Abstract
Objective
The corticotropin‐releasing factor neuropeptides (corticotropin‐releasing hormone [CRH] and urocortin [UCN]‐1,2,3) and spexin contribute to the regulation of energy balance and inhibit food intake in mammals. However, the status of these neuropeptides in children with overweight has yet to be elucidated. This study investigated the effect of increased body weight on the circulating levels of these neuropeptides.
Methods
A total of 120 children with a mean age of 12 years were enrolled in the study. Blood samples were collected to assess the circulating levels of neuropeptides and were correlated with various anthropometric, clinical, and metabolic markers.
Results
Plasma levels of UCNs were altered in children with overweight but less so in those with obesity. Furthermore, the expression pattern of UCN1 was opposite to that of UCN2 and UCN3, which suggests a compensatory effect. However, no significant effect of overweight and obesity was observed on CRH and spexin levels. Finally, UCN3 independently associated with circulating zinc‐alpha‐2‐glycoprotein and UCN2 levels, whereas UCN1 was strongly predicted by TNFα levels.
Conclusions
Significant changes in neuropeptide levels were primarily observed in children with overweight and were attenuated with increased obesity. This suggests the presence of a compensatory mechanism for neuropeptides to curb the progression of obesity.
Study Importance.
What is already known?
-
►
The corticotropin‐releasing factor (CRF) family is known to play a role in energy balance, food intake, inflammation, and stress response. However, its role and expression levels in children with overweight and obesity have yet to be investigated.
What does this study add?
-
►
Our study is the first, to our knowledge, to report an impaired expression of these neuropeptides in plasma from children with overweight and obesity.
How might these results change the direction of research or the focus of clinical practice?
-
►
The observed differential dysregulation in the levels of circulating CRF neuropeptides is indicative of early dimorphic metabolic disturbance and suggests possible compensatory mechanisms to blunt the increase in body weight.
INTRODUCTION
The rise in the prevalence of childhood obesity is an increasing public health problem worldwide, particularly in societies that have witnessed a profound change in lifestyle associated with higher food intake and decreased physical activity during the past few decades (1). The number of children aged 5 to 19 years old living with obesity in 2019 is estimated to be 158 million worldwide and is expected to reach approximately 254 million by 2030 (2). These changes are accompanied by a rise in obesity‐related metabolic disorders, including insulin resistance, dyslipidemia, and hormonal dysregulation (3). More alarmingly, nearly 80% of children and adolescents with obesity are expected to continue to have obesity in adulthood (1). Therefore, the impact of these dysregulations on morbidity, including cardiovascular disease and reduced life‐span in future generations, emphasizes the importance of understanding the pathophysiological mechanisms that occur in children with obesity (4).
Obesity induces dysregulated energy metabolism and a disturbance of hormonal system function, as reflected by insulin resistance and increased levels of leptin and adipokines in an attempt to control the energy balance by the brain and other organs such as the liver and adipose tissue (3). The corticotropin‐releasing factor (CRF) family of neuropeptides (corticotropin‐releasing hormone [CRH] and 3 urocortins [(UCN)‐1,2,3]) and their receptors (CRHR1 and CRHR2) contribute to the regulation of energy balance and inhibit food intake in mammals (5). They are concomitantly expressed in the brain, pancreas, heart, adipose tissue, and skeletal muscle (6, 7, 8). Despite the different roles for each of these peptides and their receptors in metabolic pathways (9), previous studies have indicated beneficial effects of overexpression or treatment with some of these neuropeptides, such as the administration of UCN1‐protected cardiomyocytes (10). Similar cardioprotective effects were reported for UCN2 and UCN3 (11). Moreover, UCN3 overexpression protected rodents from metabolic dysregulation induced by a high‐fat diet (HFD) (6). UCN2‐knockout mice displayed insulin sensitivity and resistance against HFD‐related effects (12), whereas UCN3 knockout increased food intake and reduced insulin sensitivity (13). The overexpression of UCN3 in the brain increased energy expenditure but reduced insulin sensitivity (14), whereas injection of UCN3 into the hypothalamus reduced food intake (15). Furthermore, we and others have reported that UCN3 expression is dysregulated in obesity, type 2 diabetes (T2D), and polycystic ovary syndrome (8, 16, 17). In an independent study, UCN3 and its receptor, CRHR2, were suggested as targets for obesity treatment because of their colocalization with quantitative trait loci for obesity (18). Recently, we reported decreased levels of UCN3 in the plasma of nondiabetic individuals with obesity, whereas increased levels were observed in subcutaneous adipose tissue from these people (16).
Spexin is another neuropeptide involved in energy homeostasis. Following injection into goldfish or a diet‐induced obesity rodent model, spexin decreases food intake (19, 20). Nevertheless, the impact of spexin levels on human obesity remains controversial, as one study reported decreased levels in both adults and children with obesity and diabetes (21), whereas another study showed no differences in serum spexin levels between adolescents with normal weight, obesity, and obesity with diabetes (22). We recently reported decreased plasma levels of spexin in adults with obesity, which were increased in response to regular moderate physical exercise (23).
Considering the available data on CRF, UCNs, and spexin and their expected protective role in metabolic disorders, we hypothesized that their expression and plasma levels are dysregulated in children with obesity, thereby affecting the cross talk between the brain and peripheral organs. Therefore, this study evaluated CRF, UCN1, UCN2, UCN3, and spexin levels in the plasma of children with normal weight, children with overweight, and children with obesity and their association with anthropometric and biochemical markers.
METHODS
Study cohort characteristics
A total of 120 boys and girls (21 with normal weight, 14 with overweight, and 85 with obesity) with a mean age of 12 years were recruited for the study (Table 1). Written informed consent was obtained from the parent(s) of all children before their enrollment, and the study was approved by the Review Board of the Dasman Diabetes Institute (Kuwait City, Kuwait) and was conducted in line with the principles of the Declaration of Helsinki. All children visited the obesity clinics at the Dasman Diabetes Institute in the Farwaniya Hospital or in the Al‐Adan Hospital between January 2014 and December 2016. The inclusion criterion was age = 6 to 17 years, whereas exclusion criteria were any chronic illnesses or endocrine diseases unrelated to diabetes. Children were divided into groups with normal weight (BMI percentile < 85th), overweight (85th ≤ BMI percentile < 95th), and obesity (BMI percentile ≥ 95th) (24).
TABLE 1.
