Abstract
Objective
To test the impact of inflammation on structural changes occurring in the sacroiliac (SI) joints and the spine detected on magnetic resonance imaging (MRI).
Methods
Patients with early axial spondyloarthritis (SpA) from the Devenir des Spondylarthropathies Indifferérenciées Récentes (DESIR) cohort were included. MRIs of the SI joints (MRI‐SI joints) and spine (MRI‐spine), obtained at baseline, 2 years, and 5 years, were scored by 3 central readers. Inflammation and structural damage on MRI‐SI joints and MRI‐spine were defined by the agreement of ≥2 of 3 readers (binary outcomes) and by the average of 3 readers (continuous outcomes). The effect of inflammation (MRI‐SI joints/MRI‐spine) on damage (MRI‐SI joints/MRI‐spine, respectively) was evaluated in 2 models: 1) a baseline prediction model (the effect of baseline inflammation on damage assessed at 5 years); and 2) a longitudinal model (the effect of inflammation on structural damage assessed during a 5‐year period).
Results
A total of 202 patients were included. Both the presence of bone marrow edema on MRI‐SI joints and on MRI‐spine at baseline were predictive of 5‐year damage (≥3 fatty lesions) on MRI‐SI joints (odds ratio [OR] 4.2 [95% confidence interval (95% CI) 2.4, 7.3]) and MRI‐spine (OR 10.7 [95% CI 2.4, 49.0]), respectively, when adjusted for C‐reactive protein level. The association was also confirmed in longitudinal models (when adjusted for Ankylosing Spondylitis Disease Activity Score) both in the SI joints (OR 5.1 [95% CI 2.7, 9.6]) and spine (OR 15.6 [95% CI 4.8, 50.3]). Analysis of other structural outcomes (i.e., erosions) on MRI‐SI joints yielded similar results. In the spine, a significant association was found for fatty lesions but not for erosions and bone spurs, which occurred infrequently over time.
Conclusion
We found a predictive and longitudinal association between inflammation detected on MRI and several types of structural damage detected on MRI in patients with early axial SpA, which adds to the evidence for a causal relationship.
INTRODUCTION
Axial spondyloarthritis (SpA) is a disease predominantly characterized by involvement of the axial skeleton. Axial involvement often translates into imaging abnormalities, which usually represent either an underlying inflammatory or structural lesion. Magnetic resonance imaging (MRI) of the sacroiliac (SI) joints (MRI‐SI joints) and spine (MRI‐spine) is a modality to detect, quantify, and evaluate (change of) axial inflammation in axial SpA. Thus far, conventional radiographs have been prescribed for assessing progression of structural damage in clinical practice and research.
Significance & Innovations.
There is a predictive and longitudinal association between inflammation detected on magnetic resonance imaging (MRI) and the development of structural damage on MRI in the sacroiliac (SI) joints (fatty lesions and erosions) and spine (fatty lesions) over 5 years in early axial spondyloarthritis (SpA).
The association between inflammation and damage is detected with more precision in the SI joints where, compared to the spine, structural damage prevails in early disease.
This is the first time that a relationship is proven between inflammation and damage when both are assessed on MRI, confirming the known relationship between inflammation and structural damage on radiographs in axial SpA.
These findings suggest that MRI, especially of the SI joints, is a valid alternative to conventional radiographs in detecting the structural consequences of axial inflammation in patients with early axial SpA.
Patients with axial SpA experience varying levels of radiographic progression (e.g., the occurrence of radiographic sacroiliitis and new syndesmophytes) (1, 2, 3, 4). Identifying patients with a higher likelihood of damage accrual is key to tailoring treatment strategies early in the disease course. Elevation of C‐reactive protein (CRP) level, disease activity as measured with the Ankylosing Spondylitis Disease Activity Score (ASDAS), and bone marrow edema (BME) on MRI‐SI joints or MRI‐spine have been shown to associate with increased probability of structural progression on conventional radiographs (3, 5, 6, 7, 8, 9, 10, 11, 12). Evidence is scarce, however, in early disease and mostly limited to studies in which structural damage was measured with conventional radiographs.
