Summary
Background
Hepatitis B virus (HBV)‐specific CD8+ cell response restoration during nucleos(t)ide analogue (NUC) treatment could lead to off‐treatment HBV control in e‐antigen‐negative chronic hepatitis B (CHBe(−)).
Aim
To predict this response with variables involved in T‐cell exhaustion for use as a treatment stopping tool.
Methods
In NUC‐treated CHBe(−) patients, we considered a functional response in cases with HBV‐specific CD8+ cells against core and polymerase HBV epitopes able to proliferate and secrete type I cytokines after antigen encounter. We performed a logistic regression model (LRM) to predict the likelihood of developing this response, based on patient age (subrogate of infection length), HBsAg level, NUC therapy starting point and duration (antigenic pressure). We discontinued treatment and assessed HBV DNA dynamics, HBsAg decline and loss during off‐treatment follow‐up according to LRM likelihood.
Results
We developed an LRM that predicted the presence of a proliferative type I cytokine‐secreting CD8+ cell response, which correlated positively with treatment duration and negatively with treatment initiation after the age of 40 years and with age adjusted by HBsAg level. We observed a positive correlation between LRM probability and intensity of proliferation, number of epitopes with the functional proliferating response and type I cytokine secretion level. Off‐treatment, HBsAg loss, HBsAg decline >50% and HBV control were more frequent in the group with >90% LRM probability.
Conclusions
Short‐term low‐level antigen exposure and early long‐term NUC treatment influence the restoration of a functional HBV‐specific CD8+ cell response. Based on these predictors, a high likelihood of detecting this response at treatment withdrawal is associated with off‐treatment HBV control and HBsAg decline and loss.
Keywords: anti‐viral therapy discontinuation, functional cure, HBV‐specific CD8+ cell response, Nucleos(t)ide analogue, predictive multivariate logistic regression model, T‐cell exhaustion
Based on therapy starting age and duration, HBsAg level and infection length, a high likelihood of a functional HBV specific CD8+ cell response during nucleos(t)ide analogue treatment foresees HBV control after treatment discontinuation.

1. INTRODUCTION
Hepatitis B virus (HBV)‐specific CD8+ cell response is essential for natural infection control but these cells become exhausted and deleted during chronic hepatitis B (CHB) due to continuous antigen (Ag)‐specific stimulation. 1 , 2 , 3 In persistent viral infections, both infection length and antigenic load correlate with the induction of T‐cell exhaustion. 4 , 5 Therefore, strategies focused on decreasing the duration and level of Ag exposure could promote restoration of this response and subsequent functional cure. 6 Long‐term CHB treatment with nucleos(t)ide analogues (NUC) could lead to this goal by a slow progressive decrease in HBV covalently closed circular (ccc) DNA level and antigenemia. 7 , 8 These changes could help restore HBV‐specific CD8+ cell response, 9 which could subsequently reduce the level of cccDNA, 10 promoting hepatitis B surface antigen (HBsAg) clearance or entry into an inactive carrier state. 11 After a prolonged period of NUC treatment, several clinical studies have shown that HBsAg loss can be achieved after stopping the medication, whereas, this hardly ever occurs while on treatment. 12 , 13 , 14 Still, there are currently no clear stopping rules for NUC‐treated e‐Ag negative (e(−)) CHB15, although the status of the adaptive immune response could be considered a promising predictor of HBV control. 16 , 17
Patient age, NUC treatment duration and end of treatment HBsAg level are predictors of relapse after treatment stops, 18 probably due to their impact on the adaptive immune response. Nevertheless, the relationship between these variables and the quality of HBV‐specific CD8+ cell response and their subsequent role in HBV control has not yet been addressed in NUC‐treated CHBe(−). To assess this issue, we developed a predictive multivariate logistic regression model (LRM) to forecast, during NUC treatment, the presence of a functional HBV‐specific CD8+ cell response against different HBV immunodominant human leucocyte antigen (HLA)‐A2 restricted epitopes, based on patient age, HBsAg level, NUC treatment duration and age at treatment initiation. Afterwards, in a cohort of CHBe(−) patients treated with NUC, we analysed HBV control, HBsAg loss and HBsAg decline by >50% during off‐treatment follow‐up as a function of the probability (prob) of detecting a restored response at the time of treatment discontinuation, according to the developed model.
2. MATERIALS AND METHODS
2.1. Patients and study design
For an initial cross‐sectional study, 41 HLA‐A2+ CHBe(−) patients on NUC treatment were recruited from the Translational Hepatology Unit HBV database at Guadalajara University Hospital (Spain) (Table 1). All enlisted cases were Caucasians, born in Mediterranean countries. We excluded patients co‐infected with hepatitis C, hepatitis D, human immunodeficiency virus or cirrhosis. Clinical diagnosis and treatment indication were performed according to the European Association for the Study of the Liver (EASL) guidelines. 14 At recruitment, we recorded age, sex, country of origin, estimated moments of HBV transmission, type of prescribed NUC, treatment duration, age at treatment start, alanine aminotransferase (ALT) (IU/ml), HBV DNA (IU/ml) and HBsAg (IU/ml) levels. Heparinised blood and serum samples were drawn for immunological and virological testing. Retrospectively, we also registered liver fibrosis (by transient hepatic elastography with FibroScan‐402 device [Echosens, France] or liver biopsy), ALT and HBV DNA levels at the time of treatment indication.
TABLE 1.
Demographic and clinical features of patients enrolled in the study
| Prob. of functional HBV‐specific CD8+ cell response a | ||||
|---|---|---|---|---|
| <0.5 (n = 28) | ≥0.5 (n = 13) | p value | All (n = 41) | |
| Age (years) | 46 (39–56) | 50 (42–53) | N.S. b | 46 (41–56) |
| Male sex (N/%) | 14/50 | 8/61 | N.S. b | 22/54 |
| Mediterranean origin (N/%) | 28/100 | 13/100 | N.S. b | 41/100 |
| Spain | 14/50 | 10/77 | 24/59 | |
| Romania | 14/50 | 3/23 | 17/41 | |
| Pre‐treatment status | ||||
| ALT (IU/ml) | 63 (46–83) | 62 (58–68) | N.S. c | 63 (47–74) |
| HBV DNA (IU/ml) [log scale] | 5.7 (4.7–6.6) | 4.6 (4.4–6.2) | N.S. c | 5.4 (4,6–6.5) |
| Liver fibrosis (N/%) | ||||
| F0–F1 | 15/53 | 8/63 | 23/56 | |
| F2–F3 | 13/47 | 5/37 | N.S. b | 18/44 |
| NUC treatment duration (years) | 5.1 (1.5–6.4) | 9 (6.6–13.7) | <0.001 c | 6.3 (3.7–8.7) |
| Age at NUC treatment start (years) | 43.7 (35–48) | 37.0 (34–44) | N.S. c | 41.7 (34–47) |
| NUC type (N/%) | ||||
| Tenofovir | 23/82 | 9/70 | N.S. b | 32/80 |
| Entecavir | 4/14 | 0/0 | 4/10 | |
| Lam. → Adef. → Tenofovir | 0/0 | 3/23 | 3/7 | |
| Lam. → Tenofovir | 1/3 | 1/7 | 2/5 | |
| Status at study recruitment | ||||
| ALT (IU/ml) | 26 (21–31) | 23 (18–33) | N.S. c | 25 (20–31) |
| HBV DNA (IU/ml) (log scale) | 0 (0–1.5) | 0 (0–0) | 0.003 c | 0 (0–1.3) |
| HBsAg (IU/ml) (log scale) | 3.7 (3.2–4.1) | 2.4 (0.6–3.4) | 0.001 c | 3.5 (2.6–4) |
Note: Data are expressed as frequency distribution for categorical variables and as median plus quartiles one and three (Q1–Q3) for quantitative variables.
Abbreviations: Adef., adefovir; Lam, lamivudine; N.A., non‐applicable; NUC, nucleos(t)ide analogue; N.S., non‐significant; Prob, probability.
Probability is calculated according to the predictive logistic regression model developed in the study.
