Abstract
Objective
To evaluate the effectiveness of 2 interventions, including the DrugFactsBox format for presenting written medication information and the SMART (Strategic Memory Advanced Reasoning Training) program designed to enhance gist (i.e., “bottom‐line” meaning) reasoning ability.
Methods
We used a 2 × 2 factorial research design. A total of 286 patients with rheumatoid arthritis were randomly assigned to 1 of 4 groups, including DrugFactsBox with the SMART program, DrugFactsBox without the SMART program, other consumer medication information (CMI) with the SMART program, and other CMI without the SMART program. Data were collected via telephone interviews and online questionnaires at 4 time points, including baseline and 6‐week, 3‐month, and 6‐month time points following baseline. The primary outcome variable was informed decision‐making, which was defined as making a value‐consistent decision concerning use of disease‐modifying antirheumatic drugs based on adequate knowledge.
Results
We found no main effects for the 2 interventions, either alone or in combination. However, there was a significant interaction between assignment to the SMART/no SMART groups and informed decision‐making at baseline. Among participants in the SMART groups who did not meet the criteria for informed decision‐making at baseline, 42.5% met the criteria at the 6‐month follow‐up, compared to 23.6% of participants in the no SMART groups (mean difference 18.9 [95% confidence interval 5.6, 32.2]; P = 0.007). This difference was driven by increased knowledge in the SMART groups. Among participants who met the criteria for informed decision‐making at baseline, the difference between the SMART and no SMART groups was not statistically significant.
Conclusion
Participation in a theory‐driven program to enhance gist reasoning may have a beneficial effect on informed decision‐making among patients with inadequate knowledge concerning therapeutic options.
INTRODUCTION
Guidelines for the management of rheumatoid arthritis (RA) endorse a treat‐to‐target strategy using disease‐modifying antirheumatic drugs (DMARDs), with achieving clinical remission (or at least low disease activity) as the primary target (1). A major issue in implementing treat‐to‐target principles in practice, however, involves patient reluctance to escalate therapy when their symptoms are tolerable despite the presence of active disease (2, 3, 4). This reluctance is understandable, because the potential benefits associated with DMARDs may be accompanied by serious risks. Obtaining accurate, personally relevant information about these risks is challenging (5, 6, 7, 8). Although the US Food and Drug Administration requires that patients receive a medication guide with most DMARDs, research suggests that many patients have difficulty understanding the information that the guides contain (9, 10, 11, 12, 13). This is likely due to both design issues (e.g., nonadherence to plain language guidelines) (14) and the prevalence of limited health literacy/numeracy skills among patients (15).
SIGNIFICANCE & INNOVATIONS.
Patients with rheumatoid arthritis (RA) are often reluctant to escalate therapy with disease‐modifying antirheumatic drugs (DMARDs) due to concern about medication risks.
Many RA patients have difficulty understanding the gist (i.e., “bottom‐line” meaning) of currently available consumer medication information, including medication guides that the US Food and Drug Administration requires for most DMARDs.
The Strategic Memory Advanced Reasoning Training (SMART) program is an innovative patient education program designed to enhance gist reasoning.
Among patients with knowledge deficits, the SMART program may facilitate informed decision‐making by helping them develop the skills needed to understand and use complex information concerning medication risks/benefits.
The present study was based on the premise that interventions designed to educate patients about the risks and benefits associated with different therapeutic options require a 2‐pronged approach, including simplification of educational materials to convey the essential gist (i.e., “bottom‐line” meaning) and assistance to patients in developing the health literacy/numeracy skills needed to process complex information (e.g., scientific uncertainty concerning medication risks/benefits) to derive that gist (16, 17). Thus, we examined the effectiveness of 2 innovative communication strategies, including DrugFactsBoxes and the Strategic Memory Advanced Reasoning Training (SMART) program. DrugFactsBoxes were developed to enhance the usability of written consumer medication information (CMI), especially among individuals with limited health literacy/numeracy skills (18, 19, 20). The SMART program was developed to enhance patients' ability to understand and extract “bottom‐line” meaning (gist) from complex information, which we view as an essential health literacy skill (21, 22, 23, 24, 25, 26, 27).
As shown in Figure 1, we hypothesized that both interventions would increase patient knowledge concerning medication risks/benefits and interest in obtaining additional information about illness self‐management. By improving gist reasoning ability, we hypothesized that the SMART program would work synergistically with accessible information such as DrugFactsBoxes to enhance informed decision‐making, which is defined as making value‐consistent decisions concerning DMARD use based on adequate knowledge.
Figure 1.
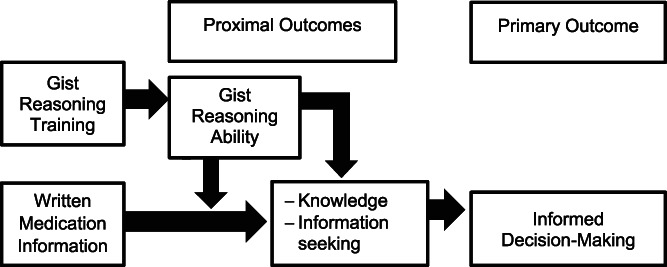
Conceptual framework for gist reasoning training and written medication information.
