Teva. Robert E. Shapiro, MD, PhD, serves as consultant, advisory board member, or has received honoraria or research support from Eli Lilly and Lundbeck. Sait Ashina, MD, serves as consultant, advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Eli Lilly, Impel NeuroPharma, Novartis, Satsuma, Supernus, and Theranica. M. Janelle Cambron‐Mellott, PhD, is an employee of Kantar Health which receives support from Eli Lilly and Company. John C. Rowland, MS, is an employee of Kantar Health which receives support from Eli Lilly and Company. Eric M. Pearlman, MD, PhD, is an employee and minor stockholder of Eli Lilly and Company.
AUTHOR CONTRIBUTIONS
Study concept and design: Richard B. Lipton, Robert A. Nicholson, Michael L. Reed, Andre B. Araujo, Dena H. Jaffe, Douglas E. Faries, Dawn C. Buse, Robert E. Shapiro, Sait Ashina, Eric M. Pearlman. Acquisition of data: Dena H. Jaffe, M. Janelle Cambron‐Mellott. Analysis and interpretation of data: Richard B. Lipton, Robert A. Nicholson, Michael L. Reed, Andre B. Araujo, Dena H. Jaffe, Douglas E. Faries, Dawn C. Buse, Robert E. Shapiro, Sait Ashina, M. Janelle Cambron‐Mellott, John C. Rowland, Eric M. Pearlman. Drafting of the manuscript: Richard B. Lipton, Robert A. Nicholson, Andre B. Araujo, Dena H. Jaffe. Revising it for intellectual content: Michael L. Reed, Douglas E. Faries, Dawn C. Buse, Robert E. Shapiro, Sait Ashina, M. Janelle Cambron‐Mellott, John C. Rowland, Eric M. Pearlman. Final approval of the completed manuscript: Richard B. Lipton, Robert A. Nicholson, Michael L. Reed, Andre B. Araujo, Dena H. Jaffe, Douglas E. Faries, Dawn C. Buse, Robert E. Shapiro, Sait Ashina, M. Janelle Cambron‐Mellott, John C. Rowland, Eric M. Pearlman.
Supporting information
Table S1‐S4
ACKNOWLEDGEMENTS
The authors sincerely appreciate the valuable contributions of Donna Golden, deMauri Mackie, Valerie Marske, Vicky Li, and Anthony Zagar.
Lipton RB, Nicholson RA, Reed ML, et al. Diagnosis, consultation, treatment, and impact of migraine in the US: Results of the OVERCOME (US) study. Headache. 2022;62:122–140. doi: 10.1111/head.14259
Andre B. Araujo’s work completed while employed with Eli Lilly and Company.
REFERENCES
- 1. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163‐2196. doi: 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496‐505. doi: 10.1111/head.13281 [DOI] [PubMed] [Google Scholar]
- 3. Hawkins K, Wang S, Rupnow MFT. Indirect cost burden of migraine in the United States. J Occup Environ Med. 2007;49(4):368‐374. doi: 10.1097/JOM.0b013e31803b9510 [DOI] [PubMed] [Google Scholar]
- 4. Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache. 2008;48(4):553‐563. doi: 10.1111/j.1526-4610.2007.00990.x [DOI] [PubMed] [Google Scholar]
- 5. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the international burden of migraine study (IBMS). Headache. 2011;51(7):1058‐1077. doi: 10.1111/j.1526-4610.2011.01945.x [DOI] [PubMed] [Google Scholar]
- 6. Raval AD, Shah A. National trends in direct health care expenditures among US adults with migraine: 2004 to 2013. J Pain. 2017;18(1):96‐107. doi: 10.1016/j.jpain.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 7. Leonardi M, Raggi A. A narrative review on the burden of migraine: when the burden is the impact on people’s life. J Headache Pain. 2019;20(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267(1):64‐69. [PubMed] [Google Scholar]
- 10. Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646‐657. doi: 10.1046/j.1526-4610.2001.041007646.x [DOI] [PubMed] [Google Scholar]
- 11. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343‐349. doi: 10.1212/01.wnl.0000252808.97649.21 [DOI] [PubMed] [Google Scholar]
- 12. Adams AM, Serrano D, Buse DC, et al. The impact of chronic migraine: the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia. 2015;35(7):563‐578. doi: 10.1177/0333102414552532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lipton RB, Munjal S, Alam A, et al. Migraine in America Symptoms and Treatment (MAST) Study: baseline study methods, treatment patterns, and gender differences. Headache. 2018;58(9):1408‐1426. doi: 10.1111/head.13407 [DOI] [PubMed] [Google Scholar]
