Abstract
Metabolic abnormalities have been associated with olanzapine treatment. We assessed if olanzapine has dose‐dependent effects on metabolic parameters with changes for weight, blood pressure, lipid and glucose profiles being modelled using linear mixed‐effects models. The risk of metabolic abnormalities including early weight gain (EWG) (≥5% during first month) was assessed using mixed‐effects logistic regression models. In 392 olanzapine‐treated patients (median age 38.0 years, interquartile range [IQR] = 26.0–53.3, median dose 10.0 mg/day, IQR = 5.0–10.0 for a median follow‐up duration of 40.0 days, IQR = 20.7–112.2), weight gain was not associated with olanzapine dose (p = 0.61) although it was larger for doses versus ≤10 mg/day (2.54 ± 5.55 vs. 1.61 ± 4.51% respectively, p = 0.01). Treatment duration and co‐prescription of >2 antipsychotics, antidepressants, benzodiazepines and/or antihypertensive agents were associated with larger weight gain (p < 0.05). Lower doses were associated with increase in total and HDL cholesterol and systolic and diastolic blood pressure (p < 0.05), whereas higher doses were associated with glucose increases (p = 0.01). Patients receiving >10 mg/day were at higher EWG risk (odds risk: 2.15, 1.57–2.97). EWG might be prominent in high‐dose olanzapine‐treated patients with treatment duration and co‐prescription of other medications being weight gain moderators. The lack of major dose‐dependent patterns for weight gain emphasizes that olanzapine‐treated patients are at weight gain risk regardless of the dose.
Keywords: antipsychotic, metabolic syndrome, obesity, olanzapine, weight gain
1. INTRODUCTION AND BACKGROUND
Consistent epidemiological data suggest that people with severe mental illnesses (SMI), such as schizophrenia spectrum and bipolar affective disorders, are at increased risk of obesity and metabolic syndrome compared to the general population. 1 , 2 In turn, metabolic syndrome and its components including central obesity, high blood pressure, low high‐density lipoprotein (HDL) cholesterol, high total cholesterol, increased triglycerides and hyperglycaemia 3 may be associated with elevated risk of cardiovascular burden. 4 The increased prevalence of metabolic abnormalities in patients with SMI compared to the general population may have various causes including sedentary lifestyle, smoking, unhealthy diets and lack of physical exercise. 5 , 6 , 7
The role of antipsychotic treatment within the increased cardiovascular and metabolic burden in patients with SMIs has also received major attention in the past decades. 1 , 8 Specifically, patients treated with almost all antipsychotic agents demonstrated essential weight gain as well as elevated risk of metabolic syndrome compared to antipsychotic‐naïve patients with SMIs. 1 , 8 Unfortunately, available evidence on predictors or moderators of the risk of metabolic abnormalities within the context of antipsychotic treatment may present some inconsistencies, 1 , 9 although low baseline body mass index (BMI) and younger age have been invariably associated with greater antipsychotic‐induced weight gain. 10 Further, the risk of metabolic alterations seems to vary between different antipsychotic medications 11 ; for instance, patients treated with olanzapine and clozapine are at higher risk of weight gain and metabolic syndrome compared to other first‐ or second‐generation antipsychotics. 1
Given that patients treated with olanzapine are considered most prone to weight gain, 12 there has been an increasing body of literature suggesting several hypotheses for the implicated central and peripheral mechanisms. 13 , 14 On a pharmacodynamic level, the affinity of olanzapine for histaminic receptors may account for some of its effects on weight, 15 although later evidence suggested that serotonergic and dopaminergic neurotransmitter pathways might be also involved. 16 Moreover, preclinical models reported that olanzapine may centrally inhibit insulin secretion and thus glucose utilization, leading to perturbation of whole‐body insulin sensitivity. 14 , 17 Data have also emphasized on behavioural mechanisms such as appetite changes following olanzapine initiation. 18 , 19
Regarding potential predictors of olanzapine‐related weight gain, some evidence suggests that patients with larger early weight gain (EWG) (weight gain within 2 weeks after olanzapine initiation) may be at higher risk of olanzapine‐induced weight gain. 20 The predictive role of EWG changes for the long‐term weight gain was also reported in a 1‐year longitudinal study of inpatients. 21 In fact, weight gain may be more rapid in short‐ versus long‐term treatment with olanzapine. 22 Data concerning risk differentiation for olanzapine‐induced weight gain between women and men may present inconsistencies. 9 , 19 There is also contrasting evidence regarding the role of olanzapine dose on its effects on weight gain; for example, higher olanzapine daily doses resulted in larger weight gain on the long term in a retrospective cohort. 22 Additionally, a recent Bayesian meta‐analysis reported a dose‐dependent olanzapine risk of clinically relevant weight (CRW; ≥7% of baseline body weight) 23 ; however, this risk only applied for olanzapine cumulative doses equivalent to 10 g chlorpromazine (= 500 mg for olanzapine) assessing joint effects of dose and duration of treatment. On the other hand, in an older systematic review, authors reported that only two out of 11 studies suggested dose‐dependent risk of olanzapine‐associated weight gain 24 ; notably, most of the prescribed olanzapine doses in the first study reporting dose‐dependent patterns were below the recommended range.
Olanzapine is an efficacious antipsychotic agent with a positive impact on social functioning. 12 In line with its efficacy, olanzapine is among the most frequently prescribed antipsychotics, 25 so there is an urgent need to profoundly understand aspects of the consistently reported olanzapine‐induced weight gain and metabolic abnormalities. Apart from the well‐known metabolic effects, treatment with olanzapine has been also linked with less frequent side effects including prolactin elevation and more rarely parasomnias. 26 , 27 However, it is the major impact of the metabolic effects for olanzapine that does not only raise concerns for the physical health of the patients but also affects efficacy outcomes. 28 The aim of this study was to provide further insight on dose‐dependent effects for the well‐established effects of olanzapine on changes of weight as well as metabolic syndrome components in a naturalistic sample treated with long‐term olanzapine‐based therapeutic regimens.