PATIENTS AND METHODS
Design
We evaluated 2 educational interventions (DrugFactsBoxes and the SMART program) using a 2 × 2 factorial research design and adhering to Consolidated Standards of Reporting Trials (CONSORT) guidelines (28). Data were collected at the following 4 time points: baseline and 6 weeks, 3 months, and 6 months following baseline. At each time point, data were collected via a combination of telephone interviews and online questionnaires. Immediately after completion of the baseline interview, we used a 1:1:1:1 allocation sequence to randomly assign participants to 1 of 4 study groups, including DrugFactsBox with the SMART program, DrugFactsBox without the SMART program, other CMI with the SMART program, and other CMI without the SMART program. Participants and all staff involved with data collection were blinded to participants' group assignment. The study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill (UNC‐CH) and is registered (ClinicalTrials.gov identifier: NCT02820038).
Participants
We recruited participants from the following resources: 1) 4 large academic rheumatology practices; 2) CreakyJoints, an online arthritis patient support community; 3) social media (e.g., Facebook, Twitter); 4) the Carolina Data Warehouse for Health, which includes patients treated at all inpatient and outpatient facilities at UNC‐CH; and 5) Join the Conquest, a website administered by the UNC Translational and Clinical Sciences Institute that allows individuals in the general public to volunteer to participate in posted research studies. To be eligible to participate, individuals had to meet the following criteria: be ≥18 years of age, have physician‐confirmed RA or be undergoing therapy with a DMARD approved for the treatment of RA, speak English, not have hearing or visual impairments that would prevent being able to complete data‐collection procedures, have an email address and internet access, have moderate or high disease activity as evidenced by a score of >6 on the 0–30 Routine Assessment of Patient Index Data 3 (RAPID3) (29, 30) scale, and not have any health problems that prevented changes in his/her RA medication regimen (e.g., ongoing serious infection). Participant recruitment began in September 2016 and ended in May 2018. Data collection was completed in December 2018. Participants received $125 for participating in the study: $25 after completing the baseline, 6‐week, and 3‐month data collection, and $50 after completing the 6‐month data collection.
At rheumatology clinic sites, clinic staff or a research assistant identified potentially eligible patients and obtained verbal consent to administer a screening interview that assessed disease activity, age, email address, and access to the internet. They then contacted the patient's rheumatologist to obtain confirmation of diagnosis and presence/absence of health problems that would prevent changes in the patient's medication regimen. If the patient was eligible to participate in the study, written informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization were obtained. The information collected via these screening procedures was then forwarded to staff in the central office at the UNC‐CH to initiate data collection. For potential participants identified via other mechanisms, research staff at the UNC‐CH administered the screening interview via telephone. If the patient appeared to be eligible to participate, he/she was mailed a consent form and HIPAA authorization to sign and return. When HIPAA authorization was obtained, staff contacted the patient's physician to obtain confirmation of diagnosis and presence/absence of health problems that would prevent medication regimen changes.
Interventions
The original DrugFactsBox format used a standardized 2‐page summary that followed plain language guidelines and clinical best practices to convey relevant facts to individuals with limited literacy or numeracy skills (18, 19, 20). For the present study, we created a website that contained 16 DrugFactsBoxes for those medications most commonly used to treat RA in the US (i.e., abatacept, adalimumab, certolizumab, etanercept, golimumab, hydroxychloroquine, infliximab, leflunomide, methotrexate pill, methotrexate subcutaneous, prednisone, rituximab, sulfasalazine, tocilizumab infusion, tocilizumab subcutaneous, and tofacitinib). A pill bottle icon for each medication appeared on the website landing page. When an icon was clicked, an overview of the medication appeared. The overview included a section labeled “Bottom Line,” which contained a narrative summary of potential medication benefits and harms, emphasizing the gist (31). The overview page also provided links to other pages within the website that contained additional information about the medication. These links were labeled trials, side effects, how to use, lifestyle changes, and interactions. The trials page provided quantitative information concerning potential medication benefits and harms, mirroring the original DrugFactsBox format. Participants in the other CMI groups were given access to a website that contained CMI for the same 16 medications. For medications that have an FDA‐approved medication guide (i.e., all biologics and tofacitinib), a link to the guide was provided. For the remaining medications, the website provided a link to CMI developed by the American Society of Health‐System Pharmacists, that are similar to the written information given to patients in the US when prescriptions are dispensed.
The SMART program is designed to enhance gist reasoning ability by training participants on the use of the following 3 metacognitive strategies: strategic attention (e.g., ignoring or eliminating distractions to facilitate single‐minded focus on understanding the specific topic at hand), integrated reasoning (e.g., strengthening integrative mental capacity to synthesize information from multiple sources), and innovation (e.g., examining multiple perspectives and information sources to best understand the information available) (21, 22, 23, 24, 25, 26, 27). The program was delivered by research personnel at the Center for BrainHealth at the University of Texas at Dallas using an online video conferencing platform that permitted synchronous, audio and visual communication between trainers and participants. In most cases, the program was delivered in small groups with 3–4 participants. Initially, the program was delivered in four 90‐minute sessions, spanning a 1‐month period. However, because many participants had difficulty committing to sessions of this length, midway through the project, we reduced the length of each session to 1 hour in an effort to increase participant engagement.