The interpretation of data stemming from the above‐mentioned studies may be jeopardized by limitations of the instruments used to measure structural progression, especially at the level of the SI joints. It is well established that radiographic sacroiliitis defined by the modified New York criteria (mNY) is poorly reliable (13, 14, 15). Investigators have been implementing strategies to improve the signal‐to‐noise ratio by, for instance, combining judgments from ≥2 trained central readers (3). Still, these strategies cannot fully eliminate the noise.
In recent years, there has been a growing interest in evaluating axial damage with other imaging modalities, such as MRI. Definitions for individual lesions (e.g., fatty lesions, erosions) have been proposed, and composite scores validated (16, 17, 18, 19). Although MRI‐detected lesions, as any outcome measure, are far from being error free, available literature shows higher reliability for MRI‐SI joints compared to pelvic radiographs in detecting structural lesions (20). A better signal‐to‐noise ratio, in theory, improves the ability to detect change and predictors thereof, especially in early disease where, at the group level, damage is known to be limited and to progress slowly (3, 21).
Thus far, no study has assessed the effect of inflammation on structural damage evaluated on MRI. We aimed to test the effect of inflammation on several types of structural lesions both assessed by MRI and at the level of the SI joints and the spine in patients with early axial SpA.
PATIENTS AND METHODS
Patients and study design
Five‐year data from patients with early axial SpA from the Devenir des Spondylarthropathies Indifferérenciées Récentes (DESIR) cohort have been used (22). Patients had to have ≥2 consecutive MRI images (either of the SI joints or spine) during the 5‐year follow‐up period to be included. The database used for the current analysis was locked on June 20, 2016. The study was conducted according to Good Clinical Practice guidelines and was approved by the appropriate local ethics committees. Written informed consent had been obtained from participating patients before inclusion.
Imaging scoring procedures
MRI‐SI joints and MRI‐spine were performed at baseline for all patients. By protocol, at 2 and 5 years of follow‐up, MRIs were only performed in participating centers in Paris (n = 9 of the 25 participating centers). Each image was independently scored by 3 trained central readers blinded to chronology and clinical data. MRI‐SI joints and MRI‐spine were performed on a 1.0–1.5T scanner providing T1‐weighted turbo spin‐echo and short tau inversion recovery sequences. Scanning was performed in a coronal oblique plane for the SI joints and in a sagittal plane for the spine, with a slice thickness of 4 mm. A detailed description of the MRI protocol in the DESIR cohort has been reported previously (23, 24).
Structural damage on MRI
The Spondyloarthritis Research Consortium of Canada (SPARCC) MRI‐SI joints structural score by Weber et al was used to define individual structural lesions on MRI‐SI joints (18). In the absence of a formal definition for structural damage on MRI‐SI joints, we considered 3 definitions previously shown most discriminatory between axial SpA and no axial SpA: ≥5 fatty lesions and/or erosions; ≥3 erosions; and ≥3 fatty lesions (25). Continuous structural lesions on MRI‐SI joints were defined as number of erosions, number of fatty lesions (range of both 0–40), number of fatty lesions and/or erosions (range 0–80), and as the total number of lesions including fatty lesions, erosions, and partial ankylosis/total ankylosis with the addition of sclerosis (not in the original score) (range 0–144).
Structural lesions on MRI‐spine were scored according to the Canada–Denmark (CANDEN) method, modified to include only corner lesions (16, 17). Similar to MRI‐SI joints, in the absence of a formal definition, we defined structural damage on MRI‐spine as ≥5 fatty lesions, which has been previously shown highly specific for axial SpA (25, 26). In addition, we also considered ≥5 fatty lesions and/or erosions; ≥3 erosions; ≥3 fatty lesions; and ≥3 bone spurs. The total number of fatty lesions, erosions, bone spurs (range 0–92 for each), fatty lesions and/or erosions (range 0–184), and the total number of structural lesions (fatty lesions, erosions, bone spurs, including also ankylosis; range 0–322) was assessed as continuous structural outcomes.