2. MATERIALS AND METHODS
2.1. Study design
Data were acquired from a longitudinal cohort study running at the Department of Psychiatry of the University Hospital of Lausanne, in collaboration with a private mental healthcare centre (Les Toises; Lausanne). 29 Reporting adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Table S1). Ethical approval was warranted by the Ethics Committee of the Canton of Vaud (CER‐VD); informed consent was obtained for the inclusion of patients in the PsyMetab study, which allows the use of clinical data (for the present study, data from 21/11/2006 to 27/01/2020). In addition, the Ethics Committee of the Canton of Vaud (CER‐VD) granted access to clinical data of followed‐up patients in the Department of Psychiatry of the Lausanne University Hospital until the end of 2015 due to the non‐interventional post hoc analysis design (PsyClin; for the present study, data from 29/04/2007 to 31/12/2015). We included in‐ and outpatients treated with oral olanzapine having at least two available measurements of weight (including baseline measures). Patients were previously either drug naïve or had received antipsychotic treatments other than olanzapine. Patients were not subject to any specific lifestyle and/or pharmaceutical intervention to reduce cardiometabolic risk.
2.2. Measurements
Data on age, sex, height, olanzapine daily dose and date of first olanzapine intake were included in our analysis. Further, weight, waist circumference, systolic and diastolic blood pressure, fasting plasma glucose and lipids were measured at baseline and at least at one follow‐up time point. For metabolic syndrome, we used the criteria of the International Diabetes Federation (IDF), which include central obesity plus any two of the following metabolic abnormalities: hyperglycaemia (≥5.6 mmol/L), elevated blood pressure (systolic blood pressure ≥130 or diastolic blood pressure ≥85 mmHg), hypertriglyceridaemia (≥1.7 mmol/L) and low HDL cholesterol levels (<1.03 mmol/L in males and <1.29 mmol/L in females). 30 We also included data on psychiatric and somatic co‐medications (Table S2; list of co‐medications). Olanzapine plasma levels were quantified by ultra‐high‐performance liquid chromatography (Waters ACQUITY UPLC I‐Class) coupled to electrospray ionization‐tandem mass spectrometry (Waters Xevo TQ‐S). The method was validated according to international guidelines using a stable isotope‐labelled internal standard. 31
2.3. Statistical analysis
Low‐ versus high‐dose olanzapine‐treated patients were compared using an olanzapine dose cut‐off of 10 mg daily, which is the target dose for its licensed indications according to the package insert. 32 Comparisons of demographic and metabolic baseline variables were performed using the χ 2 test for categorical variables and the Wilcoxon test for continuous variables.
The effects of olanzapine daily dose on weight change was modelled using a linear mixed‐effects model, accounting for baseline BMI, age, sex, smoking status, co‐prescription of lipid‐lowering, hypertensive, antidiabetic, second or third antipsychotic agent, antidepressants, benzodiazepines, olanzapine treatment duration and setting of care (in‐ vs. outpatient). As for the majority of the patients, the treatment duration was shorter than 3 months, and we restricted our analysis to a time window of 3 months (90 days). Only patients with follow‐up data were included in our analysis. Weight changes were included in the model as the percentage of change from baseline value over time. Dose effects were also assessed in a categorical scale (high vs. low olanzapine dose; >10 mg/day vs. ≤10 mg/day). In a subsample of patients with available data, the analysis was replicated using olanzapine trough plasma concentrations instead of daily dose as a measure of drug exposition. We also assessed associations using olanzapine levels in a categorical scale based on the upper threshold of the therapeutic reference range of olanzapine (>80 ng/mL vs. ≤80 ng/mL). 33 Considering previous literature on the association between baseline BMI and weight gain, 34 , 35 we also tested the effects of interactions between baseline BMI and olanzapine dose on weight changes.
Linear mixed‐effects models with the same covariates were also used to assess dose effects on changes of monitored metabolic parameters including fasting plasma glucose, systolic and diastolic blood pressure, triglycerides, total, HDL and low‐density lipoprotein (LDL) cholesterol.
Additionally, we applied mixed‐effects logistic regression models, including the above‐mentioned covariates, to assess the risk of developing metabolic abnormalities such as CRW (≥7%), EWG (≥5% weight gain during first month), 36 obesity, dyslipidaemia and elevated blood pressure, elevated fasting plasma glucose and metabolic syndrome. All analyses were performed using the R environment for statistical computing Version 3.6.0.
3. RESULTS
A total of 392 patients were included with baseline characteristics provided in Table 1. The median olanzapine dose was 10 mg/day (interquartile range [IQR] = 5.0–10.0), with approximately one‐fourth of the cohort receiving daily doses higher than 10 mg per day. The percentages of men and smokers in the group of patients receiving olanzapine daily doses >10 mg/day was higher compared to patients receiving olanzapine daily doses ≤10 mg/day (p < 0.001 and 0.04, respectively).
TABLE 1.
Clinical and demographic parameters of the study sample based on olanzapine dose
| Total sample | Low‐dose group (≤10 mg/day) | High‐dose group (>10 mg/day) | p‐value | |
|---|---|---|---|---|
| N | 392 | 297 | 95 | |
| Age, median (IQR), years | 38.0 (26.0–53.3) | 38.0 (26.0–54.0) | 37.0 (27.0–51.0) | 0.64 |
| Men, n (%) | 208 (53.1) | 149 (50.2) | 59 (62.1) | 0.04 |