Measures
Our primary outcome variable was informed decision‐making regarding the use of DMARDs. Informed decision‐making is typically conceptualized as making a value‐consistent decision that is based on adequate knowledge (32, 33, 34). To use this approach, the online questionnaires included items asking participants to indicate the extent to which they agreed or disagreed with 10 value statements pertaining to the management of RA (e.g., “It is important to accept the risk of side effects now in order to improve my chances of being healthy in the future”), which were developed based on theory and empirically validated (35). Responses were recorded on a 4‐point scale ranging from 1 (strongly agree) to 4 (strongly disagree). Responses were summed and transformed to a composite score ranging from –15 to 15, where positive numbers reflected values favoring aggressive treatment. Participants were classified as meeting the criteria for informed decision‐making if they: 1) answered at least 85% of the knowledge items (described below) correctly, scored >0 on the values measure and were taking ≥1 DMARD or 2) answered 85% of the knowledge items correctly, scored ≤0 on the values measure, and were not taking a DMARD. All other individuals were classified as not meeting the criteria for informed decision‐making.
Knowledge was assessed by 3 separate instruments administered via telephone interview, including an 8‐item measure assessing knowledge concerning methotrexate (which is often first‐line therapy for RA) (36), a 20‐item measure assessing knowledge concerning biologic treatment options (35), and an 8‐item measure assessing knowledge of RA and RA treatment options more generally (37). Correct answers were summed across all 3 measures and transformed to a 100‐point scale, reflecting the percentage of questions answered correctly.
DMARD use was assessed via a checklist of 19 RA medications (abatacept, adalimumab, azathioprine, certolizumab pegol, cyclosporine, etanercept, golimumab, gold, hydroxycholoroquine, infliximab, leflunomide, methotrexate pill, methotrexate shot, minocycline, rituximab, sulfasalazine, tocilizumab infusion, tocilizumab shot, and tofacitinib) included in the online questionnaires. Participants were asked to check all of those medications that they were currently being treated with or to check an option labeled “none of the above.”
Gist reasoning ability was assessed by the Test of Strategic Learning (TOSL). The TOSL was developed to systematically quantify participants' capacity to abstract gist meanings from complex input (26, 38). The TOSL consists of nonmedical text passages varying in length (from 291 to 575 words) and complexity. At each time point, participants read one of the text passages presented via an online questionnaire. After reading the passage, participants clicked on a link to the next page of the questionnaire, which included a single item asking participants to summarize the original text, focusing on bottom‐line meaning (i.e., “the moral of the story”) rather than specific details. Participants had up to 5 minutes to complete this task and were not allowed to return to the page on which the passage appeared while writing the summary.
The next page of the questionnaire asked participants to take up to 3 minutes to list the lessons learned (i.e., take‐home messages) from the text. A total of 4 different text passages were used. These were balanced across participants over the course of the study such that each participant viewed a different passage at each time point, with the order in which passages were viewed randomized across participants. Participants' responses were scored using a manualized, objective scoring system by a trained and experienced rater (MK) who was blinded to participants' group assignment and time of testing. Two separate scores, including complex abstraction and lesson quality, were derived from participants' responses. To assess interrater reliability prior to the initiation of coding, 2 raters scored 25 responses for each of the 4 text passages. The mean intraclass correlation coefficient for a single score was 0.74 for complex abstraction (range 0.43–0.94) and 0.95 for lesson quality (range 0.84–0.99), indicating good reliability.
Information seeking was assessed using behavioral measures. First, we created a website that provided easy access to information about RA, treatment options, and illness self‐management. All participants were emailed a link to the website following the 6‐week follow‐up, regardless of their group assignment. We used Google analytics to track whether participants accessed the website. Second, after the 6‐week follow‐up, we also emailed all participants an invitation to participate (free of charge) in BetterChoices, BetterHealth, an online chronic illness self‐management program, and tracked class enrollment. Finally, we assessed the following sociodemographic characteristics: age, sex (male, female), race (White, other), ethnicity (Hispanic, non‐Hispanic), education (less than bachelor's degree, bachelor's degree or more), marital status (currently married, other), and difficulty affording RA medications (no trouble, a little trouble, a lot of trouble).
Analyses
Characteristics of study participants are presented using means and percentages, depending on the measurement properties of the variables. We used logistic regression to assess the effects of the 2 interventions on our primary outcome: informed decision‐making at the 6‐month follow‐up. A separate regression model was performed at each follow‐up time point (i.e., 6‐week, 3‐month, and 6‐month). Each model controlled for informed decision‐making at baseline (0 = did not meet criteria, 1 = met criteria) and indicator variables for each intervention indexing assignment to the SMART program (0 = no, 1 = yes) and the DrugFactsBox group (0 = no, 1 = yes). We also included three two‐way interaction terms in each model. The first interaction term assessed whether the effects of the 2 interventions were dependent on one another. The other interaction terms assessed whether the effects of the interventions varied as a function of informed decision‐making at baseline. Interaction terms that were not statistically significant (P < 0.05) were dropped from the models and the models were re‐run to examine main effects.
When significant interactions were observed, we used stratified analyses to determine the nature of the interaction. Statistical significance was evaluated with alpha (2‐tailed test) set at 0.05. Missing baseline data were imputed by substituting the mean or mode in the full sample for continuous variables and categorical variables, respectively. Missing follow‐up data were imputed using multiple imputation methods, via PROC MI and PROC MIANALYZE in SAS. Because the pattern of missing data was not monotonic, we used the Markov chain Monte Carlo method. As recommended by Sullivan and colleagues (39), imputation procedures were carried out separately for each randomized group. In PROC MIANALYZE, we used the EDF option to specify the complete‐data degrees of freedom for parameter estimates. Power analyses conducted a priori indicated that a sample of 300 would provide 80% power to detect a between‐group difference of 25% in the percentage of participants meeting the criteria for informed decision‐making (e.g., 35% versus 60%). This anticipated effect size is based on previous research (35) and corresponds to a moderate‐sized effect (40). Power calculations were performed with alpha (2‐tailed test) set at 0.05 and allowed for 15% attrition from baseline to final follow‐up. All analyses were performed using SAS PC, version 9.4.