Chi‐square test.
Mann–Whitney U test.
Patient age was considered as a surrogate of HBV infection duration since in the Mediterranean basin, the infection is usually acquired during childhood through horizontal transmission. 19 , 20 Those cases with a known time of infection after 18 years of age were excluded from the study.
First, we performed a multivariate cross‐sectional analysis to develop a predictive LRM to forecast the presence of a functional peripheral HBV‐specific CD8+ cell response against HBV‐core18‐27 and ‐polymerase (pol)455–63 in NUC‐treated HLA‐A2+ CHBe(−) patients. We considered a functional anti‐HBV cytotoxic T‐cell response, in those cases with proliferating type I cytokine‐secreting HBV‐specific CD8+ cells against core and polymerase. 11 Variables potentially involved in T‐cell exhaustion were included in the model. Specifically, the effect of antigenic exposure on HBV‐specific CD8+ cells was estimated by the product of patient age (decades) and HBsAg log (IU/ml) level, the NUC therapy starting point (before or after the age of 40 years) and treatment duration (years). Patient age was adjusted by HBsAg level to control the interaction between these two variables, in order to define the effect of infection duration on immunological response, according to the level of HBsAg. Therefore, this generated variable would represent the level of antigenic pressure to which HBV‐specific CD8+ cells would have been subjected during infection, assuming that HBsAg level is fairly stable throughout the infection in CHBe(−). 21
Secondly, we compared the functional features of HBV‐specific CD8+ cells according to the probability level of having a functional response, calculated by the LRM. The value of the LRM defines the probability of having a functional response but it could be also considered as a score of the exhaustion level of HBV‐specific CD8+ cell response. Table 1 summarises the main demographic and clinical characteristics of the enlisted cases as a function of the LRM probability.
Finally, we discontinued NUC treatment in a cohort of 22 CHBe(−) patients with liver fibrosis <F3 and more than 3.5 years of therapy to assess the utility of the LRM as a treatment stopping tool. We carried out a longitudinal study to evaluate the HBV control, HBsAg loss and the rate of >50% HBsAg decline without the need for retreatment, according to the LRM likelihood of detecting a functional response. Table 2 summarises the baseline characteristics of this longitudinal cohort. This group of CHBe(−) patients underwent off‐treatment follow‐up every 3 months for a median period of 36 months until the presence of an event (HBsAg loss or retreatment). HBsAg, HBV DNA and ALT levels were tested at each appointment and a liver transient elastography was performed at the end of the follow‐up. Treatment was reintroduced according to the FINITE study criteria. 12
TABLE 2.
Baseline characteristics and outcomes of the eAg(−) chronic hepatitis B cohort undergoing NUC treatment discontinuation
| Prob. of functional HBV‐specific CD8+ cell detection at treatment stop a | |||
|---|---|---|---|
| >0.9 (n = 7) | ≤0.9 (n = 15) | p value | |
| Age (years) | 44 (38–53) | 45 (35–53) | N.S. c |
| Male sex (%) | 70 | 53 | N.S. b |
| NUC treatment duration (years) | 10 (9.5–11) | 6 (4.5–7.5) | 0.002 c |
| Age at NUC treatment start (years) | 32.3 (29–44) | 37.1 (30–44) | N.S. c |
| HBsAg (IU/ml) [log scale] | 2.8 (2.2–3.3) | 3.6 (3.2–4.1) | 0.012 c |
| HBsAg (IU/ml) > 3 logs (n/N) | (3/7) | (13/15) | 0.034 b |
| Follow‐up (months) | 30 (6–36) | 30 (18–36) | N.S. c |
| HLA‐A2 (%) | 43 | 73 | N.S. c |
| Liver fibrosis (%) | |||
| F1 | 43 | 80 | N.S. b |
| F2 | 57 | 20 | |
| Prob. of functional HBV‐specific CD8+ cell response (%) | 98 (97–99) | 35 (18–69) | <0.001 c |
| Status at follow‐up end (n/N), (%) | 0.036 b | ||
| Retreatment | 1/7 (14%) | 5/15 (33%) | N.S. b |
| HBV DNA < 20,000 IU/ml and ALT <2 × ULN [GZ] | 3/7 (43%) | 4/15 (27%) | N.S. b |
| HBV DNA ≤2000 IU/ml & ALT <2 × ULN [IC] | 0/7 (0%) | 6/15 (40%) | N.S. b |
| HBsAg loss | 3/7 (43%) | 0/15 (0%) | 0.006 b |
| HBsAb seroconversion | 2/7 (29%) | 0/15 (0%) | 0.019 b |
| HBsAg decline >50% and no retreatment (n/N), (%) | 6/7 (86%) | 3/15 (20%) | 0.004 b |
| Liver stiffness at the end of follow‐up (KPa) | 4.4 (3.8–6.8) | 5.1 (4.3–5.7) | N.S. c |
Note: Data are expressed as frequency distribution for categorical variables and as median plus quartiles one and three (Q1–Q3) for quantitative variables.
Abbreviations: GZ, grey zone; IC, inactive carrier; N.S., non‐significant; Prob., probability; ULN, upper limit normal.
Probability is calculated according to the predictive logistic regression model developed in the study.
Chi‐square test.
Mann–Whitney U test.
The Research Ethical Committee of the Guadalajara University Hospital (Spain) approved the study protocol. All the patients enrolled in the study gave written informed consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Full materials and methods regarding the assessment of virological parameters, immune responses and statistical analysis are available in Supplementary Material.
3. RESULTS
3.1. Quantification of interferon‐γ and tumour necrosis factor‐α on T‐cell culture supernatants
Based on the report by Rivino et al., we defined an HLA‐A2+ CHBe(−) patient with an HBV‐specific CD8+ functional response, potentially capable of controlling the virus, as those cases with proliferative HBVcore18‐27 and HBVpol455‐63‐specific CD8+ cells able to secrete interferon (IFN)‐γ and/or tumour necrosis factor (TNF)‐α after Ag encounter. Due to the T‐cell receptor downregulation after Ag‐specific stimulation, we could not test in the whole cohort for cytokine secretion at the single‐cell level by phycoerythrin‐conjugated HLA‐A2/peptide pentameric complex (pentamer) staining and intracellular cytokine staining (ICS). For this reason, in cases with visible HBVcore18‐27 CD8+ pentamer+ cells after restimulation, we analysed whether IFN‐γ mean fluorescence intensity by ICS correlated with the cytokine level measured by enzyme‐linked immunosorbent assay (ELISA) in supernatants of cell cultures challenged with HBV‐core18‐27 peptide. We observed a significant positive correlation between these two assays (Figure 1A) that encouraged us to use the ELISA assay to estimate the ability of HBV‐specific CD8+ cells to secrete type I cytokines in the entire NUC‐treated CHBe(−) cohort. Those cases without expansion were taken as controls to define the cytokine production by bystander activation. According to the upper limit of the cytokine concentration in the non‐expansion group, we defined a supernatant cytokine level >150 pg/ml as the cut‐off point for considering a CD8+ cell culture with positive expansion as a functional type I cytokine‐secreting response after HBV‐Ag encounter (Figure 1B). Those cases with HBV‐specific CD8+ cell expansion but with a supernatant cytokine level ≤150 pg/ml were considered as having partial exhaustion grade II or full exhaustion, according to Wherry’s model for hierarchical loss of T‐cell function during chronic viral infection. 4 IFN‐γ secretion >150 pg/ml was observed in 90% and 61% of pol455‐63 and core18‐27 T‐cell cultures with Ag‐specific expansion respectively. TNFα level>150 pg/ml was detected in 55% of pol455‐63 and 61% of core18‐27 T‐cell cultures with Ag‐specific proliferation (Figure 1B).
FIGURE 1.
