Abstract
Objective
To evaluate the efficacy and safety of eptinezumab versus placebo in patients ≥50 years old with episodic (EM) or chronic migraine (CM).
Materials and Methods
This post hoc analysis included data from two phase 3, parallel‐group, randomized, double‐blind, placebo‐controlled studies in adults with EM (PROMISE‐1) or CM (PROMISE‐2). Patients ≥50 years at baseline treated with eptinezumab 100 mg, 300 mg, or placebo were pooled from both studies to evaluate efficacy and safety.
Results
A total of 385/1960 (19.6%) EM and CM patients who were ≥50 years old at baseline (range, 50–71 and 50–65 years, respectively) received eptinezumab 100 mg (n = 132), 300 mg (n = 127), or placebo (n = 126) over Weeks 1–12. Reductions in mean monthly migraine days (MMDs) in ≥50‐year‐old EM patients were –3.8 (100 mg) and –4.4 (300 mg) with eptinezumab versus –2.6 with placebo. In ≥50‐year‐old CM patients, mean changes in MMDs were –7.7 (100 mg) and –8.6 (300 mg) with eptinezumab versus –6.0 with placebo. Changes in MMDs were comparable to total study results. A ≥50% MMD reduction over Weeks 1–12 was achieved by 57.9% of eptinezumab‐treated versus 35.7% of patients who received placebo, and a ≥75% reduction by 30.5% versus 13.5%, respectively. The incidence of treatment‐emergent adverse events (TEAEs) in EM and CM patients ≥50 years old was similar across treatment groups, with ≥96% of TEAEs mild or moderate in severity.
Conclusions
Treatment with eptinezumab was efficacious, tolerable, and safe in patients ≥50 years with EM or CM, congruent with results from the overall study population.
Keywords: CGRP monoclonal antibody, chronic migraine, episodic migraine, eptinezumab, subgroup analysis
1. INTRODUCTION
Migraine—characterized by recurrent episodes of moderate to severe headache associated with disruptions of neurological, gastrointestinal, and sensory function 1 —often occurs over decades of life, with symptoms that can cause substantial functional disability. 2 , 3 , 4 Older individuals with migraine may be at an increased risk of dementia (in adults aged ≥65 years), 5 cognitive complaints (aged ≥50 years), 6 medication overuse (aged ≥55 years), 7 and sleep apnea (aged ≥45 years). 8 Management of migraine in older persons can be complicated by the presence of comorbidities and age‐related changes in drug disposition that may result in altered pharmacokinetics and response. 9 , 10 Older patients are also likely to be taking medications that can further complicate drug treatment. It is, therefore, essential to investigate the efficacy, tolerability, and safety of preventive medications in this population.
In the phase 3 PROMISE trials, 11 , 12 , 13 , 14 the calcitonin gene‐related peptide (CGRP) antagonist eptinezumab significantly reduced migraine frequency at approved doses (100 mg and 300 mg intravenously [IV] 15 ) in adults up to the age of 65 (PROMISE‐2) or 75 years (PROMISE‐1). In both studies, the onset of effect was rapid (within 1 day) and sustained for the duration of the studies (48 weeks in PROMISE‐1; 24 weeks in PROMISE‐2). Approximately one‐fifth of all patients in PROMISE‐1 and PROMISE‐2 were 50–71 years old at baseline. Because patients of higher age with migraine pose unique challenges to acute and preventive treatment, particularly complexities related to comorbidity and polypharmacy, we performed a post hoc subgroup analysis to evaluate the efficacy and safety of eptinezumab for the preventive treatment of migraine in this clinically important subgroup of migraine patients.
2. MATERIALS AND METHODS
Detailed methodologies of the PROMISE studies have been published. 11 , 12 Independent ethics committee or institutional review board approval was obtained for each study site, with all patients providing written informed consent prior to study participation. PROMISE‐1 (NCT02559895) and PROMISE‐2 (NCT02974153) were pivotal phase 3, parallel‐group, randomized, double‐blind, placebo‐controlled trials that evaluated the preventive migraine efficacy, tolerability, and safety of eptinezumab in adults with episodic migraine (EM) and chronic migraine (CM), respectively. PROMISE‐1 included adults with EM up to 75 years of age; PROMISE‐2 included adults with CM up to 65 years of age. In both studies, patients received eptinezumab or placebo, administered IV over 30 minutes to 1 hour every 12 weeks. Patients in PROMISE‐1 received up to four doses of study medication, and patients in PROMISE‐2 received up to two doses.