Characteristics of the study population
| Bonferroni post hoc p value | Overall p value | ||||||
|---|---|---|---|---|---|---|---|
| NW (n = 21) | OW (n = 14) | OB (n = 85) | NW vs. OW | NW vs. OB | OW vs. OB | All children | |
| Physical and clinical characteristics | |||||||
| Sex (M/F) | 13/8 | 4/10 | 50/35 | 0.829 | 0.054 | 0.032 | 0.085 |
| Age (y) | 11.9 ± 4.0 | 13.1 ± 3.0 | 13.0 ± 2.9 | 0.766 | 0.368 | 0.983 | 0.288 |
| Weight (kg) | 45.4 ± 19.1 | 61.7 ± 13.4 | 87.9 ± 26.5 | 0.04 | <0.001 | 0.001 | <0.001 |
| Height (m) | 1.50 ± 0.21 | 1.57 ± 0.12 | 1.60 ± 0.15 | 0.720 | 0.060 | 0.857 | 0.065 |
| BMI (kg/m2) | 18.9 ± 3.5 | 24.7 ± 2.8 | 33.8 ± 6.4 | 0.012 | <0.0001 | <0.0001 | <0.001 |
| Percentile | 54.9 ± 19.8 | 92.0 ± 1.6 | 98.5 ± 1.1 a | <0.0001 | <0.0001 | 0.045 | <0.0001 |
| Body fat (%) | 7.3 ± 6.0 | 18.4 ± 6.7 | 32.8 ± 13.0 | 0.018 | <0.001 | <0.001 | <0.001 |
| Metabolic and hormonal markers | |||||||
| Cholesterol (mmol/L) | 3.9 ± 0.5 | 4.3 ± 0.6 | 4.3 ± 0.7 | 0.507 | 0.170 | 1.000 | 0.150 |
| HDL (mmol/L) | 1.48 ± 0.27 | 1.35 ± 0.24 | 1.20 ± 0.45 | 0.453 | 0.016 | 0.709 | 0.017 |
| LDL (mmol/L) | 2.20 ± 0.49 | 2.60 ± 0.61 | 2.65 ± 0.72 | 0.286 | 0.022 | 1.000 | 0.026 |
| TGL (mmol/L) | 0.65 ± 0.30 | 0.92 ± 0.45 | 1.00 ± 0.43 | 0.172 | 0.002 | 1.000 | 0.003 |
| GLU (mmol/L) | 4.8 ± 0.4 | 5.4 ± 1.7 | 5.1 ± 0.4 | 0.040 | 0.463 | 0.240 | 0.046 |
| Total protein (mmol/L) | 72.7 ± 3.5 | 74.4 ± 3.4 | 74.3 ± 3.3 | 0.588 | 0.282 | 1.000 | 0.221 |
| Albumin (mmol/L) | 41.7 ± 3.2 | 41.8 ± 3.2 | 40.7 ± 2.8 | 1.000 | 0.600 | 0.728 | 0.283 |
| Total bilirubin (mmol/L) | 10.4 ± 5.8 | 10.8 ± 3.9 | 8.1 ± 2.9 | 1.000 | 0.078 | 0.086 | 0.017 |
| ALT (mmol/L) | 26.1 ± 6.8 | 23.1 ± 6.6 | 37.2 ± 22.3 | 0.240 | 0.080 | 0.060 | 0.014 |
| AST (mmol/L) | 27.5 ± 7.2 | 21.0 ± 5.8 | 25.2 ± 8.8 | 0.011 | 0.901 | 0.328 | 0.111 |
| Alkaline phosphatase (mmol/L) | 161.8 ± 78.1 | 170.7 ± 115.3 | 169.7 ± 77.4 | 0.807 | 0.702 | 0.977 | 0.931 |
| GGT (mmol/L) | 21.0 ± 4.4 | 25.5 ± 8.5 | 30.5 ± 17.5 | 0.168 | 0.085 | 0.500 | 0.182 |
| HbA1c (%) | 5.2 ± 0.3 | 5.56 ± 1.7 | 5.62 ± 2.3 | 0.443 | 0.141 | 0.932 | 0.712 |
| Insulin (mIU/L) | 5.5 ± 3.0 | 9.3 ± 3.9 | 18.2 ± 13.7 | 0.003 | <0.001 | 0.037 | <0.001 |
| HOMA‐IR | 1.23 ± 0.74 | 1.95 ± 0.75 | 4.32 ± 3.42 | 0.016 | <0.001 | 0.014 | <0.001 |
| Adiponectin (µg/mL) | 9.8 ± 1.4 | 8.9 ± 1.4 | 8.1 ± 2.0 | 0.065 | 0.001 | 0.411 | 0.001 |
| TNFα (ng/mL) | 4.3 ± 12.8 | 32.8 ± 8.8 | 35.0 ± 33.0 | 0.014 | <0.001 | 1.000 | <0.001 |
| Other markers | |||||||
| Leptin (ng/mL) | 7.5 ± 2.6 | 13.3 ± 7.0 | 16.1 ± 7.5 | 0.049 | <0.001 | 0.466 | <0.001 |
| C‐pep (ng/mL) | 0.26 ± 0.17 | 0.18 ± 0.08 | 0.27 ± 1.06 | 0.120 | 1.000 | 1.000 | 0.787 |
| Glucagon (ng/mL) | 3.59 ± 1.53 | 1.92 ± 1.20 | 2.35 ± 0.98 | <0.001 | <0.001 | 0.576 | <0.001 |
| NGAL (ng/mL) | 22.9 ± 6.1 | 35.7 ± 15.1 | 33.5 ± 13.9 | 0.018 | 0.005 | 1.000 | 0.004 |
| RBP4 (µg/mL) | 20.2 ± 5.5 | 55.30 ± 25.1 | 63.76 ± 103.2 | 0.011 | 0.152 | 1.000 | 0.147 |
| sICAM‐1 (ng/mL) | 128.3 ± 78.8 | 255.2 ± 199.4 | 217.3 ± 252.2 | 0.015 | 0.019 | 1.000 | 0.204 |
| ZAG (µg/mL) | 1.6 ± 0.2 | 4.9 ± 0.6 | 3.3 ± 2.0 | <0.001 | 0.001 | 0.005 | <0.001 |
| Resistin (ng/mL) | 4.9 ± 2.7 | 4.6 ± 1.4 | 5.4 ± 2.2 | 0.706 | 0.507 | 0.280 | 0.487 |
Note: Data are presented as mean ± SD. One‐way ANOVA followed by Bonferroni post hoc test.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; C‐pep, connecting peptide; F, female; GGT, gamma‐glutamyltransferase; GLU, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment for insulin resistance; LDL, low‐density lipoprotein; M, male; NGAL, neutrophil gelatinase‐associated lipocalin; NW, normal weight; OB, obesity; OW, overweight; TGL, triglyceride; TNFα, tumor necrosis factor alpha; RBP4, retinol‐binding protein 4; sICAM‐1, soluble intercellular adhesion molecule‐1; ZAG, zinc‐alpha‐2‐glycoprotein.