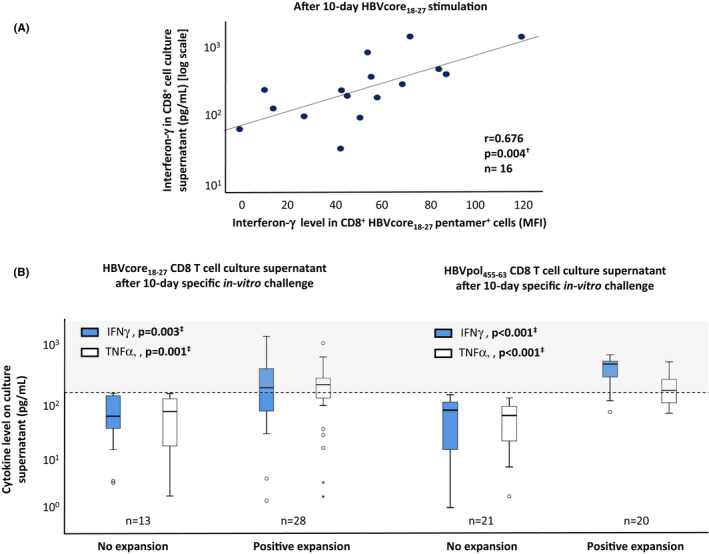
Strategy to detect proliferative type I cytokine‐secreting HBVcore18–27 and HBVpol455‐63 CD8+ cells. In NUC‐treated chronic hepatitis B e‐Ag negative: (A) Scatterplot showing the correlation between interferon (IFN)‐γ mean fluorescence intensity in the whole pool of HBVcore18‐27 CD8+ cells tested by intracellular cytokine staining and IFN‐γ concentration in CD8+ cell culture supernatant tested by enzyme‐linked immunosorbent assay after 10‐day Ag‐specific stimulation. (B) Boxplot depicting the IFN‐γ/tumour necrosis factor (TNF‐α) level in CD8+ cell culture supernatant after 10‐day Ag‐specific in vitro challenge in cases with and without Ag‐specific CD8+ cell expansion. Cases without expansion were used as a negative control to quantify the cytokine level due to bystander activation. The dashed line represents the cut‐off level for an experiment to be considered as having type I cytokine‐secreting cells after HBV‐Ag‐specific stimulation. IFN, interferon; MFI, mean fluorescence intensity; TNF, tumour necrosis factor. †Pearson correlation test. ‡ Mann–Whitney U test
3.2. Predictive model to forecast the presence of a peripheral functional HBV‐specific CD8 + cell response in NUC‐treated CHBe(−)
In a cohort of HLA‐A2+ CHBe(−) patients on NUC treatment (Table 1), we developed a predictive multivariate LRM to forecast the probability of detecting a functional HBV‐core18‐27 and ‐pol455‐63‐specific CD8+ cell response, able to proliferate and to secrete IFN‐γ and/or TNF‐α, as a function of patient’s age adjusted by the HBsAg level (), age at NUC therapy initiation and duration of NUC treatment. A functional response was considered when HBV‐specific CD8+ cells had an expansion >0.1% out of total CD8+ cells after Ag encounter, aggrouped in a cluster form, and the IFN‐γ and/or TNF‐α level in culture supernatants was above the level due to bystander activation (Figure 1B). The model significantly predicted the likelihood of detecting a functional cytotoxic T‐cell response against at least these two HBV epitopes, explaining ≈76% of the observed variability (Figure 2A). Patient age adjusted by the level of HBsAg negatively correlated with detection of functional cells, with an odds ratio (OR) of 1.5 (1/0.649) for non‐detection of functional cells for every one‐decade increase in age adjusted by HBsAg level (Figure 2A, Figure S1). In addition, initiation of NUC treatment after the age of 40 years was also negatively associated with the presence of proliferative cytokine‐secreting cells with an OR of 27 (1/0.036) for the absence of these functional cells relative to cases aged less than 40 years at treatment start (Figure 2A, Figure S1). In contrast, NUC treatment duration correlated positively with the detection of functional CD8+ cells, with an OR of 1.9 for the detection of these cells per year of treatment (Figure 2A, Figure S1). The overall accuracy of the derivation model to correctly classify functional and non‐functional cases in our cohort, using 0.5 as a cut‐off point was 85.4%, detecting 92% of cases without and, 73% of patients with functional response against both core and pol epitopes (Figure 2B). The area under the receiver operating characteristics (ROC) curve for the developed model was 0.95 (95% Confidence Interval [CI]: 0.84–0.99) (Figure 2C). The jack‐knife resampling strategy internally validated the model with similar results across the 41 models generated with n − 1 patient each. Although the 0.5 probability cut‐off point showed an excellent positive predictive value (PPV) of 85% and a negative predictive value of 86%, the 0.90 cut‐off point had a 100% PPV (Figure 2D). For this reason, we selected this point to split the patients into two groups to assess the clinical outcome after NUC treatment discontinuation, as will be discussed later. Figure 2E shows the resulting probabilities after the application of the model to different hypothetical CHBe(−) patients, with a wide range of values in the predictors. This “Metroticket” chart shows a decrease in the mean probability of detecting a functional response in relation to the initiation of NUC treatment after the age of 40 years and with increasing age and HBsAg levels. On the contrary, a prolonged NUC treatment results in a progressive increase in the probability of detecting functional cells.
FIGURE 2.
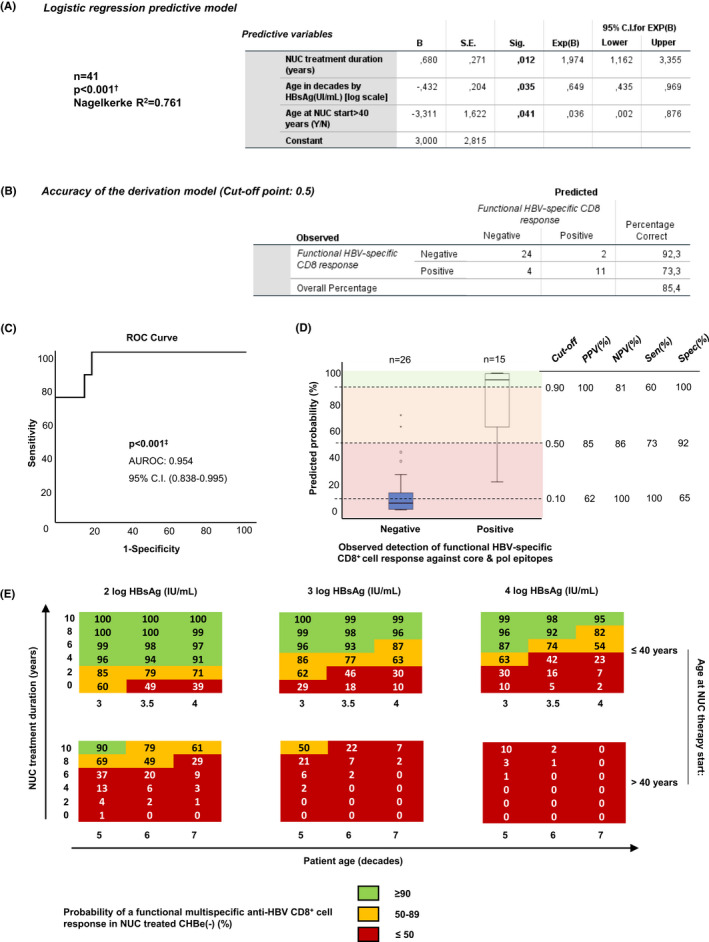
Logistic regression predictive model to forecast the probability of detecting a peripheral functional HBV‐specific CD8+ cell response against core and pol epitopes during eAg(−) chronic hepatitis B treatment with nucleos(t)ide analogues. (A) Table with the coefficient values of the significant logistic regression model developed to predict the presence of functional HBV‐specific CD8+ cell response against core18‐27 and pol455‐63, including as independent variables: patient age in decades, duration of treatment with nucleos(t)ide analogues (NUC) in years, log HBsAg level (IU/ml) and NUC treatment initiation after the age of 40 years (positive vs negative). A functional response was considered in those cases with CD8+ cell proliferation and secretion of IFN‐γ and/or TNF‐α after HBVcore18‐27 and polymerase455‐63‐specific stimulation. (B) Accuracy of the model to classify patients with functional response in the study cohort, using 50% probability as the cut‐off point. (C) The area under the receiver operating characteristic curve of the model. (D) Boxplots showing the probability distribution of detecting a functional response calculated by the predictive model in the groups with observed presence and absence of this kind of response. The dashed lines indicate the sensitivity (Sen), Specificity (Spec), Positive Predictive Value (PPV) and Negative Predictive Value (NPV), according to different cut‐off points in the probability distribution of detecting functional cells. (E) “Metroticket” charts showing the mean probability of detecting peripheral functional HBV‐specific CD8+ cell responses against HBVcore18‐27 and HBVpol455‐63 epitopes after applying the logistic regression model to a wide range of possible scenarios on the independent variables. AUROC, area under receiver operative characteristics; B, coefficient of the independent variables; CI, confidence interval; NUC, nucleos(t)ide analogue; OR, odds ratio; pol, polymerase; ROC, receiver operative characteristics; S.E., standard error; Sig, significance; Y/N, yes/no. †Chi‐square test. ‡Mann–Whitney U test