Patients in each study completed a daily electronic diary (eDiary) from screening through the end of treatment to capture the incidence and characteristics of headache and migraine days, as well as acute headache medication use (i.e., triptans, ergots, opioids, simple analgesics, and combination analgesics). In both studies, the primary outcome measure was the reduction in monthly migraine days (MMDs) over the first 12 weeks of the study. Secondary outcomes included ≥50% and ≥75% migraine responder rates and acute headache medication days/month over Weeks 1–12. The ≥50% or ≥75% migraine responder rates were defined as patients achieving ≥50% or ≥75% reduction from baseline in average MMDs, respectively. Acute headache medication days/month were measured as both total medication days and any medication days. Total acute headache medication days/month was defined as the average sum of the days of use of triptans, ergots, opioids, simple analgesics, or combination analgesics; if a patient used two classes of medication on the same day, they were counted twice. Any acute headache medication days/month were defined as days of any acute medication use; if a patient used two classes of medication on the same day, they were counted once. Safety, including adverse event monitoring, was assessed throughout each study.
This post hoc analysis evaluated the efficacy and safety of eptinezumab in the subgroup of patients ≥50 years of age at baseline in PROMISE‐1 and PROMISE‐2. For the efficacy analyses, patient results were summarized within the treatment group to which they were randomly assigned. In the safety subpopulation, patients were analyzed according to the randomized treatment they received. Patients from PROMISE‐1 and PROMISE‐2 were pooled for all analyses, apart from the change from baseline in MMDs, due to the differences in patient populations at baseline.
All results from this post hoc analysis are descriptive statistics, such as means, standard deviations, and rates, analyzed using the methods detailed in the statistical analysis plans previously published 11 , 12 and conducted using SAS software (SAS Institute, Inc., Cary, NC) v9.2 or higher. Consequently, no formal statistical evaluation of group difference was performed. Missing data for migraine days were imputed depending on patient compliance with the eDiary. If the eDiary was completed for ≥21 days in a 28‐day study month, the observed frequency was normalized to 28 days. If the eDiary was completed for <21 days, the results were a weighted function of the observed data for the current interval and the results for the previous interval, with the weight being proportional to the number of completed eDiary days. For acute headache medication days/month, a simplified imputation algorithm was employed. If the eDiary was completed for ≥14 days in a 28‐day study month, the observed frequency was normalized to 28 days; if the eDiary was completed for <14 days, missing data were not imputed, and these patients were excluded from the acute headache medication analysis.
3. RESULTS
A total of 385/1960 (19.6%) patients in PROMISE‐1 and PROMISE‐2 were ≥50 years old at baseline (range, 50–71 years and 50–65 years, respectively). Of these, 132 received eptinezumab 100 mg (42 EM, 90 CM), 127 received eptinezumab 300 mg (52 EM, 75 CM), and 126 received placebo (49 EM, 77 CM).
Demographic and baseline clinical characteristics of this patient subset are summarized in Table 1. The mean age of all patients in this analysis was 55.7 years. Patients were predominantly female (336/385 [87.3%]) and white (360/385 [93.5%]). Nearly all (382/385 [99.2%]) were taking ≥1 medication at baseline; the most commonly reported baseline medications (those used by ≥10% of patients in any treatment arm) were sumatriptan (153/385 [39.7%]), ibuprofen (114/385 [29.6%]), acetylsalicylic acid +paracetamol + caffeine (106/385 [27.5%]), paracetamol (53/385 [13.8%]), rizatriptan (50/385 [13.0%]), topiramate (35/385 [9.1%], and vitamins (33/385 [8.6%]). Nearly all patients were taking acute migraine medication (≤7 days per month within two months prior to trial screening) at baseline (375/385 [97.4%]), and 33% (127/385) of all patients reported using preventive migraine medication at baseline. The majority of patients had ≥1 cardiovascular risk factor at baseline (268/385 [69.6%]), and approximately one‐fourth had ≥2 cardiovascular risk factors (90/385 [23.4%]) (Table 2). No patient had a medical history of coronary artery disease or stroke.
TABLE 1.