Inflammation on MRI
Inflammation on MRI‐SI joints was assessed using the Assessment of SpondyloArthritis international Society (ASAS) definition (positive/negative) and the SPARCC score (range 0–72) (27, 28, 29). BME on MRI‐spine was defined according to the ASAS definition (≥3 vertebral corner lesions; positive/negative) (30). In addition, a cutoff of at least 5 lesions was assessed, as it has been shown to be highly specific of axial SpA (25). The total spine SPARCC score was used as a continuous inflammatory outcome (range 0–414) (31). The interreader reliability of the MRI scores used in this study has been reported elsewhere (32).
Statistical analysis
Structural progression of binary scores was assessed in clinically relevant subgroups according to the CRP level and BME status at baseline and defined by the agreement of ≥2 of 3 readers as the percentage of net progression: the number of progressors (change from negative to positive) minus the number of regressors (change from positive to negative) divided by the total number of patients, a method previously described in detail (33).
The effect of inflammation, both on MRI‐SI joints and MRI‐spine, on structural outcomes, again both on MRI‐SI joints and MRI‐spine, respectively, was evaluated by 2 types of generalized estimating equation (GEE) models: 1) a baseline model (the effect of baseline inflammation on 5 years of structural damage incorporating measurements from all readers [1‐level GEE model adjusted for the reader]); and 2) a longitudinal model (the effect of BME at t on structural outcomes at t + 1 over 5 years [longitudinal time‐lagged, 2‐level GEE models with autoregression]). Binary variables of inflammation (i.e., BME) were modeled using binary damage outcomes (binomial GEE), while continuous variables of inflammation (i.e., SPARCC score) were modeled using continuous outcomes of damage (linear GEE).
The final multivariable models included variables that were found to confound the association of interest (i.e., that importantly changed the effect of inflammation on structural outcomes). The following variables were tested as possible confounders: age (in years), sex (male versus female), HLA–B27 (positive versus negative), smoking status (smoker versus nonsmoker), CRP level (mg/liter), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, the ASDAS (BASDAI score plus CRP level and ASDAS tested in separate models to avoid collinearity), treatment with nonsteroidal antiinflammatory drugs (yes/no), and treatment with tumor necrosis factor inhibitors (TNFi) (yes/no). Variables with a potential to change over time were modeled as such (i.e., all the above except sex and HLA–B27) in the longitudinal models.
RESULTS
Baseline characteristics
Of the total 708 patients from the DESIR cohort, 262 could have imaging at follow‐up according to the protocol, and 202 had at least 2 consecutive visits with data available either on MRI‐SI joints or MRI‐spine (196 had both modalities, 3 had MRI‐SI joints only, and 3 had MRI‐spine only) and were therefore included. No significant baseline differences were found between patients included and not included in this study (Table 1). The presence of BME at baseline was more frequent in the SI joints (29%) than in the spine (7% [ASAS definition]; 5% for ≥5 BME lesions). Likewise, structural damage was higher in the SI joints (e.g., ≥3 fatty lesions on MRI‐SI joints: 12%) than in the spine (e.g., ≥3 fatty lesions on MRI‐spine: 2%).
Table 1.