3.3. Features of peripheral HBV‐specific CD8 + cells according to the predictive model probability of detecting a functional response
In the same HLA‐A2+ NUC‐treated CHBe(−) cohort, we described the frequency of cases with ex vivo peripheral core18‐27, pol455‐63 and envelope (env)183‐91‐specific CD8+ cells and with proliferating type I cytokine‐secreting cells after Ag‐specific stimulation according to the LRM probability of detecting functional cells (<0.5 vs ≥0.5). In these two groups, we also analysed by flow cytometry the IFN‐γ secretion and degranulation capacity of HBVcore18‐27‐specific CD8+ cells after a 10‐day‐specific in vitro challenge. Almost all cases had no detectable response ex vivo or after stimulation against HBV‐env183‐91 (Figure 3A,B–F), indicating the common deletion of this specificity during persistent HBV infection. The frequency of cases with directly ex vivo detectable pol455‐63‐ and core18‐27‐specific CD8+ cells was higher in NUC‐treated CHBe(−) cases with prob ≥0.5 than in those with prob <0.5. Globally, we found fewer cases with expansion after Ag encounter for pol (49%) than for core (68%) stimulation. We also observed less commonly an intense proliferative functional response after Ag encounter for pol (36%) than for core epitope (54%), (p = 0.016, Wilcoxon test). Anyhow, those CHBe(−) cases with prob ≥0.5 had an expansion of functional HBV‐specific CD8+ cells after stimulation with core18‐27 and pol455‐63 more frequently and more intense than the group with less probability (Figure 3B–F). Eighty‐five percent of CHBe(−) patients with prob ≥0.5 exhibited a functional response against two HBV epitopes, whereas this was only observed in 14% of cases in the group with prob <0.5 (Figure 3C). Furthermore, we demonstrated a positive correlation between the probability of having a proliferating type I cytokine‐secreting response and both the real number of epitopes with functional response (Figure 3D) and the intensity of the Ag‐specific CD8+ cell proliferation (Figure 3E).
FIGURE 3.
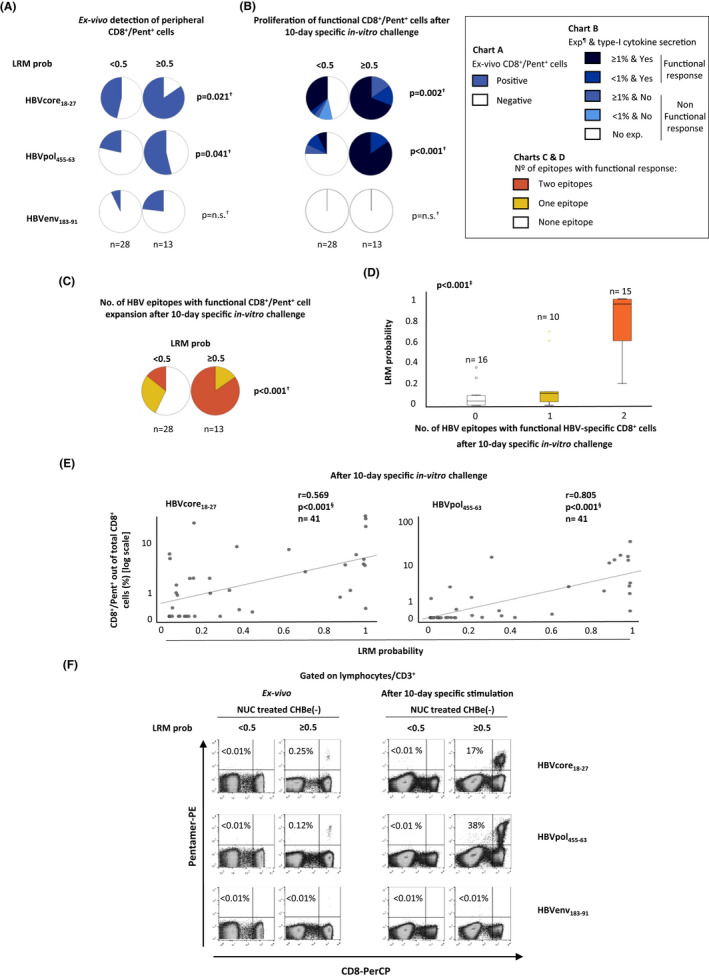
Peripheral ex vivo detectability and expansion ability of functional HBV‐specific CD8+ cells after specific in vitro challenge according to the model probability of having a functional response against core and pol epitopes. Pie charts depicting the percentage of cases with (A) peripheral HBV‐specific CD8+ cells detectable ex vivo and (B) with proliferating type I cytokine‐secreting CD8+ cells after Ag encounter in eAg(−) chronic hepatitis B (CHBe(−)), according to the model probability of detecting a functional response against core and pol epitopes (≤0.5 vs >0.5). (C) Pie charts showing the percentage of cases with functional response against none, one or two HBV epitopes as a function of the model probability of detecting a functional response. (D) Boxplot chart showing the linear trend between the number of HBV epitopes with proliferating type I cytokine‐secreting CD8+ cell response in NUC‐treated CHBe(−) and the model probability. (E) Scatterplots depicting the correlation between the model probability of detecting a functional response and the intensity of HVB‐specific CD8+ cell proliferation after a 10‐day‐specific in vitro challenge. (F) Representative dot plots showing the CD8+/pentamer+ cells frequency out of total CD8+ cells directly ex vivo and after Ag‐specific expansion as a function of the model probability of detecting a functional response. CHB, chronic hepatitis B; env, envelope, e(−): e antigen‐negative; Exp, expansion; HBV, hepatitis B virus; n.s., non‐significant; LRM, logistic regression model; NUC, nucleos(t)ide analogue; Pent, pentamer; pol, polymerase; prob, probability. †Chi‐square test. ‡Jonckheere‐Terpstra test. §Spearman correlation test. ¶Expansion is given as the percentage of CD8+ Pentamer+ cells out of total CD8+ cells
In the cases with expanding CD8+ cells after 10‐day HBVcore18‐27 in vitro challenge, the percentage of IFN‐γ‐secreting cells was slightly higher in the cohort with a probability of ≥0.5 but it did not reach statistical significance. Nevertheless, we found a positive correlation between the mean fluorescence intensity level of IFN‐γ secretion by the whole pool of HBVcore18‐27‐specific CD8+ cells tested by ICS and the LRM probability of having a functional response (Figure 4A,B). On the contrary, the degranulation capacity was preserved in all cases with expanding cells independently of the level of LRM probability (Figure 4A,B). We also observed a positive correlation between the IFN‐γ and TNF‐α levels on the HBVcore18‐27 and HBVpol455‐463 culture supernatant after Ag‐specific stimulation and the probability of detecting a functional response calculated by the LRM (Figure 5). This significant correlation translated into a higher IFN‐γ and TNF‐α level in HBVcore18‐27 and HBVpol455‐463 culture supernatant in the group with LRM prob >90% of having a functional response (Figure 5), which has a 100% PPV of detecting functional cells (Figure 2D).
