Abstract
Aims
To evaluate the clinical and genetic virulence characteristics of critically ill patients with hypervirulent Klebsiella pneumoniae (hvKP) and classic KP (cKP) infection.
Methods and Results
The patients included in this retrospective study (n = 225) were grouped according to their hvKP (n = 114) or cKP (n = 111) status, and their clinical characteristics were analysed and compared. Cox multivariate analysis was conducted to determine the risk factors for hvKP infection. Length of hospital stay, length of intensive care unit stay, duration of mechanical ventilation and 28‐day survival rate were similar between the groups. However, the incidence of septic shock was higher in the hvKP group (16.7%) than in the cKP group (8.1%).
Conclusions
There was a high rate of hvKP infection in this population. Compared to patients with cKP infection, those with hvKP infection showed a higher probability of having septic shock; nevertheless, survival and length of hospital stay were similar between the groups. Risk factors for hvKP infection included hospital‐acquired infection and renal insufficiency.
Significance and Impact of the Study
This study presents relevant information on the characteristics of hvKP infection in a Chinese population, and this promotes early diagnosis and supports the view that the prevalence of hvKP is high in China.
Keywords: classic Klebsiella pneumoniae , hypervirulent Klebsiella pneumoniae , infection, risk factors, septic shock
INTRODUCTION
Klebsiella pneumoniae (KP) is a Gram‐negative, lactose‐fermenting, aerobic rod‐shaped bacterium (Martin & Bachman, 2017). It was first isolated and identified at the end of the 19th century and is a known common human pathogen since then (Martin & Bachman, 2017).
Classic KP (cKP) infection is usually hospital‐acquired, mainly occurs in patients with impaired host defence function, and frequently causes pneumonia or urinary tract infection (Chew et al., 2017). However, a more serious hypervirulent variant of KP (hvKP) can produce invasive infections even in apparently healthy adults (Catalán‐Nájera et al., 2017).
The hvKP strains have high viscosity and can be identified on an agar plate using a positive string test (Vila et al., 2011). KP clinical isolates can be identified using pulsed‐field gel electrophoresis, multi‐locus sequence typing and capsular polysaccharide characterization (K typing) (Brisse et al., 2013). The K1, and to a lesser degree K2, capsular serotype was first described as producing mucous colonies and being the most pathogenic strain affecting humans (Fang et al., 2007).
However, hvKP clones with a hypermucoviscous phenotype and different capsular serotypes have been described (Cubero et al., 2016; Zhang et al., 2015). Community‐acquired invasive primary liver abscess syndrome caused by KP was first reported in Taiwan in 1986 (Liu et al., 1986; Wang et al., 1998). Patients with concurrent KP liver abscess (KLA) usually have no history of hepatobiliary disease. In the subsequent 30 years, continuous research confirmed that primary KLA was mainly caused by hvKP (Fang et al., 2004). In 2012, KLA invasive syndrome was defined as liver abscess and its associated distant infections caused by a KP infection (Siu et al., 2012). hvKP strains have been described worldwide, but they are most common in Asian countries (Cubero et al., 2016). Compared with cKP infection, hvKP infection spreads easily, shows metastasis and progresses rapidly (Decré et al., 2011). The lungs, central nervous system and eyes are the most common metastatic sites, although only a third of them are diagnosed on admission (Sánchez‐López et al., 2019). hvKP infections can develop into septic shock and multiple organ failure within a short time (Rafat et al., 2018). Complications such as meningitis and endophthalmitis are associated with poorer outcomes and high mortality rates (Sánchez‐López et al., 2019). However, early diagnosis and treatment minimize the development of these complications and improve the clinical outcomes (Siu et al., 2012). Therefore, studies that present the clinical characteristics and factors related to the virulence of KP infection are important in raising awareness of this infection and increasing the chance of an early diagnosis.