| Follow‐up duration, median (IQR), days | 40.0 (20.7–112.2) | 36.0 (18.0–101.0) | 47.0 (28.0–137.0) | 0.01 |
| Olanzapine dose, median (IQR), mg/d | 10.0 (5.0–10.0) | 10.0 (5.0–10.0) | 20.0 (15.0–20.0) | |
| Smoking, n (%) | 212 (54.1) | 143 (48.1) | 69 (72.6) | <0.001 |
| Main diagnosis (%) a | 0.80 | |||
| Schizophrenia spectrum disorders (F20–29) | 211 (53.8) | 151 (50.8) | 60 (63.2) | |
| Mood disorders (F30–39) | 104 (26.5) | 82 (27.6) | 22 (23.2) | |
| Other | 67 (17.1) | 56 (18.9) | 11 (11.6) | |
| Missing | 10 (2.6) | 8 (2.7) | 2 (2.1) | |
| Baseline metabolic parameters | ||||
| Weight, median (IQR) kg | ♂ 74.0 (65.0–82.0) | ♂ 72.4 (65.0–81.0) | ♂ 74.7 (69.7–83.7) | ♂ 0.04 |
| ♀ 59.0 (50.7–71.2) | ♀ 58.1 (50.7–70.2) | ♀ 61.5 (52.0–75.2) | ♀ 0.35 | |
| BMI, median (IQR) kg/m2 | n = 327, 22.7 (20.2–26.2) | n = 260, 22.6 (20.1–26.1) | n = 67, 22.9 (21.1–26.5) | 0.24 |
| Obesity (>30 kg/m2), n/total (%) | 36/327 (11.0) | 27/260 (10.4) | 9/67 (13.4) | 0.51 |
| Hypertension, n/total (%) | 34/70 (48.6) | 27/60 (45.0) | 7/10 (70.0) | 0.18 |
| Raised fasting plasma glucose, n/total (%) | 55/252 (21.8) | 40/197 (20.3) | 15/55 (27.3) | 0.27 |
| Fasting hypertriglyceridaemia, n/total (%) | 54/223 (24.2) | 43/173 (24.9) | 11/50 (22.0) | 0.85 |
| HDL hypocholesterolaemia, n/total (%) | 83/261 (31.8) | 61/197 (31.0) | 22/64 (34.4) | 0.64 |
| Total hypercholesterolaemia, n/total (%) | 114/264 (43.1) | 87/199 (43.7) | 27/65 (41.5) | 0.77 |
| Metabolic syndrome IDF, n/total (%) | 15/323 (4.6) | 10/256 (3.9) | 5/67 (7.5) | 0.21 |
| Co‐medication at baseline b | ||||
| Second antipsychotic | 74/386 (19.2) | 62/294 (21.1) | 12/92 (13.0) | 0.10 |
| Third antipsychotic | 32/386 (8.3) | 19/294 (6.5) | 13/92 (14.1) | 0.03 |
| Antidepressant | 65/392 (16.6) | 47/297 (15.8) | 18/95 (18.9) | 0.53 |
| Benzodiazepine | 288/392 (73.5) | 209/297 (70.4) | 79/95 (83.2) | 0.02 |
| Hypertensive drug | 32/392 (8.2) | 28/297 (9.4) | 4/95 (4.2) | 0.13 |
| Antidiabetic drug | 6/392 (1.5) | 5/297 (1.9) | 1/95 (1.0) | 1.0 |
| Lipid‐lowering drug | 16/392 (4.1) | 13/297 (4.4) | 3/95 (3.1) | 0.77 |
Note: Significant p‐values are indicated in bold.
Abbreviations: BMI, body mass index; HDL, high‐density lipoprotein; IDF, International Diabetes Federation; IQR, interquartile range.
Based on international Classification of Diseases (ICD‐10).
List of co‐medication detailed in Table S2.
There were no baseline differences for metabolic parameters between patients receiving high‐ versus low‐dose of olanzapine except for baseline weight among men (p = 0.04). Moreover, patients receiving high‐ versus low‐doses of olanzapine were more likely to be prescribed up to two more antipsychotics apart from olanzapine as well as benzodiazepines (p = 0.03 and 0.02, respectively).
After accounting for age, sex, smoking status, baseline BMI, co‐prescription of lipid‐lowering, hypertensive, antidiabetic, second or third antipsychotic agent, antidepressants, benzodiazepines and setting of care (in‐ vs. outpatient status), weight gain over treatment time was not associated with olanzapine daily dose (p = 0.62), although weight gain was larger in patients with olanzapine doses higher versus lower than or equal to 10 mg/day (weight gain percentage from baseline value over time: 2.54 ± 5.55 vs. 1.61 ± 4.51%, p = 0.01) (Figure 1 and Table 2). Treatment duration and co‐prescription of >2 antipsychotics, antidepressants, benzodiazepines and antihypertensive agents were positively associated with the increase in weight gain (p < 0.001, p < 0.001. p < 0.001, p < 0.001 and p = 0.01). Baseline BMI and prescription of lipid‐lowering agents were negatively associated with weight changes (p < 0.001 for both). There was also a negative interaction between baseline BMI and olanzapine daily dose (p < 0.001, data not shown). No significant associations were found between trough olanzapine plasma levels with weight change (n = 227, ES = 0.0, 95%CI = −0.01, –0.02, p = 0.67). However, using the upper threshold of the therapeutic reference range, olanzapine levels above 80 ng/mL were associated with increased weight gain (ES = 1.85, 95%CI = −0.45, –3.26, p = 0.01).
FIGURE 1.
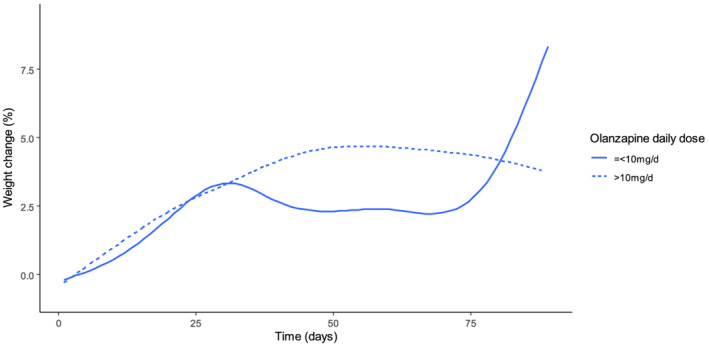
Weight change (%) over treatment duration in high‐ versus low‐dose olanzapine daily dose groups
TABLE 2.
Associations of olanzapine daily dose with weight changes as primary outcome
| 5 mg olanzapine daily dose increase | High dose of olanzapine a | ||||
|---|---|---|---|---|---|
| E (95% CI) | p value | E (95% CI) | p‐value | ||
| Olanzapine dose | 5 mg/day increase | 0.03 (−0.07 to 0.12) | 0.62 | ||
| High dose a | 0.31 (0.07 to 0.55) | 0.01 | |||
| Age | −0.06 (−0.28 to 0.17) | 0.63 | −0.06 (−0.29 to 0.17) | 0.63 | |
| Sex b | −0.23 (−1.04 to 0.58) | 0.58 | −0.24 (−1.05 to 0.57) | 0.56 | |
| Baseline BMI | −0.20 (−0.28 to −0.13) | <0.001 | −0.20 (−0.28 to −0.13) | <0.001 | |
| Smoking status c | 0.46 (−0.24 to 1.16) | 0.20 | 0.43 (−0.27 to 1.13) | 0.23 | |
| Olanzapine treatment duration | 1.98 (1.85 to 2.12) | <0.001 | 1.96 (1.82 to 2.09) | <0.001 | |
| In‐ vs. outpatient treatment | 0.10 (−0.42 to 0.62) | 0.71 | 0.11 (−0.41 to 0.63) | 0.68 | |
| Co‐medication with | |||||
| Lipid‐lowering agent | −1.59 (−2.32 to −0.87) | <0.001 | −1.57 (−2.30 to −0.85) | <0.001 | |
| Hypertensive agent | 0.76 (0.18 to 1.34) | 0.01 | 0.77 (0.19 to 1.36) | 0.01 | |
| Antidiabetic agent | 0.11 (−1.37 to 1.58) | 0.89 | 0.10 (1.37 to 1.57) | 0.89 | |
| Second antipsychotic | −0.31 (−0.64 to 0.03) | 0.076 | −0.29 (−0.63 to 0.05) | 0.09 | |
| Third antipsychotic | 1.61 (1.14 to 2.08) | <0.001 | 1.62 (1.16 to 2.09) | <0.001 | |
| Antidepressant | 0.78 (0.37 to 1.18) | <0.001 | 0.77 (0.37 to 1.18) | <0.001 | |
| Benzodiazepine | 0.68 (0.39 to 0.98) | <0.001 | 0.68 (0.38 to 0.97) | <0.001 | |
Note: Significant p‐values are indicated in bold.