FIGURE 4.
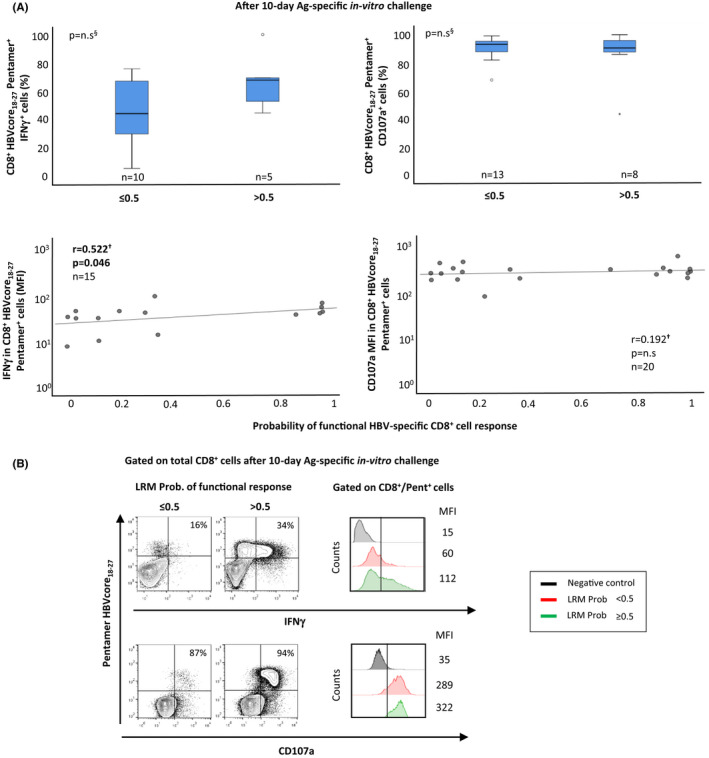
Type I cytokine secretion and degranulation capacity of HBVcore18‐27‐specific CD8+ cells after a 10‐day‐specific in vitro challenge. In nucleos(t)ide analogue‐treated eAg(−) chronic hepatitis B: (A) Boxplots showing the percentage of IFN‐γ‐secreting and CD107a‐expressing HBV‐core18‐27‐pentamer+ CD8+ cells out of total CD8+ Pentamer+ cells and scatterplots depicting the correlation between interferon (IFN)γ/CD107a mean fluorescence intensity level in HBVcore18‐27‐pantamer+ CD8+ cells and the model probability of detecting proliferating type I cytokine‐secreting HBV‐specific CD8+ cells. (B) Representative FACS dot plots and histograms showing the IFN‐γ secretion and CD107a expression in CD8+/HBVcore18‐27‐pentamer+ cells. The frequency of IFN‐γ‐secreting/CD107a‐expressing CD8+/pentamer+ out of total CD8+/pentamer+ cells is shown in the upper right quadrant of each dot plot. LRM, logistic regression model; MFI, mean fluorescence intensity; n.s., non‐significant; Prob, probability. †Spearman correlation test. § Mann–Whitney U test
FIGURE 5.
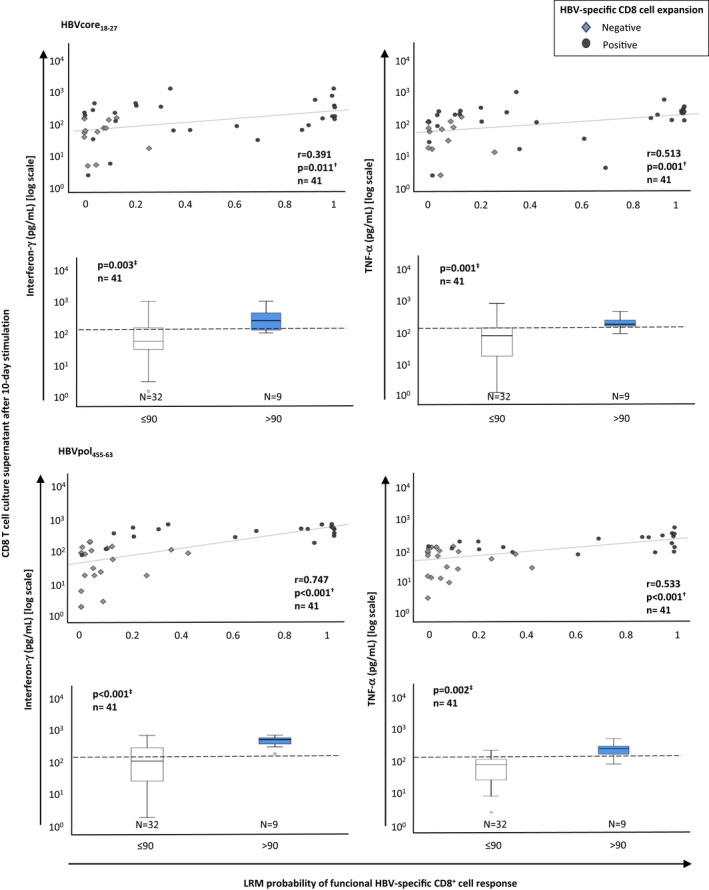
Correlation between the probability of detecting a functional HBV‐specific CD8+ response and type I cytokine secretion by HBV‐specific CD8+ cells after Ag encounter. Scatterplots and boxplots depicting the relationship between the logistic regression model probability of detecting a functional HBV‐specific CD8+ cell response and interferon‐γ and tumour necrosis factor‐α level in CD8+ cell culture supernatants after 10‐day‐specific in vitro challenge with HBVcore18‐27 or HBVpol455‐63 peptides. To compare the type I cytokine secretion according to the LRM level, the 90% probability cut‐off point was chosen due to the 100% predictive positive value of this point for detection of a functional response. HBV, hepatitis B virus; LRM, logistic regression model probability;, MFI, mean fluorescence intensity; n.s., non‐significant; pol, polymerase. †Spearman correlation test. ‡Mann–Whitney U test
3.4. Clinical application of the model
Although the model was developed to predict the presence of a functional cytotoxic T‐cell response against HBV‐specific HLA‐A2‐restricted epitopes, we assumed that in other haplotypes, there should also be specific CD8+ cells that would behave similar to HBV‐pol455‐63‐ and ‐core18‐27‐specific CD8+ cells. Under this premise, we applied the predictive model to a cohort of NUC‐treated CHBe(−) patients, candidates for NUC treatment withdrawal. In 22 cases (63% HLA‐A2+) with liver fibrosis <F3 and NUC treatment duration >3.5 years, NUC treatment was discontinued (Table 2). In this selected cohort, treatment discontinuation was safe and effective, as only in six cases (27%, 95% CI: 7%–47%), treatment had to be reintroduced according to FINITE criteria 12 and neither severe adverse events nor liver fibrosis worsening was observed after the 36‐month follow‐up (Table 2). Using the predictive LRM developed in this study, we split this cohort into two groups, based on the probability of detecting a peripheral functional HBV‐specific CD8+ cell response against at least two HBV epitopes (>0.9 vs ≤0.9). Figure 6A shows the clinical outcomes of both cohorts during 36‐month follow‐up after NUC treatment discontinuation. The cohort with a >90% probability of detecting a functional response lost HBsAg in three out of seven patients and developed anti‐HBs Ab in two out of seven cases (Table 2) while this did not occur in any case in the cohort with a probability ≤90% (Figure 6A,B). HBV control was achieved in 86% (6/7) of cases in the ≥90% prob group but only in 66% (10/15) of patients in the <90% prob cohort (Table 2). In addition, the >90% probability patients showed a more rapid HBsAg decline, with HBsAg decreasing >50% from baseline without the need for retreatment in six out of seven (86%) cases while this occurred in only 3 out of 15 patients (20%) in the cohort with probability ≤0.9 (Figure 6B).
FIGURE 6.