MATERIALS AND METHODS
Patients
From January 2015 to January 2019, patients with severe infection treated at the surgical intensive care unit (ICU) of Fujian Provincial Hospital were considered for inclusion in this single‐centre retrospective study. Patients were identified through the Intelligent Database platform (Yidu Cloud Technology Ltd.). The inclusion criteria were as follows: (a) patients with KP infection that was detected using cultures from the original smear epithelial cell secretions in a <10/low power field and (b) the KP strains retained for analysis; if they were repeatedly isolated from the same patient and from the same location, only the first strain was retained, but all strains isolated from different locations of the same patient were retained. The patients were excluded if they: (a) were aged <16 years; (b) had common sputum specimens; (c) had a community infection colonized with KP or (d) had a tuberculosis infection. The project was approved by the clinical scientific research section of the Ethics Committee of Fujian Provincial Hospital (K2018‐04‐026). All patients signed an informed consent form approved by the Institutional Review Board.
Clinical data collection and examination methods
The clinical and demographical data were collected for all study participants. The diagnostic criteria for a pyogenic liver abscess were as follows: (a) The patient had clinical manifestations such as fever, chills, nausea, liver discomfort, fatigue, liver tenderness or percussion pain; (b) Abdominal ultrasound, computed tomography or magnetic resonance imaging and other imaging findings found a liver abscess; (c) Clinical bacteriological examination results were positive or antibiotic treatment was effective; (d) Pyogenic liver abscess confirmed by percutaneous liver puncture or surgical treatment and (e) Amoebic, tuberculous liver abscess, hepatic echinococcosis and liver liquefaction infarction were excluded. On the basis of 1 and 2, a bacterial liver abscess could be diagnosed in accordance with any one or more of 3, 4 and 5.
Sampling and experimental methods
Strains were isolated from lower respiratory tract secretions, blood, thoracic and abdominal drainage fluid and wound secretions which were collected from patients with severe infection.
Strain identification and drug susceptibility analysis
The string test was performed using an inoculating loop to touch a freshly cultured colony that had been grown overnight on an agar plate. The loop was pulled outward, and if there was a mucinous filament with a traction length larger than 5 mm, it was judged to be a positive high‐viscosity phenotype. The strain was identified using a VITEK 2 Compact automatic microbial identification system (BioMérieux SA). Drug sensitivity testing was carried out according to CLSI M100‐S27 (Wayne, PA, 2017). The sensitivity of the strain to piperacillin, piperacillin/tazobactam, cefoxitin, cefazolin, ceftazidime, cefotaxime, cefepime, imipenem, meropenem, amikacin and ciprofloxacin was tested; the quality control strain used was Escherichia coli ATCC 25922.
Statistical methods
Data were analysed using SPSS 19.0 software (IBM Corp.). The sensitivity, intermediation and drug resistance rate of bacterial strains to antimicrobial agents were calculated using WHONET software (World Health Organization). Normal distribution was tested using the Kolmogorov–Smirnov test. Continuous variables are presented as means ± standard deviations or medians (interquartile range) and compared using Student's t‐test or the Mann–Whitney U test, as appropriate. Categorical variables are presented as numbers (percentages) and were compared using chi‐square or Fisher's exact probability tests. Kaplan–Meier survival curves were used to analyse patient survival. Multivariate analysis was performed to identify factors related to hvKP infection using Cox proportional hazards model.
Initially, a univariate Cox proportional hazard regression was performed, and variables with p < 0.2 in the univariate analysis were included in the multivariate analysis (backward method). p < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the patients
There were 589 cases of KP infection identified using microbiological culture; 225 cases of KP infection were included in the study, including 114 cases (50.67%) in the hvKP group and 111 cases (49.33%) in the cKP group. The baseline demographical and clinical data of the patients, including their treatment and prognosis, are shown in Table 1. The two groups had a similar sex ratio and mean age (p > 0.05). All clinical evaluations were similar except albumin level, which was lower (p = 0.003), procalcitonin level, which was higher (p = 0.002) and sequential organ failure assessment score, which was higher (p < 0.001) in the hvKP group compared to the cKP group. There were no significant differences in length of hospital stay, length of ICU stay, duration of mechanical ventilation, duration of antibiotic use or liver and kidney function between the two groups (all p > 0.05).