Only percentiles between 95 and 99 were included for the children with obesity.
Anthropometric measurements
Children underwent a standard physical examination by a pediatrician endocrinologist, and anthropometric measurements were obtained based on World Health Organization (WHO) recommendations. Body weight and percentage of body fat were measured by Pediatric Body Composition Analyzer/Segmental (GAIA KIKO Jawon Medical) while children were wearing light clothes without footwear. Height was measured using a stadiometer. BMI percentile was calculated according to the Centers for Disease Control and Prevention (CDC) growth chart, and the measured BMI was used to classify children as having normal weight (BMI percentile < 85th), overweight (85th ≥ BMI percentile < 95th), and obesity (≥95th percentile) (24). Children were further grouped by BMI percentile based on cutoff points, as defined by Cole et al. (25).
Sample collection and blood analysis
Venous blood was collected in EDTA tubes after overnight fasting. Peripheral blood mononuclear cells were isolated using ready‐to‐use, sterile Ficoll‐Hypaque density gradient medium and a centrifugation method. Plasma was aliquoted and stored at −80°C. Lipid and glucose profiles were measured using a Siemens Dimension RXL chemistry analyzer (Diamond Diagnostics). Hemoglobin A1c was measured with a Variant device (Bio‐Rad Laboratories). Insulin concentration and liver function enzymes were measured using Access 2 and AU480 Systems (Beckman Coulter) respectively. The homeostatic model assessment of insulin resistance (HOMA‐IR) index was calculated using the following formula: HOMA‐IR = (glucose × insulin)/22.5.
Plasma levels of UCN1 (#LS‐F6155, Lifespan Biosciences, Inc.), UCN2 (#LS‐F39013, Lifespan Biosciences), UCN3 (#LS‐F12902, Lifespan Biosciences), CRH (#LS‐F5352, Lifespan Biosciences), spexin (#EK‐023‐81, Phoenix Pharmaceuticals), insulin, and ultrasensitive connecting (C)‐peptide (Mercodia AB) were measured with enzyme‐linked immunosorbent assay (ELISA) kits. Sample dilutions were determined through optimization, and ELISA was performed according to the manufacturer’s instructions. Absorbance was measured using a Synergy H4 plate reader (BioTek Instruments).
Obesity and diabetes markers were measured using the following panels and the bead‐based multiplexing technology Bio‐Plex 200 system: obesity (Metabolism/Obesity 5‐Plex Human ProcartaPlex Panel 1 [EPX09A‐15804‐901], Metabolism/Obesity 9‐Plex Human ProcartaPlex Panel 2 [EPX09A‐15804‐901]) and diabetes (MILLIPLEX MAP Human Diabetes Panel, Premixed 5‐Plex Assay, Millipore HDIAB‐34K‐PMX5). Fluorescence intensities were measured using Bio‐Plex manager software version 6 (Bio‐Rad Laboratories).
Statistical analysis
All statistical analyses were performed using SPSS Statistics software version 25.0 (IBM Corp.). Descriptive statistics were used to report the mean and standard deviation for continuous variables, and frequency statistics were used to calculate the number and percentage for categorical variables. Categorical parameters are described as number and percentage. The neuropeptide variables were log transformed using SPSS. Post log‐transformation variables were checked for normality. To evaluate the effects of groups, we conducted a 1 way ANOVA test with post hoc Bonferroni correction that included the entire study population. Bonferroni post hoc p values based on ANOVA for each outcome, as well as the entire study population combined, are included in Table 1. Partial correlation was used for all population analyses adjusted for age and gender, and Spearman rank correlation was performed for gender‐specific analysis. Univariate and stepwise multivariate linear regression analyses were performed to examine the predictive effect of selected factors, with and without adjustment for age and sex. P values <0.05 were considered statistically significant.
RESULTS
Characteristics of study population
The study population consisted of 120 children (21 with normal weight, 14 with overweight, and 85 with obesity; 67 male individuals and 53 female individuals) with a mean age of 12 years. Blood samples were collected and processed for plasma and peripheral blood mononuclear cells. Table 1 summarizes the anthropometric, clinical, and biochemical characteristics of the cohort.
When compared with children with normal weight, the group with overweight exhibited a higher body fat percentage (p < 0.05). The glycemic markers insulin, fasting plasma glucose (GLU), and HOMA‐IR were also markedly increased, whereas glucagon levels were decreased in this group (p < 0.05). Circulating levels of the inflammatory marker tumor necrosis factor α (TNFα) were significantly higher in the children with overweight and were associated with increased levels of the obesity markers leptin, retinol‐binding protein 4 (RBP4), neutrophil gelatinase‐associated lipocalin (NGAL), soluble intercellular adhesion molecule‐1 (sICAM‐1), and zinc‐alpha‐2‐glycoprotein (ZAG; p < 0.05) (Table 1).
In the group with obesity, there was a marked increase in insulin and HOMA‐IR levels (p < 0.001) compared with children with normal weight. Similarly, lipid profiles were impaired with significantly reduced high‐density lipoprotein (HDL) and increased low‐density lipoprotein (LDL) and triglyceride levels (p < 0.05). In addition, a significant increase (p < 0.05) in adiposity‐related markers (leptin, TNFα, NGAL, sICAM‐1, and ZAG) was also observed, whereas adiponectin and glucagon levels were decreased (p < 0.05). When compared with the group with overweight, the group with obesity exhibited persistent increased levels of insulin and HOMA‐IR (p < 0.05). The children with obesity were further stratified into metabolically healthy obesity (MHO) and metabolically unhealthy obesity (MUO) groups based on classification by HOMA‐IR levels, with HOMA‐IR < 3.16 for MHO and ≥3.16 for MUO (26). In addition to the glycemic parameters (GLU, insulin, and HOMA‐IR), there was a significant increase in BMI and body fat percentage (p < 0.05) in the MUO group compared with the MHO group, but not with other tested markers (Supporting Information Table S1).