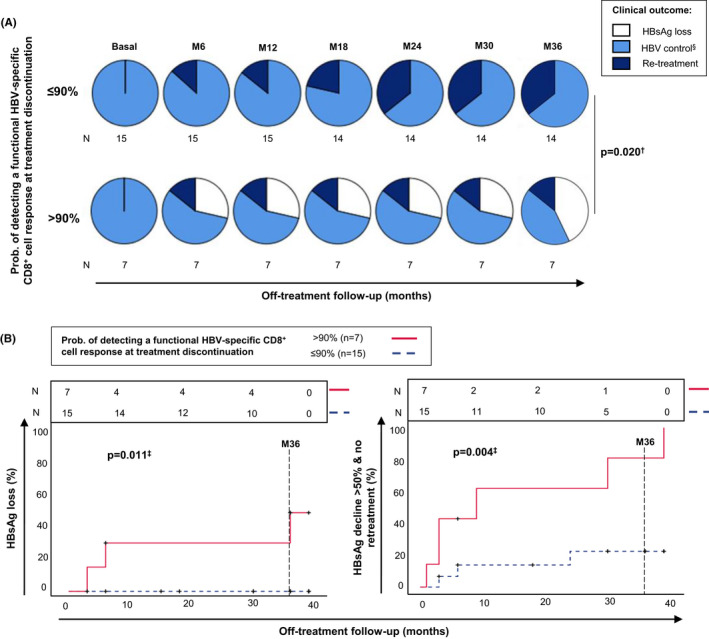
Follow‐up of an eAg(−) chronic hepatitis B cohort after NUC therapy discontinuation according to the probability of the presence of a functional HBV‐specific CD8+ cell response at the time of treatment withdrawal. (A) Dynamics of patient outcomes during off‐treatment follow‐up according to the probability level of detecting a functional HBV‐specific CD8+ cell response at the time of treatment discontinuation, calculated using the predictive model developed in the study. (B) Kaplan–Meier curves showing the frequency of eAg(−) chronic hepatitis B cases with HBsAg loss and > 50% HBsAg decline with respect to baseline without need for retreatment, according to the level of probability of detecting peripheral functional HBV‐specific CD8+ cells at discontinuation of treatment, based on the predictive model developed in the study. N, number of cases exposed during the different periods; M, month; Prob, probability. §HBV control comprises an inactive carrier and grey‐zone status. †Chi‐square test. ‡Log‐rank test (Mantel‐Cox)
In two HLA‐A2 prototypical cases, with high and low prob of detecting a functional response according to the LRM, HBV‐specific CD8+ cell expansion ability and IFN‐γ/TNF‐α secretion were tested longitudinally off‐treatment to check whether LRM prob correlated with an actual T cell function restoration after treatment withdrawal. The low probability case had no proliferating HBV‐specific CD8+ cells after NUC discontinuation at any point tested, whereas the high probability case had a functional response during the first‐year follow‐up, with increased proliferative capacity and cytokine secretion after HBV DNA peak. This patient eventually developed HBsAb seroconversion after 2 years of stopping NUC treatment (Figure S2).
4. DISCUSSION
NUC treatment is highly effective in inhibiting HBV replication and decreasing long‐term liver damage. 14 Loss of HBsAg is considered the hallmark of HBV functional cure. 22 Nevertheless, this event rarely occurs during treatment and subsequently, CHBe(−) patients are subjected to lifelong therapy. 14 In some cases, the restoration of HBV‐specific cytotoxic T‐cell response could be achieved during treatment, 11 , 16 which could lead to functional cure after treatment discontinuation. 12 , 17 Thus, the detection of a functional HBV multi‐specific CD8+ cell pool could be considered a reasonable predictor of long‐term HBV control after a finite therapy. 11 , 17 However, experimental procedures to test for the presence of specific T‐cell responses are complex, time consuming and not easy to apply in daily clinical practice. To surpass this issue, we developed a predictive LRM to forecast the presence of this response, based on easy obtainable clinical variables, as a potential tool in the treatment withdrawal decision process.
As predictors of the functionality of HBV‐specific cytotoxic T‐cell response, we chose variables potentially involved in T‐cell exhaustion. The level of antigenemia and the duration of Ag exposure could be implicated in the impairment of Ag‐specific T‐cell function. 4 , 23 On the other hand, a prolonged NUC treatment could progressively decrease the antigenic pressure, 7 , 8 which could favour the restoration of the cellular response. However, delaying the initiation of treatment with NUC could decrease the likelihood of reaching this target due to the progressive deletion of these cells. 5 Although HBsAg level does not correlate accurately with cccDNA transcription in CHBeAg(−) patients, 24 a low HBsAg level remains a good predictor of achieving functional cure, whereas a high level is a marker of potential relapse after NUC treatment discontinuation. 18 The HBsAg level in CHBe(−) patients show a slow but continuous decay throughout the patient’s life, with a median decline of about 0.6 logs (IU/ml) between the third and the seventh decade of age. 21 Because of this slow dynamic, a spot HBsAg measurement could be a surrogate for global antigenic pressure. We considered these four previously described prognostic variables (infection length, HBsAg level, NUC treatment duration and age at treatment initiation) as a basis for carrying out a predictive model, defining the probability of developing a functional HBV‐specific CD8+ cell response during NUC treatment in CHBe(−). In our model, we considered a positive result in the dependent variable (functional response) in those cases with a peripheral proliferating type I cytokine‐secreting CD8+ cell response specific against core and pol epitopes, which could be potentially able to achieve the functional HBV control as previously reported. 11
The developed model significantly defined the probability of a patient having proliferative HBV‐core18‐27‐ and ‐pol455‐63‐specific CD8+ cells with the ability to secrete type I cytokines. The model was developed with 41 cases and 15 events, which could seem initially an insufficient sample size. Nevertheless, this predictive model explained ≈76% of the observed variability with a p value of <0.001. Discounting the observed statistically significant predictive model does not appear to be justified based only on the sample size as stated by Vittinghoff E. & McCulloch C.E. 25 According to these authors when a statistically significant association is found in a model with ≥5 events per variable (EPV), only a minor degree of caution should be taken to interpret the results, in particular for plausible and highly significant associations hypothesised a priori as it occurs in our study. In our cohort, we found 15 events and we included three variables in the model (NUC treatment duration [years], age at treatment start [<40 vs >40] and age [decades] × HBsAg log [IU/ml]), which means an EPV = 5. Therefore, although we should be cautious in the interpretation of the results, we should not expect severe problems with the model or at least they should be similar to those found in models with EPV ≥10. 25 The duration of NUC treatment increased ≈two‐fold the likelihood of having functional HBV‐specific CD8+ cells per year of treatment. On the other hand, each decade increase in patient age adjusted by HBsAg level meant a ≈50% decrease in the possibility of having a functional response. Late initiation of treatment was the worst prognostic factor for detecting a restored response. Initiation of NUC treatment after the age of 40 years decreased ≈27‐fold the chance of restoring an HBV‐specific CD8+ cell response during NUC treatment.
Current clinical guidelines suggest discontinuing treatment in those CHBe(−) patients who have achieved complete virological suppression for more than 3 years. 14 Nevertheless, based on our model’s predictions, this minimum treatment duration (≈3.5 years) may not be sufficient in many cases to achieve HBV‐specific cytotoxic T‐cell restoration. Our model showed a 100% PPV of having a functional response for a probability cut‐off point >90%. According to this model and using this cut‐off point to consider a case as having a functional HBV‐specific CD8+ response, a prototypical CHBe(−) patient initiating treatment before 40 years with an HBsAg level of 3 logs (IU/ml) would need about 6 years of NUC treatment to restore a functional response. In contrast, a patient starting treatment at the age 60 years with the same HBsAg level of 3 logs (IU/ml) would have a very low probability of having restored cells, even after 10 years of treatment. Based on our model, the minimum 3.5 years of NUC therapy length indicated by clinical guidelines 14 would be enough to restore a functional HBV‐specific CD8+ T‐cell response in a patient starting NUC treatment before the age of 40 years and with an HBsAg level of 2 logs (IU/ml) at treatment discontinuation.