Baseline demographics and disease characteristics in patients ≥50 years of age (full analysis set)
| Eptinezumab 100 mg (n = 132) | Eptinezumab 300 mg (n = 127) | Placebo (n = 126) | |
|---|---|---|---|
| Age (years), mean (SD) | 55.9 (4.37) | 55.7 (4.93) | 55.5 (4.91) |
| Sex: Female, n (%) | 111 (84.1%) | 115 (90.6%) | 110 (87.3%) |
| Race, n (%) | |||
| White | 124 (93.9%) | 121 (95.3%) | 115 (91.3%) |
| Black | 5 (3.8%) | 4 (3.1%) | 8 (6.3%) |
| Other | 3 (2.3%) | 2 (1.6%) | 3 (2.4%) |
| Body mass index (kg/m2), mean (SD) | 27.5 (4.79) | 26.5 (5.18) | 28.0 (6.52) |
| Age at migraine diagnosis (years), mean (SD) | 28.4 (12.93) | 26.3 (10.97) | 28.0 (12.01) |
| Duration of migraine diagnosis (years), mean (SD) | 27.5 (13.65) | 29.4 (11.79) | 27.6 (12.86) |
| Duration of chronic migraines (years), mean (SD) | 17.3 (16.03) | 19.7 (15.29) | 16.9 (14.85) |
| Baseline migraine days, mean (SD) | 13.5 (5.06) | 12.9 (5.42) | 12.7 (5.30) |
| Baseline headache days, mean (SD) | 16.9 (5.59) | 16.2 (5.69) | 16.1 (5.95) |
| Diagnosis, n (%) | |||
| Episodic migraine | 42 (31.8%) | 52 (40.9%) | 49 (38.9%) |
| Chronic migraine | 90 (68.2%) | 75 (59.1%) | 77 (61.1%) |
| Chronic migraine and MOH a | 34 (25.8%) | 35 (27.6%) | 31 (24.6%) |
| Baseline medication use in ≥10% of patients, n (%) | |||
| Sumatriptan | 41 (31.1%) | 49 (38.6%) | 63 (50.0%) |
| Ibuprofen | 38 (28.8%) | 41 (32.3%) | 35 (27.8%) |
| Acetylsalicylic acid+paracetamol+caffeine | 38 (28.8%) | 36 (28.3%) | 32 (25.4%) |
| Paracetamol | 20 (15.2%) | 19 (15.0%) | 14 (11.1%) |
| Rizatriptan | 21 (15.9%) | 15 (11.8%) | 14 (11.1%) |
| Topiramate | 8 (6.1%) | 13 (10.2%) | 14 (11.1%) |
| Vitamins NOS | 10 (7.6%) | 13 (10.2%) | 10 (7.9%) |
| Baseline preventive or acute medication use, n (%) | |||
| Preventive | 46 (34.8%) | 39 (30.7%) | 42 (33.3%) |
| Acute | 126 (95.5%) | 127 (100.0%) | 122 (96.8%) |
Patients in PROMISE‐2 (chronic migraine) were allowed to have a secondary diagnosis of MOH at enrollment. 30 MOH, medication overuse headache; NOS, not otherwise specified; and SD, standard deviation.
TABLE 2.
Medical history and cardiovascular (CV) risk factors in patients ≥50 years of age (full analysis set)
| Eptinezumab 100 mg (n = 132) | Eptinezumab 300 mg (n = 127) | Placebo (n = 126) | |
|---|---|---|---|
| Medical history in ≥10% of total patients, n (%) | |||
| Hysterectomy | 19 (14.4%) | 28 (22.0%) | 27 (21.4%) |
| Gastroesophageal reflux disease | 25 (18.9%) | 20 (15.7%) | 15 (11.9%) |
| Depression | 19 (14.4%) | 20 (15.7%) | 20 (15.9%) |
| Drug hypersensitivity | 16 (12.1%) | 26 (20.5%) | 17 (13.5%) |
| Insomnia | 20 (15.2%) | 19 (15.0%) | 17 (13.5%) |
| Menopause | 26 (19.7%) | 14 (11.0%) | 14 (11.1%) |
| Postmenopausal | 18 (13.6%) | 19 (15.0%) | 17 (13.5%) |
| Seasonal allergy | 19 (14.4%) | 19 (15.0%) | 12 (9.5%) |
| Tonsillectomy | 16 (12.1%) | 15 (11.8%) | 15 (11.9%) |
| Osteoarthritis | 9 (6.8%) | 18 (14.2%) | 12 (9.5%) |
| CV risk factors, n (%) | |||
| Hypertension‐related | 15 (11.4%) | 13 (10.2%) | 7 (5.6%) |
| Diabetes‐related | 1 (0.8%) | 0 | 3 (2.4%) |
| Prior history of ischemic CV events or procedures | 1 (0.8%) | 1 (0.8%) | 0 |
| Obesity (body mass index ≥30 kg/m2) | 37 (28.0%) | 25 (19.7%) | 43 (34.1%) |
| Male and ≥45 years of age | 21 (15.9%) | 12 (9.4%) | 16 (12.7%) |
| Female and ≥55 years of age | 61 (46.2%) | 54 (42.5%) | 49 (38.9%) |
| Black or African American race | 5 (3.8%) | 4 (3.1%) | 8 (6.3%) |
| ≥ 1 CV risk factor | 100 (75.8%) | 81 (63.8%) | 87 (69.0%) |
| ≥ 2 CV risk factors | 33 (25.0%) | 25 (19.7%) | 32 (25.4%) |
Monthly migraine days among patients who were ≥50 years old at baseline are summarized in Table 3. In patients with EM who were ≥50 years of age, least squares mean changes from baseline in MMDs over Weeks 1–12 were –3.6 (100 mg) and –4.4 (300 mg) with eptinezumab versus –2.8 with placebo. These changes were comparable to those in the total study (PROMISE‐1) population (–3.9 [100 mg], –4.3 [300 mg], and –3.2 [placebo]). 11 In patients with CM who were ≥50 years old, least squares mean changes from baseline in MMDs over Weeks 1–12 were –7.6 (100 mg) and –8.4 (300 mg) with eptinezumab versus –6.0 with placebo. Again, these changes were comparable to those in the total study (PROMISE‐2) population (–7.7 [100 mg], –8.2 [300 mg], and –5.6 [placebo]). 12
TABLE 3.