RESULTS
A total of 634 patients were screened for eligibility (see Supplementary Figure 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24421/abstract). Of these, 309 met study eligibility criteria, provided written informed consent, completed the baseline interview, and were randomized to a group. However, 23 of the patients randomized either withdrew from the study or were lost to follow‐up before completing the baseline questionnaire. Therefore, only 286 (93%) of the 309 individuals who completed the baseline interview received the email giving them access to intervention materials. Characteristics of these study participants, assessed at baseline, are shown in Table 1.
Table 1.
Baseline characteristics of study participants (n = 286)*
| Characteristic | Other CMI only (n = 78) | Other CMI w/ SMART (n = 77) | DrugFactsBox only (n = 65) | DrugFactsBox w/ SMART (n = 66) |
|---|---|---|---|---|
| Age, mean ± SD years | 55.5 ± 10.8 | 54.7 ± 11.8 | 56.2 ± 10.1 | 54.9 ± 14.7 |
| White race† | 76.3 (58) | 81.6 (62) | 73.4 (47) | 71.2 (47) |
| Married | 66.7 (52) | 52.0 (40) | 60.0 (39) | 65.2 (43) |
| Female sex | 89.7 (70) | 89.6 (69) | 92.3 (60) | 89.4 (59) |
| College graduate‡ | 52.0 (40) | 62.3(48) | 49.2 (32) | 57.6 (38) |
| Disease activity, mean ± SD | 4.6 ± 1.7 | 4.3 ± 1.6 | 4.8 ± 1.7 | 4.5 ± 1.7 |
| Reported having a lot of trouble affording medications | 16.7 (13) | 10.4 (8) | 13.9 (9) | 21.2 (14) |
| Met criteria for informed decision‐making‡ | 37.7 (29) | 36.4 (28) | 38.5 (25) | 30.3 (20) |
| Not taking any DMARDs§ | 5.2 (4) | 10.7 (8) | 10.8 (7) | 9.1 (6) |
| Knowledge, mean ± SD | 77.1 ± 15.1 | 79.3 ± 14.3 | 77.2 ± 15.7 | 75.8 ± 17.2 |
| Values, mean ± SD | 5.0 ± 3.5 | 5.2 ± 4.2 | 5.7 ± 4.0 | 4.7 ± 3.5 |
| Gist reasoning ability¶ | ||||
| Complex abstraction, mean ± SD | 2.0 ± 1.6 | 2.0 ± 1.5 | 2.0 ± 1.4 | 2.0 ± 1.5 |
| Lesson quality, mean ± SD | 1.0 ± 1.1 | 1.0 ± 1.2 | 1.0 ± 1.1 | 0.7 ± 1.0 |
Values are the percent (number) unless indicated otherwise. For all variables, higher values reflect higher levels of the attribute measured. CMI = consumer medical information; DMARDs = disease‐modifying antirheumatic drugs; SMART = Strategic Memory Advanced Reasoning Training.
Due to missing data, the total number of study participants with the characteristic of White race was n = 282.
Due to missing data, the total number of study participants with the characteristic of college graduate and who met criteria for informed decision‐making was n = 285.
Due to missing data, the total number of study participants with the characteristic of not taking any DMARDs was n = 283.
Due to missing data, the total number of study participants with scores for Test of Strategic Learning complex abstraction and lesson quality was n = 270.
Informed decision‐making
None of the interaction terms in the logistic regression models predicting informed decision‐making were statistically significant at the 6‐week follow‐up. However, there was a significant interaction between assignment to the SMART program and baseline informed decision‐making at both the 3‐month (P = 0.05) and 6‐month (P = 0.01) follow‐ups. To follow up on these interactions, we stratified the sample by whether participants were classified as meeting the criteria for informed decision‐making at baseline. Of note is that the stratified analyses examined the main effects of each intervention (i.e., SMART/no SMART, DrugFactsBox/other CMI), because we found no statistically significant interactions between the interventions. Thus, with respect to the SMART program, data were pooled across participants regardless of whether they received DrugFactsBoxes or other CMI. As shown in Table 2, 42.5% of participants in the SMART group who did not meet the criteria for informed decision‐making at baseline met the criteria at the 6‐month follow‐up, compared to 23.6% of participants in the no SMART group (P = 0.007). A similar difference was observed among these individuals at the 3‐month follow‐up. In contrast, among participants classified as meeting the criteria for informed decision‐making at baseline, none of the differences between the SMART and no SMART groups were statistically significant. Finally, none of the interactions or main effects involving whether participants were assigned to receive DrugFactBoxes versus other CMI were statistically significant.
Table 2.