Plasma UCN1, UCN2, and UCN3 levels are associated with obesity and sex
We assessed the circulating levels of the neuropeptides UCN1, UCN2, UCN3, CRH, and spexin in the plasma of our study population and stratified children according to body weight groups (Supporting Information Figure S1). The results showed that UCN1 levels were significantly decreased (p < 0.05) in the plasma of the group with overweight (15.6 [6.4] pg/mL) compared with the group with normal weight (37.7 [7.6] pg/mL) but increased in the group with obesity to a similar level to that of the group with normal weight (43.1 [9.5] pg/mL; Supporting Information Figure S1A). Circulating UCN3 levels were significantly increased with increased body weight (p < 0.001), with a marked increase in the group with overweight compared with the group with obesity (Supporting Information Figure S1C). A similar trend was observed for UCN2, in which UCN2 levels were significantly increased in the group with overweight (1,789 [103] ng/mL) compared with the group with normal weight (1,477 [101] ng/mL; p < 0.043), but only a statistically nonsignificant increase was observed in the group with obesity (Supporting Information Figure S1B). However, the circulating levels of CRH and spexin were not significantly affected by increased body weight, as shown in Supporting Information Figure S1D,E.
Considering the observed differential profiles of UCN1, UCN2, and UCN3 with body weight, we further evaluated these neuropeptide levels with respect to gender (Figure 1). UCN1 levels were globally lower in girls than boys regardless of their body weight, particularly in children with normal weight (19.3 [25.7] pg/mL and 49.1 [34.8] pg/mL, respectively; p < 0.05 Figure 1A). On the contrary, significantly higher levels of UCN2 and UCN3 were observed in girls compared with boys with overweight and obesity. In children with obesity, the levels of UCN2 in girls and boys were 1,838 (351) ng/mL and 1,498 (39) ng/mL, respectively, and the levels of UCN3 were 16.5 (22.4) ng/mL and 6.2 (8.0) ng/mL, respectively (p < 0.01; Figure 1B,C). Although not statistically significant, trends of higher levels of CRH were observed in all girls than boys, irrespective of body weight (Figure 1D). However, no differences were observed by sex in spexin levels (Figure 1E). In children with obesity, no significant differences existed between the MHO and MUO groups in the levels of all studied neuropeptides (Supporting Information Table S1).
FIGURE 1.
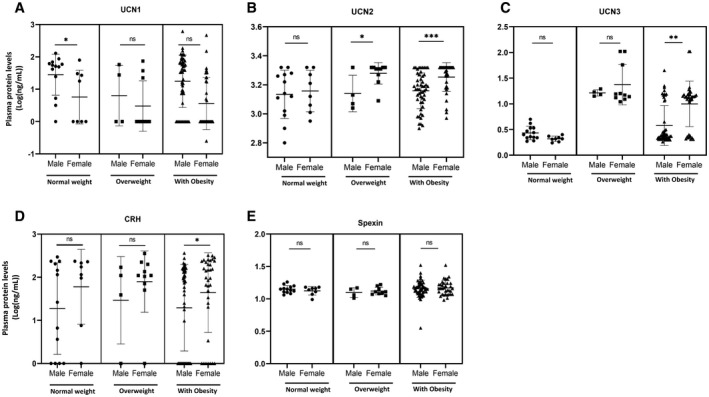
Urocortin (UCN) and spexin levels in plasma based on body weight groups and sex. Circulating levels of (A) UCN1, (B) UCN2, (C) UCN3, (D) CRH, and (E) spexin were measured by ELISA using plasma samples from boys and girls in the normal weight group (n = 23 and n = 8, respectively), the overweight group (n = 4 and n = 10, respectively), and the obesity group (n = 50 and n = 35, respectively). A 1‐way ANOVA test was used to determine significance of the difference in means between the groups, and data were log transformed. Statistical significances are as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. CRH, corticotropin‐releasing hormone; ns, not significant
Correlation analysis
Partial correlation analysis was performed on the entire cohort to evaluate the association of circulating UCN1, UCN2, UCN3, CRF, and spexin with various clinical parameters (Table 2). Overall, UCN1 and UCN3 were correlated with more variables compared with UCN2, CRH, and spexin. UCN1 levels were positively correlated with height, albumin, and total protein. UCN3 correlated positively and significantly with body percentile, TNFα, RBP4, and ZAG concentrations but was negatively correlated with C‐peptide (Table 2). In this adjusted analysis, UCN2 correlated only with CRH, whereas the latter marker only displayed a negative correlation with aspartate aminotransferase (AST) in all children.
TABLE 2.
Partial correlation ranking of neuropeptides with selected study characteristics
| All children | UCN1 | UCN2 | UCN3 | CRH | Spexin |
|---|---|---|---|---|---|
| Height (m) | 0.75* | −0.11 | 0.26 | −0.11 | 0.45 |
| Percentiles | −0.06 | 0.23 | 0.56* | 0.44 | −0.17 |
| Total protein (mmol/L) | 0.36* | −0.61 | −0.06 | −0.35 | 0.06 |
| Albumin (mmol/L) | 0.88** | −0.25 | −0.01 | −0.35 | −0.39 |
| AST (mmol/L) | 0.45 | −0.53 | −0.17 | −0.62* | −0.05 |
| TNFα (ng/mL) | −0.33 | 0.33 | 0.87** | 0.06 | 0.21 |
| C‐pep (ng/mL) | 0.11 | −0.20 | −0.57* | −0.07 | 0.04 |
| RBP4 (µg/mL) | −0.19 | 0.00 | 0.70* | −0.20 | 0.02 |
| ZAG (µg/mL) | −0.21 | 0.24 | 0.96** | 0.09 | 0.22 |
| CRH (pg/mL) | −0.01 | 0.68* | −0.01 | – | −0.10 |
| UCN1 (pg/mL) | – | −0.12 | −0.10 | −0.01 | 0.27 |
| UCN2 (ng/mL) | −0.12 | – | 0.16 | 0.68* | 0.16 |
| UCN3 (ng/mL) | −0.10 | 0.16 | – | −0.01 | 0.20 |
| Spexin (ng/mL) | 0.27 | 0.16 | 0.20 | −0.10 | – |
Note: Adjusted for age and sex. Data are presented as the R values.
Abbreviations: AST, aspartate aminotransferase; C‐pep, connecting peptide; CRH, corticotropin‐releasing hormone; RBP4, retinol‐binding protein 4; TNFα, tumor necrosis factor alpha; UCN, urocortin; ZAG, zinc‐alpha‐2‐glycoprotein.
p < 0.05
p < 0.01.