Monthly migraine days (MMDs) before and after treatment in patients ≥50 years of age in PROMISE‐1 and PROMISE‐2 (full analysis set)
| Eptinezumab 100 mg | Eptinezumab 300 mg | Placebo | |
|---|---|---|---|
| PROMISE−1, a n | 42 | 52 | 49 |
| Baseline, mean (SD) | 8.9 (2.23) | 8.5 (2.49) | 8.3 (2.67) |
| Weeks 1–12, mean (SD) | 5.1 (3.49) | 4.1 (3.14) | 5.7 (3.26) |
| Change from baseline, LS mean (95% CI) | –3.6 (–4.5, –2.8) | –4.4 (–5.2, –3.7) | –2.8 (–3.6, –1.9) |
| Difference from placebo (95% CI) | –0.9 (–2.1, 0.3) | –1.7 (–2.8, –0.6) | |
| PROMISE−2, b n | 90 | 75 | 77 |
| Baseline, mean (SD) | 15.6 (4.56) | 15.9 (4.80) | 15.5 (4.63) |
| Weeks 1–12, mean (SD) | 7.9 (6.24) | 7.3 (6.11) | 9.5 (5.91) |
| Change from baseline, LS mean (95% CI) | –7.6 (–8.8, –6.4) | –8.4 (–9.7, –7.1) | –6.0 (–7.3, –4.7) |
| Difference from placebo (95% CI) | –1.6 (–3.4, 0.2) | –2.4 (–4.2, –0.5) |
Abbreviations: CI, confidence interval; LS, least squares; and SD, standard deviation.
Mean changes from baseline in the total PROMISE‐1 population were –3.9 (100 mg [n =221]), –4.3 (300 mg [n =222]), and –3.2 (placebo [n =222]). 11
Mean changes from baseline in the total PROMISE‐2 population were –7.7 (100 mg [n =356]), –8.2 (300 mg [n =350]), and –5.6 (placebo [n =366]). 12
The proportion of patients 50 years or older (EM and CM pooled) achieving ≥50% and ≥75% response at Weeks 1–12 is illustrated in Figure 1. Both thresholds were achieved by more patients treated with eptinezumab than by patients who received placebo, with 150/259 (57.9%) patients treated with eptinezumab and 45/126 (35.7%) patients who received placebo attaining a ≥50% reduction in migraine frequency and 79/259 (30.5%) patients treated with eptinezumab and 17/126 (13.5%) patients who received placebo attaining a ≥75% reduction in migraine frequency during this time period. Greater decreases in both total and any acute headache medication days/month over Weeks 1–12 were noted for patients treated with eptinezumab than those receiving placebo (Figure 2).
FIGURE 1.
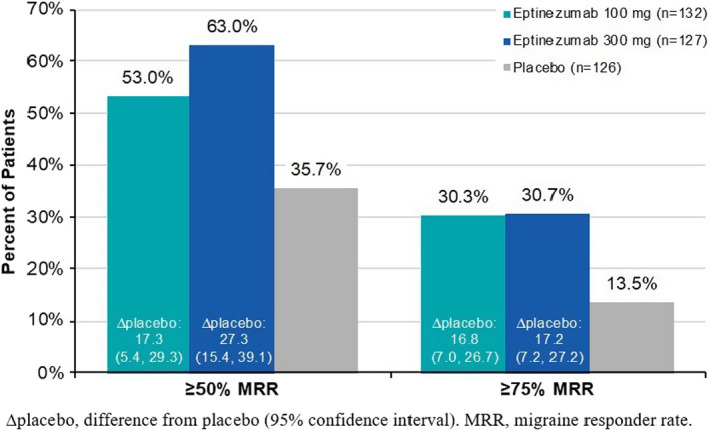
Migraine responder rates over Weeks 1–12 in patients ≥50 years of age (full analysis set). ∆placebo, difference from placebo (95% confidence interval). MRR, migraine responder rate
FIGURE 2.