Effect of SMART program on informed decision‐making at 6‐week, 3‐month, and 6‐month follow‐ups, stratified by informed decision‐making at baseline*
| 6‐week follow‐up | 3‐month follow‐up | 6‐month follow‐up | |
|---|---|---|---|
| Did not meet criteria for informed decision‐making at baseline (n = 184)† | |||
| SMART program, % | 23.8 | 40.6 | 42.5 |
| No SMART program, % | 24.7 | 21.7 | 23.6 |
| Difference (95% CI) | –0.9 (–13.3, 11.5) | 18.9 (5.8, 32.0) | 18.9 (5.6, 32.2) |
| P | 0.89 | 0.006 | 0.007 |
| Met criteria for informed decision‐making at baseline (n = 102)‡ | |||
| SMART program, % | 77.1 | 75.4 | 63.0 |
| No SMART program, % | 80.0 | 83.3 | 78.2 |
| Difference (95% CI) | –2.8 (–18.8, 13.1) | –7.8 (–23.6, 7.9) | –15.2 (–32.7, 2.4) |
| P | 0.73 | 0.33 | 0.09 |
Data in the Strategic Memory Advanced Reasoning Training (SMART) and no SMART program groups were pooled across participants regardless of whether they received DrugFactsBoxes or other consumer medication information. Percentages in the body of the table are averaged across 50 imputations used to estimate values for missing data at the follow‐up assessments. 95% CI = 95% confidence interval.
N = 95 for SMART program; n = 89 for no SMART program.
N = 48 for SMART program; n = 54 for no SMART program.
We used the complete case data (without imputed values for missing data) to identify the factors that caused participants to transition from not meeting the criteria for informed decision‐making at baseline to meeting these criteria at the 6‐month follow‐up. A total of 45 participants (27 in the SMART group and 18 in the no SMART group) made this transition. Among these participants, 41 (23 in the SMART group, 18 in the no SMART group) exhibited knowledge gains that moved them above the 85% threshold required to meet the criteria for informed decision‐making; 3 individuals who used a DMARD at baseline (all in the SMART group) had shifts in values that moved them above the threshold to be classified as valuing aggressive therapy; and 6 (3 in the SMART group, 3 in the no SMART group) began using a DMARD during the follow‐up period, consistent with their values favoring aggressive therapy. All 45 participants who transitioned from not meeting the criteria for informed decision‐making at baseline to meeting these criteria at the 6‐month follow‐up were being treated with at least 1 DMARD at the 6‐month follow‐up.
Components of informed decision‐making and other proximal outcomes
Table 3 presents the results of analyses assessing differences between the SMART and no SMART groups with respect to the components of informed decision‐making (i.e., knowledge, values, and DMARD use) and other proximal outcome variables. Compared to individuals in the no SMART group, those in the SMART group exhibited greater knowledge at the 6‐month follow‐up and higher scores on the measure of complex abstraction at the 3‐month follow‐up. No other differences were statistically significant (no statistically significant differences between the DrugFactsBox and other CMI groups) (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24421/abstract).
Table 3.
Components of informed decision‐making and proximal outcome variables by assignment to the SMART or no SMART program groups*
| Outcome variable, by follow‐up time period† | SMART program | Difference (95% CI) | P | |
|---|---|---|---|---|
| Yes | No | |||
| Not using any DMARDs, % (no.) | ||||
| 6‐week | 10.6 (15) | 7.8 (11) | 2.8 (–3.9, 9.5) | 0.42 |
| 3‐month | 11.9 (17) | 7.7 (11) | 4.3 (–2.6, 11.2) | 0.22 |
| 6‐month | 10.9 (16) | 7.7 (11) | 3.2 (–3.5, 9.9) | 0.35 |
| Knowledge | ||||
| 6‐week | 81.4 ± 0.8 | 80.6 ± 0.7 | 0.9 (–1.2, 2.9) | 0.42 |
| 3‐month | 83.8 ± 0.8 | 81.8 ± 0.7 | 2.0 (–0.1, 4.1) | 0.06 |
| 6‐month | 84.0 ± 0.7 | 81.7 ± 0.7 | 2.2 (0.3, 4.2) | 0.03 |
| Values | ||||
| 6‐week | 5.6 ± 0.3 | 5.3 ± 0.3 | 0.4 (–0.5, 1.2) | 0.39 |
| 3‐month | 5.4 ± 0.4 | 6.0 ± 0.3 | –0.6 (–1.5. 0.4) | 0.24 |
| 6‐month | 5.7 ± 0.3 | 5.9 ± 0.3 | –0.3 (–1.1, 0.6) | 0.55 |
| Gist reasoning ability | ||||
| Complex abstraction | ||||
| 6‐week | 1.8 ± 0.2 | 1.8 ± 0.1 | 0.04 (–0.4, 0.5) | 0.87 |
| 3‐month | 2.2 ± 0.2 | 1.7 ± 0.1 | 0.5 (0.1, 0.9) | 0.02 |
| 6‐month | 1.8 ± 0.2 | 2.0 ± 0.1 | –0.10 (–0.5, 0.3) | 0.53 |
| Lesson quality | ||||
| 6‐week | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.04 (–0.3, 0.4) | 0.79 |
| 3‐month | 1.2 ± 0.1 | 0.9 ± 0.1 | 0.30 (–0.0, 0.6) | 0.08 |
| 6‐month | 1.0 ± 0.2 | 1.1 ± 0.1 | –0.04 (–0.4, 0.3) | 0.86 |
| Information seeking, % (no.) | ||||
| Viewed RA self‐management website | 15.2 (22) | 22.3 (32) | –7.1 (–16.1, 1.9) | 0.14 |
| Participated in BetterChoices, BetterHealth | 15.2 (22) | 21.0 (30) | –5.9 (–14.8, 3.1) | 0.21 |
Values are the adjusted mean ± SE, unless indicated otherwise. All mean values are adjusted for the baseline value of the dependent variable and group assignment. For use of disease‐modifying antirheumatic drugs (DMARDs), raw percentages are shown. Frequencies and percentages are averaged across 50 imputations used to estimate values for missing data. The average frequencies are rounded to the nearest integer. 95% CI = 95% confidence interval; RA = rheumatoid arthritis; SMART = Strategic Memory Advanced Reasoning Training.
