ABSTRACT
Gender‐affirming hormone therapy aligns physical characteristics with an individual's gender identity, but sex hormones regulate bone remodeling and influence bone morphology. We hypothesized that trans men receiving testosterone have compromised bone morphology because of suppression of ovarian estradiol production, whereas trans women receiving estradiol, with or without anti‐androgen therapy, have preserved bone microarchitecture. We compared distal radial and tibial microarchitecture using high‐resolution peripheral quantitative computed tomography images in a cross‐sectional study of 41 trans men with 71 cis female controls, and 40 trans women with 51 cis male controls. Between‐group differences were expressed as standardized deviations (SD) from the mean in age‐matched cisgender controls with 98% confidence intervals adjusted for cross‐sectional area (CSA) and multiple comparisons. Relative to cis women, trans men had 0.63 SD higher total volumetric bone mineral density (vBMD; both p = 0.01). Cortical vBMD and cortical porosity did not differ, but cortices were 1.11 SD thicker (p < 0.01). Trabeculae were 0.38 SD thicker (p = 0.05) but otherwise no different. Compared with cis men, trans women had 0.68 SD lower total vBMD (p = 0.01). Cortical vBMD was 0.70 SD lower (p < 0.01), cortical thickness was 0.51 SD lower (p = 0.04), and cortical porosity was 0.70 SD higher (p < 0.01). Trabecular bone volume (BV/TV) was 0.77 SD lower (p < 0.01), with 0.57 SD fewer (p < 0.01) and 0.30 SD thicker trabeculae (p = 0.02). There was 0.56 SD greater trabecular separation (p = 0.01). Findings at the distal radius were similar. Contrary to each hypothesis, bone microarchitecture was not compromised in trans men, perhaps because aromatization of administered testosterone prevented bone loss. Trans women had deteriorated bone microarchitecture either because of deficits in microstructure before treatment or because the estradiol dosage was insufficient to offset reduced aromatizable testosterone. Prospective studies are needed to confirm these findings. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: BONE, ESTRADIOL, TESTOSTERONE, HRpQCT, MICROARCHITECTURE, TRANSGENDER
Introduction
Transgender individuals comprise 0.5% to 2.8% of the population.( 1 ) Gender‐affirming hormone therapy aligns physical characteristics with a person's gender identity. Masculinizing hormone therapy given to trans men typically aims to increase serum testosterone into the male reference range (10–35 nmol/L, 2.88–10.09 ng/mL),( 2 ) which in turn increases muscle mass, decreases fat mass, alters fat distribution, deepens the voice, and increases facial and body hair.( 3 , 4 ) Feminizing hormone therapy for trans women usually increases serum estradiol to the female reference range (211–400 pmol/L, 57–109 pg/mL) and lowers testosterone (2–4 nmol/L, 0.57–1.15 ng/mL),( 2 ) thereby decreasing muscle mass, increasing fat mass, inducing a more gynoid fat distribution, decreasing libido, and increasing breast growth.( 3 , 4 ) In the absence of gonadectomy, anti‐androgens (such as cyproterone acetate, spironolactone, gonadotropin‐releasing hormone [GnRH] analogues, and progesterone) may be required to suppress testosterone.( 4 )
Hormone therapy may improve psychological outcomes and quality of life but may adversely influence bone microstructure because sex hormones regulate bone remodeling.( 5 ) Estradiol, whether of ovarian origin, administered in pharmacological doses, or produced by aromatization of testosterone systemically or locally within bone, is a major regulator of the birth rate of bone remodeling units and the net balance between the volumes of bone resorbed and formed by each remodeling event.
In trans men, testosterone administration inhibits the hypothalamic‐pituitary‐ovarian axis and so reduces estrogen synthesis. Serum estradiol concentrations decrease but by only approximately 50 to 60 pmol/L (13.6–17.1 pg/mL).( 4 ) Nevertheless, as the potency of estradiol exceeds that of testosterone in activating their respective receptors, lower estradiol levels are likely to increase in the birth rate of remodeling units and produce remodeling imbalance, resulting in bone loss and microarchitectural deterioration. In trans women, estradiol administration produces hypothalamic‐pituitary‐testicular axis suppression and so reduces testosterone synthesis. If exogenous estradiol administration is sufficient, remodeling may remain slow and balanced, preserving bone morphology.