Previous studies have related advanced age, short treatment duration and high HBsAg level at end of treatment to relapse after treatment discontinuation. 18 , 26 Our model links these three variables to a low probability of having a functional HBV‐specific CD8+ cell response, which could explain the outcomes observed in these clinical studies. The developed predictive LRM showed high accuracy in detecting those cases without HBV‐specific CD8+ cell restoration during NUC treatment, which could make this model very useful to avoid ineffective treatment discontinuations. The accuracy in detecting cases with functional cells was somewhat lower, perhaps due to the low correlation between antigenic pressure and HBsAg level as a consequence of HBV S gene integration into the host genome in CHBe(−). 24 Therefore, we think that this model could be improved by using other markers of HBV antigenemia that better express the antigenic pressure such as the HBV core‐related Ag or pre‐genomic HBV RNA. 27 , 28 Furthermore, detection of type I cytokine‐secreting HBV‐specific CD8+ cells could be improved by using a more sensitive test than the enzyme‐linked immunosorbent assay utilised in the study such as the enzyme‐linked immunospot (ELISPOT) assay. 29 Another issue to note is that we only tested for the presence of a peripheral functional CD8+ T‐cell response, assuming that the intrahepatic HBV‐specific T‐cell compartment would have similar functional behaviour, but this was not confirmed. However, the correlation between the peripheral compartment data and the clinical outcome after NUC treatment discontinuation suggests that intrahepatic T‐cell functionality would also be correlated with peripheral pool effector abilities. In addition, another important issue, before using this model clinically, would be the need for its external validation.
Globally, pol‐specific proliferating CD8+ T‐cell response was less frequently observed than core‐specific one, probably due to more severe exhaustion in the pol subset. This finding is in agreement with the recent report stating that the dysregulation of TCF1/Bcl2 expression limits the expansion capacity of pol‐specific CD8+ cells in chronic patients. 30 Nevertheless, a long‐term NUC treatment was also able to restore pol‐specific responses in our study. The probability level of detecting a functional response during NUC treatment according to our model correlated with the intensity of HBV‐specific CD8+ cell expansion and with the level of type I cytokine secretion but not with the cytotoxic potential. Although we did not directly check granzyme‐B and perforin expression, testing the CD107a level could be considered a reliable subrogate for the secretion of these cytolytic molecules. The observed preservation of cytolytic ability suggests that the most exhausted cells can lose non‐cytolytic functions, but they maintain the direct killing capability, as has been previously shown in murine lymphocytic choriomeningitis virus chronic infection. 4
Finally, we tested whether our model’s predictions about the development of a functional core‐ and pol‐specific CD8+ cell response would correlate with HBV control, HBsAg loss and HBsAg decline after treatment discontinuation. Previously, Rivino et al. demonstrated that the presence of residual PD‐1‐expressing type I cytokine‐secreting core‐ and pol‐specific T‐cell responses were associated with viral control upon NUC therapy discontinuation. 11 Accordingly, we assumed that in cases with a high probability of developing a functional response during NUC therapy, the balance between the virus and the adaptive immune response could be modified during the treatment in favour of the T‐cell response, allowing for indefinite HBV control by these cells after NUC discontinuation. According to EASL guidelines, 14 we withdrew NUC treatment in a cohort of 22 patients with not‐advanced liver fibrosis and more than 3.5 years of therapy. Treatment discontinuation was safe, as only in a few cases treatment had to be restarted due to virus‐dominating reactivations, but without neither severe adverse events nor liver fibrosis worsening. 15 , 31
A high probability of detecting a functional HBV multi‐specific CD8+ cell response was detected in 32% of CHBe(−) cases at the time of treatment discontinuation. This value was similar to the off‐treatment sustained viral response reported in CHBe(−) at a 36‐month follow‐up in a recent meta‐analysis. 26 Both the group with the highest probability of detecting functional cells and the group with the lowest probability were treated mostly with tenofovir. Cases that started treatment with first‐ or second‐generation NUC were switched to third‐generation drugs after the appearance of tenofovir or entecavir. The timing of treatment with first‐ or second‐generation NUC was also considered to have a positive effect on T‐cell response, as has been previously shown. 32 , 33 After treatment withdrawal, the cohort with a high probability of detecting functional cells had a higher rate of HBsAg loss and a faster overall decline in HBsAg level during the follow‐up. In contrast, in the group with a low probability of having a functional HBV‐specific CD8+ cell response, no cases developed HBsAg loss, and only a minority experienced a significant decline in HBsAg level. Therefore, our predictive LRM agrees with Rivino et al. findings, 11 and it could be a good tool to select those cases with a high probability of having this type of response as candidates for NUC discontinuation. Moreover, due to this positive correlation between effector abilities and the model probability level, this LRM could also provide an approach to the degree of HBV‐specific cytotoxic T‐cell response exhaustion from easily obtainable clinical data (prob >0.9: low exhaustion; prob 0.9–0.5: intermediate exhaustion and prob <0.5: intense exhaustion). From a practical point of view, those cases with a high likelihood of having functional cells could stop treatment, whereas patients with a low probability, such as those starting treatment after the age of 40 years, should continue on‐treatment. Patients with an intermediate likelihood could undergo a potential future test to rapidly assess the immune response against HBV, such as the QuantiFERON (QIAGEN, MD) used for CMV or tuberculosis. 34
Our model’s predictions are consistent with previous reports stating the correlation between the intensity of HBV‐specific CD8+ T‐cell response and HBV control during persistent infection 3 and the association between HBV‐specific T‐cell restoration and long‐term NUC treatment. 9 Moreover, another recent paper has also shown a relationship between the presence of functional HBV‐specific T cells at treatment stop and a successful outcome after treatment withdrawal. 17 Similarly, our LRM’s prediction of a high likelihood of a functional response correlates with the achievement of HBV control and functional cure. In line with our LRM estimations, it has recently been published that those cases with HBsAg loss after treatment withdrawal display less‐exhausted T cells with higher levels of activation and proliferation. 16 Therefore, our work and these previous studies 11 , 16 , 17 support the correlation between functional cure after treatment withdrawal and restoration of a functional HBV‐specific CD8+ cell response. Finally, our LRM could also be useful for selecting those cases with a very low likelihood of achieving a functional response during NUC treatment, as occurs with cases starting treatment after the age of 40 years, to be candidates for potential synergic immunotherapeutic strategies to boost anti‐HBV immune responses in combination with NUC therapy. 35 , 36
Our LRM only predicts the restoration of an essential part of the immune response against HBV, which is the cytotoxic T‐cell response, but the HBV immune control is an orchestrated effort between many different cell types, such as CD4 T cells, B cells, innate cells and innate‐like cells. 37 Therefore, the development of a model capable of predicting the restoration of a global functional response against HBV, not only focused on the functional CD8+ T‐cell response, could be an even more powerful tool in the decision‐making process for stopping NUC treatment.
To sum up, the restoration of a functional HBV multi‐specific cytotoxic T‐cell response can be achieved during NUC treatment in CHBe(−) patients from Mediterranean origin. Our model can predict the probability of detecting this response during treatment, based on the duration of infection (age) adjusted by HBsAg level, NUC therapy length and age at treatment initiation. According to our predictive model, a high likelihood of a functional HBV‐specific CD8+ cell response at the time of treatment discontinuation translates into HBV control and a rapid decline and loss of HBsAg, which could be used as an NUC treatment stopping rule in daily clinical practice after validating these results.
AUTHORSHIP
Guarantor of the article: Juan‐R Larrubia
Author contributions: JRL and JPA contributed to the study concept and design. JRL, JM, and ESV were involved in patient recruitment, follow‐up, and clinical data collection. JPA and AGP carried out the immunological and virological analysis. JRL, MT, HC and JPA contributed to analysis and interpretation of data. JRL and JPA drafted the manuscript. All authors contributed to critical revisions and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Appendix S1. Supporting information
Figure S1
Figure S2
Table S1
Table S2
ACKNOWLEDGEMENTS
We are very grateful to the patients for their generous participation in the study and to the Nursing staff from the Endoscopy Unit at Guadalajara University Hospital for taking the patients’ samples.
