Abstract
Aims
We aimed to summarize existing evidence from published randomized trials that assessed atrial fibrillation (AF) screening for stroke prevention.
Methods and results
We searched MEDLINE for randomized trials that enrolled patients without known AF, screened for AF using electrocardiogram-based methods, and reported stroke outcomes. For this analysis, we excluded studies that focused on post-stroke populations. We combined data using a random-effects model and performed trial sequential meta-analysis using an O’Brien-Fleming alpha-spending function.
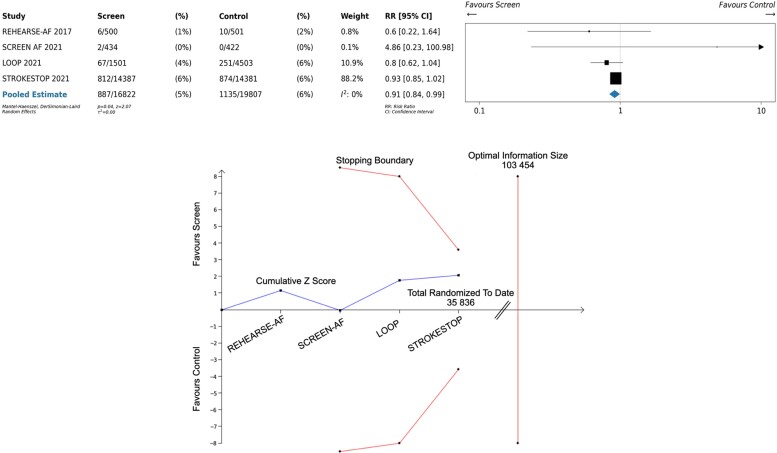
We identified four randomized clinical trials with a total of 35 836 participants. The populations, screening intervention, and definition of stroke varied markedly. As compared with no screening, AF screening was associated with a reduction in stroke (relative risk 0.91; 95% confidence interval: 0.84–0.99]. Trial sequential meta-analysis found that the cumulative z-score did not cross the stopping boundary.
After polling members of the AF-SCREEN and AFFECT-EU consortia, we identified a further 12 trials that are complete but have not yet reported stroke outcomes or are ongoing and expected to collect stroke outcomes. These consortia are planning an individual participant data meta-analysis which will permit the exploration of methodological heterogeneity.
Conclusions
If and how to screen for AF is an important public health concern. The body of evidence published to date suggests that AF could be effective to prevent strokes in some settings. The AF-SCREEN/AFFECT-EU individual patient data meta-analysis aims to comprehensively assess the benefits and risks of AF screening, and determine how population, screening method, and health-system factors influence stroke prevention.
Keywords: Atrial fibrillation, Screening, Stroke, Meta-analysis
Graphical Abstract
Graphical Abstract.
Atrial fibrillation (AF) is a major cause of disabling stroke worldwide. Once AF is identified, stroke risk can be substantially reduced with oral anticoagulation (OAC). Many medical and consumer-facing technologies can now detect AF, and there is widespread interest in screening for AF, as a means of preventing stroke.1 However, advisory panels, like the United States Preventative Services Task Force, have concluded that there is currently insufficient evidence to endorse AF screening.2 We undertook a focused review to summarize existing evidence from published randomized controlled trials (RCTs) that assessed AF screening for stroke prevention.
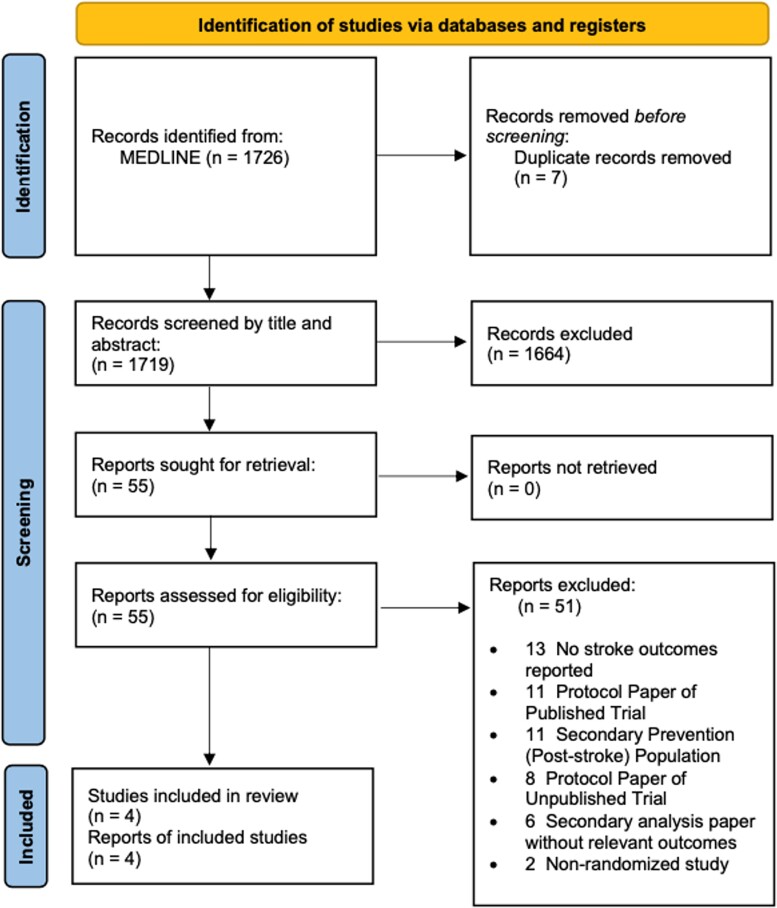
We searched MEDLINE for randomized trials that enrolled patients without known AF, screened for AF using electrocardiogram (ECG)-based methods, and reported stroke outcomes. We excluded studies that focused on post-stroke populations. We identified four randomized clinical trials with a total of 35 836 participants (Table 1 and Figure 1).3–6 Figure 2’s lower panel shows the results of a random-effects meta-analysis of stroke outcomes, following the intention-to-treat principle. While the point estimate [relative risk 0.91; 95% confidence interval (CI): 0.84–0.99] is modestly in favour of AF screening, published trials are heterogenous in their populations, their definition of stroke (Figure 2 footnote), and their screening methodology (from single time-point ECG to years of invasive monitoring).