TABLE 1.
Baseline demographical and clinical data of patients in the two groups
| Variates | hvKP group (n = 114) | cKP group (n = 111) | p |
|---|---|---|---|
| Sex (male) | 74 (64.9%) | 66 (59.5%) | 0.413 |
| Age (years) | 63 (55, 73) | 66 (53, 72) | 0.294 |
| White blood cell (*109/L) | 11.9 (8.8, 15.9) | 12.6 (11.0, 16.6) | 0.27 |
| Percentage of neutrophil (%) | 85 (79, 90) | 84 (78, 88) | 0.291 |
| Platelet (*109/L) | 226 (129, 311) | 194 (142, 295) | 0.465 |
| Albumin (g/L) | 28 (18, 32) | 30 (26, 33) | 0.003 |
| Total bilirubin (μmol/L) | 23 (12, 31) | 22 (13, 33) | 0.396 |
| Serum creatinine (μmol/L) | 44 (33, 67) | 48 (36, 65) | 0.859 |
| PCT (ng/ml) | 17 (3, 38) | 5 (1, 20) | 0.002 |
| CRP (mg/L) | 126 (46, 174) | 120 (43, 200) | 0.423 |
| Troponin I (ng/ml) | 0.1 (0.1, 0.3) | 0.1 (0.1, 0.6) | 0.524 |
| NT‐proBNP (Pg/ml) | 464 (232, 868) | 288 (223, 810) | 0.112 |
| APACHE II (points) | 6 (5, 9) | 7 (5, 8) | 0.226 |
| SOFA (points) | 3 (2, 5) | 2 (1, 3) | <0.001 |
| NUTRIC (points) | 2 (1, 2) | 2 (1, 2) | 0.378 |
| Total length of hospital stay (d) | 21 (12, 31) | 21 (14, 28) | 0.227 |
| Length of ICU stay (d) | 15 (8, 17) | 18 (10, 18) | 0.386 |
| Duration of mechanical ventilation (h) | 12 (8, 10) | 13 (11, 15) | 0.462 |
| Time using antibiotics (d) | 13 (7, 16) | 14 (9, 18) | 0.333 |
| Hospital infection | 28 (60.9%) | 18 (39.1%) | 0.138 |
| Targeted single drug anti‐infection | 60 (52.6%) | 9 (8.1%) | <0.001 |
| Combined with sepsis | 34 (29.8%) | 43 (38.7%) | 0.164 |
| Combined with septic shock | 19 (16.7%) | 9 (8.1%) | 0.04 |
| 28‐day survival | 98 (86.0%) | 104 (93.7%) | 0.077 |
Notes: The length of ICU stay, duration of mechanical ventilation and troponin were normally distributed. However, the standard deviation was greater than the mean value; hence, a nonparametric test was used.
Abbreviations: APACHE, Acute Physiology, Age, Chronic Health Evaluation; cKP, classic Klebsiella pneumoniae PCT = procalcitonin; CRP, C‐reactive protein; hvKP, hypervirulent Klebsiella pneumoniae; ICU, intensive care unit; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NUTRIC, Nutrition Risk in the Critically Ill; SOFA, sequential organ failure assessment.
Underlying diseases in the study population
The underlying diseases were diabetes in 96 cases (46.5%), hypertension in 74 (37.7%), chronic liver disease in 35 (16.7%), malignant tumour in 28 (16.7%), renal insufficiency in 17 (7.0%), coronary heart disease in one (4.4%) and immunosuppression in six (4.4%). There was no significant difference in the underlying disease between the two patient groups (Table 2). Among them, 33 (14.5%) patients had ≥3 underlying diseases, 43 (18.9%) patients used ≥2 antibiotic drugs, the targeted medication and empirical drug use were consistent in 182 (81%) patients and 79 (35.2%) patients used antibiotics for ≥2 weeks. The top five antibiotic drugs used were cefoperazone/sulbactam, tazocin, imipenem, meropenem and ceftazidime.