Consistent information concerning the effects of gender‐affirming hormone therapy on bone morphology is lacking because of challenges in study design and execution. For example, there are no prospective randomized double‐blind trials evaluating the efficacy and safety of hormonal therapy in transgender individuals relative to respective cis controls. Inferences are based on cross‐sectional( 6 , 7 , 8 , 9 , 10 ) or longitudinal observational cohort studies,( 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 ) many lacking a cisgender control group.( 6 , 8 , 11 , 12 , 15 , 16 , 17 , 18 , 19 ) Systematic reviews and meta‐analyses also produce inconsistent observations.( 20 , 21 , 22 )
Moreover, most studies use bone densitometry and report normal,( 6 , 8 , 9 , 11 , 13 , 15 , 16 , 17 , 18 , 19 ) reduced, 7 , 8 , 10 , 12 , 14 ) or increased( 6 , 9 , 11 , 12 , 13 , 18 ) bone mineral density (BMD). The BMD measurement captures bone mass, not microarchitecture, a limitation in fragility fracture assessment because of the nonlinear relationship between microarchitecture and bone strength. Bone stiffness decreases as a seventh and third power of the increase in cortical porosity and decrease in trabecular density, respectively.( 23 ) This sensitivity may partly account for the greater predictive strength of measures of microarchitectural deterioration than BMD in detecting persons at imminent risk of fracture.( 24 )
We therefore sought to determine whether measurement of microarchitecture will identify any association between gender‐affirming hormone therapy and bone morphology. We hypothesized that in trans men, testosterone administration is associated with deficits in bone microarchitecture, whereas in trans women, estradiol administration is associated with preservation of bone microarchitecture.
Materials and Methods
Participants
We conducted a cross‐sectional study between April 1, 2017, and April 30, 2018, in trans individuals aged 18 years and older receiving continuous gender‐affirming hormone therapy for 12 months or more and a group of age‐matched cisgender controls. Trans men receiving either intramuscular or transdermal testosterone were compared with cis female controls. Trans women receiving oral or transdermal estradiol were compared with cis male controls. Trans individuals were recruited from endocrinology outpatient clinics and primary care general practice clinics specializing in trans health in Melbourne, Australia. All 41 trans men received testosterone therapy: intramuscular (IM) testosterone undecanoate (1000 mg 8 to 14 weekly, n = 30), IM testosterone enanthate (250 mg fortnightly, n = 9), and transdermal testosterone gel (1%, 5 g/d, n = 2). All 40 trans women received estradiol (oral estradiol valerate, dose range 1–6 mg daily, n = 33), transdermal estradiol (100 mcg/24 hours, n = 4), and oral ethinyl estradiol (dose range 30–100 mcg daily, n = 3). A total of 78% (n = 31) of the feminizing hormone therapy group were taking androgen‐blocking therapy in addition to estradiol therapy (cyproterone acetate n = 21, spironolactone n = 4, progesterone n = 5, GnRH n = 1) and 28% (n = 11) had undergone orchidectomy. No individuals in the masculinizing hormone therapy group had undergone oophorectomy. The age of gender‐affirming hormone therapy initiation was variable, but no participants underwent treatment with GnRH agonists for puberty blockade. In terms of ethnicity, trans men were of White (n = 32) and Asian (n = 7) background, and trans women were of White (n = 38) and Asian (n = 2) background. All control participants (n = 122) were White. Control groups were derived from a database comprising healthy cis females and males in the community who responded to local advertisements.
Exclusion criteria for all participants were the presence of metabolic bone disease or receiving therapy that affects bone (glucocorticoids, bisphosphonates, anti‐epileptic medication, use of HIV pre‐exposure prophylaxis). Participants presumed to be menopausal (trans men and cis females age >50 years) were excluded. All participants provided written informed consent and the protocol was approved by the Austin Health Human Research Ethics Committee (approval reference HREC/17/Austin/74).