Baseline patient and disease characteristics comparing patients with magnetic resonance imaging (MRI) results available for ≥2 consecutive (included) visits to those without (excluded)*
| Characteristic | MRI on ≥2 consecutive visits (n = 202) | MRI on <2 consecutive visits (n = 60) |
|---|---|---|
| Age at baseline, mean ± SD years | 34 ± 9 | 33 ± 8 |
| Male sex | 96 (48) | 27 (45) |
| Symptom duration, mean ± SD years | 2 ± 1 | 1 ± 1 |
| HLA–B27 | 125 (62) | 32 (53) |
| ASAS axial SpA criteria | 133 (66) | 35 (60) |
| Sacroiliitis on MRI‐SI joints (ASAS)† | 58 (29) | 15 (28) |
| BME on MRI‐spine (ASAS)† | 14 (7) | 3 (6) |
| ≥5 BME lesions on MRI‐spine | 10 (5) | 2 (4) |
| Radiographic sacroiliitis (mNY)† | 25 (13) | 8 (14) |
| ≥3 fatty lesions on MRI‐SI joints | 23 (12) | 7 (14) |
| ≥3 erosions on MRI‐SI joints | 29 (15) | 9 (17) |
| ≥3 fatty lesions on MRI‐spine | 3 (2) | 0 (0) |
| ≥3 erosions on MRI‐spine | 0 (0) | 0 (0) |
| ≥3 bone spurs on MRI‐spine | 0 (0) | 0 (0) |
| BASDAI score, mean ± SD (range 0–10) | 4 ± 2 | 47 ± 21 |
| ASDAS‐CRP score, mean ± SD | 3 ± 1 | 3 ± 1 |
| Elevated CRP (≥6 mg/liter) | 52 (27) | 12 (21) |
| BASFI score, mean ± SD (range 0–10) | 3 ± 2 | 33 ± 28 |
| Treatment with NSAIDs | 192 (95) | 57 (95) |
| Treatment with TNFi | 0 (0) | 0 (0) |
Values are the number (%) unless indicated otherwise. The following variables had <5% missing data: radiographic sacroiliitis (mNY), bone marrow edema (BME) on MRI‐spine (ASAS), ≥5 BME lesions on MRI‐spine, ≥3 fatty lesions on MRI‐spine, ≥3 erosions on MRI‐spine, ≥3 bone spurs on MRI‐spine, and ASDAS‐CRP score. The following categories had <1% missing data: sacroiliitis on MRI‐SI joints (ASAS), ≥3 fatty lesions on MRI‐SI joints, ≥3 erosions on MRI‐SI joints, BASDAI score, and BASFI score. ASAS = Assessment of SpondyloArthritis international Society; ASDAS = Ankylosing Spondylitis Disease Activity Score; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; BASFI = Bath Ankylosing Spondylitis Functional Index; CRP = C‐reactive protein; mNY = modified New York criteria for radiographic sacroiliitis; MRI‐SI joints = MRI of the sacroiliac joints; MRI‐spine = MRI of the spine; NSAIDs = nonsteroidal antiinflammatory drugs; SpA = spondyloarthritis; TNFi = tumor necrosis factor inhibitors.
Agreement between 2 of 3 readers.
Structural progression according to the presence of objective inflammation at baseline
In total, 155 patients had complete MRI data at baseline and 5 years (141 both modalities, 10 MRI‐SI joints only, and 4 MRI‐spine only). Net progression, defined by ≥5 fatty lesions and/or erosions, ≥3 fatty lesions, and ≥3 erosions on MRI‐SI joints, according to baseline objective inflammatory markers, is shown in Figure 1. Patients with BME on MRI‐SI joints present at baseline had higher net progression rates compared to those who were BME‐negative for all outcomes, irrespective of the CRP status (range if BME positive: 7–24%; range if BME negative: 0–4%). On MRI‐spine overall, net progression was –0.7% both for ≥5 fatty lesions and/or erosions and for ≥5 fatty lesions; 0.7% for ≥3 fatty lesions, and 0% for ≥3 erosions and for ≥3 bone spurs. These low numbers precluded further analysis according to the presence of inflammatory markers at baseline.
Figure 1.