When separating all children into male and female subgroups, additional correlations were evident despite the relatively low number of children in each subgroup (Table 3). Accordingly, strong negative correlations were observed between UCN1 with albumin in boys and with HDL, TNFα, and ZAG in girls. However, UCN2 levels correlated negatively with alkaline phosphatase and positively with C‐peptide and glucagon levels in boys (Table 3). In girls, UCN2 only correlated negatively with leptin levels. Furthermore, UCN3 correlated positively with TNFα, leptin, RBP4, and ZAG in boys and with percentile in the female group but correlated negatively with glucagon in the latter group. Nevertheless, CRH displayed a negative correlation with albumin and spexin displayed a positive correlation with AST in girls, respectively. No correlation was observed in boys for these markers.
TABLE 3.
Spearman correlation coefficient ranking of neuropeptides with all study variables by sex
| Boys | Girls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UCN1 | UCN2 | UCN3 | CRH | Spexin | UCN1 | UCN2 | UCN3 | CRH | Spexin | |
| Height (m) | 0.83* | −0.076 | 0.145 | −0.066 | 0.723 | −0.811 | 0.730 | 0.98* | 0.129 | −0.397 |
| Percentiles | 0.006 | 0.163 | 0.255 | 0.623 | −0.046 | −0.779 | 0.553 | 0.98* | −0.050 | −0.625 |
| HDL (mmol/L) | 0.150 | 0.180 | −0.006 | −0.561 | 0.645 | −0.94* | 0.387 | 0.715 | 0.792 | 0.014 |
| LDL (mmol/L) | −0.110 | −0.305 | 0.310 | 0.335 | −0.524 | 0.94* | −0.051 | −0.748 | −0.504 | 0.431 |
| Albumin (mmol/L) | −0.95** | −0.438 | −0.046 | −0.403 | −0.411 | 0.382 | −0.340 | −0.053 | −0.96* | −0.751 |
| Total bilirubin (mmol/L) | 0.67* | −0.191 | −0.113 | 0.139 | 0.003 | −0.843 | 0.094 | 0.859 | 0.065 | −0.768 |
| AST (mmol/L) | 0.334 | −0.145 | 0.280 | −0.563 | 0.080 | 0.493 | 0.191 | −0.614 | 0.365 | 0.98* |
| Alkaline phosphatase (mmol/L) | −0.268 | −0.74* | 0.348 | −0.128 | −0.219 | −0.029 | 0.896 | 0.181 | 0.239 | 0.602 |
| TNFα (ng/mL) | −0.166 | −0.160 | 0.92** | −0.256 | 0.119 | −0.96* | 0.196 | 0.879 | 0.345 | −0.551 |
| Leptin (ng/mL) | −0.092 | −0.365 | 0.88* | −0.089 | −0.012 | 0.357 | −0.97* | −0.479 | −0.369 | −0.430 |
| C‐pep (ng/mL) | −0.099 | 0.77* | 0.002 | 0.279 | 0.009 | 0.652 | −0.781 | −0.923 | 0.067 | 0.414 |
| Glucagon (ng/mL) | 0.173 | 0.82* | −0.258 | 0.873 | −0.065 | 0.937 | −0.651 | −0.97* | −0.390 | 0.287 |
| RBP4 (µg/mL) | −0.221 | 0.016 | 0.84* | −0.196 | 0.144 | −0.249 | 0.224 | 0.624 | −0.669 | −0.863 |
| sICAM‐1 (ng/mL) | −0.381 | 0.150 | 0.268 | −0.083 | 0.049 | −0.480 | 0.437 | 0.183 | 0.95* | 0.686 |
| ZAG (µg/mL) | −0.066 | −0.160 | 0.97** | −0.136 | 0.197 | −0.96* | 0.600 | 0.956 | 0.439 | −0.284 |
| CRH (pg/mL) | 0.160 | 0.610 | −0.241 | 0.000 | −0.333 | −0.547 | 0.187 | 0.157 | 1.000 | 0.559 |
| UCN1 (pg/mL) | 1.000 | 0.215 | 0.047 | 0.160 | 0.482 | 1.000 | −0.378 | −0.882 | −0.547 | 0.305 |
| UCN2 (ng/mL) | 0.215 | 1.000 | −0.249 | 0.610 | −0.133 | −0.378 | 10.000 | 0.591 | 0.187 | 0.230 |
| UCN3 (ng/mL) | 0.047 | −0.249 | 1.000 | −0.241 | 0.308 | −0.882 | 0.591 | 1.000 | 0.157 | −0.499 |
| Spexin (ng/mL) | 0.482 | −0.133 | 0.308 | −0.333 | 1.000 | 0.305 | 0.230 | −0.499 | 0.559 | 1.000 |
Note: Data are presented as the R values.
Abbreviations: AST, aspartate aminotransferase; C‐pep, connecting peptide; CRH, corticotropin‐releasing hormone; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RBP4, retinol‐binding protein 4; sICAM‐1, soluble intercellular adhesion molecule‐1; TNFα, tumor necrosis factor alpha; UCN, urocortin; ZAG, zinc‐alpha‐2‐glycoprotein.
p < 0.05
p < 0.01.
An univariate linear regression analysis (Supporting Information Table S2), followed by a multivariable stepwise linear regression analysis (Table 4), was performed, with each neuropeptide marker as a dependent variable, in the whole study population before and after adjustment for age and sex. In both unadjusted and adjusted models, independent associations were observed for UCN1 levels with TNFα and UCN3 (p < 0.05), whereas UCN3 and CRH were the only predictors for UCN2 (Table 4). However, the UCN2 and ZAG markers were independently associated with circulating UCN3 levels (p < 0.05). CRH was independently predicted by UCN2 and spexin (p < 0.05).
TABLE 4.
Multivariable linear regression analysis in all children, with neuropeptides as the dependent variables a
| Neuropeptide | Independent variables | β coefficient | p value |
|---|---|---|---|
| UCN1 | TNFα | −0.349 | 0.005 |
| UCN3 | 0.316 | 0.035 | |
| UCN2 | UCN3 | 0.426 | 0.042 |
| CRH | 0.373 | 0.029 | |
| UCN3 | ZAG | 0.292 | 0.030 |
| UCN2 | 0.121 | 0.03 | |
| CRH | UCN2 | 0.547 | 0.002 |
| Spexin | −0.245 | 0.018 | |
| Spexin | CRH | −0.242 | 0.027 |
Abbreviations: CRH, corticotropin‐releasing hormone; TNFα, tumor necrosis factor alpha; UCN, urocortin; ZAG, zinc‐alpha‐2‐glycoprotein.
Adjusted for age and sex.