TABLE 2.
The underlying diseases in the two groups of patients infected with Klebsiella pneumoniae
| Variates | hvKP group (n = 114) | cKP group (n = 111) | p |
|---|---|---|---|
| Diabetes | 53 (46.5%) | 43 (38.7%) | 0.281 |
| Hypertension | 43 (37.7%) | 31 (27.9%) | 0.122 |
| Chronic liver disease | 19 (16.7%) | 16 (14.4%) | 0.714 |
| Malignant tumour | 19 (16.7%) | 9 (8.1%) | 0.068 |
| Malnutrition status | 12 (10.5%) | 9 (8.1%) | 0.648 |
| Renal insufficiency | 8 (7.0%) | 9 (8.1%) | 0.805 |
| Coronary heart disease | 5 (4.4%) | 5 (4.5%) | >0.99 |
| Immunosuppressive diseases | 5 (4.4%) | 1 (0.9%) | 0.213 |
Abbreviations: cKP, classic Klebsiella pneumoniae; hvKP, hypervirulent Klebsiella pneumoniae.
Source of the bacterial strains
The sources of hvKP and cKP infections are shown in Table 3. There were similar numbers of hospital‐acquired infections in the hvKP and cKP groups (28 [24.6%] vs. 18 [16.2%], p = 0.138). Regarding primary infection, the rate of lung infection was higher in the hvKP group than in the cKP group (48 [42.1%] vs. 31 [27.9%], p = 0.018), whereas the rate of liver infection was lower in the hvKP group (79 [69.3%] vs. 90 [81.1%], p = 0.046). The sources of secondary infection were similar between the two groups (all p > 0.05).
TABLE 3.
Source of the bacterial strains in the hvKP and cKP groups
| Variates | hvKP group (n = 114) | cKP group (n = 111) | p |
|---|---|---|---|
| Hospital infection | 28 (24.6%) | 18 (16.2%) | 0.138 |
| Primary infection | |||
| Liver | 79 (69.3%) | 90 (81.1%) | 0.046 |
| Lung | 48 (42.1%) | 31 (27.9%) | 0.018 |
| Bone and soft tissue | 4 (3.5%) | 2 (1.8%) | 0.683 |
| Unitary system | 5 (4.4%) | 5 (4.5%) | 1 |
| Gastrointestinal tract | 2 (1.8%) | 3 (2.7%) | 0.681 |
| Blood | 0 (0%) | 3 (2.7%) | 0.118 |
| Secondary infection | |||
| Lung | 11 (9.6%) | 5 (4.5%) | 0.107 |
| Unitary system | 4 (3.5%) | 2 (1.8%) | 0.683 |
| Brain | 4 (3.5%) | 2 (1.8%) | 0.683 |
| Blood | 3 (2.6%) | 1 (0.9%) | 0.622 |
| Liver | 1 (0.9%) | 2 (1.8%) | 0.618 |
| Gastrointestinal tract | 0 (0%) | 1 (0.9%) | 0.493 |
Abbreviations: cKP, classic Klebsiella pneumoniae; hvKP, hypervirulent Klebsiella pneumoniae.
Patient outcomes
The occurrence of septic shock caused by severe infection attributed to KP was significantly higher in the hvKP group than in the cKP group (19 [16.7%] vs. 9 [8.1%], p = 0.040) (Table 1). The 28‐day mortality rate in the patients was similar between the hvKP and cKP groups (86.0% vs. 93.7%, p = 0.199) (Figure 1).
FIGURE 1.