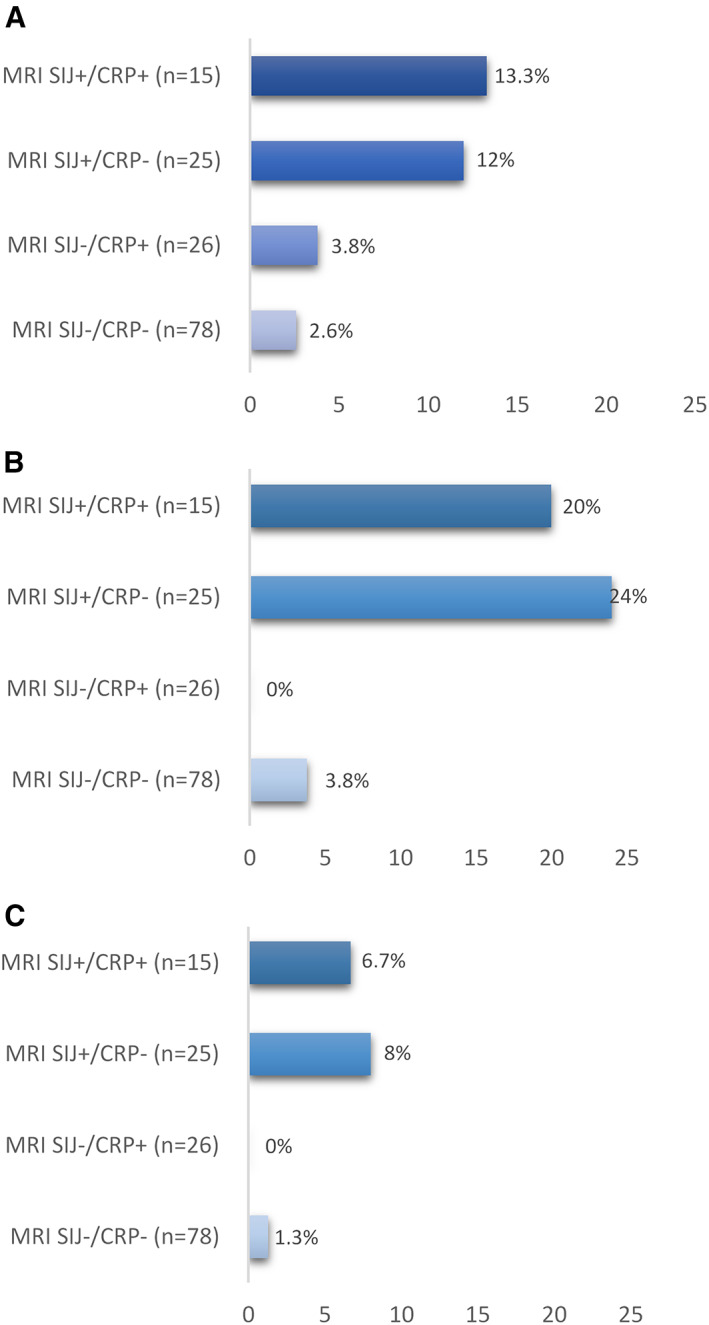
Net progression from magnetic resonance imaging (MRI) of the sacroiliac (SI) joints (MRI‐SI joints) without structural lesions (MRI SIJ–) to MRI‐SI joints with structural lesions (MRI SIJ+) defined by ≥5 fatty lesions and/or erosions (A), ≥3 fatty lesions (B), and ≥3 erosions (C) according to baseline objective inflammatory markers (MRI‐SI joints inflammation and C‐reactive protein [CRP] level). MRI‐SIJ+ is defined as the presence of bone marrow edema on MRI‐SI joints according to the Assessment of SpondyloArthritis international Society definition. CRP+ is defined as a CRP level ≥6 mg/liter at baseline. Net progression from MRI SIJ– to MRI SIJ+ at year 5 is defined as the number of progressors minus the number of regressors divided by the total number of patients in each category (n = 144; MRI‐SI joints available both at baseline and year 5, and CRP level available at baseline).
Effect of inflammation on structural progression (multivariable models)
Sacroiliac joints
The presence of BME on MRI‐SI joints at baseline was predictive of the development of fatty lesions and erosions on MRI‐SI joints 5 years later for all binary definitions (range odds ratio [OR] 4.1–5.6) after adjustment for CRP at baseline (Table 2). Similar results were found in the longitudinal models (after adjustment for ASDAS). On average, patients with BME on MRI‐SI joints had a 5 times higher likelihood of having at least 3 fatty lesions in the subsequent visit as compared to those without BME (OR 5.1 [95% confidence interval (95% CI) 2.7, 9.6]) (Figure 2). The association between the continuous SPARCC score on MRI‐SI joints and the various continuous structural outcomes was also always statistically significant and present in both models.
Table 2.