Measurements
Both transgender and control participants had imaging of the nondominant distal radius and distal tibia using high‐resolution peripheral quantitative computed tomography (HR‐pQCT, XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland). One hundred ten slices (82 μm isotropic voxel size) were obtained at 9.5 and 22.5 mm from a reference line at the endplate of the distal radius and distal tibia, respectively. Strax software, a nonthreshold‐based method, was used to quantify microarchitecture in vivo (StraxCorp, Melbourne, Australia). The coefficients of variation (CV) ranged 0.6% to 0.9% for volumetric BMD (vBMD) and 1.4% to 7.4% for structural parameters.
Trans participants provided a fasting early morning blood sample to measure estradiol, testosterone, luteinizing hormone (LH), follicle‐stimulating hormone (FSH), sex hormone binding globulin (SHBG), 25‐hydroxy‐vitamin D (vitamin D), and estimated glomerular filtration rate (eGFR). Blood tests were not collected in controls. Estradiol was measured using immunoassay (Cobas E801, Roche Diagnostics, interassay variation 25% at level of 100 pnmol/L or less and 25% at a level of greater than 100 pmol/L). Testosterone was measured using electrochemiluminescent immunoassay (Cobas E801, Roche Diagnostics, Indianapolis, IN, USA; interassay CV is 5.3% at a level of 3.4 nmol/L, 4.5% at 13.3 nmol/L, and 4.0% at 27.2 nmol/L). SHBG was measured on immunoassay (Cobas E801, Roche Diagnostics, interassay variation 6% at a level of 21 nmol/L, and 6% at a level of 40 nmol/L). Twenty‐five hydroxy vitamin D was measured using chemiluminescent immunoassay (Liaison XL, DiaSorin Inc., Stillwater, MN, USA; interassay variation ±7.98 <75 nmol/L and 11.56% >75 nmol/L). In participants taking IM testosterone, blood samples were timed and collected as a “trough level.”
Statistical analysis
Age, duration of hormonal therapy, and blood results are presented as median (interquartile range). Differences in bone microarchitecture in cases and controls are presented in absolute terms (Supplemental Tables S1 and S2) and as the number of standard deviation (SD) of the mean in cases relative to age‐matched cisgender controls (Table 2). Linear regression, adjusted for cross‐sectional area (CSA) of entire bone was used to compare group differences in bone microarchitecture. Post hoc tests were performed using custom contrasts comparing between trans and cisgender controls, with Benjamini‐Hochberg procedure to adjust for multiple comparisons. Normality of residuals were assessed by normal Q‐Q plots. A two‐tailed alpha of 0.05 was the chosen level of significance for all analyses, where an alpha level of 0.02 (ie, 98% confidence intervals) was reported to account for multiple comparisons. Statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Results are reported as SD or Z‐scores relative to cisgender controls, with 98% confidence intervals after adjusting for age and cross‐sectional area.
Table 2.
Distal Tibia Cortical and Trabecular Microstructure in Trans Men and Trans Women Expressed as SD Relative to Age‐Matched Controls (Trans Men Were Compared With Cis Female Controls and Trans Women Were Compared With Cis Male Controls)
| Distal tibia | Trans men Z‐score a SD (98% CI) | p Value | Trans women Z‐score a SD (98% CI) | p Value |
|---|---|---|---|---|
| Total CSA | 0.85 (0.50, 1.20) | <0.01 * | −0.21 (−0.61, 0.19) | 0.05 * |
| Total vBMD (mg/cc) | 0.63 (0.23, 1.02) | 0.01 * | −0.68 (−1.11, −0.26) | 0.01 * |
| Cortical | ||||
| vBMD (mg/cc) | 0.18 (−0.19, 0.55) | 0.32 | −0.70 (−1.10, −0.30) | <0.01 * |
| Thickness (mm) | 1.11 (0.64, 1.57) | <0.01 * | −0.51 –1.01, −0.01) | 0.04 * |
| Porosity (%) | −0.18 (−0.55, 0.19) | 0.33 | 0.70 (0.30, 1.10) | <0.01 * |
| Trabecular | ||||
| BV/TV (%) | 0.09 (−0.33, 0.50) | 0.67 | −0.77 (−1.21, −0.33) | <0.01 * |
| Number (mm2) | 0.05 (−0.36, 0.45) | 0.82 | −0.57 (−1.01, −0.14) | <0.01 * |
| Thickness (mm) | 0.38 (−0.03, 0.79) | 0.05 * | 0.30 (−0.14, 0.74) | 0.02 * |
| Separation (mm) | −0.21 (−0.61, 0.19) | 0.28 | 0.56 (0.12, 0.99) | 0.01 * |
SD = standard deviation; CI = confidence interval; CSA = cross‐sectional area; vBMD = volumetric bone mineral density; BV/TV = bone volume/tissue volume (bone volume fraction).