Peña‐Asensio J, Calvo H, Miquel J, et al. Model to predict on‐treatment restoration of functional HBV‐specific CD8 + cell response foresees off‐treatment HBV control in eAg‐negative chronic hepatitis B. Aliment Pharmacol Ther. 2022;55:1545–1559. doi: 10.1111/apt.16850
The Handling Editor for this article was Professor Geoffrey Dusheiko, and it was accepted for publication after full peer‐review.
Funding informationThis study was sponsored by the “Instituto de Salud Carlos III” (ISCIII), Spain, through the “State Plan for Scientific and Technical Research and Innovation 2017‐2020,” grant PI19/00206 (JRL), co‐funded by the European Regional Development Fund (ERDF), E.U., “A way of making Europe.” The founders did not have any role in data analysis, writing the manuscript or deciding about the submission for publication.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Maini MK, Boni C, Ogg GS, et al. Direct ex vivo analysis of hepatitis B virus‐specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117(6):1386‐1396. [DOI] [PubMed] [Google Scholar]
- 2. Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)‐specific T‐cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215‐4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Webster GJ, Reignat S, Brown D, et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78(11):5707‐5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wherry EJ, Blattman JN, Murali‐Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T‐cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911‐4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Bert N, Gill US, Hong M, et al. Effects of Hepatitis B surface antigen on virus‐specific and global T cells in patients with chronic Hepatitis B virus infection. Gastroenterology. 2020;159(2):652‐664. [DOI] [PubMed] [Google Scholar]
- 6. Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18(11):827‐844. [DOI] [PubMed] [Google Scholar]
- 7. Lai CL, Wong D, Ip P, et al. Reduction of covalently closed circular DNA with long‐term nucleos(t)ide analogue treatment in chronic hepatitis B. J Hepatol. 2017;66(2):275‐281. [DOI] [PubMed] [Google Scholar]
- 8. Liao H, Liu Y, Li X, et al. Monitoring of serum HBV RNA, HBcrAg, HBsAg and anti‐HBc levels in patients during long‐term nucleoside/nucleotide analogue therapy. Antivir Ther. 2019;24(2):105‐115. [DOI] [PubMed] [Google Scholar]
- 9. Boni C, Laccabue D, Lampertico P, et al. Restored function of HBV‐specific T cells after long‐term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143(4):963‐973 e969. [DOI] [PubMed] [Google Scholar]
- 10. Xia Y, Stadler D, Lucifora J, et al. Interferon‐gamma and tumor necrosis factor‐alpha produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology. 2016;150(1):194‐205. [DOI] [PubMed] [Google Scholar]
- 11. Rivino L, Le Bert N, Gill US, et al. Hepatitis B virus‐specific T cells associate with viral control upon nucleos(t)ide‐analogue therapy discontinuation. J Clin Invest. 2018;128(2):668‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berg T, Simon KG, Mauss S, et al. Long‐term response after stopping tenofovir disoproxil fumarate in non‐cirrhotic HBeAg‐negative patients—FINITE study. J Hepatol. 2017;67(5):918‐924. [DOI] [PubMed] [Google Scholar]
- 13. Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg‐negative patients with chronic hepatitis B who stop long‐term treatment with adefovir. Gastroenterology. 2012;143(3):629‐636 e621. [DOI] [PubMed] [Google Scholar]
- 14. EASL . EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370‐398. [DOI] [PubMed] [Google Scholar]
- 15. Tout I, Lampertico P, Berg T, Asselah T. Perspectives on stopping nucleos(t)ide analogues therapy in patients with chronic hepatitis B. Antivir Res. 2021;185:104992. [DOI] [PubMed] [Google Scholar]
- 16. Rinker F, Zimmer CL, Siederdissen C, et al. Hepatitis B virus‐specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg‐negative chronic hepatitis B. J Hepatol 2018;69(3):584–593. [DOI] [PubMed] [Google Scholar]
- 17. Garcia‐Lopez M, Lens S, Pallett LJ, et al. Viral and immune factors associated with successful treatment withdrawal in HBeAg‐negative chronic hepatitis B patients. J Hepatol. 2021;74(5):1064‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Jia M, Wu S, Jiang W, Feng Y. Predictors of relapse after cessation of nucleos(t)ide analog treatment in HBeAg‐negative chronic hepatitis B patients: a meta‐analysis. Int J Infect Dis. 2019;86:201‐207. [DOI] [PubMed] [Google Scholar]
- 19. Liaw YF, Brunetto MR, Hadziyannis S. The natural history of chronic HBV infection and geographical differences. Antivir Ther. 2010;15(Suppl 3):25‐33. [DOI] [PubMed] [Google Scholar]
- 20. Hadziyannis SJ. Natural history of chronic hepatitis B in Euro‐Mediterranean and African countries. J Hepatol. 2011;55(1):183‐191. [DOI] [PubMed] [Google Scholar]
- 21. Jang JW, Yoo SH, Kwon JH, et al. Serum hepatitis B surface antigen levels in the natural history of chronic hepatitis B infection. Aliment Pharmacol Ther. 2011;34(11–12):1337‐1346. [DOI] [PubMed] [Google Scholar]
- 22. Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology. 2017;66(4):1296‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLane LM, Abdel‐Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457‐495. [DOI] [PubMed] [Google Scholar]
- 24. Pollicino T, Caminiti G. HBV‐integration studies in the clinic: role in the natural history of infection. Viruses. 2021;13(3):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165(6):710‐718. [DOI] [PubMed] [Google Scholar]
- 26. Papatheodoridis G, Vlachogiannakos I, Cholongitas E, et al. Discontinuation of oral antivirals in chronic hepatitis B: a systematic review. Hepatology. 2016;63(5):1481‐1492. [DOI] [PubMed] [Google Scholar]
- 27. Testoni B, Lebosse F, Scholtes C, et al. Serum hepatitis B core‐related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70(4):615‐625. [DOI] [PubMed] [Google Scholar]
- 28. Gao Y, Li Y, Meng Q, et al. Serum Hepatitis B virus DNA, RNA, and HBsAg: which correlated better with intrahepatic covalently closed circular DNA before and after Nucleos(t)ide analogue treatment? J Clin Microbiol. 2017;55(10):2972‐2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyahira Y, Murata K, Rodriguez D, et al. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181(1):45‐54. [DOI] [PubMed] [Google Scholar]
- 30. Schuch A, Salimi Alizei E, Heim K, et al. Phenotypic and functional differences of HBV core‐specific versus HBV polymerase‐specific CD8+ T cells in chronically HBV‐infected patients with low viral load. Gut. 2019;68(5):905‐915. [DOI] [PubMed] [Google Scholar]
- 31. Liaw YF, Hepatitis B. Flare after cessation of Nucleos(t)ide analogue therapy in HBeAg‐negative chronic Hepatitis B: to retreat or not to retreat. Hepatology. 2021;73(2):843‐852. [DOI] [PubMed] [Google Scholar]
- 32. Boni C, Bertoletti A, Penna A, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998;102(5):968‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cooksley H, Chokshi S, Maayan Y, et al. Hepatitis B virus e antigen loss during adefovir dipivoxil therapy is associated with enhanced virus‐specific CD4+ T‐cell reactivity. Antimicrob Agents Chemother. 2008;52(1):312‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yong MK, Lewin SR, Manuel O. Immune monitoring for CMV in transplantation. Curr Infect Dis Rep. 2018;20(4):4. [DOI] [PubMed] [Google Scholar]
- 35. Boni C, Barili V, Acerbi G, et al. HBV immune‐therapy: from molecular mechanisms to clinical applications. Int J Mol Sci. 2019;20(11):2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maini MK, Burton AR. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat Rev Gastroenterol Hepatol. 2019;16(11):662‐675. [DOI] [PubMed] [Google Scholar]
- 37. Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22(1):19‐32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Figure S1
Figure S2
Table S1
Table S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