Abbreviations: BMI, body mass index (kg/m2); CI, confidence interval; E, effect size.
High dose defined as >10 mg/day. A 5‐mg increase of olanzapine dose would correspond to a decreased 0.2 kg/m2 of baseline BMI, increased 2 days of olanzapine treatment duration, decreased 1.59‐ and 0.31‐fold co‐prescription rates of lipid‐lowering agents and second antipsychotic and increased 0.76‐, 1.61‐, 0.78‐ and 0.68‐fold co‐prescription rates of hypertensive, third antipsychotic, antidepressants and benzodiazepines, respectively.
Males using females as reference.
Smokers using non‐smokers as reference.
Table 3 summarizes the associations between olanzapine daily dose and the evolution of monitored metabolic parameters. Briefly, higher olanzapine doses were associated with decreases in total and HDL cholesterol and systolic and diastolic blood pressure (p = 0.03, p = 0.01, p = 0.03 and p = 0.01) and with increases in fasting glucose (p = 0.01). Among covariates, the co‐prescription of a second antipsychotic was associated with decrease for triglyceride and systolic and diastolic blood pressure (p = 0.03, p = 0.02 and p < 0.001; Tables S6, S8 and S9). The co‐prescription of a third antipsychotic was associated with lower total cholesterol levels (p = 0.02; Table S3). Co‐prescription of benzodiazepines was associated with increases of LDL cholesterol and triglyceride levels (p = 0.01 and p = 0.03; Tables S4 and S6). The co‐prescription of a hypertensive agent was negatively associated with increases of triglycerides (p = 0.01; Table S6). Smoking was negatively associated with glucose elevations, total, HDL, LDL cholesterol and triglyceride levels (p < 0.001 in all cases; Tables S3–S7). When estimating the effect of a dose lower or higher than 10 mg per day, no associations were found with any metabolic parameters (p > 0.05) except for increase in fasting glucose and decrease in HDL cholesterol in patients with olanzapine doses ≤10 mg/day (p < 0.001 and 0.01; Table 3).
TABLE 3.
Associations of olanzapine daily dose with metabolic parameters
| Metabolic parameter change % | n | 5 mg olanzapine daily dose increase | High dose of olanzapine a | ||
|---|---|---|---|---|---|
| E (95% CI) | p‐value | E (95% CI) | p‐value | ||
| Systolic blood pressure (mmHg) | 55 | −1.1 (−2.0 to −0.01) | 0.03 | 0.1 (−1.9 to 2.1) | 0.92 |
| Diastolic blood pressure (mmHg) | 55 | −1.8 (−3.0 to −0.7) | 0.01 | −1.7 (−4.1 to 0.6) | 0.16 |
| Fasting plasma glucose (mmol/L) | 200 | 0.4 (0.1 to 0.6) | 0.01 | 1.8 (1.3 to 2.3) | <0.001 |
| Triglycerides (mmol/L) | 182 | 0.2 (−0.2 to 0.6) | 0.25 | 0.0 (−0.8 to 0.8) | 0.95 |
| Total cholesterol (mmol/L) | 209 | −0.1 (−0.2 to −0.01) | 0.03 | −0.1 (−0.3 to 0.1) | 0.33 |
| LDL cholesterol (mmol/L) | 175 | −0.1 (−0.4 to 0.2) | 0.50 | 0.3 (−0.5 to 1.0) | 0.49 |
| HDL cholesterol (mmol/L) | 205 | −0.2 (−0.3 to 0.0) | 0.01 | −0.4 (−0.6 to −0.1) | 0.01 |
Note: Significant p‐values are indicated in bold.
Abbreviations: CI, confidence interval; E, effect size; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
High‐dose defined as >10 mg/day. A 5‐mg increase of olanzapine doses would correspond to a decreased 1.1 and 1.8 mmHg of systolic and diastolic blood pressure, to an increased 0.4 mmol/L of fasting glucose and to a decreased 0.1 and 0.2 mmol/L of total and HDL cholesterol, respectively.
As shown in Table 4, the odds of experiencing CRW (≥7%) were not increased with a higher dose of olanzapine, although a trend was observed (OR = 1.55, 95%CI = 0.93–2.59, p = 0.09). Odds of experiencing EWG were increased for higher olanzapine doses (OR = 12.77, 95%CI = 3.78–43.07, p < 0.001) as well as in patients receiving high versus low olanzapine daily doses (OR = 2.15, 95%CI = 1.57–2.97, p < 0.001). Regarding the onset of metabolic traits, no differences between patients receiving high and low daily doses of olanzapine were reported. We could not model the odds ratios of new‐onset elevated fasting glucose, hypertriglyceridaemia and metabolic syndrome in our sample because of the small number of new‐onset cases.
TABLE 4.