Adjusted for total cross‐sectional area with corresponding p value shown.
p values in bold were ≤0.05.
Results
We recruited 41 trans men receiving testosterone therapy, 40 trans women receiving estradiol therapy, and 122 controls (71 cis females, 51 cis males) (Table 1). Z‐scores for tibial bone microarchitecture with 98% CI are shown in Table 2 and Fig. 1. Absolute values are presented in Supplemental Table S1.
Table 1.
Age, Duration of Hormone Therapy, and Biochemistry in Trans Men, Trans Women, and Cisgender Controls
| Trans men (n = 41) | Cis women (n = 71) | Trans women (n = 40) | Cis men (n = 51) | |
|---|---|---|---|---|
| Age (years) | 28.6 (24.6, 30.9) | 28.2 (24.2, 31.7) | 37.6 (26.3, 52.7) | 41.6 (32.4, 54.4) |
| Duration of hormone therapy (months) | 42.5 (21.4, 65.0) | NA | 39.1 (21.8, 60.0) | NA |
| eGFR | 90.0 (90.0, 90.0) | – | 88.0 (67.5, 90.0) | – |
| Vitamin D (nmol/L) | 51.5 (34.8, 64.2) | – | 55.0 (42.0, 68.0) | – |
| FSH (IU/L) | 2.9 (1.1, 5.6) | – | 1.4 (0.3, 13.2) | – |
| LH (IU/L) | 1.9 (0.6, 5.7) | – | 2.9 (0.3, 8.0) | – |
| Estradiol (pmol/L) | 115.0 (93.0, 164.0) | – | 335.0 (157.0, 468.0) a | – |
| Testosterone (nmol/L) | 15.6 (13.2, 19.7) | – | 0.6 (0.4, 0.9) | – |
| SHBG (nmol/L) | 31.5 (21.0, 41.0) | – | 82.0 (58.5, 116.5) | – |
Data are expressed as median (interquartile range).
eGFR = estimated glomerular filtration rate; FSH = follicle‐stimulating hormone; LH = luteinizing hormone; SHBG = sex hormone binding globulin.
Those taking ethinyl estradiol, n = 3, were excluded.
Fig. 1.
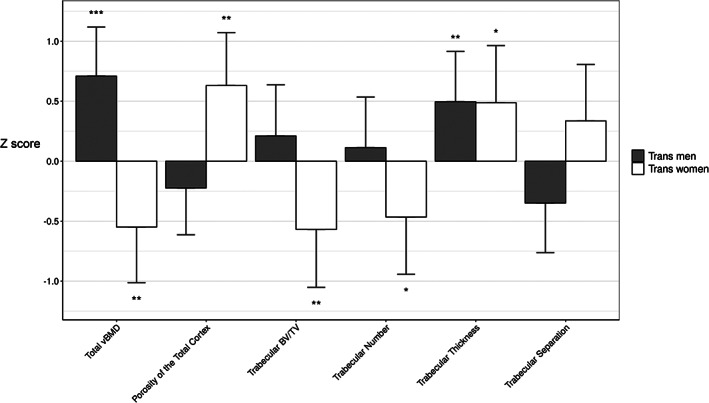
Distal tibia microstructure in trans men and trans women relative to age and birth‐assigned sex‐matched cisgender controls. Mean differences in morphology expressed as the number of standard deviations with 98% confidence interval in cases relative to controls. vBMD = volumetric bone density; BV/TV = trabeculae bone volume/tissue volume. *p < 0.05; **p < 0.02; ***p < 0.01.
Compared with cis women, trans men had 0.85 SD higher total CSA and 0.63 SD higher total vBMD (both p = 0.01). Cortical vBMD and cortical porosity did not differ from controls, but cortices were 1.11 SD thicker (p < 0.01). Trabeculae were 0.38 SD thicker (p = 0.05). Trabecular morphology was otherwise no different from controls.