Engagement in intervention activities
Table 4 shows information concerning the extent to which individuals actively engaged in intervention activities, stratified by the 4 study groups. Overall, about half of the participants assigned to the SMART group completed at least 1 training session and about 40% of participants viewed at least 1 page on either the DrugFactsBox or other CMI website. Among those who had access to the DrugFactsBox website, 18.3% (n = 24) viewed at least 1 of the trials pages included on the website. These pages set DrugFactBoxes apart from other CMI in that they provide quantitative information concerning the probability of experiencing medication benefits and harms.
Table 4.
Engagement in intervention activities*
| Variable | Other CMI only (n = 78) | Other CMI with SMART (n = 77) | DrugFactBox only (n = 65) | DrugFactBox with SMART (n = 66) | P |
|---|---|---|---|---|---|
| No. of SMART sessions attended, mean ± SD | NA | 1.60 ± 1.8 | NA | 1.41 ± 1.8 | 0.53 |
| Attended 1+ SMART sessions | NA | 48.1 (37) | NA | 40.9 (27) | 0.39 |
| Attended 3+ SMART sessions | NA | 41.6 (32) | NA | 34.9 (23) | 0.41 |
| No. of DrugFactsBox/other CMI pages viewed, mean ± SD | 1.63 ± 2.8 | 1.39 ± 4.1 | 2.63 ± 3.99 | 2.06 ± 4.0 | 0.22 |
| Viewed 1+ DrugFactsBox/other CMI pages | 38.5 (30) | 23.4 (18) | 53.9 (35) | 42.4 (28) | 0.003 |
| Viewed DrugFactsBox trials page | NA | NA | 21.5 (14) | 15.2 (10) | 0.34 |
Values are the % (no.) unless indicated otherwise. CMI = consumer medication information; NA = not applicable; SMART = Strategic Memory Advanced Reasoning Training.
DISCUSSION
Enhancing patients' ability to understand and use information about medication risks and benefits to make informed decisions concerning treatment alternatives remains an important goal. Although more than half of the participants in our sample were college graduates, nearly two‐thirds (n = 184) did not meet the criteria for informed decision‐making at baseline. In our full sample, neither of the interventions that were evaluated improved informed decision‐making, either alone or in combination. However, although not hypothesized a priori, our analyses revealed a statistically significant interaction between the SMART program and informed decision‐making at baseline. Specifically, the SMART program had a positive impact on informed decision‐making in the subset of participants who did not meet the criteria for informed decision‐making at baseline. This finding is consistent with previous research that has demonstrated benefits of the SMART program on performance on cognitive, neural, and functional measures immediately post‐training and 3–6 months post‐training (24, 27, 41, 42, 43).
The improvements in informed decision‐making in this study were driven by increases in knowledge, which was the only component of informed decision‐making that differed between the SMART and no SMART groups at the 6‐month follow‐up. This finding is noteworthy because the SMART program did not provide any content that would have increased patient knowledge concerning RA treatment options directly. Rather, the program is designed to enhance gist reasoning ability, which we view as an essential health literacy skill (24). We observed transient improvements in our measures of gist reasoning ability (i.e., complex abstraction and lesson quality) at the 3‐month follow‐up. Although these differences were not sustained at the 6‐month follow‐up, they may have been sufficient to facilitate uptake of medication information at earlier time points and facilitate decision‐making.
The lack of any differences between the DrugFactsBox and other CMI group is surprising given that considerable research has demonstrated the superiority of the DrugFactsBox format compared to other types of CMI (18, 19, 20, 44). Lack of participant engagement in intervention activities may have contributed to these null findings, as well as the null findings for the SMART program in the full sample. Less than 40% of study participants visited the DrugFactsBox/other CMI websites, and only about 50% of those who were randomized to SMART took part in any training sessions. Participant engagement with both interventions might have been higher if we had limited the study to patients who were actively contemplating a medication regimen change. These patients are more likely than others to be interested in obtaining information about treatment options and gaining the skills needed to better understand the information they obtain.
We focused on individuals with moderate‐to‐severe active RA because current guidelines call for a treat‐to‐target strategy, with remission or low disease activity being the primary target. Therefore, we expected most of our participants to be contemplating medication regimen changes to better control disease activity. However, the RAPID3 may overestimate RA disease activity in people who experience pain or functional impairment due to other health conditions (e.g., fibromyalgia, osteoarthritis). Thus, reliance on this measure likely resulted in some participants being inaccurately classified as candidates for escalation of DMARD therapy, which accounts for, at least in part, the lack of more active participant engagement with the interventions being evaluated. Participant engagement in the SMART program might also be enhanced by offering the program asynchronously, allowing participants greater flexibility when accessing program materials and completing required activities (45).