Odds ratios (OR) of clinically relevant weight gain (≥7%), early weight change (≥5% within 30 days) and new onset of other metabolic abnormalities in function of olanzapine doses
| Metabolic disturbance onset | n | Associations with olanzapine daily dose, OR (95% CI) | Associations with high daily dose of olanzapine (>10 mg/day), OR (95% CI) |
|---|---|---|---|
| CRW | 377 | 2.21 (0.35–14.1) | 1.55 (0.93–2.59) |
| EWC | 377 | 12.7 (3.78–43.1) * | 2.15 (1.57–2.94) * |
| Obesity (>30 kg/m2) | 283 | 1.38 (0.01–152) | 2.37 (0.81–6.89) |
| Elevated fasting plasma glucose (≥5.6 mmol/L) | NC | NC | |
| Hypertriglyceridaemia (>1.7 mmol/L) | NC | NC | |
| Total hypercholesterolaemia (≥5 mmol/L) | 149 | 0.00 (0.00–574) | 1.06 (0.05–21.5) |
| HDL hypocholesterolaemia (≥1.29 mmol/L in women/≥1.03 mmol/L in men) | 170 | 4.81 (0.00–42 857) | 0.53 (0.06–4.80) |
| Hypertension (SBP ≥ 85mmgHg & DBP ≥ 130 mmHg) | 38 | 0.53 (0.00–59.2) | 0.68 (0.15–3.02) |
| Metabolic Syndrome | NC | NC |
Abbreviations: CI, confidence interval; CRW, clinically relevant weight gain (≥7%); DBP, diastolic blood pressure; EWC, early weight change (≥5% within 30 days); HDL, high‐density lipoprotein; NC, not calculated; OR, odds ratio; SBP, systolic blood pressure.
p < 0.001.
4. DISCUSSION
In light of long‐term efficacy and tolerability of antipsychotic treatment, there is a vivid ongoing debate regarding the prescription of reduced versus standard antipsychotic doses in maintenance treatment. 37 , 38 , 39 Our analysis of 392 patients in a follow‐up period of 3 months after olanzapine initiation suggests minor differences between metabolic effects of low versus high daily doses of olanzapine with few exceptions of metabolic alterations. The size of our sample and the longitudinal design support the generalizability of our findings. In our cohort, weight gain was larger in patients receiving more than 10 mg/day olanzapine compared to patients receiving ≤10 mg/day, although no association between weight changes and olanzapine dose as continuous variable was observed. This contrasting finding may be driven by the twofold higher risk to develop EWG in patients receiving >10 mg/day compared to patients receiving olanzapine doses ≤10 mg/day. Thus, we may hypothesize a different temporal course of weight changes following olanzapine initiation for low‐ versus high‐dose olanzapine treatment, although the risk of CRW may ultimately not be dose dependent on the long term. In other words, despite early differences, olanzapine‐associated weight gain may not be moderated by the dose on the long term. This lack of dose‐dependent pattern may also reflect the lack of dose‐dependent olanzapine effects on gut microbial composition, 40 which seems to be part of the pathway underlying olanzapine‐associated metabolic alterations. 41
Previous data on dose‐dependent effects for olanzapine‐associated weight gain and metabolic abnormalities present severe discrepancies, mainly due to methodological heterogeneity. In two samples of schizophrenia patients followed up for 6 weeks, no dose‐dependent patterns regarding olanzapine‐related weight gain were observed. 42 In alignment with these results, a study of 573 schizophrenia patients reported no effects for olanzapine dose on weight gain over 39 weeks. 35 However, in a larger retrospective cohort (n = 38 865) with various psychiatric diagnoses, patients receiving more than 5 mg/day gained 3.2 kg after 6 weeks compared to 1.9 kg in patients receiving ≤5 mg/day of olanzapine. 22 Likewise, in patients with borderline personality disorder, patients with olanzapine 2.5 mg/day had a weight gain of 2.09 kg versus 3.17 kg in patients with 5–10 mg/day over 12 weeks. 43 Nevertheless, since doses lower than 5 mg/day do not reflect approved doses for schizophrenia or mania, this risk stratification for weight gain may be less useful. This strong limitation had been highlighted by a review of earlier research of antipsychotic‐associated weight gain suggesting no dose‐dependent pattern for olanzapine‐related weight gain in a total of nine studies with the exception of two studies, one of which investigated olanzapine doses below the therapeutic reference range. 24
On the other hand, in a cohort of patients with psychotic depression, authors reported a positive association between olanzapine cumulative dose and weight gain for a minimum of 4 weeks of olanzapine treatment. 44 In fact, previous works have highlighted different risk profiles of olanzapine for patients with different diagnoses; for example, weight gain is considered more prominent in patients with schizophrenia spectrum disorders compared to patients with affective disorders, 45 suggesting underlying mechanisms included differences in incidence of related lifestyle risk factors. 45
Further, we reported a negative interaction effect of baseline BMI with olanzapine dose on weight change (Table 2), meaning that when baseline BMI is lower, the effect of an increase of olanzapine dose has a greater impact on weight. Previous literature has consistently reported a negative association between baseline BMI and weight gain 34 , 35 ; in other words, patients with lower baseline BMI or weight values may demonstrate larger weight gain. Nevertheless, the role of interplay between baseline BMI and olanzapine dose has received disproportionately less attention. Our results suggest that larger weight gain in patients with lower baseline BMI may be mediated at least partially by higher daily doses.
When modelling effects on weight gain, co‐prescription of >2 antipsychotics, antidepressants and benzodiazepines was positively associated with the increase in weight gain. Previous pharmacoepidemiological data suggested an association between polypharmacy and increased risk of obesity in the youth. 46 As previous works suggested, 46 it is particularly difficult to unravel olanzapine effects from potential synergies between olanzapine and other psychotropic agents on weight gain. It is also impossible to exclude the possibility of some confounding effects of the potentially more severe clinical picture in patients receiving polypharmacy versus monotherapy.
In line with previous literature on the association between olanzapine levels and weight gain, we did not report an association between olanzapine levels and weight gain. 47 , 48 , 49 Nevertheless, we detected an association between weight gain and olanzapine levels >80 ng/ml, which is the upper threshold of the therapeutic reference range. 33 It is difficult to compare this association with previous findings due to differences in follow‐up duration or sample of patients. For example, a previous study of first‐episode olanzapine‐treated patients suggested a threshold of 23.28 ng/ml for CRW (>7%) after 2 months of olanzapine treatment. 50 A potential explanation for the gap between this threshold and the threshold adopted by us (80 ng/ml) may be the fact that our patients were not first‐episode patients and thus not antipsychotic exposure‐free. Additionally, a study of chronic patients titrated up to 20 mg/day olanzapine reported a threshold for dose‐weighted plasma levels of 20.6 ng/ml for CRW after 6 weeks, 51 which is a shorter follow‐up window compared to ours.