Compared with cis men, trans women had 0.21 SD lower total CSA (p = 0.05) and 0.68 SD lower total vBMD (p = 0.01). Cortical vBMD was 0.70 SD lower (p < 0.01), cortical thickness was 0.51 SD lower (p = 0.04), and cortical porosity was 0.70 SD higher (p < 0.01). Trabecular BV/TV was 0.77 SD lower (p < 0.01), with 0.57 SD fewer (p < 0.01) and 0.30 SD thicker trabeculae (p = 0.02). There was 0.56 SD greater trabecular separation (p = 0.01). Findings at the distal radius were similar (Supplemental Tables S2 and S3).
Discussion
Contrary to the proposed hypotheses, bone morphology was not compromised in trans men receiving testosterone relative to cis female controls; total vBMD was higher with higher trabecular thickness and no difference in cortical morphology. Bone morphology was compromised in trans women receiving estradiol relative to cis male controls; cortical vBMD was lower, cortical porosity was higher, and trabecular density was lower.
Trans men
Relative to cis female controls, trans men had total vBMD and greater trabecular thickness. Our findings are consistent with preservation of bone microstructure and in keeping with Lips and colleagues, who used histomorphometry in 15 trans men compared with controls.( 25 ) If sufficient testosterone was aromatized to estradiol, this may maintain trabecular thickness because estradiol is antiresorptive. Estradiol slows bone remodeling, preserving or slowing microarchitectural deterioration; it is not anabolic. Alternatively, as trans men had a median hormone therapy duration of 3 years, it is possible that the greater trabecular thickness relative to cis female controls was attributable to bone loss in the controls. Substantial trabecular bone loss is reported in cis males before the age of 50 years and cis females before menopause.( 26 )Whether testosterone has antiresorptive action directly or via aromatization to estradiol is not known. However, testosterone plus aromatase inhibitor administration is associated with lower BMD than administration of testosterone alone, consistent with an estradiol‐mediated action.( 27 ) Alternatively, the greater trabecular thickness could be attributable to an anabolic effect of testosterone, but there is no evidence to support this.
Trans women
Trans women taking estradiol had lower total vBMD, lower cortical vBMD, higher cortical porosity and reduced trabecular bone volume fraction relative to cis male controls. Finding fewer trabeculae with greater trabecular separation is consistent with bone loss. The higher mean trabecular thickness is also consistent with preferential loss (complete obliteration) of thinner trabeculae.( 26 )
It is plausible that estradiol administration was insufficient to suppress bone remodeling. The importance of the dosage of estradiol is supported by a study of 711 trans women followed for 10 years. Those with estradiol in the lower tertile lost bone, whereas those with serum estradiol in the higher tertile had higher BMD.( 28 ) Higher doses of estradiol may be needed to offset bone loss, but given the potential for adverse cardiovascular and venous thromboembolic effects, an alternative approach might be administration of bisphosphonate therapy, which reduces fracture risk in cis men and cis women.( 29 ) However, because this study was cross‐sectional, we cannot exclude the possibility that these deficits are due to sampling whereby trans women had a lower pretreatment baseline level( 19 , 30 ) or that cis male controls had higher values or both.
Gender‐affirming hormone therapy and bone morphology
Most associations between gender‐affirming hormone therapy and bone morphology are based on studies using BMD. The findings are inconsistent—normal, reduced, or increased BMD relative to baseline or relative to controls reported in single studies, meta‐analyses, and systematic reviews.( 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 ) Many factors may account for these disparate reports. For example, between‐group differences depend on changes in controls as well as the treated group. Changes in body composition accompanying hormone therapy influence photon attenuation independent of bone matrix volume. Testosterone administration increases lean mass and decreases fat mass.( 3 ) These soft tissue changes attenuate photon transmission artifactually overestimating BMD. This may obscure a true detrimental effect of hormone therapy in trans men. Estradiol and androgen blockade reduce lean mass and increase fat mass, resulting in greater photon transmission artifactually underestimating BMD.