The effect of inflammation detected by magnetic resonance imaging (MRI) on structural damage detected by MRI in the sacroiliac joints (multivariable models)*
| ≥5 fatty lesions/erosions, OR (95% CI) | ≥3 fatty lesions, OR (95% CI) | ≥3 erosions, OR (95% CI) | Fatty lesions/erosions, β (95% CI) | Fatty lesions, β (95% CI) | Erosions β (95% CI) | |
|---|---|---|---|---|---|---|
| Binary scores | ||||||
| BME at baseline (range 144–151)† | 5.6 (3.1, 10.0)‡ | 4.2 (2.4, 7.3)‡ | 4.1 (2.1, 7.8) | – | – | – |
| BME over 5 years (range 197–199)§ | 7.7 (4.5, 13.4)¶ | 5.1 (2.7, 9.6)¶ | 3.2 (1.9, 5.3) | – | – | – |
| Continuous scores | ||||||
| SPARCC at baseline (range 144–151)† | – | – | – | 0.23 (0.15, 0.31)‡ | 0.12 (0.05, 0.19)‡ | 0.12 (0.06, 0.18) |
| SPARCC over 5 years (range 197–199)§ | – | – | – | 0.13 (0.07, 0.19)¶ | 0.10 (0.04, 0.16)¶ | 0.04 (0.01, 0.06) |
95% CI = 95% confidence interval; ASDAS = Ankylosing Spondylitis Disease Activity Score; BME = bone marrow edema (according to the Assessment of SpondyloArthritis international Society definition [positive/negative]); OR = odds ratio; SPARCC = Spondyloarthritis Research Consortium of Canada.
Multilevel generalized estimating equation (GEE) models (i.e., effect of inflammation at baseline on the outcome at 5 years, taking the scores from the individual readers into account).
Adjusted for C‐reactive protein (CRP) level at baseline.
Longitudinal multilevel time‐lagged GEE models with autoregression (i.e., effect of inflammation at t on the outcome at t + 1, adjusted for the outcome at t, taking the scores from the individual readers into account).
Adjusted for time‐lagged ASDAS‐CRP score.
Figure 2.
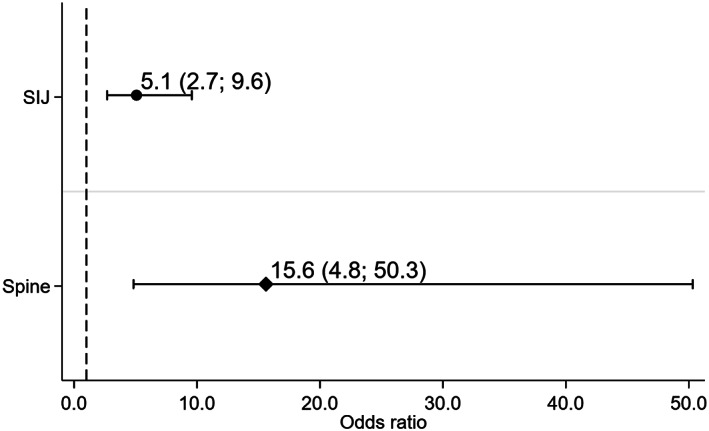
The effect of bone marrow edema (according to the Assessment of SpondyloArthritis international Society definition) on structural damage (defined as ≥3 fatty lesions) over 5 years both in the sacroiliac joints (SIJ) and spine (longitudinal time‐lagged models with autoregression). Circles represent ≥3 fatty lesions on MRI of the SI joints. Diamonds represent ≥3 fatty lesions on MRI of the spine. Bars show the 95% confidence interval. MRI = magnetic resonance imaging.
Spine
Testing the association of interest on MRI‐spine was hampered by a low number of lesions, leading to imprecise estimates and, for some outcomes (i.e., ≥3 erosions and ≥5 fatty lesions/erosions), precluded the estimation of the effect (Table 3). Only the association between BME and ≥3 fatty lesions was statistically significant. The presence of baseline BME (ASAS definition) on MRI‐spine was positively associated with ≥3 fatty lesions at 5 years on MRI‐spine (OR 10.7 [95% CI 2.4, 49]). This effect was also positive in the longitudinal model (OR 15.6 [95% CI 4.8, 50.3]) (Figure 2). As in MRI‐SI joints, CRP level (baseline models), and ASDAS (longitudinal models) have been found to confound the association of interest. Testing the effect of ≥5 BME lesions yielded similar results, but with wider a 95% CI (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24449/abstract). For continuous variables, a positive association could be found for fatty lesions alone or in combination with erosions, but not for erosions alone and bone spurs, both in baseline and longitudinal models.
Table 3.