Concerning changes for other metabolic traits, lower olanzapine doses were associated with increases in total and HDL cholesterol and blood pressure. These counter‐intuitive findings might be understood in light of the role of potential confounders; for example, increase of total cholesterol was also observed for longer treatment duration and in younger patients as well as non‐smokers. This association may imply that patients potentially receiving olanzapine for longer time may have been more exposed to increase of total cholesterol. In younger patients, cumulative exposure to olanzapine may have been smaller compared to older patients. In general, the time window following first antipsychotic exposure is considered as a critical period where metabolic changes are most prominent. 52 We also reported HDL cholesterol increases in patients treated with lower doses of olanzapine; when modelling changes in HDL cholesterol, smoking also had a negative association, but not age. Smoking contributes directly to HDL cholesterol decrease. 53 Further, low‐dose‐treated patients might have been patients with shorter illness duration or less likely to be treatment resistant. Thus, they might have previously been more exposed to smaller metabolic burden explaining higher total and HDL cholesterol levels as well as blood pressure as compared to high‐dose‐treated patients. The association between lower blood pressure and higher olanzapine doses may be understood in light of the olanzapine‐related hypotension in schizophrenia patients with new or recent onset. 54
There was also a clear association between fasting plasma glucose and olanzapine dose. A previous work suggested slightly higher odds ratio of elevated haemoglobin A1C (HbA1c) in patients receiving ≥10 versus <10 mg/day. 55 Nevertheless, an assessment of 25 patients with schizophrenia suggested no olanzapine daily dose effects on oral glucose tolerance test (OGTT) performance 56 ; however, the contrast between our findings and the results of this, potentially underpowered, study may be explained by the cross‐sectional design, 15 which included only non‐diabetic patients. Additionally, OGTT is a more sensitive screening tool for dysglycaemic status than single measures such as fasting glucose or HbA1c. 57
The main limitation of our exploratory analysis refers to the assessment of associations, which, however, cannot be used as to infer causality. Moreover, the use of more sensitive screening tools for metabolic parameters in larger samples of olanzapine‐treated patients may have provided further insight. Further, using 10 mg daily olanzapine dose was clinically driven, but it led to two comparison groups that were not equally powered. Some bias might have been introduced by the non‐randomized design of the study with the choice of olanzapine dose being made by the clinicians, who might have considered metabolic alterations during the dose selection. Last, the data were not primarily collected for this study, which may have impacted the results.
5. CONCLUSION
In our large sample, the lack of major dose‐dependent patterns for weight gain emphasizes that all olanzapine‐treated patients are at risk of weight gain regardless of the dose. Even doses below the minimal effective dose may not result in reduced potential harm on metabolic parameters. Metabolic monitoring should be integral part of olanzapine‐based treatment regardless of the olanzapine dose, the setting of care or treatment duration.
CONFLICT OF INTEREST
The funding sources had no role in the writing of the manuscript or in the decision to submit it for publication. Dr Eap received honoraria for conferences or teaching CME courses from Janssen‐Cilag, Lundbeck, Otsuka, Sandoz, Servier, Sunovion, Vifor‐Pharma and Zeller in the past 3 years. Dr Crettol received honoraria for conferences or teaching CME courses from Forum für Medizinische Fortbildung. Dr Schoretsanitis has served as a consultant for HLS Therapeutics. Dr Seifritz has received educational grants, consulting fees and lecture honoraria from Janssen‐Cilag, Lundbeck, Angelini, Otsuka, Servier, Ricordati, Vifor, Sunovion, Schwabe and Mepha. Dr Vandenberghe received honoraria for conferences or teaching CME courses from Forum für Medizinische Fortbildung and Sysmex Suisse AG. Dr von Gunten received honoraria for a conference or workshop participation from Vifor and Schwabe in the previous 3 years. All other authors report no conflicts of interest.
ETHICS STATEMENT
Ethical approval was warranted by the Ethics Committee of the Canton of Vaud (CER‐VD); informed consent was obtained for the inclusion of patients in the PsyMetab study, which allows the use of clinical data (for the present study, data from 21/11/2006 to 27/01/2020). The Ethics Committee of the Canton of Vaud (CER‐VD) also granted access to clinical data of followed‐up patients in the Department of Psychiatry of the Lausanne University Hospital until the end of 2015 due to the non‐interventional post hoc analysis design (PsyClin; for the present study, data from 29/04/2007 to 31/12/2015).
Supporting information
Table S1. STROBE Statement—checklist of items that should be included in reports of observational studies
Table S2. List of psychotropic medications and treatments for cardiometabolic diseases identified in the PsyMetab and PsyClin cohort
Table S3. Associations of olanzapine daily dose with total cholesterol levels
Table S4. Associations of olanzapine daily dose with LDL cholesterol levels
Table S5. Associations of olanzapine daily dose with HDL cholesterol levels
Table S6. Associations of olanzapine daily dose with triglyceride levels
Table S7. Associations of olanzapine daily dose with fasting glucose levels
Table S8. Associations of olanzapine daily dose with systolic blood pressure
Table S9. Associations of olanzapine daily dose with diastolic blood pressure
ACKNOWLEDGEMENTS
The authors thank the staff at the University Hospital of Lausanne and at private mental healthcare centre Les Toises who participated in the metabolic monitoring program and all the participants in PsyMetab and PsyClin. This work has been funded in part by the Swiss National Research Foundation (CE and PC: 320030‐120686, 324730‐144064 and 320030‐173211; CBE, PC and KJP: 320030_200602). Open Access Funding provided by Universitat Zurich.