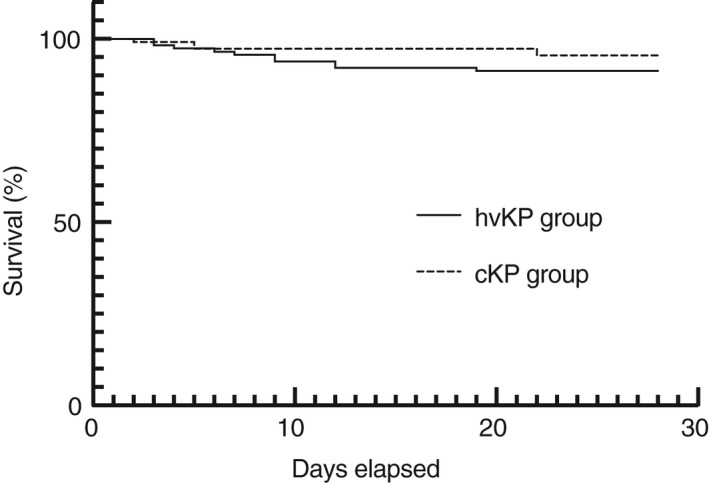
Comparison of survival rates between the hvKP and cKP groups The 28‐day mortality rate was similar between the hvKP and cKP groups (86.0% vs. 93.7%, p = 0.199). hvKP, hypervirulent Klebsiella pneumoniae; cKP, classic Klebsiella pneumoniae
Factors related to hvKP infection
Table 4 shows the Cox proportional hazards model analysis to identify factors related to hvKP infection. Hospital‐acquired infection (hazard ratio [HR] = 4.245, 95% confidence interval [CI]: 1.407–12.812, p = 0.010) and renal insufficiency (HR = 4.017, 95% CI: 1.072–15.059, p = 0.039) were risk factors for clinical infection with hvKP.
TABLE 4.
Analysis of factors related to hvKP infection
| Variates | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| hvKP | 0.502 | 0.172–1.469 | 0.208 | |||
| Age | 1 | 0.962–1.039 | 0.991 | |||
| Sex | 1.936 | 0.614–6.102 | 0.26 | |||
| Hospital‐acquired infection | 4.113 | 1.462–11.568 | 0.007 | 4.25 | 1.407–12.812 | 0.01 |
| Malignant tumour | 1.612 | 0.452–5.743 | 0.461 | |||
| Hypertension | 1.717 | 0.622–4.735 | 0.297 | |||
| Coronary heart disease | 0.046 | 0.129–1.867 | 0.555 | |||
| Diabetes | 0.454 | 0.144–1.426 | 0.176 | |||
| Renal insufficiency | 3.091 | 0.871–10.971 | 0.081 | 4.02 | 1.072–15.059 | 0.039 |
| Chronic liver diseases | 0.365 | 0.048–2.777 | 0.33 | |||
| Immunosuppressive diseases | 7.817 | 1.758–34.766 | 0.007 | 4.37 | 0.934–20.405 | 0.061 |
| Malnutrition status | 0.754 | 0.099–5.737 | 0.785 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio; hvKP, hypervirulent Klebsiella pneumoniae.
DISCUSSION
This study aimed to analyse the clinical characteristics of critically ill patients infected with KP in our hospital and identify factors related to hvKP infection. Of the 225 patients, 50.7% had hvKP infection. Most outcomes were similar in patients with hvKP and cKP infections, including the length of hospital stay, length of ICU stay, duration of mechanical ventilation and 28‐day survival rate.
However, more patients had a septic shock in the hvKP group than in the cKP group. Results of Cox multivariate analysis indicated that hospital‐acquired infection and renal insufficiency were risk factors for bacterial clinical infection with hvKP.