Table 1.
Ongoing and completed randomized trials assessing atrial fibrillation screening
| Study | Number randomized | Screening intervention | Population |
|---|---|---|---|
| Studies cited in the current report | |||
| LOOP3 | 6004 | Implanted monitor | Age ≥70 with risk factors, Denmark |
| REHEARSE-AF4 | 1001 | Hand-held ECG, BID for 1 year | Age 65 + risk factors, UK/Wales |
| SCREEN-AF5 | 822 | 14-day ECG Patch, Twice | Age ≥75 with hypertension, Canada/Germany |
| STROKESTOP6 | 28 768 | Hand-held ECG, BID for 14 days | Age 75 and 76, Sweden |
| Completed trials without published stroke outcomes and/or conducted in post-stroke population | |||
| Find-AF NCT01855035 | 398 | 10-day Holter, 0, 3, and 6 months | Post-stroke, Germany |
| MonDAFIS NCT02204267 | 3470 | 7-day Holter, once | Post-stroke, Germany |
| mSTOPS NCT02506244 | 2659 | 12-day ECG Patch, twice | Age >75 or <75 with risk factors, USA |
| PerDIEM NCTT02428140 | 300 | Implanted monitor | Post-stroke, Canada |
| VITAL-AF NCT03515057 | 35 308 | Hand-held ECG, once | Age ≥65, USA |
| Ongoing trials | |||
| AMALFI ISRCTN15544176 | 5029 | 14-day ECG patch, once | Age ≥65 with risk factors, UK |
| DANCAVAS ISRCTN12157806 | 79 000 | 3-lead ECG, once | Men, age 60–74, Denmark |
| FIND-AF2 (high risk) NCT04371055 | 1040 | Implanted monitor | Post-stroke, Germany |
| FIND-AF2 (low risk) NCT04371055 | 4160 | 7-day Holter, once | Post-stroke, Germany |
| GUARD-AF NCT04126486 | 11 931 | 14-day ECG Patch, once | Age ≥70, USA |
| Heartline NCT04276441 | 150 000 | ECG Watch | Age ≥65, USA |
| SAFER-Internal Pilot ISRCTN16939438 | 14 082 | Hand-held ECG, QID for 21 days | Age ≥70, UK |
| SAFER-UK ISRCTN72104369 | 100 418 | Hand-held ECG, QID for 21 days | Age ≥70, UK |
| SAFER-AUS ISRCTN72104369 | 2100 | Hand-held ECG, QID for 21 days | Age ≥70, Australia |
| STROKESTOP II NCT02743416 | 28 712 | Hand-held ECG, QID for 14 days | Age 75/76 with elevated NT-ProBNP, Sweden |
Figure 1.
Study selection diagram.
Figure 2.
Forest plot (upper panel) and trial sequential analysis (lower panel) of published RCTs of AF screening for stroke prevention. Stroke was defined in REHEARSE-AF as stroke, transient ischaemic attack, or systemic embolism; stroke was defined in SCREEN-AF as ischaemic stroke; stroke was defined in LOOP as stroke or systemic embolism; stroke was defined in STROKESTOP as ischaemic stroke or systemic embolism. *REHEARSE-AF was not penalized at interim look due to low information use (<1%).
Figure 2 ’s upper panel shows a trial sequential analysis of reported studies. The boundary in red is calculated using the observed event rates of studies to date, a two-sided Type-1 error of 5%, 80% power, 50% heterogeneity, and an O’Brien-Fleming alpha-spending function. The trial sequential analysis shows that the cumulative z-score from published data (blue line) is insufficient to conclude the benefits of screening and calculates an optimal sample size of a total of 103 454 participants randomized, indicating that further trials should be performed.