Gender‐affirming hormone therapy and fracture risk
Attributing fracture prevalence or incidence to the use of gender‐affirming hormone therapy is also challenging because no randomized prospective controlled trials have been done. Cross‐sectional or prospective cohort studies are usually based on small sample sizes limiting the ability to adjust for confounders that may contribute to fracture risk independent of the hormonal therapy. In the largest study of fractures in trans men and trans women, Wiepjes and colleagues report only 18 trans men with fractures in a cohort of 1036 trans men, a sample size insufficient for robust multivariable analysis.( 28 ) There was no overall increase in fracture prevalence in trans women compared with cis men (or cis women). However, in a subanalysis, there was a 4.4% higher fracture prevalence in trans women older than 50 years compared with cis men (odds ratio [OR] = 1.9, 98% CI 1.32–2.74). Whether this risk is attributable to insufficient feminizing hormone therapy or measured and unmeasured confounders (ie, physical activity, tobacco use) is unclear because of the observational nature of the data.
Limitations
This study has several limitations. The cross‐sectional study design precludes establishment of causation between hormone therapy and bone morphology. We did not quantify bone microarchitecture before treatment and so we cannot exclude the possibility that the absence of microarchitectural deterioration in trans men is proof of safety of testosterone administration. Nor can we establish whether deteriorated microarchitecture in trans women was the result of insufficient dosage of estradiol or baseline differences. Other potential determinants of bone health, including physical activity estimates, dietary calcium intake estimates, and smoking status, were not measured. We did not measure testosterone and estradiol levels using liquid chromatography mass spectrometry. Clinically available immunoassay was used. Prospective studies are needed to address the effects of sex hormones on bone remodeling and microstructure, the effects of treatment on BMD independent of effects on body composition, and whether between‐group differences are due to changes in controls, trans persons, or both.
Estradiol, whether of ovarian origin, synthesized by aromatization of testosterone, or administered pharmacologically, plays a central role in bone health in both trans men and trans women. Within the constraints of this cross‐sectional design, we infer that in trans men, estradiol produced by aromatization of administered testosterone preserves bone microarchitecture. In trans women, microarchitectural deterioration may not be prevented if estradiol replacement is insufficient to offset the reduced testosterone substrate for aromatization locally in bone.
Disclosures
AGZ has an association with StraxCorp, receiving remuneration as an image analyst, having an entitlement to future royalties, and as a shareholder. ES has an association with StraxCorp, receiving renumeration as scientific officer, having an entitlement to future royalties, and as a shareholder. All other authors have no financial or personal relationships with other people or organizations to declare.
Author Contributions
Ingrid Bretherton: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing‐original draft, Writing‐review & editing; Ali Ghasem‐Zadeh: Data curation, Writing‐review & editing; Shalem Y Leemaqz: Formal analysis, Writing‐review & editing; Ego Seeman: Formal analysis, Writing‐original draft, Writing‐review & editing; Xiaofang Wang: Data curation, Writing‐review & editing; Thomas McFarlane: Data curation, Writing‐review & editing; Cassandra Spanos: Data curation, Writing‐review & editing; Mathis Grossmann: Conceptualization, Supervision, Writing‐review & editing; Jeffrey D Zajac: Conceptualization, Supervision, Writing‐review & editing; Ada S Cheung: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing‐review & editing.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4497.
Supporting information
Supplemental Table S1. Distal Tibia Cortical and Trabecular Microstructure in Trans Women Expressed in Absolute Terms Relative to the Mean in Age‐Matched Cis Male Controls
Supplemental Table S2. Distal Radial Cortical and Trabecular Microstructure in Trans Women Expressed in Absolute Terms Relative to the Mean in Age‐Matched Cis Male Controls
Supplemental Table S3. Distal Radial Cortical and Trabecular Microstructure in Trans Men and Trans Women Expressed as Standardized Deviations Relative to Age‐Matched Controls (Trans Men Were Compared With Cis Female Controls and Trans Women Were Compared With Cis Male Controls)
Acknowledgments
IB is supported by an Australian Government Research Training Program Scholarship and a Rowden White Scholarship (application ref: 184409), The University of Melbourne. ASC is supported by an Australian Government National Health and Medical Research Council Early Career Fellowship (#1143333) and receives research support by the Viertel Charitable Foundation Clinical Investigator Award, Endocrine Society of Australia, Austin Medical Research Foundation, and the Royal Australasian College of Physicians Foundation. Study data were collected and managed using REDCap electronic data capture tools hosted at University of Melbourne. No sponsor or funding source was involved in the study design, collection, analysis, and interpretation of data, in the decision to submit the manuscript for publication, or any other aspect of the study.