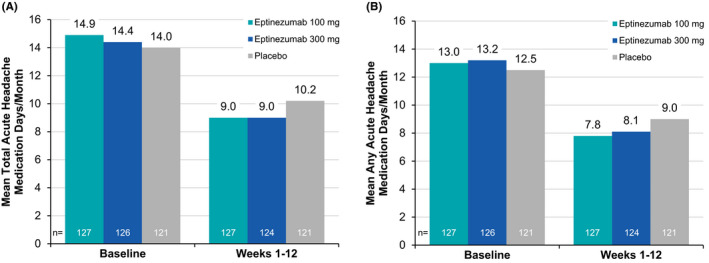
Acute headache medication days of use before and after treatment in patients ≥50 years of age (full analysis set): calculated as (A) total medication daysa and (B) any medication daysb. aTotal acute headache medication days are the sum of days when a patient took triptan, ergotamine, opioid, simple analgesic, or combination analgesic. If a patient used 2 classes of medication on the same day, they were counted twice. bAny acute headache medication days are defined as the number of days a patient used ≥1 class of medication. If a patient used 2 classes of medication on the same day, they were counted once. Missing data were not imputed
Treatment‐emergent adverse events (TEAEs) in patients ≥50 years of age (EM and CM pooled) are summarized in Table 4. The incidence of TEAEs was similar across treatment groups (100 mg, 46.6%; 300 mg, 53.5%; placebo, 52.4%). The most common TEAEs were nasopharyngitis (100 mg, 5.3%; 300 mg, 8.7%; placebo, 4.8%) and upper respiratory tract infection (100 mg, 4.5%; 300 mg, 7.9%; placebo, 6.3%). Most TEAEs were mild or moderate in severity (≥96% of patients across arms). Only 7/476 (1.8%) patients had TEAEs that led to treatment discontinuation (100 mg, n = 2; 300 mg, n = 1; placebo, n = 4). It is notable that central nervous system and cardiovascular events commonly considered potentially problematic in patients ≥50 years of age, such as syncope (eptinezumab, n = 2; placebo, n = 1), somnolence (eptinezumab, n = 0; placebo, n = 2), blood pressure increased (eptinezumab, n = 2; placebo, n = 0), and hypertension (eptinezumab, n = 1; placebo, n = 2) occurred infrequently, as did falls (eptinezumab, n = 0; placebo, n = 1). There were no reports of dementia. A total of 13/260 (5.0%) patients treated with eptinezumab and 6/126 (4.8%) patients who received placebo experienced psychiatric events, including insomnia (eptinezumab, n = 3; placebo, n = 3), depression (eptinezumab, n = 3; placebo, n = 1), and anxiety (eptinezumab, n = 3; placebo, n = 0). One patient treated with eptinezumab reported anorgasmia; no other TEAEs related to sexual dysfunction were reported. Only 2 patients experienced constipation (eptinezumab n = 1; placebo, n = 1), and 1 (eptinezumab 300 mg) developed nephrolithiasis. One patient treated with placebo reported sleep apnea syndrome.
TABLE 4.