Atrial fibrillation screening can only prevent strokes in patients who are found to have the disease, and then take OAC as a result of positive screening. Furthermore, AF is only one of many important risk factors for stroke. This means that the relative risk reduction for screening could be small and large numbers of patients need to be studied to demonstrate the efficacy of AF screening for stroke prevention. Still, the number of patients worldwide who are at risk of AF-related stroke is very large, and the absolute benefit of AF screening could be large. Given this potential public health impact of AF screening on stroke, there is a need to systematically collate data on RCTs of AF screening in a variety of healthcare settings.
The International AF-SCREEN collaboration has been working since 2015 to assess the efficacy of AF screening for the prevention of stroke.1 Members of the group secured a European Union Horizons 2020 grant (Digital, risk-based screening for atrial fibrillation in the European community, agreement No 847770), which supports a prospective, individual patient data meta-analysis of RCTs (PROSPERO, Protocol Under Review).7 The primary outcome of the meta-analysis is stroke. Secondary outcomes include AF detection, OAC prescription, hospitalization, mortality, and bleeding. Anonymized participant data from individual RCTs are being translated into a common format and collated in a central database. Individual participant data will permit pre-specified subgroup and meta-regression analyses to explore heterogeneity in populations, healthcare settings, screening modalities, and uptake of OAC. To date, study teams from 16 RCTs including nearly 300 000 participants are contributing to the effort; any group conducting an eligible trial is invited to join (Table 1).
Conclusion
If and how to screen for AF is an important public health concern. The AF-SCREEN/AFFECT-EU individual patient data meta-analysis aims to comprehensively assess the benefits and risks of AF screening, and determine how population, screening method, and health-system factors influence stroke prevention.
Contributor Information
William F McIntyre, McMaster University, Hamilton L8S 4L8, Canada.
Søren Z Diederichsen, Copenhagen University Hospital, Copenhagen 21003, Denmark.
Ben Freedman, University of Sydney, Sydney 2006, Australia.
Renate B Schnabel, University Heart Centre, Hamburg 20251, Germany.
Emma Svennberg, Karolinska Institutet, Stockholm 171 77, Sweden.
Jeff S Healey, McMaster University, Hamilton L8S 4L8, Canada.
Data availability
All data were abstracted from the referenced publications.
References
- 1. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Albert CM, Anderson CS, Antoniou S, Benjamin EJ, Boriani G, Brachmann J, Brandes A, Chao T-F, Conen D, Engdahl J, Fauchier L, Fitzmaurice DA, Friberg L, Gersh BJ, Gladstone DJ, Glotzer TV, Gwynne K, Hankey GJ, Harbison J, Hillis GS, Hills MT, Kamel H, Kirchhof P, Kowey PR, Krieger D, Lee VWY, Levin L-Å, Lip GYH, Lobban T, Lowres N, Mairesse GH, Martinez C, Neubeck L, Orchard J, Piccini JP, Poppe K, Potpara TS, Puererfellner H, Rienstra M, Sandhu RK, Schnabel RB, Siu C-W, Steinhubl S, Svendsen JH, Svennberg E, Themistoclakis S, Tieleman RG, Turakhia MP, Tveit A, Uittenbogaart SB, Van Gelder IC, Verma A, Wachter R, Yan BP, Al Awwad A, Al-Kalili F, Berge T, Breithardt G, Bury G, Caorsi W, Chan N, Chen S, Christophersen I, Connolly S, Crijns H, Davis S, Dixen U, Doughty R, Du X, Ezekowitz M, Fay M, Frykman V, Geanta M, Gray H, Grubb N, Guerra A, Halcox J, Hatala R, Heidbuchel H, Jackson R, Johnson L, Kaab S, Keane K, Kim Y, Kollios G, Løchen M, Ma C, Mant J, Martinek M, Marzona I, Matsumoto K, McManus D, Moran P, Naik N, Ngarmukos T, Prabhakaran D, Reidpath D, Ribeiro A, Rudd A, Savalieva I, Schilling R, Sinner M, Stewart S, Suwanwela N, Takahashi N, Topol E, Ushiyama S, Walker N, Wijeratne T, Verbiest van Gurp N . Screening for atrial fibrillation: a Report of the AF-SCREEN international collaboration. Circulation 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 2. Force USPST, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Epling JW Jr, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for atrial fibrillation: US preventive services task force recommendation statement. JAMA 2022;327:360–367. [DOI] [PubMed] [Google Scholar]
- 3. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C, Olesen MS, Nielsen JB, Holst AG, Brandes A, Haugan KJ, Køber L. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. The Lancet 2021;398:1507–1516. [DOI] [PubMed] [Google Scholar]
- 4. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of remote heart rhythm sampling using the alivecor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 5. Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N, Marsden T, Thornton A, Djuric A, Suerbaum J, von Grunhagen D, McIntyre WF, Benz AP, Wong JA, Merali F, Henein S, Nichol C, Connolly SJ, Healey JS, SCREEN-AF Investigators and Coordinators . Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol 2021;6:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. The Lancet 2021;398:1498–1506. [DOI] [PubMed] [Google Scholar]
- 7. Engler D, Heidbuchel H, Schnabel RB. Digital, risk-based screening for atrial fibrillation in the European community – the AFFECT-EU project funded by the European Union. Eur Heart J 2021;42:2625–2627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were abstracted from the referenced publications.