In conclusion, although we found no support for the study hypotheses in our full sample, our findings suggest that the SMART program may help support informed decision‐making when targeted toward individuals with inadequate knowledge concerning the risks/benefits associated with different treatment options. This conclusion is consistent with prior research investigating gist‐based interventions (46); however, because our findings emerged from unplanned, subsample analyses, more research is needed to assess the replicability and generalizability of our findings and evaluate other approaches to enhance patients' health literacy/numeracy skills. More research is also needed to evaluate the effectiveness of DrugFactsBoxes in real‐world settings, incorporating procedures to enhance utilization and focusing on patients actively contemplating either initiating DMARD therapy for the first time or making a medication regimen change.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Blalock had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Blalock, Solow, Reyna, Keebler, Carpenter, O'Neill, Chapman.
Acquisition of data
Blalock, Solow, Keebler, Hunt, Hickey, Curtis, Chapman.
Analysis and interpretation of data
Blalock, Solow, Reyna, Keebler, Carpenter, Hunt, Hickey, O'Neill, Curtis, Chapman.
Supporting information
Supplementary Table 1 Components of Informed Decision‐Making and Proximal Outcome Variables by Assignment to the DrugFactsBox® or Other CMI Group (n = 286)
Supplementary Figure 1: Consort Figure (n = 286)
ClinicalTrials.gov identifier: NCT02820038.
The statements in this work are solely the responsibility of the authors and do not necessarily represent the views of Patient‐Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Supported by a PCORI award (PCS‐1409‐24099).
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Review: treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol 2014;66:775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fraenkel L, Cunningham M. High disease activity may not be sufficient to escalate care. Arthritis Care Res (Hoboken) 2014;66:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Hulst LT, Kievit W, van Bommel R, van Riel PL, Fraenkel L. Rheumatoid arthritis patients and rheumatologists approach the decision to escalate care differently: results of a maximum difference scaling experiment. Arthritis Care Res (Hoboken) 2011;63:1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, et al. Systematic review: comparative effectiveness and harms of disease‐modifying medications for rheumatoid arthritis. Ann Intern Med 2008;148:124–34. [DOI] [PubMed] [Google Scholar]
- 6. Le Blay P, Mouterde G, Barnetche T, Morel J, Combe B. Risk of malignancy including non‐melanoma skin cancers with anti‐tumor necrosis factor therapy in patients with rheumatoid arthritis: meta‐analysis of registries and systematic review of long‐term extension studies. Clin Exp Rheumatol 2012;30:756–64. [PubMed] [Google Scholar]
- 7. Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta‐analysis. Clin Infect Dis 2014;58:1649–57. [DOI] [PubMed] [Google Scholar]
- 8. Williams M, Chakravarty EF. Rheumatoid arthritis and pregnancy: impediments to optimal management of both biologic use before, during and after pregnancy. Curr Opin Rheumatol 2014;26:341–6. [DOI] [PubMed] [Google Scholar]
- 9. Wolf MS, King J, Wilson EA, Curtis LM, Bailey SC, Duhig J, et al. Usability of FDA‐approved medication guides. J Gen Intern Med 2012;27:1714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolf MS, Davis TC, Shrank WH, Neuberger M, Parker RM. A critical review of FDA‐approved medication guides. Patient education and counseling 2006;62:316–22. [DOI] [PubMed] [Google Scholar]
- 11. Shiffman S, Gerlach KK, Sembower MA, Rohay JM. Consumer understanding of prescription drug information: an illustration using an antidepressant medication. Ann Pharmacother 2011;45:452–8. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz L, Mazzola N, Hoffman RS, Howland MA, Mercurio‐Zappala M, Nelson LS. Evaluating patients' understanding of printed warfarin medication information. J Pharm Pract 2015;28:518–22. [DOI] [PubMed] [Google Scholar]
- 13. Wolf MS, Bailey SC, Serper M, Smith M, Davis TC, Russell AL, et al. Comparative effectiveness of patient‐centered strategies to improve FDA medication guides. Med Care 2014;52:781–9. [DOI] [PubMed] [Google Scholar]
- 14. Bailey SC, Navaratnam P, Black H, Russell AL, Wolf MS. Advancing best practices for prescription drug labeling. Ann Pharmacother 2015;49:1222–36. [DOI] [PubMed] [Google Scholar]
- 15. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97–107. [DOI] [PubMed] [Google Scholar]
- 16. Reyna VF. A new intuitionism: meaning, memory, and development in fuzzy‐trace theory. Judgm Decis Mak 2012;7:332–59. [PMC free article] [PubMed] [Google Scholar]
- 17. Reyna VF, Brainerd CJ. Dual processes in decision making and developmental neuroscience: a fuzzy‐trace model. Dev Rev 2011;31:180–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med 2009;150:516–27. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz LM, Woloshin S. The Drug Facts Box: improving the communication of prescription drug information. Proc Natl Acad Sci U S A 2013;110 Suppl 3:14069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz LM, Woloshin S, Welch HG. The drug facts box: providing consumers with simple tabular data on drug benefit and harm. Med Decis Making 2007;27:655–62. [DOI] [PubMed] [Google Scholar]