Summary of treatment‐emergent adverse events (TEAEs) in patients ≥50 years of age (safety population)
| Eptinezumab 100 mg (n = 132) | Eptinezumab 300 mg (n = 127) | Placebo (n = 126) | |
|---|---|---|---|
| Patients with any TEAE, n (%) | 62 (46.6%) | 68 (53.5%) | 66 (52.4%) |
| Mild | 26 (19.5%) | 24 (18.9%) | 25 (19.8%) |
| Moderate | 32 (24.1%) | 42 (33.1%) | 36 (28.6%) |
| Severe | 4 (3.0%) | 2 (1.6%) | 4 (3.2%) |
| Life‐threatening | 0 | 0 | 1 (0.8%) |
| Most common TEAEs (≥ 2% of total patients), n (%) | |||
| Nasopharyngitis | 7 (5.3%) | 11 (8.7%) | 6 (4.8%) |
| Upper respiratory tract infection | 6 (4.5%) | 10 (7.9%) | 8 (6.3%) |
| Urinary tract infection | 4 (3.0%) | 7 (5.5%) | 3 (2.4%) |
| Dizziness | 4 (3.0%) | 5 (3.9%) | 3 (2.4%) |
| Fatigue | 5 (3.8%) | 4 (3.1%) | 2 (1.6%) |
| Sinusitis | 2 (1.5%) | 3 (2.4%) | 5 (4.0%) |
| Back pain | 3 (2.3%) | 3 (2.4%) | 3 (2.4%) |
| Patients with any TEAE leading to treatment discontinuation, n (%) | 2 (1.5%) | 1 (0.8%) | 4 (3.2%) |
| Patients with any TEAE related to study drug, n (%) | 14 (10.5%) | 18 (14.2%) | 13 (10.3%) |
| Patients with ≥1 serious adverse event, n (%) | 2 (1.5%) | 1 (0.8%) | 3 (2.4%) |
| Syncope | 1 (0.8%) | 0 | 1 (0.8%) |
| Apnea | 0 | 0 | 1 (0.8%) |
| Breast cancer | 0 | 1 (0.8%) | 0 |
| Cellulitis | 0 | 0 | 1 (0.8%) |
| Chronic obstructive pulmonary disease | 0 | 0 | 1 (0.8%) |
| Depression suicidal | 1 (0.8%) | 0 | 0 |
| Gastric ulcer | 1 (0.8%) | 0 | 0 |
| Hematemesis | 1 (0.8%) | 0 | 0 |
| Migraine | 0 | 0 | 1 (0.8%) |
| Tibia fracture | 1 (0.8%) | 0 | 0 |
4. DISCUSSION
The results of this post hoc analysis suggest that the efficacy of eptinezumab in patients with migraine who are 50 years or older is comparable to that observed in the overall trial populations, with an equally favorable safety and tolerability profile, suggesting that eptinezumab is also a valid option for migraine prevention in this subpopulation of patients. These findings are important for clinicians managing patients of 50 years or older, for whom traditional migraine preventives may be more problematic. Indeed, older patients may be more susceptible to side effects of traditional migraine preventives such as tricyclic antidepressants (e.g., anticholinergic and arrhythmogenic effects, drowsiness, weight gain), selective norepinephrine reuptake inhibitors (e.g., drowsiness, fatigue), beta blockers (e.g., cardiovascular effects, dizziness, insomnia), topiramate (e.g., paraesthesia, central nervous system side effects), and sodium valproate (e.g., weight gain, hair loss, and liver function disturbances). 16 , 17 , 18 , 19 They may also be at greater risk for drug–drug interactions, due to more frequent medication use related to the increased presence of comorbidities in this population. 20 In the current analysis, more than one‐fourth of patients were obese (105/385 [27.3%]), more than two‐thirds had ≥1 cardiovascular risk factor (268/385 [69.6%]), and nearly all (382/385 [99.2%]) were using ≥1 medication at baseline.
No impact on cardiovascular safety was observed. This finding is consistent with the collective evidence from the clinical trials with CGRP‐targeting monoclonal antibodies (mAbs), which has failed to identify any clinically meaningful increase in cardiovascular side effects in the general migraine population. 21 Because CGRP has a physiologic role in cardiac function, this aspect of the safety of anti‐CGRP mAbs in older patients warrants further study.
Nervous system adverse events were uncommon, and no falls were reported for eptinezumab‐treated patients.
To our knowledge, this is only the second published analysis exploring the efficacy and safety of anti‐CGRP mAbs specifically in patients of increased age. Our findings are consistent with those described in a pooled analysis of data from galcanezumab trials, which concluded that age had no meaningful impact on efficacy and safety outcomes. 22
The selection of 50 years of age was considered a reasonable cutoff to account for cardiovascular risk and menopause (87% of analysis population was female) based on the literature and clinical practice guidelines. Regarding cardiovascular risk, for primary prevention of cardiovascular disease in those with no history but risk factors (i.e., most of the current analysis population), the US Preventive Services Task Force provided a Grade B recommendation (i.e., offer or provide this service) for statin use in those ages 40–75 years and a Grade B recommendation for aspirin use in those ages 50–59 years. 23 , 24 Data from the World Health Organization also indicate that the proportion of those with ≥30% 10‐year total cardiovascular disease risk in North America and Europe was higher beginning at age 50 years than <50 years across both men and women, particularly in men. 25 Though not well represented in these studies, a study in Taiwanese adults found that the risk of stroke was particularly increased beginning at age 50 years compared with those younger, especially in males. 26 An analysis supporting age‐based screening (vs Framingham screening) for cardiovascular disease found that 50–55 years represents the minimum age for the recommendation to initiate treatment to prevent cardiovascular disease. 27 Regarding menopause, using a cutoff of 50 years of age includes women that were mostly likely in the late perimenopausal, early postmenopausal, and possibly later postmenopausal time periods, given that the average age of menopause is 51 years in the United States. 28 These time periods can comprise months to years of amenorrhea (i.e., low serum levels of estradiol). It has been demonstrated that estrogen can moderate the synthesis and release of CGRP from trigeminal neurons 29 ; therefore, documenting the efficacy and safety of anti‐CGRP mAbs in such a subpopulation is necessary as it cannot be assumed that anti‐CGRP mAbs will work in women during the menopausal transition and postmenopausal time periods.