- 14. Goadsby PJ. Primary headache disorders: five new things. Neurol Clin Pract. 2019;9(3):233‐240. doi: 10.1212/CPJ.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1‐18. doi: 10.1111/head.13456 [DOI] [PubMed] [Google Scholar]
- 16. Reuter U, McClure C, Liebler E, Pozo‐Rosich P. Non‐invasive neuromodulation for migraine and cluster headache: a systematic review of clinical trials. J Neurol Neurosurg Psychiatry. 2019;90(7):796‐804. doi: 10.1136/jnnp-2018-320113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1‐211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 18. Serrano D, Buse DC, Reed ML, Runken MC, Lipton RB. Development of the migraine symptom severity score (MSSS): a latent variable model for migraine definition. Headache. 2010;50:40. [Google Scholar]
- 19. Lipton RB, Stewart WF, Sawyer J, Edmeads JG. Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2001;41(9):854‐861. doi: 10.1111/j.1526-4610.2001.01156.x [DOI] [PubMed] [Google Scholar]
- 20. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache‐related disability. Neurology. 2001;56(suppl 1):S20‐S28. doi: 10.1212/wnl.56.suppl_1.s20 [DOI] [PubMed] [Google Scholar]
- 21. Stewart WF, Lipton RB, Whyte J, et al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology. 1999;53(5):988‐994. doi: 10.1212/wnl.53.5.988 [DOI] [PubMed] [Google Scholar]
- 22. Hahn SR, Lipton RB, Sheftell FD, et al. Healthcare provider‐patient communication and migraine assessment: results of the American Migraine Communication Study, phase II. Curr Med Res Opin. 2008;24(6):1711‐1718. doi: 10.1185/03007990802122388 [DOI] [PubMed] [Google Scholar]
- 23. Lipton RB, Hahn SR, Cady RK, et al. In‐office discussions of migraine: results from the American migraine communication study. J Gen Intern Med. 2008;23(8):1145‐1151. doi: 10.1007/s11606-008-0591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holmes WF, Anne MacGregor E, Sawyer JPC, Lipton RB. Information about migraine disability influences physicians’ perceptions of illness severity and treatment needs. Headache. 2001;41(4):343‐350. doi: 10.1046/j.1526-4610.2001.111006343.x [DOI] [PubMed] [Google Scholar]
- 25. Nicholson RA. Chronic headache: the role of the psychologist. Curr Pain Headache Rep. 2010;14(1):47‐54. doi: 10.1007/s11916-009-0087-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make a difference in conversations between physicians and patients: a systematic review of the evidence. Med Care. 2007;45(4):340‐349. doi: 10.1097/01.mlr.0000254516.04961.d5 [DOI] [PubMed] [Google Scholar]
- 27. Haskard Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826‐834. doi: 10.1097/MLR.0b013e31819a5acc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buse DC, Lipton RB. Facilitating communication with patients for improved migraine outcomes. Curr Pain Headache Rep. 2008;12(3):230‐236. doi: 10.1007/s11916-008-0040-3 [DOI] [PubMed] [Google Scholar]
- 29. Buse DC, Gillard P, Arctander K, Kuang AW, Lipton RB. Assessing physician‐patient dialogues about chronic migraine during routine office visits. Headache. 2018;58(7):993‐1006. doi: 10.1111/head.13314 [DOI] [PubMed] [Google Scholar]
- 30. Mauser ED, So RNL, Migraines M. So few subspecialists: analysis of the geographic location of United Council for Neurologic Subspecialties (UCNS) certified headache subspecialists compared to United States headache demographics. Headache. 2014;54(8):1347‐1357. doi: 10.1111/head.12406 [DOI] [PubMed] [Google Scholar]
- 31. Steiner TJ, Jensen R, Katsarava Z, et al. Aids to management of headache disorders in primary care (2nd edition): on behalf of the European Headache Federation and Lifting the Burden: The Global Campaign against Headache. J Headache Pain. 2019;20(1). doi: 10.1186/s10194-018-0899-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Can J Neurol Sci. 1993;20(2):131‐137. doi: 10.1017/S0317167100047697 [DOI] [PubMed] [Google Scholar]
- 33. WHO . Atlas of Headache Disorders and Resources in the World 2011. WHO; 2012. [Google Scholar]
- 34. Lipton RB, Stewart WF, Simon D. Medical consultation for migraine: results from the American Migraine Study. Headache. 1998;38(2):87‐96. doi: 10.1046/j.1526-4610.1998.3802087.x [DOI] [PubMed] [Google Scholar]