DISCUSSION
This study is the first one, to our knowledge, assessing the status of the main neuropeptides of the CRF family in children. The results showed a disturbed plasma profile of UCN neuropeptides with increased body weight in children. The main findings of this study are as follows: 1) major changes in UCN levels occurred in children with overweight, whereas these changes were less significant in children with obesity; 2) the variation in the pattern of UCN1 expression was opposite to that of UCN2 and UCN3, suggesting that the increase in UCN2 and UCN3 in children with overweight may be a compensatory effect; 3) no significant effect of overweight and obesity was observed on CRH and spexin levels; and 4) UCN3 was independently associated with circulating UCN1 and UCN2, whereas UCN1 was strongly predicted by TNFα levels.
In addition to the expected trends of dysregulated glycemic and lipid profiles, we observed that there were increased levels of leptin and other adipokines, as well as inflammatory markers, in children with overweight and children with obesity compared with children with normal weight, which is in line with previous studies (27, 28). This included TNFα, NGAL, RBP4, ZAG, and sICAM1, along with a significant decrease in adiponectin levels.
These expected observations suggest disturbed food intake and energy balance, glucose and fat metabolism, and metabolic stress response in these children. Moreover, we observed elevated alanine transferase (ALT) and an increased trend of γ‐glutamyl transferase (γGT) with increased weight. Both markers are surrogate markers for liver dysfunction and nonalcoholic fatty liver, as well as factors of metabolic syndrome. Increased ALT and γGT are considered indicators of the development of prediabetes and T2D in apparently healthy individuals (28). ZAG is involved in lipolysis stimulation and reduction in body fat in mice, whereas its levels rise with the onset of T2D (29). Our data showed a strong increase in ZAG levels concomitant with increased BMI.
CRH family members are involved in the regulation of various physiological processes through autocrine/paracrine mechanisms (8, 16), including food intake and energy balance (5, 6, 13, 14, 15), inflammation (9, 30), and stress response (5). The present study demonstrated an altered pattern of UCNs in children associated with children’s body weight. Interestingly, the major changes were observed in children who had overweight compared with children with normal weight and children with obesity. Indeed, plasma levels of UCN1 levels decreased to half, whereas UCN3 levels increased nearly fivefold in children with overweight compared with the normal‐weight control children.
These observations suggest a critical role for UCNs at the early stages of increased body weight and metabolic syndrome development and may indicate that UCNs could function as early sensors or be involved in an early response to excessive body weight increases and insulin resistance. Consistent with this hypothesis, UCN3‐transgenic mice demonstrated a metabolically favorable phenotype that was resistant to obesity and hyperglycemia under controlled conditions following an obesogenic HFD challenge (9). Also, agonizing CRHR2 with UCN3 and UCN2 was shown to have beneficial effects not only on heart function, but also on glucose control, weight loss, and reduction in liver fat (5). In contrast, mice deficient in UCN3 and UCN3‐null mice exhibited increased feeding, weight gain, and lower circulating insulin, likely resulting from white adipose tissue accumulation, along with reduced free fatty acid and glycerol levels (13). We recently reported that UCN3, the most recently discovered member of the CRF family, was differentially expressed in the circulation and subcutaneous adipose tissue of people with normal weight and those with overweight, although this pattern was opposite to that observed here, in children (16). In these adults, UCN3 levels were more affected in adults with overweight with a higher insulin secretion than in people with diabetes. This suggests that UCN3 is involved in a feedback loop linking insulin secretion and glucose that seems to be disrupted, not only in established diabetes, but also in conditions in which glucose utilization and energy homeostasis are partially impaired, such as in nondiabetic individuals with obesity. Interestingly, circulating UCN3 was increased in people with early overweight with T2D compared with healthy control individuals (31). Even with an increased duration of T2D and with increased BMI, we observed augmented plasma UCN3 levels (16). These results suggest that circulating UCN3 critically regulates feeding, energy homeostasis, and is potentially involved in metaflammation status modulation in obesity. The latter assumption is supported by the positive correlation that we observed in our study between UCN3 and the TNFα, RBP4, and ZAG proteins. Therefore, the increased levels of UCN2, and particularly UCN3, observed in our study may reflect a compensatory mechanism to suppress further food intake in individuals with overweight. In support for this hypothesis, CRHR2 knockout mice were found to eat larger meals as opposed to smaller meals consumed in CRHR2 agonist‐treated rats (32). Also, UCN2 peptide decreased nocturnal feeding and caused rats to consume smaller meals less frequently (32). Accordingly, an increase in UCN2 and UCN3 may be beneficial in regulating gastric emptying and meal satiation.
In previous studies, UCN1 has been associated with inflammatory and hypertensive conditions. For instance, circulating maternal and fetal UCN1 levels were increased, along with gestational hypertension, preeclampsia, and other hypertensive disorders, during pregnancy (33), whereas decreased plasma UCN1 levels were observed in intrahepatic cholestasis pregnancy, potentially leading to fatal outcomes (34). As UCN1 has vasoactive properties, its increased levels may represent an adaptive stress response (33). UCN1 also increases neuronal activation related to the reduction of ghrelin secretion and food intake (35). Furthermore, increased plasma UCN2 following UCN2 gene transfer improved total body glucose disposal in HFD‐fed mice with associated insulin resistance (36). However, nonischaemic cardiomyopathy exhibited an 8‐fold‐increased plasma UCN2 level compared with healthy controls (37). Also, serum UCN2 levels in hypertensive patients were significantly higher when compared with non‐hypertensive patients (38). In summary, these studies demonstrate a context‐related status for UCNs.
In contrast to the UCNs, reduced circulating levels of CRH were reported in people with T2D, and its secretion was not stimulated by glucose (39). Shortly after inducing stress, increased plasma CRH may be partly responsible for stress‐induced adrenocorticotropic hormone (ACTH) secretion (40). Consistent with this, a reduction in plasma CRH levels was observed with decreased neurotoxicity in an antioxidant‐treated Alzheimer’s disease rat model (41). Therefore, reduced CRH levels are mostly associated with dysregulated homeostasis and related disorders. In our study, circulating CRH levels were not affected with increased BMI in children. This may result from the relatively lower cellular and metabolic stress in this age group compared with adult individuals who frequently exhibit insulin resistance and other metabolic disorders. Notably, our data revealed a strong positive correlation between CRH and UCN2, but not with UCN1 and UCN3, across the weight groups. These data are interesting because UCN2 and UCN3 are homologous to one another and bind to the same receptor (CRHR2), whereas CRH binds to the CRFR1 receptor in the same family.