Interpretation of these results is limited by the analysis type (post hoc), between‐study differences in enrollment criteria (PROMISE‐1 enrolled patients up to 75 years of age; PROMISE‐2 capped the age of enrollment at 65 years), and the potential lack of generalizability of the trial population, which excluded patients with conditions that might be encountered in a clinic setting, such as active, progressive, or unstable cardiovascular or neurologic disorders and uncontrolled hypertension.
5. CONCLUSION
This post hoc subgroup analysis of the pivotal PROMISE‐1 and PROMISE‐2 clinical studies suggests eptinezumab may be useful in patients 50 years or older who experience migraine, as the efficacy, safety, or tolerability of eptinezumab in this subpopulation was in line with what was demonstrated in the overall study populations.
CONFLICT OF INTEREST
V. Martin has been a consultant for Abbvie, Alder, Amgen, Biohaven, Lilly, Teva, and Theranica, and has been a speaker for Abbvie, Amgen, Biohaven, Lilly, and Teva. C. Tassorelli has been a principal investigator or collaborator for clinical trials sponsored by Alder, Allergan/Abbvie, IBSA, Lilly, Lundbeck, Novartis, and Teva; has been a consultant or speaker for Allergan/Abbvie, Biohaven, Lilly, Lundbeck, Novartis, and Teva; has been an advisory board member for Allergan/AbbVie, Lilly, Lundbeck, Novartis, and Teva; and is an associate editor with The Journal of Headache and Pain. A. Ettrup is a full‐time employee of H. Lundbeck A/S or one of its subsidiary companies. J. Hirman is an employee of Pacific Northwest Statistical Consulting, Inc., a contracted service provider of biostatistical resources for H. Lundbeck A/S. R. Cady was an employee of Lundbeck or one of its subsidiary companies at the time of study and manuscript development.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ane.13603.
ACKNOWLEDGMENTS
The authors thank Mary Tom, PharmD, and Nicole Coolbaugh, CMPP, of The Medicine Group (New Hope, PA, USA), for providing medical writing support, in accordance with Good Publication Practice guidelines. Funding for manuscript preparation was provided by H. Lundbeck A/S (Copenhagen, Denmark).
Martin V, Tassorelli C, Ettrup A, Hirman J, Cady R. Eptinezumab for migraine prevention in patients 50 years or older. Acta Neurol Scand. 2022;145:698–705. doi: 10.1111/ane.13603
Trial registration: ClinicalTrials.gov (Identifier: NCT02559895, NCT02974153)
Funding information
Sponsored and funded by H. Lundbeck A/S. The sponsor participated in the design and conduct of the study; data collection, management, analysis, and interpretation; and preparation, review, and approval of the manuscript. All statistical analyses were performed by a contracted research organization and were directed or designed by Pacific Northwest Statistical Consulting under contractual agreement with Lundbeck Seattle BioPharmaceuticals, Inc. All authors and H. Lundbeck A/S and Lundbeck Seattle BioPharmaceuticals, Inc. prepared, reviewed, and approved the manuscript and made the decision to submit the manuscript for publication. Editorial support for the development of this manuscript was funded by H. Lundbeck A/S
REFERENCES
- 1. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders. Cephalalgia. 2018;38(1):1‐211. [DOI] [PubMed] [Google Scholar]
- 2. Buse DC, Rupnow MF, Lipton RB. Assessing and managing all aspects of migraine: migraine attacks, migraine‐related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. 2009;84(5):422‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burton WN, Landy SH, Downs KE, Runken MC. The impact of migraine and the effect of migraine treatment on workplace productivity in the United States and suggestions for future research. Mayo Clin Proc. 2009;84(5):436‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301‐315. [DOI] [PubMed] [Google Scholar]
- 5. Morton RE, St John PD, Tyas SL. Migraine and the risk of all‐cause dementia, Alzheimer's disease, and vascular dementia: A prospective cohort study in community‐dwelling older adults. Int J Geriatr Psychiatry. 2019;34(11):1667‐1676. [DOI] [PubMed] [Google Scholar]