Authors’ roles: Conceptualization: IB, MG, JDZ, and ASC. Data curation: IB, AG‐Z, X‐FW, TM, and CS. Formal analysis: IB, SYL, ES, and ASC. Investigation: IB. Methodology: IB and ASC. Project administration: IB. Supervision: MG, JDZ, and ASC. Writing—original draft: IB and ES. Writing—review and editing: all authors.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, ASC, upon reasonable request.
References
- 1. Åhs JW, Dhejne C, Magnusson C, et al. Proportion of adults in the general population of Stockholm County who want gender‐affirming medical treatment. PLoS One. 2018;13(10):e0204606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheung AS, Lim HY, Cook T, et al. Approach to interpreting common laboratory pathology tests in transgender individuals. J Clin Endocrinol Metab. 2021;106(3):893‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klaver M, de Blok CJM, Wiepjes CM, et al. Changes in regional body fat, lean body mass and body shape in trans persons using cross‐sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol. 2018;178(2):165‐173. [DOI] [PubMed] [Google Scholar]
- 4. Cheung AS, Wynne K, Erasmus J, Murray S, Zajac JD. Position statement on the hormonal management of adult transgender and gender diverse individuals. Med J Aust. 2019;211(3):127‐133. [DOI] [PubMed] [Google Scholar]
- 5. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19(9):1323‐1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruetsche AG, Kneubuehl R, Birkhaeuser MH, Lippuner K. Cortical and trabecular bone mineral density in transsexuals after long‐term cross‐sex hormonal treatment: a cross‐sectional study. Osteoporos Int. 2005;16(7):791‐798. [DOI] [PubMed] [Google Scholar]
- 7. Lapauw B, Taes Y, Simoens S, et al. Body composition, volumetric and areal bone parameters in male‐to‐female transsexual persons. Bone. 2008;43(6):1016‐1021. [DOI] [PubMed] [Google Scholar]
- 8. Wierckx K, Mueller S, Weyers S, et al. Long‐term evaluation of cross‐sex hormone treatment in transsexual persons. J Sex Med. 2012;9(10):2641‐2651. [DOI] [PubMed] [Google Scholar]
- 9. Broulik PD, Urbánek V, Libanský P. Eighteen‐year effect of androgen therapy on bone mineral density in trans(gender) men. Horm Metab Res. 2018;50(2):133‐137. [DOI] [PubMed] [Google Scholar]
- 10. Chrisostomo KR, Skare TL, Chrisostomo HR, Barbosa EJL, Nisihara R. Transwomen and bone mineral density: a cross‐sectional study in Brazilian population. Br J Radiol. 2020;93(1111):20190935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Kesteren P, Lips P, Deville W, et al. The effect of one‐year cross‐sex hormonal treatment on bone metabolism and serum insulin‐like growth factor‐1 in transsexuals. J Clin Endocrinol Metab. 1996;81(6):2227‐2232. [DOI] [PubMed] [Google Scholar]
- 12. van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J. Long‐term follow‐up of bone mineral density and bone metabolism in transsexuals treated with cross‐sex hormones. Clin Endocrinol (Oxf). 1998;48(3):347‐354. [DOI] [PubMed] [Google Scholar]
- 13. Turner A, Chen TC, Barber TW, Malabanan AO, Holick MF, Tangpricha V. Testosterone increases bone mineral density in female‐to‐male transsexuals: a case series of 15 subjects. Clin Endocrinol (Oxf). 2004;61(5):560‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. T'Sjoen G, Weyers S, Taes Y, et al. Prevalence of low bone mass in relation to estrogen treatment and body composition in male‐to‐female transsexual persons. J Clin Densitom. 2009;12(3):306‐313. [DOI] [PubMed] [Google Scholar]
- 15. Mueller A, Haeberle L, Zollver H, et al. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female‐to‐male transsexuals. J Sex Med. 2010;7(9):3190‐3198. [DOI] [PubMed] [Google Scholar]
- 16. Mueller A, Zollver H, Kronawitter D, et al. Body composition and bone mineral density in male‐to‐female transsexuals during cross‐sex hormone therapy using gonadotrophin‐releasing hormone agonist. Exp Clin Endocrinol Diabetes. 2011;119(2):95‐100. [DOI] [PubMed] [Google Scholar]