- 21. Vas AK, Spence J, Chapman SB. Abstracting meaning from complex information (gist reasoning) in adult traumatic brain injury. J Clin Exp Neuropsychol 2015;37:152–61. [DOI] [PubMed] [Google Scholar]
- 22. Motes MA, Gamino JF, Chapman SB, Rao NK, Maguire MJ, Brier MR, et al. Inhibitory control gains from higher‐order cognitive strategy training. Brain Cogn 2014;84:44–62. [DOI] [PubMed] [Google Scholar]
- 23. Gamino JF, Motes MM, Riddle R, Lyon GR, Spence JS, Chapman SB. Enhancing inferential abilities in adolescence: new hope for students in poverty. Front Hum Neurosci 2014;8:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chapman SB, Mudar RA. Enhancement of cognitive and neural functions through complex reasoning training: evidence from normal and clinical populations. Front Syst Neurosci 2014;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cook LG, Chapman SB, Elliott AC, Evenson NN, Vinton K. Cognitive gains from gist reasoning training in adolescents with chronic‐stage traumatic brain injury. Front Neurol 2014;5:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anand R, Chapman SB, Rackley A, Keebler M, Zientz J, Hart J Jr. Gist reasoning training in cognitively normal seniors. Int J Geriatr Psychiatry 2011;26:961–8. [DOI] [PubMed] [Google Scholar]
- 27. Chapman SB, Aslan S, Spence JS, Hart JJ Jr, Bartz EK, Didehbani N, et al. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb Cortex 2015;25:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28–55. [DOI] [PubMed] [Google Scholar]
- 29. Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol 2008;35:2136–47. [DOI] [PubMed] [Google Scholar]
- 30. Pincus T, Yazici Y, Bergman MJ. RAPID3, an index to assess and monitor patients with rheumatoid arthritis, without formal joint counts: similar results to DAS28 and CDAI in clinical trials and clinical care. Rheum Dis Clin North Am 2009;35:773–8. [DOI] [PubMed] [Google Scholar]
- 31. Reyna VF. A theory of medical decision making and health: fuzzy trace theory. Med Decis Making 2008;28:850–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect 2001;4:99–108.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dormandy E, Tsui EY, Marteau TM. Development of a measure of informed choice suitable for use in low literacy populations. Patient Educ Couns 2007;66:278–95. [DOI] [PubMed] [Google Scholar]
- 34. Sepucha K, Ozanne E, Silvia K, Partridge A, Mulley AG Jr. An approach to measuring the quality of breast cancer decisions. Patient Educ Couns 2007;65:261–9. [DOI] [PubMed] [Google Scholar]
- 35. Fraenkel L, Peters E, Charpentier P, Olsen B, Errante L, Schoen RT, et al. Decision tool to improve the quality of care in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fayet F, Savel C, Rodere M, Pereira B, Abdi D, Mathieu S, et al. The development of a questionnaire to evaluate rheumatoid arthritis patient's knowledge about methotrexate. J Clin Nurs 2016;25:682–9. [DOI] [PubMed] [Google Scholar]
- 37. Barton JL, Trupin L, Schillinger D, Evans‐Young G, Imboden J, Montori VM, et al. Use of low‐literacy decision aid to enhance knowledge and reduce decisional conflict among a diverse population of adults with rheumatoid arthritis: results of a pilot study. Arthritis Care Res (Hoboken) 2016;68:889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vas AK, Chapman SB, Cook LG, Elliott AC, Keebler M. Higher‐order reasoning training years after traumatic brain injury in adults. J Head Trauma Rehabil 2011;26:224–39. [DOI] [PubMed] [Google Scholar]
- 39. Sullivan TR, White IR, Salter AB, Ryan P, Lee KJ. Should multiple imputation be the method of choice for handling missing data in randomized trials? Stat Methods Med Res 2018;27:2610–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale, NJ: Erlbaum;1988. [Google Scholar]
- 41. Mudar RA, Chapman SB, Rackley A, Eroh J, Hsueh‐Sheng C, Perez A, et al. Enhancing latent cognitive capacity in mild cognitive impairment with gist reasoning training: a pilot study. Int J Geriatr Psychiatry 2017;32:548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Motes MA, Yezhuvath US, Aslan S, Spence JS, Rypma B, Chapman SB. Higher‐order cognitive training effects on processing speed‐related neural activity: a randomized trial. Neurobiol Aging 2018;62:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chapman SB, Spence JS, Aslan S, Keebler MW. Enhancing innovation and underlying neural mechanisms via cognitive training in healthy older adults. Front Aging Neurosci 2017;9:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woloshin S, Schwartz LM. Communicating data about the benefits and harms of treatment: a randomized trial. Ann Intern Med 2011;155:87–96. [DOI] [PubMed] [Google Scholar]
- 45. Ackerman IN, Bucknill A, Page RS, Broughton NS, Roberts C, Cavka B, et al. Preferences for disease‐related education and support among younger people with hip or knee osteoarthritis. Arthritis Care Res (Hoboken) 2017;69:499–508. [DOI] [PubMed] [Google Scholar]
- 46. Wolfe CR, Reyna VF, Widmer CL, Cedillos‐Whynott EM, Brust‐Renck PG, Weil AM, et al. Understanding genetic breast cancer risk: processing loci of the BRCA gist intelligent tutoring system. Learn Individ Differ 2016;49:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Components of Informed Decision‐Making and Proximal Outcome Variables by Assignment to the DrugFactsBox® or Other CMI Group (n = 286)
Supplementary Figure 1: Consort Figure (n = 286)