Schoretsanitis G, Dubath C, Grosu C, et al. Olanzapine‐associated dose‐dependent alterations for weight and metabolic parameters in a prospective cohort. Basic Clin Pharmacol Toxicol. 2022;130(4):531-541. doi: 10.1111/bcpt.13715
Funding information Swiss National Research Foundation, Grant/Award Numbers: 320030_200602, 320030‐173211, 324730‐144064, 320030‐120686
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry. 2015;14(3):339‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fountoulakis KN, Siamouli M, Panagiotidis P, et al. Obesity and smoking in patients with schizophrenia and normal controls: a case‐control study. Psychiatry Res. 2010;176(1):13‐16. [DOI] [PubMed] [Google Scholar]
- 3. Diabetes Canada Clinical Practice Guidelines Expert C , Punthakee Z, Goldenberg R, Definition KP. Classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10‐S15. [DOI] [PubMed] [Google Scholar]
- 4. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J am Coll Cardiol. 2010;56(14):1113‐1132. [DOI] [PubMed] [Google Scholar]
- 5. de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ. Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital. Schizophr Res. 2002;56(1–2):55‐65. [DOI] [PubMed] [Google Scholar]
- 6. McCreadie RG, Scottish Schizophrenia Lifestyle G . Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534‐539. [DOI] [PubMed] [Google Scholar]
- 7. Roick C, Fritz‐Wieacker A, Matschinger H, et al. Health habits of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2007;42(4):268‐276. [DOI] [PubMed] [Google Scholar]
- 8. Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta‐analysis. PLoS ONE. 2014;9(4):e94112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schoretsanitis G, Drukker M, Van Os J, Schruers KRJ, Bak M. No differences in olanzapine‐ and risperidone‐related weight gain between women and men: a meta‐analysis of short‐ and middle‐term treatment. Acta Psychiatr Scand. 2018;138(2):110‐122. [DOI] [PubMed] [Google Scholar]
- 10. de Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114‐126. [DOI] [PubMed] [Google Scholar]
- 11. Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU. Long‐term effectiveness of oral second‐generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta‐analysis of direct head‐to‐head comparisons. World Psychiatry. 2019;18(2):208‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huhn M, Nikolakopoulou A, Schneider‐Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi‐episode schizophrenia: a systematic review and network meta‐analysis. Lancet. 2019;394(10202):939‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shamshoum H, Medak KD, Wright DC. Peripheral mechanisms of acute olanzapine induced metabolic dysfunction: a review of in vivo models and treatment approaches. Behav Brain Res. 2021;400:113049 [DOI] [PubMed] [Google Scholar]
- 14. Kowalchuk C, Castellani L, Kanagsundaram P, et al. Olanzapine‐induced insulin resistance may occur via attenuation of central KATP channel‐activation. Schizophr Res. 2021;228:112‐117. [DOI] [PubMed] [Google Scholar]
- 15. He M, Deng C, Huang XF. The role of hypothalamic H1 receptor antagonism in antipsychotic‐induced weight gain. CNS Drugs. 2013;27(6):423‐434. [DOI] [PubMed] [Google Scholar]
- 16. Weston‐Green K, Huang XF, Deng C. Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine‐induced weight gain. PLoS ONE. 2012;7(3):e33548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ninagawa S, Tada S, Okumura M, et al. Antipsychotic olanzapine‐induced misfolding of proinsulin in the endoplasmic reticulum accounts for atypical development of diabetes. Elife. 2020;9:e60970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang J, Hei GR, Yang Y, et al. Increased appetite plays a key role in olanzapine‐induced weight gain in first‐episode schizophrenia patients. Front Pharmacol. 2020;11:739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choong E, Bondolfi G, Etter M, et al. Psychotropic drug‐induced weight gain and other metabolic complications in a Swiss psychiatric population. J Psychiatr Res. 2012;46(4):540‐548. [DOI] [PubMed] [Google Scholar]
- 20. Lin CH, Lin SC, Huang YH, Wang FC, Huang CJ. Early prediction of olanzapine‐induced weight gain for schizophrenia patients. Psychiatry Res. 2018;263:207‐211. [DOI] [PubMed] [Google Scholar]
- 21. Vandenberghe F, Gholam‐Rezaee M, Saigi‐Morgui N, et al. Importance of early weight changes to predict long‐term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76(11):e1417‐e1423. [DOI] [PubMed] [Google Scholar]
- 22. Bazo‐Alvarez JC, Morris TP, Carpenter JR, Hayes JF, Petersen I. Effects of long‐term antipsychotics treatment on body weight: a population‐based cohort study. J Psychopharmacol. 2020;34(1):79‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spertus J, Horvitz‐Lennon M, Abing H, Normand SL. Risk of weight gain for specific antipsychotic drugs: a meta‐analysis. NPJ Schizophr. 2018;4(1):12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simon V, van Winkel R, de Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041‐1050. [DOI] [PubMed] [Google Scholar]
- 25. Claassen JN, Park JS. Examining the dispensing patterns of antipsychotics in Australia from 2006 to 2018 ‐ a pharmacoepidemiology study. Res Social Adm Pharm. 2021;17(6):1159‐1165. [DOI] [PubMed] [Google Scholar]
- 26. Hoekstra S, Bartz‐Johannessen C, Sinkeviciute I, et al. Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: results from the BeSt InTro study. NPJ Schizophr. 2021;7(1):39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Filippis R, Guinart D, Rania M, Carbone EA, Gaetano R, Segura‐Garcia C. Olanzapine‐related somnambulism: a systematic review of literature and a case report of anorexia nervosa. J Clin Psychopharmacol. 2021;41(6):658‐666. [DOI] [PubMed] [Google Scholar]
- 28. Bellavia A, Centorrino F, Jackson JW, Fitzmaurice G, Valeri L. The role of weight gain in explaining the effects of antipsychotic drugs on positive and negative symptoms: an analysis of the CATIE schizophrenia trial. Schizophr Res. 2019;206:96‐102. [DOI] [PubMed] [Google Scholar]
- 29. Dubath C, Delacretaz A, Glatard A, et al. Evaluation of cardiometabolic risk in a large psychiatric cohort and comparison with a population‐based sample in Switzerland. J Clin Psychiatry. 2020;81(3):2272 [DOI] [PubMed] [Google Scholar]
- 30. International Diabetes Federation (IDF) . IDF consensus worldwide definition of the metabolic syndrome. Brussels, Belgium: International Diabetes Federation29.07.2020; 2006.
- 31. Ansermot N, Brawand‐Amey M, Kottelat A, Eap CB. Fast quantification of ten psychotropic drugs and metabolites in human plasma by ultra‐high performance liquid chromatography tandem mass spectrometry for therapeutic drug monitoring. J Chromatogr a. 2013;1292:160‐172. [DOI] [PubMed] [Google Scholar]
- 32. Lilly E. ZYPREXA (olanzapine) tablet for oral use. Indianapolis, USA. 2009.