- 17. Pelusi C, Costantino A, Martelli V, et al. Effects of three different testosterone formulations in female‐to‐male transsexual persons. J Sex Med. 2014;11(12):3002‐3011. [DOI] [PubMed] [Google Scholar]
- 18. Wiepjes CM, Vlot MC, Klaver M, et al. Bone mineral density increases in trans persons after 1 year of hormonal treatment: a multicenter prospective observational study. J Bone Miner Res. 2017;32(6):1252‐1260. [DOI] [PubMed] [Google Scholar]
- 19. Wiepjes CM, de Jongh RT, de Blok CJ, et al. Bone safety during the first ten years of gender‐affirming hormonal treatment in transwomen and transmen. J Bone Miner Res. 2019;34(3):447‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh‐Ospina N, Maraka S, Rodriguez‐Gutierrez R, et al. Effect of sex steroids on the bone health of transgender individuals: a systematic review and meta‐analysis. J Clin Endocrinol Metabol. 2017;102(11):3904‐3913. [DOI] [PubMed] [Google Scholar]
- 21. Delgado‐Ruiz R, Swanson P, Romanos G. Systematic review of the long‐term effects of transgender hormone therapy on bone markers and bone mineral density and their potential effects in implant therapy. J Clin Med. 2019;8(6):784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fighera TM, Ziegelmann PK, Rasia da Silva T, Spritzer PM. Bone mass effects of cross‐sex hormone therapy in transgender people: Updated systematic review and meta‐analysis. J Endocr Soc. 2019;3(5):943‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schaffler MB, Burr DB. Stiffness of compact bone: effects of porosity and density. J Biomech. 1988;21(1):13‐16. [DOI] [PubMed] [Google Scholar]
- 24. Chapurlat R, Bui M, Sornay‐Rendu E, et al. Deterioration of cortical and trabecular microstructure identifies women with osteopenia or normal bone mineral density at imminent and long‐term risk for fragility fracture: a prospective study. J Bone Miner Res. 2020;35(5):833‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lips P, van Kesteren PJ, Asscheman H, Gooren LJ. The effect of androgen treatment on bone metabolism in female‐to‐male transsexuals. J Bone Miner Res. 1996;11(11):1769‐1773. [DOI] [PubMed] [Google Scholar]
- 26. Riggs BL, Melton LJ, Robb RA, et al. A population‐based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23(2):205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meriggiola MC, Armillotta F, Costantino A, et al. Effects of testosterone undecanoate administered alone or in combination with letrozole or dutasteride in female to male transsexuals. J Sex Med. 2008;5(10):2442‐2453. [DOI] [PubMed] [Google Scholar]
- 28. Wiepjes CM, de Blok CJ, Staphorsius AS, et al. Fracture risk in trans women and trans men using long‐term gender‐affirming hormonal treatment: a nationwide cohort study. J Bone Miner Res. 2020;35(1):64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367(18):1714‐1723. [DOI] [PubMed] [Google Scholar]
- 30. Van Caenegem E, Taes Y, Wierckx K, et al. Low bone mass is prevalent in male‐to‐female transsexual persons before the start of cross‐sex hormonal therapy and gonadectomy. Bone. 2013;54(1):92‐97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Distal Tibia Cortical and Trabecular Microstructure in Trans Women Expressed in Absolute Terms Relative to the Mean in Age‐Matched Cis Male Controls
Supplemental Table S2. Distal Radial Cortical and Trabecular Microstructure in Trans Women Expressed in Absolute Terms Relative to the Mean in Age‐Matched Cis Male Controls
Supplemental Table S3. Distal Radial Cortical and Trabecular Microstructure in Trans Men and Trans Women Expressed as Standardized Deviations Relative to Age‐Matched Controls (Trans Men Were Compared With Cis Female Controls and Trans Women Were Compared With Cis Male Controls)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, ASC, upon reasonable request.