An epidemiological cross‐sectional survey of KP infection in 10 Chinese cities led by the Peking University in 2013 showed that hvKP accounted for 37.8% of all KP infections, and the detection rate showed regional differences fluctuating between 8.33% (Zhejiang) to 73.91% (Wuhan) (Zhang et al., 2016). None of the cities included were from Fujian province, the location of our study. The results of this study showed that hvKP accounted for 50.7% of the KP infections in patients with serious infections, within the range presented above. Therefore, the results of this study support the view that the prevalence of hvKP is high in China (Zhang et al., 2016).
Risk factors for hvKP identified in this study were hospital‐acquired infection and renal insufficiency. These seem to be specific to this population since other studies have found different risk factors, most commonly diabetes and sometimes cancer (Zhang et al., 2016). A retrospective study from Taiwan, China, found that 70%–78% of KLA patients had diabetes or impaired fasting glucose. The statistics showed that this was a major risk factor for KLA (Wang et al., 1998; Yang et al., 2004). Other studies have also found a relationship between diabetes and hvKP infection (Guo et al., 2017; Liu & Guo, 2019; Zhang et al., 2016). This study found that 46.9% of hvKP‐infected patients had diabetes but did not find diabetes to be a risk factor for hvKP infection. However, one study suggested that diabetes was directly related to the invasiveness of KP (Lee et al., 2016). It suggested that the mechanism is related to high glucose levels, which may stimulate capsular polysaccharide biosynthesis and gene expression of hvKP to increase resistance to phagocytosis and contribute to the development of invasive syndrome (Lee et al., 2016). Why diabetes was not a risk factor for hvKP in this study and whether it is a direct risk factor for KLA requires further study.
This study showed no differences in the length of hospital stay, length of ICU stay, duration of mechanical ventilation, duration of antibiotic use and 28‐day survival rate between patients with cKP infection and those with hvKP infection. However, there was a higher rate of septic shock among hvKP patients than that reported by other studies (Liu & Guo, 2018). The rate of hvKP‐targeted drug use inconsistency and empirical drug use was 7.8%, and the combined use of antibiotics in targeted medication accounted for 17.9%. Therefore, timely, effective and accurate antibiotic selection may have impacted the results of this study. There is an increasing concern that KP may be developing antibiotic resistance, making this infection more difficult to treat (Chung, 2016). An increasing rate of antibiotic resistance of KP has been identified in China, with cKP strains having wider resistance (Li et al., 2014). The results of the present study suggested a relatively low level of antibiotic resistance in the infections in this population. Although all hvKP infections were resistant to ampicillin, the rate was lower in cKP infections (24%). However, cKP infections did show some resistance to several other antibiotics as well; therefore, this situation should be monitored further.
Colonization of hvKP is probably a critical step that leads to infection, with the most common site of colonization being the gastrointestinal tract; however, it is also found in the oropharynx and skin of healthy carriers (Shon et al., 2013). Studies have shown that KP strains isolated from liver abscesses and the intestine of healthy carriers had the same pulse gel electrophoresis band, the same virulence‐related genes and a similar median lethal dose (Fung et al., 2012). A mouse model of hvKP infection demonstrated that hvKP could cross the intestinal mucosal barrier leading to liver abscess (Tu et al., 2009), suggesting that hvKP gastrointestinal colonization is an important source of infection. However, the results of this study suggested that primary gastrointestinal infection rates were low in this population at 1.8% in the hvKP group and 2.7% in the cKP group.
This study has some limitations. No genetic analysis was performed, such as that for identifying the K type, and hvKP was identified using string assay. Because this was a retrospective study, there may have been some bias in the inclusion of the patients. The patients were all from one hospital in Fujian, China; therefore, these results cannot be used to represent the Chinese population.
In conclusion, the number of patients infected with hvKP is gradually increasing; therefore, this study presents important information on the characteristics of hvKP infection in a Chinese population. This report shows a high rate of infection with hvKP in this population. Compared to patients with cKP infection, those with hvKP infection showed a higher probability of having septic shock, although survival and length of hospital stay were similar between the two groups. Risk factors for hvKP infection were hospital‐acquired infection and renal insufficiency.