- 33. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–02):9‐62. [DOI] [PubMed] [Google Scholar]
- 34. Gentile S. Contributing factors to weight gain during long‐term treatment with second‐generation antipsychotics. A systematic appraisal and clinical implications. Obes Rev. 2009;10(5):527‐542. [DOI] [PubMed] [Google Scholar]
- 35. Kinon BJ, Basson BR, Gilmore JA, Tollefson GD. Long‐term olanzapine treatment: weight change and weight‐related health factors in schizophrenia. J Clin Psychiatry. 2001;62(2):92‐100. [PubMed] [Google Scholar]
- 36. Dubath C, Piras M, Gholam M, et al. Effect of quetiapine, from low to high dose, on weight and metabolic traits: results from a prospective cohort study. Pharmacopsychiatry. 2021;54(6):279‐286. [DOI] [PubMed] [Google Scholar]
- 37. Dellva MA, Tran P, Tollefson GD, Wentley AL, Beasley CM Jr. Standard olanzapine versus placebo and ineffective‐dose olanzapine in the maintenance treatment of schizophrenia. Psychiatr Serv. 1997;48(12):1571‐1577. [DOI] [PubMed] [Google Scholar]
- 38. Hojlund M, Kemp AF, Haddad PM, Neill JC, Correll CU. Standard versus reduced dose of antipsychotics for relapse prevention in multi‐episode schizophrenia: a systematic review and meta‐analysis of randomised controlled trials. Lancet Psychiatry. 2021;8(6):471‐486. [DOI] [PubMed] [Google Scholar]
- 39. Zhou Y, Li G, Li D, Cui H, Ning Y. Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: a single‐blinded, 52‐week, randomized controlled study. J Psychopharmacol. 2018;32(5):524‐532. [DOI] [PubMed] [Google Scholar]
- 40. Pelka‐Wysiecka J, Kaczmarczyk M, Baba‐Kubis A, et al. Analysis of gut microbiota and their metabolic potential in patients with schizophrenia treated with olanzapine: results from a six‐week observational prospective cohort study. J Clin Med. 2019;8(10):1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang Y, Long Y, Kang D, et al. Effect of Bifidobacterium on olanzapine‐induced body weight and appetite changes in patients with psychosis. Psychopharmacology (Berl). 2021;238(9):2449‐2457. [DOI] [PubMed] [Google Scholar]
- 42. Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J Clin Psychiatry. 2001;62(4):231‐238. [DOI] [PubMed] [Google Scholar]
- 43. Zanarini MC, Schulz SC, Detke HC, et al. A dose comparison of olanzapine for the treatment of borderline personality disorder: a 12‐week randomized, double‐blind, placebo‐controlled study. J Clin Psychiatry. 2011;72(10):1353‐1362. [DOI] [PubMed] [Google Scholar]
- 44. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130‐137. [DOI] [PubMed] [Google Scholar]
- 45. Moteshafi H, Zhornitsky S, Brunelle S, Stip E. Comparing tolerability of olanzapine in schizophrenia and affective disorders: a meta‐analysis. Drug Saf. 2012;35(10):819‐836. [DOI] [PubMed] [Google Scholar]
- 46. Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2011;21(6):517‐535. [DOI] [PubMed] [Google Scholar]
- 47. Lu ML, Chen CH, Kuo PT, Lin CH, Wu TH. Application of plasma levels of olanzapine and N‐desmethyl‐olanzapine to monitor metabolic parameters in patients with schizophrenia. Schizophr Res. 2018;193:139‐145. [DOI] [PubMed] [Google Scholar]
- 48. Kelly DL, Richardson CM, Yu Y, Conley RR. Plasma concentrations of high‐dose olanzapine in a double‐blind crossover study. Hum Psychopharmacol. 2006;21(6):393‐398. [DOI] [PubMed] [Google Scholar]
- 49. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278‐283. [DOI] [PubMed] [Google Scholar]
- 50. Arnaiz JA, Rodrigues‐Silva C, Mezquida G, et al. The usefulness of olanzapine plasma concentrations in monitoring treatment efficacy and metabolic disturbances in first‐episode psychosis. Psychopharmacology (Berl). 2021;238(3):665‐676. [DOI] [PubMed] [Google Scholar]
- 51. Perry PJ, Argo TR, Carnahan RM, et al. The association of weight gain and olanzapine plasma concentrations. J Clin Psychopharmacol. 2005;25(3):250‐254. [DOI] [PubMed] [Google Scholar]
- 52. Perez‐Iglesias R, Martinez‐Garcia O, Pardo‐Garcia G, et al. Course of weight gain and metabolic abnormalities in first treated episode of psychosis: the first year is a critical period for development of cardiovascular risk factors. Int J Neuropsychopharmacol. 2014;17(1):41‐51. [DOI] [PubMed] [Google Scholar]
- 53. He BM, Zhao SP, Peng ZY. Effects of cigarette smoking on HDL quantity and function: implications for atherosclerosis. J Cell Biochem. 2013;114(11):2431‐2436. [DOI] [PubMed] [Google Scholar]
- 54. Choure BK, Gosavi D, Nanotkar S. Comparative cardiovascular safety of risperidone and olanzapine, based on electrocardiographic parameters and blood pressure: a prospective open label observational study. Indian J Pharm. 2014;46(5):493‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guo Z, L'Italien GJ, Jing Y, et al. A real‐world data analysis of dose effect of second‐generation antipsychotic therapy on hemoglobin A1C level. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1326‐1332. [DOI] [PubMed] [Google Scholar]
- 56. Guina J, Roy S, Gupta A, Langleben DD, Elman I. Oral glucose tolerance test performance in olanzapine‐treated schizophrenia‐spectrum patients is predicted by BMI and triglycerides but not olanzapine dose or duration. Hum Psychopharmacol. 2017;32(4):e2604 [DOI] [PubMed] [Google Scholar]
- 57. Thewjitcharoen Y, Jones Elizabeth A, Butadej S, et al. Performance of HbA1c versus oral glucose tolerance test (OGTT) as a screening tool to diagnose dysglycemic status in high‐risk Thai patients. BMC Endocr Disord. 2019;19(1):23 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. STROBE Statement—checklist of items that should be included in reports of observational studies
Table S2. List of psychotropic medications and treatments for cardiometabolic diseases identified in the PsyMetab and PsyClin cohort
Table S3. Associations of olanzapine daily dose with total cholesterol levels
Table S4. Associations of olanzapine daily dose with LDL cholesterol levels
Table S5. Associations of olanzapine daily dose with HDL cholesterol levels
Table S6. Associations of olanzapine daily dose with triglyceride levels
Table S7. Associations of olanzapine daily dose with fasting glucose levels
Table S8. Associations of olanzapine daily dose with systolic blood pressure
Table S9. Associations of olanzapine daily dose with diastolic blood pressure
Data Availability Statement
Data are available upon reasonable request.