- 35. Friedman BW, Serrano D, Reed M, Diamond M, Lipton RB. Use of the emergency department for severe headache. A population‐based study. Headache. 2009;49(1):21‐30. doi: 10.1111/j.1526-4610.2008.01282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang JE, Brundage SC, Chokshi DA. Convenient ambulatory care—promise, pitfalls, and policy. N Engl J Med. 2015;373(4):382‐388. doi: 10.1056/NEJMhpr1503336 [DOI] [PubMed] [Google Scholar]
- 37. Loder E, Weizenbaum E, Frishberg B, Silberstein S, American Headache Society Choosing Wisely Task Force . Choosing wisely in headache medicine: the American Headache Society’s list of five things physicians and patients should question. Headache. 2013;53(10):1651‐1659. doi: 10.1111/head.12233 [DOI] [PubMed] [Google Scholar]
- 38. Mazer‐Amirshahi M, Dewey K, Mullins PM, et al. Trends in opioid analgesic use for headaches in US emergency departments. Am J Emerg Med. 2014;32(9):1068‐1073. 10.1016/j.ajem.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 39. Colman I, Rothney A, Wright SC, Zilkalns B, Rowe BH. Use of narcotic analgesics in the emergency department treatment of migraine headache. Neurology. 2004;62(10):1695‐1700. doi: 10.1212/01.WNL.0000127304.91605.BA [DOI] [PubMed] [Google Scholar]
- 40. Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55(1):21‐34. doi: 10.1111/head.12482 [DOI] [PubMed] [Google Scholar]
- 41. Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47(3):355‐363. doi: 10.1111/j.1526-4610.2006.00631.x [DOI] [PubMed] [Google Scholar]
- 42. Lipton RB, Buse DC, Friedman BW, et al. Characterizing opioid use in a US population with migraine: results from the CaMEO study. Neurology. 2020;95(5):e457‐e468. doi: 10.1212/WNL.0000000000009324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bigal ME, Buse DC, Chen Y‐T, et al. Rates and predictors of starting a triptan: results from the American Migraine Prevalence and Prevention Study. Headache. 2010;50(9):1440‐1448. doi: 10.1111/j.1526-4610.2010.01703.x [DOI] [PubMed] [Google Scholar]
- 44. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3‐20. doi: 10.1111/head.12499 [DOI] [PubMed] [Google Scholar]
- 45. Silberstein SD, Marmura MJ. Acute migraine treatment. Headache. 2015;55(1):1‐2. [DOI] [PubMed] [Google Scholar]
- 46. Lipton RB, Reed ML, Kurth T, Fanning KM, Buse DC. Framingham‐based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2017;57(10):1507‐1521. doi: 10.1111/head.13179 [DOI] [PubMed] [Google Scholar]
- 47. Buse DC, Reed ML, Fanning KM, Kurth T, Lipton RB. Cardiovascular events, conditions, and procedures among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2017;57(1):31‐44. doi: 10.1111/head.12962 [DOI] [PubMed] [Google Scholar]
- 48. Guy GP, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697‐704. doi: 10.15585/mmwr.mm6626a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. U.S. Opioid Prescribing Rate Maps | Drug Overdose | CDC Injury Center . Accessed October 13, 2020. https://www.cdc.gov/drugoverdose/maps/rxrate‐maps.html
- 50. Buse DC, Pearlman SH, Reed ML, Serrano D, Ng‐Mak DS, Lipton RB. Opioid use and dependence among persons with migraine: results of the AMPP study. Headache. 2012;52(1):18‐36. doi: 10.1111/j.1526-4610.2011.02050.x [DOI] [PubMed] [Google Scholar]
- 51. Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population‐based study. Headache. 2008;48(8):1157‐1168. doi: 10.1111/j.1526-4610.2008.01217.x [DOI] [PubMed] [Google Scholar]
- 52. Lipton RB, Seng EK, Chu MK, et al. The effect of psychiatric comorbidities on headache‐related disability in migraine: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2020;60(8):1683‐1696. doi: 10.1111/head.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scher AI, Stewart WF, Liberman J, Lipton RB. Prevalence of frequent headache in a population sample. Headache. 1998;38(7):497‐506. doi: 10.1046/j.1526-4610.1998.3807497.x [DOI] [PubMed] [Google Scholar]
- 54. Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population‐based study. Pain. 2003;106(1‐2):81‐89. doi: 10.1016/S0304-3959(03)00293-8 [DOI] [PubMed] [Google Scholar]
- 55. Burch RC, Buse DC, Migraine LRB. Epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631‐649. doi: 10.1016/j.ncl.2019.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S4