- 6. Martins IP, Maruta C, Alves PN, et al. Cognitive aging in migraine sufferers is associated with more subjective complaints but similar age‐related decline: a 5‐year longitudinal study. J Headache Pain. 2020;21(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Müller B, Dresler T, Gaul C, et al. More attacks and analgesic use in old age: self‐reported headache across the lifespan in a German sample. Front Neurol. 2019;10:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buse DC, Rains JC, Pavlovic JM, et al. Sleep disorders among people with migraine: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache. 2019;59(1):32‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wijeratne T, Tang HM, Crewther D, Crewther S. Prevalence of migraine in the elderly: a narrated review. Neuroepidemiology. 2019;52(1–2):104‐110. [DOI] [PubMed] [Google Scholar]
- 10. Curto M, Capi M, Martelletti P, Lionetto L. How do you choose the appropriate migraine pharmacotherapy for an elderly person? Expert Opin Pharmacother. 2019;20(1):1‐3. [DOI] [PubMed] [Google Scholar]
- 11. Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double‐blind, placebo‐controlled study (PROMISE‐1). Cephalalgia. 2020;40(3):241‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine. PROMISE‐2. Neurology. 2020;94(13):e1365‐e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith TR, Janelidze M, Chakhava G, et al. Eptinezumab for the prevention of episodic migraine: sustained effect through 1 year of treatment in the PROMISE‐1 study. Clin Ther. 2020;42(12):2254‐2265. [DOI] [PubMed] [Google Scholar]
- 14. Silberstein S, Diamond M, Hindiyeh NA, et al. Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE‐2 (Prevention of migraine via intravenous ALD403 safety and efficacy–2) study. J Headache Pain. 2020;21(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. VYEPTI . In. Bothell WA. Lundbeck Seattle BioPharmaceuticals, Inc.; 2021.
- 16. Silberstein SD. Preventive migraine treatment. Continuum. 2015;21:973‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathew S, Ailani J. Traditional and novel migraine therapy in the aging population. Curr Pain Headache Rep. 2019;23(6):42. [DOI] [PubMed] [Google Scholar]
- 18. Sarchielli P, Mancini ML, Calabresi P. Practical considerations for the treatment of elderly patients with migraine. Drugs Aging. 2006;23(6):461‐489. [DOI] [PubMed] [Google Scholar]
- 19. Whyte CA, Tepper SJ. Adverse effects of medications commonly used in the treatment of migraine. Expert Rev Neurother. 2009;9(9):1379‐1391. [DOI] [PubMed] [Google Scholar]
- 20. Ansari H, Ziad S. Drug‐drug interactions in headache medicine. Headache. 2016;56(7):1241‐1248. [DOI] [PubMed] [Google Scholar]
- 21. Favoni V, Giani L, Al‐Hassany L, et al. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain. 2019;20(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bangs ME, Kudrow D, Wang S, et al. Safety and tolerability of monthly galcanezumab injections in patients with migraine: integrated results from migraine clinical studies. BMC Neurol. 2020;20(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/statin‐use‐in‐adults‐preventive‐medication Accessed 12/22/2021
- 24. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/aspirin‐to‐prevent‐cardiovascular‐disease‐and‐cancer Accessed 12/22/2021
- 25. World Health Organization . Prevention of Cardiovascular Disease Guidelines for assessment and management of cardiovascular risk. 2007. https://apps.who.int/iris/handle/10665/43685 Accessed 12/22/2021.
- 26. Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66(12):1339‐1347. [DOI] [PubMed] [Google Scholar]
- 27. Wald NJ, Simmonds M, Morris JK. Screening for future cardiovascular disease using age alone compared with multiple risk factors and age. PLoS One. 2011;6(5):e18742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The North American Menopause Society . Menopause 101: A primer for the perimenopausal. https://www.menopause.org/for‐women/menopauseflashes/menopause‐symptoms‐and‐treatments/menopause‐101‐a‐primer‐for‐the‐perimenopausal Accessed 12/22/2021
- 29. Warfvinge K, Krause DN, Maddahi A, Edvinsson JCA, Edvinsson L, Haanes KA. Estrogen receptors α, β and GPER in the CNS and trigeminal system ‐ molecular and functional aspects. J Headache Pain. 2020;21(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diener H‐C, Marmura MJ, Tepper SJ, et al. Efficacy, tolerability, and safety of eptinezumab in patients with a dual diagnosis of chronic migraine and medication‐overuse headache: subgroup analysis of PROMISE‐2. Headache. 2021;61(1):125‐136. [DOI] [PubMed] [Google Scholar]