Our data revealed striking sex dimorphic differences in the levels of UCNs between male and female children. Although UCN1 was lower in girls, UCN2 and UCN3 were higher in girls than boys. Comparable observations have been previously reported in CRHR2‐ and CRFR1‐deficient mice that exhibited sexually dichotomous anxiety‐like behavior (42). CRHR2 knockout mice developed impaired glucose tolerance in males fed with a chow diet, but not in females. Therefore, CRFR dysregulation is a sexually dimorphic factor associated with the development of diabetes and other metabolic syndromes (43). As the hypothalamic–pituitary–adrenal axis interacts with many other physiological pathways, the changes in endocrine function are also sex‐specific and age‐dependent. However, the implication of such observations on the role of UCNs and their receptors in sex‐related vulnerability or resistance to metabolic diseases requires further investigation. Furthermore, the low number of participants in the groups with normal weight and overweight should be taken into consideration before generalizing this conclusion.
Spexin is another neuropeptide that has similar functionalities to the CRH neuropeptide family. In previous studies, chronic subcutaneous injection with spexin reduced food intake and led to weight loss in diet‐induced‐obesity mice and rats (20). Consistent with these findings, we recently reported that circulating levels of spexin were decreased with obesity and diabetes in adults and inversely correlated with adiposity indicators (BMI, waist circumference, and hip circumference), blood pressure, and lipid markers (LDL, triglycerides, and total cholesterol), but positively correlated with HDL levels (23). However, regular moderate physical exercise for 3 months was able to normalize spexin in people with obesity. No change in BMI was observed, which highlights the reversibility of the spexin secretion activity (23). Taken together, these data support a role for spexin in energy metabolism and weight regulation, with a potential link to obesity and diabetes (21). Despite these previous studies, including ours, that have reported decreased spexin levels with obesity and T2D in adults, we did not find significant changes in children with overweight and obesity when compared with matched control children with normal weight. Consistent with this finding, Hodges et al. [22] did not find any variation in spexin levels in adolescents with obesity or diabetes and there was no correlation with body composition or blood measurements, indicating that spexin may not act as a metabolic regulator in adolescents. These findings suggest that spexin may have different functions in obesity and diabetes depending on age, as both disorders are associated with age.
To our knowledge, the present study is the first to evaluate the status of circulating levels of the main neuropeptides of the CRF family in children with overweight and obesity. However, the study had some limitations. First, the cross‐sectional study design did not allow us to determine whether the attenuated neuropeptide levels contributed to the development of obesity. Second, the low number of participants in the groups with normal weight and overweight limited the power of correlations between neuropeptide levels and other clinical parameters. On the other hand, owing to multiple comparisons, the statistical significances must be interpreted with caution. Furthermore, no data regarding diet or physical activity of the participants was collected. Also, the family history or psychological status of these children were not included and were beyond the scope of this study. In addition, the measured levels of the neuropeptides in the plasma may not reflect their central and peripheral bioavailability.
CONCLUSION
Our study showed a disturbed plasma profile of UCN neuropeptides with increased body weight and obesity in children. The significant changes in neuropeptide levels were primarily observed in participants with overweight and were attenuated with increasing body weight reaching the level of obesity. This suggests that a compensatory mechanism may be involved in neuropeptides to curb the development and progression of obesity and its comorbidities.O
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
SK, AK, and AT designed the study. SW, HA, and MA enrolled the children and collected data and blood samples. SK and DM performed the experiments. SK, SD, and AT analyzed the data. SK and AT wrote and revised the manuscript. AK, HK, and JT contributed to the discussion and the revision of the manuscript. FA and JA revised the manuscript.
Supporting information
Fig S1
Table S1‐S2
ACKNOWLEDGMENTS
We thank the staff at the Medical Division Clinics, the Clinical Laboratory and Biobank at the Dasman Diabetes Institute, and the staff at Al‐Farwaniya and Al‐Aden Hospitals for their help throughout the present study. We also thank all participants in this study and their parents. We thank Enago (www.enago.com) for the English language review.
Kavalakatt S, Khadir A, Madhu D, et al. Circulating levels of urocortin neuropeptides are impaired in children with overweight. Obesity (Silver Spring). 2022;30:472–481. doi: 10.1002/oby.23356
Funding information
This research was funded by the Kuwait Foundation for the Advancement of Sciences under projects RA‐2011‐018.
Contributor Information
Jehad Abubaker, Email: jehad.abubakr@dasmaninstitute.org.
Ali Tiss, Email: ali.tiss@dasmaninstitute.org.
REFERENCES
- 1. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988‐1994 through 2013‐014. JAMA. 2016;315:2292‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lobstein T, Brinsden H. Atlas of Childhood Obesity. World Obesity Federation; 2019. [Google Scholar]
- 3. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860‐867. [DOI] [PubMed] [Google Scholar]
- 4. Bjerregaard LG, Adelborg K, Baker JL. Change in body mass index from childhood onwards and risk of adult cardiovascular disease. Trends Cardiovasc Med. 2020;30:39‐45. [DOI] [PubMed] [Google Scholar]
- 5. Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roustit MM, Vaughan JM, Jamieson PM, Cleasby ME. Urocortin 3 activates AMPK and AKT pathways and enhances glucose disposal in rat skeletal muscle. J Endocrinol. 2014;223:143‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dermitzaki E, Liapakis G, Androulidaki A, et al. Corticotrophin‐Releasing Factor (CRF) and the Urocortins are potent regulators of the inflammatory phenotype of human and mouse white adipocytes and the differentiation of mouse 3T3L1 pre‐adipocytes. PLoS One. 2014;9:e97060. doi: 10.1371/journal.pone.0097060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Meulen T, Donaldson CJ, Caceres E, et al. Urocortin3 mediates somatostatin‐dependent negative feedback control of insulin secretion. Nat Med. 2015;21:769‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jamieson PM, Cleasby ME, Kuperman Y, et al. Urocortin 3 transgenic mice exhibit a metabolically favourable phenotype resisting obesity and hyperglycaemia on a high‐fat diet. Diabetologia. 2011;54:2392‐2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brar BK, Railson J, Stephanou A, Knight RA, Latchman DS. Urocortin increases the expression of heat shock protein 90 in rat cardiac myocytes in a MEK1/2‐dependent manner. J Endocrinol. 2002;172:283‐293. [DOI] [PubMed] [Google Scholar]