CONFLICT OF INTEREST
No conflict of interest declared.
ACKNOWLEDGEMENTS
We are grateful to all those who have participated in our research and to those who have funded our work. We thank Editage (www.editage.com) for English language editing. This work was supported by the Fujian Natural Science Foundation [grant no. 2020J05258], High‐level hospital foster grants from the Fujian Provincial Hospital [grant no. 2020HSJJ09], Fujian Province Intensive Care Medical Center Construction Project grants from Fujian Provincial Hospital [grant no. (2017)510#] and Health Science and Technology Program of Fujian Province [grant no. 2020GGA006].
Chen, D, Zhang, Y, Wu, J, Li, J, Chen, H, Zhang, X et al. (2022) Analysis of hypervirulent Klebsiella pneumoniae and classic Klebsiella pneumoniae infections in a Chinese hospital. Journal of Applied Microbiology. 132:3883–3890. 10.1111/jam.15476
Dongjie Chen, Yingrui Zhang and Jiafang Wu co‐first authors.
REFERENCES
- Brisse, S. , Passet, V. , Haugaard, A.B. , Babosan, A. , Kassis‐Chikhani, N. , Struve, C. et al. (2013) wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. Journal of Clinical Microbiology, 51, 4073–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán‐Nájera, J.C. , Garza‐Ramos, U. & Barrios‐Camacho, H. (2017) Hypervirulence and hypermucoviscosity: Two different but complementary Klebsiella spp. phenotypes? Virulence, 8, 1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, K.L. , Lin, R. & Teo, J. (2017) Klebsiella pneumoniae in Singapore: hypervirulent infections and the carbapenemase threat. Frontiers in Cellular and Infection Microbiology, 7, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, P.Y. (2016) The emerging problems of Klebsiella pneumoniae infections: carbapenem resistance and biofilm formation. FEMS Microbiology Letters, 363, fnw219. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) . (2017) Performance standards for antimicrobial susceptibility testing 27th ed CLSI supplement M100‐S27. Wayne, PA: CLSI. [Google Scholar]
- Cubero, M. , Grau, I. , Tubau, F. , Pallarés, R. , Dominguez, M.A. , Liñares, J. et al. (2016) Hypervirulent Klebsiella pneumoniae clones causing bacteraemia in adults in a teaching hospital in Barcelona, Spain (2007–2013). Clinical Microbiology and Infection, 22, 154–160. [DOI] [PubMed] [Google Scholar]
- Decré, D. , Verdet, C. , Emirian, A. , Le Gourrierec, T. , Petit, J.C. , Offenstadt, G. et al. (2011) Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. Journal of Clinical Microbiology, 49, 3012–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, C.T. , Chuang, Y.P. , Shun, C.T. , Chang, S.C. & Wang, J.T. (2004) A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. The Journal of Experimental Medicine, 199, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, C.T. , Lai, S.Y. , Yi, W.C. , Hsueh, P.R. , Liu, K.L. & Chang, S.C. (2007) Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clinical Infectious Diseases, 45, 284–293. [DOI] [PubMed] [Google Scholar]
- Fung, C.P. , Lin, Y.T. , Lin, J.C. , Chen, T.L. , Yeh, K.M. , Chang, F.Y. et al. (2012) Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerging Infectious Diseases, 18, 1322–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y. , Wang, S. , Zhan, L. , Jin, Y. , Duan, J. , Hao, Z. et al. (2017) Microbiological and clinical characteristics of hypermucoviscous Klebsiella pneumoniae isolates associated with invasive infections in China. Frontiers in Cellular and Infection Microbiology, 7, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C.H. , Chen, I.L. , Chuah, S.K. , Tai, W.C. , Chang, C.C. , Chen, F.J. et al. (2016) Impact of glycemic control on capsular polysaccharide biosynthesis and opsonophagocytosis of Klebsiella pneumoniae: Implications for invasive syndrome in patients with diabetes mellitus. Virulence, 7, 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Sun, G. , Yu, Y. , Li, N. , Chen, M. , Jin, R. et al. (2014) Increasing occurrence of antimicrobial‐resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clinical Infectious Diseases, 58, 225–232. [DOI] [PubMed] [Google Scholar]