- 11. Brar BK, Jonassen AK, Egorina EM, et al. Urocortin‐II and urocortin‐III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24‐35. [DOI] [PubMed] [Google Scholar]
- 12. Chen A, Brar B, Choi CS, et al. Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc Natl Acad Sci U S A. 2006;103:16580‐16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chao H, Digruccio M, Chen P, Li C. Type 2 corticotropin‐releasing factor receptor in the ventromedial nucleus of hypothalamus is critical in regulating feeding and lipid metabolism in white adipose tissue. Endocrinology. 2012;153:166‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuperman Y, Issler O, Regev L, et al. Perifornical Urocortin‐3 mediates the link between stress‐induced anxiety and energy homeostasis. Proc Natl Acad Sci U S A. 2010;107:8393‐8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen P, Vaughan J, Donaldson C, Vale W, Li C. Injection of Urocortin 3 into the ventromedial hypothalamus modulates feeding, blood glucose levels, and hypothalamic POMC gene expression but not the HPA axis. Am J Physiol Endocrinol Metab. 2010;298:E337‐E345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kavalakatt S, Khadir A, Madhu D, et al. Urocortin 3 levels are impaired in overweight humans with and without type 2 diabetes and modulated by exercise. Front Endocrinol (Lausanne). 2019;10:762. doi: 10.3389/fendo.2019.00762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gozal D, Jortani S, Snow AB, et al. Two‐dimensional differential in‐gel electrophoresis proteomic approaches reveal urine candidate biomarkers in pediatric obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180:1253‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang Z, Michal JJ, Williams GA, Daniels TF, Kunej T. Cross species association examination of UCN3 and CRHR2 as potential pharmacological targets for antiobesity drugs. PLoS One. 2006;1:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong MK, Sze KH, Chen T, et al. Goldfish Spexin: solution structure and novel function as a satiety factor in feeding control. Am J Physiol Endocrinol Metab. 2013;305:E348‐366. [DOI] [PubMed] [Google Scholar]
- 20. Walewski JL, Ge F, Lobdell HT, et al. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet‐induced obesity. Obesity (Silver Spring). 2014;22:1643‐1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lv SY, Zhou YC, Zhang XM, Chen WD, Wang YD. Emerging roles of NPQ/Spexin in physiology and pathology. Front Pharmacol. 2019;10:457. doi: 10.3389/fphar.2019.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodges SK, Teague AM, Dasari PS, Short KR. Effect of obesity and type 2 diabetes, and glucose ingestion on circulating Spexin concentration in adolescents. Pediatr Diabetes. 2018;19:212‐216. [DOI] [PubMed] [Google Scholar]
- 23. Khadir A, Kavalakatt S, Madhu D, et al. Spexin as an indicator of beneficial effects of exercise in human obesity and diabetes. Sci Rep. 2020;10:10635. doi: 10.1038/s41598-020-67624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1‐190. [PubMed] [Google Scholar]
- 25. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prince RL, Kuk JL, Ambler KA, Dhaliwal J, Ball GD. Predictors of metabolically healthy obesity in children. Diabetes Care. 2014;37:1462‐1468. [DOI] [PubMed] [Google Scholar]
- 27. Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J Clin Invest. 2017;127:65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel DA, Srinivasan SR, Chen W, Berenson GS. Serum alanine aminotransferase and its association with metabolic syndrome in children: the bogalusa heart study. Metab Syndr Relat Disord. 2011;9:211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vann i H, Kazeros A, Wang R, et al. Cigarette smoking induces overexpression of a fat‐depleting gene AZGP1 in the human. Chest. 2009;135:1197‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Temur M, Yilmaz O, Aksun S, et al. Increased circulating urocortin‐3 levels is associated with polycystic ovary syndrome. Gynecol Endocrinol. 2016;32:218‐222. [DOI] [PubMed] [Google Scholar]
- 31. Alarslan P, Unal Kocabas G, Demir I, et al. Increased urocortin 3 levels are associated with the risk of having type 2 diabetes mellitus. J Diabetes. 2020;12:474‐482. [DOI] [PubMed] [Google Scholar]
- 32. Tabarin A, Diz‐Chaves Y, Consoli D, et al. Role of the corticotropin‐releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. Eur J Neurosci. 2007;26:2303‐2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Florio P, Torricelli M, De Falco G, et al. High maternal and fetal plasma urocortin levels in pregnancies complicated by hypertension. J Hypertens. 2006;24:1831‐1840. [DOI] [PubMed] [Google Scholar]
- 34. Zhang L, Sun Q, Wang XD, Zhou F, Gao BX. Decreased maternal plasma urocortin level in intrahepatic cholestasis of pregnancy. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015;46:263‐266. [PubMed] [Google Scholar]
- 35. Yakabi K, Noguchi M, Ohno S, et al. Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am J Physiol Endocrinol Metab. 2011;301:E72‐E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim YC, Truax AD, Giamouridis D, et al. Significant alteration of liver metabolites by AAV8.Urocortin 2 gene transfer in mice with insulin resistance. PLoS One. 2019;14:e0224428. doi: 10.1371/journal.pone.0224428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsuda T, Takefuji M, Wettschureck N, et al. Corticotropin releasing hormone receptor 2 exacerbates chronic cardiac dysfunction. J Exp Med. 2017;214:1877‐1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aslan G, Aytekin S. Evaluation of serum urocortin 2 levels in patients with hypertension. High Blood Press Cardiovasc Prev. 2020;27:35‐42. [DOI] [PubMed] [Google Scholar]
- 39. Hashimoto K, Nishioka T, Takao T, Numata Y. Low plasma corticotropin‐releasing hormone (CRH) levels in patients with non‐insulin dependent diabetes mellitus (NIDDM). Endocr J. 1993;40:705‐709. [DOI] [PubMed] [Google Scholar]
- 40. Hashimoto K, Murakami K, Takao T, Makino S, Sugawara M, Ota Z. Effect of acute ether or restraint stress on plasma corticotropin‐releasing hormone, vasopressin and oxytocin levels in the rat. Acta Med Okayama. 1989;43:161‐167. [DOI] [PubMed] [Google Scholar]
- 41. Ooi TC, Ahmad Munawar M, Mohd Rosli NH, et al. Neuroprotection of tropical fruit juice mixture via the reduction of iNOS expression and CRH level in β‐amyloid‐induced rats model of Alzheimer’s disease. Evid Based Complement Alternat Med. 2020;2020:5126457. doi: 10.1155/2020/5126457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin‐releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety‐like behavior. J Neurosci. 2002;22:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paruthiyil S, Hagiwara SI, Kundassery K, Bhargava A. Sexually dimorphic metabolic responses mediated by CRF(2) receptor during nutritional stress in mice. Biol Sex Differ. 2018;9:49. doi: 10.1186/s13293-018-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S2