- Liu, C. & Guo, J. (2018) Characteristics of ventilator‐associated pneumonia due to hypervirulent Klebsiella pneumoniae genotype in genetic background for the elderly in two tertiary hospitals in China. Antimicrobial Resistance and Infection Control, 7, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. & Guo, J. (2019) Hypervirulent Klebsiella pneumoniae (hypermucoviscous and aerobactin positive) infection over 6 years in the elderly in China: antimicrobial resistance patterns, molecular epidemiology and risk factor. Annals of Clinical Microbiology and Antimicrobials, 18, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.C. , Cheng, D.L. & Lin, C.L. (1986) Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Archives of Internal Medicine, 146, 1913–1916. [PubMed] [Google Scholar]
- Martin, R.M. & Bachman, M.A. (2017) Colonization, infection, and the accessory genome of Klebsiella pneumonia . Frontiers in Cellular and Infection Microbiology, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafat, C. , Messika, J. , Barnaud, G. , Dufour, N. , Magdoud, F. , Billard‐Pomarès, T. et al. (2018) Hypervirulent Klebsiella pneumoniae, a 5‐year study in a French ICU. Journal of Medical Microbiology, 67, 1083–1089. [DOI] [PubMed] [Google Scholar]
- Sánchez‐López, J. , García‐Caballero, A. , Navarro‐San Francisco, C. , Quereda, C. , Ruiz‐Garbajosa, P. , Navas, E. et al. (2019) Hypermucoviscous Klebsiella pneumoniae: a challenge in community acquired infection. IDCases, 17, e00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon, A.S. , Bajwa, R.P. & Russo, T.A. (2013) Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence, 4, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu, L.K. , Yeh, K.M. , Lin, J.C. , Fung, C.P. & Chang, F.Y. (2012) Klebsiella pneumoniae liver abscess: a new invasive syndrome. The Lancet Infectious Diseases, 12, 881–887. [DOI] [PubMed] [Google Scholar]
- Tu, Y.C. , Lu, M.C. , Chiang, M.K. , Huang, S.P. , Peng, H.L. , Chang, H.Y. et al. (2009) Genetic requirements for Klebsiella pneumoniae‐induced liver abscess in an oral infection model. Infection and Immunity, 77, 2657–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila, A. , Cassata, A. , Pagella, H. , Amadio, C. , Yeh, K.M. , Chang, F.Y. et al. (2011) Appearance of Klebsiella pneumoniae liver abscess syndrome in Argentina: case report and review of molecular mechanisms of pathogenesis. Open Microbiol J, 5, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.H. , Liu, Y.C. , Lee, S.S. , Yen, M.Y. , Chen, Y.S. , Wang, J.H. et al. (1998) Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clinical Infectious Diseases, 26, 1434–1438. [DOI] [PubMed] [Google Scholar]
- Yang, C.C. , Yen, C.H. , Ho, M.W. & Wang, J.H. (2004) Comparison of pyogenic liver abscess caused by non‐Klebsiella pneumoniae and Klebsiella pneumoniae . Journal of Microbiology, Immunology, and Infection, 37, 176–184. [PubMed] [Google Scholar]
- Zhang, Y. , Sun, J. , Mi, C. , Li, W. , Zhao, S. , Wang, Q. et al. (2015) First report of two rapid‐onset fatal infections caused by a newly emerging hypervirulent K. Pneumonia ST86 strain of serotype K2 in China. Frontiers in Microbiology, 6, 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Zhao, C. , Wang, Q. , Wang, X. , Chen, H. , Li, H. et al. (2016) High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrobial Agents and Chemotherapy, 60, 6115–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]