The effect of inflammation detected by magnetic resonance imaging (MRI) on structural damage detected by MRI in the spine (multivariable models)*
| ≥5 fatty lesions/erosions | ≥5 fatty lesions | ≥3 fatty lesions | ≥3 erosions | ≥3 bone spurs | |
|---|---|---|---|---|---|
| Binary scores | |||||
| BME at baseline (n = 139)† | ‡ | ‡ | 10.7 (2.4, 49.0)§ | ‡ | 3.2 (0.4, 27.8)§ |
| BME over 5 years (n = 197)¶ | ‡ | 0.9 (0.8, 1.2)# | 15.6 (4.8, 50.3)# | ‡ | 2.8 (0.8, 9.6)# |
| Continuous scores | |||||
| SPARCC at baseline (range 139–145)† | 0.10 (0.01, 0.18)§ | 0.08 (0.02, 0.14) | 0.08 (0.02, 0.14) | 0.02 (0.00, 0.03)† | 0.01 (–0.01, 0.03)† |
| SPARCC over 5 years (n = 197)¶ | 0.06 (0.02, 0.11)# | 0.07 (0.02, 0.11)# | 0.07 (0.02, 0.11)# | 0.00 (–0.01, 0.01)# | 0.01 (0.00, 0.02) |
Values are the odds ratio (95% confidence interval). ASDAS = Ankylosing Spondylitis Disease Activity Score; BME = bone marrow edema (according to the Assessment of SpondyloArthritis international Society definition [≥3 lesions; positive/negative]); SPARCC = Spondyloarthritis Research Consortium of Canada.
Multilevel generalized estimating equation (GEE) model (i.e., effect of inflammation at baseline on the outcome at 5 years, taking the scores from the individual readers into account).
Model fails to find a mathematical solution due to low number of events.
§ Adjusted for C‐reactive protein (CRP) level at baseline.
Longitudinal multilevel time‐lagged GEE models with autoregression (i.e., effect of inflammation at t on the outcome at t + 1, adjusted for the outcome at t, taking the scores from the individual readers into account).
Adjusted for time‐varying lagged ASDAS‐CRP score.
DISCUSSION
In this prospective observational cohort study, we have shown that axial inflammation detected on MRI predicts subsequent development of structural lesions (especially fatty lesions) also on MRI over 5 years in patients with early axial SpA. This effect is independent of systemic inflammation and is seen at the level of both the SI joints and the spine but is measured more precisely in the SI joints where damage prevails in early disease. Our results add to the existing evidence by showing that the association between axial inflammation and some lesions reflecting structural damage can be measured with MRI in patients with early axial SpA.
In the current study, we have demonstrated an association between local inflammation and structural damage both measured on MRI in patients with early axial SpA. Involvement of the axial skeleton in axial SpA usually starts at the level of the SI joints (21, 34, 35). In line with the literature, we found that 6 times more patients showed structural damage (e.g., ≥3 fatty lesions) on MRI‐SI joints (12%) than on MRI‐spine (2%) at baseline. Consequently, the longitudinal association between BME and structural damage (e.g., ≥3 fatty lesions) on MRI‐SI joints (OR 5.1 [95% CI 2.7, 9.6]) was found with a substantially higher precision (narrower confidence intervals) compared to the same effect in the spine (OR 15.6 [95% CI 4.8, 50.3]). Although it may seem that the effect of inflammation on damage is stronger on the spine than on the SI joints (OR 16 versus 5), this is not necessarily the case. It is well known that imprecise estimates tend to overestimate effect sizes (36).
Evidence that inflammation on MRI drives structural damage in early axial SpA is relevant to the practicing rheumatologist because it argues in favor of its use for prognostic stratification. In addition, if inflammation drives damage, it is logical to expect that interventions targeting the former will prevent, or at least inhibit, the latter. However, thus far, trial data do not support this claim (37). The complex, and yet not fully understood, pathophysiology of new bone formation in axial SpA may, at least in part, explain this disappointing result. For instance, it has been shown that systemic inflammation, measured by the ASDAS, predicts spinal radiographic progression in radiographic axial SpA (6, 8). However, progression was still found in patients with inactive disease. Similarly, in another study, inflammation at the level of vertebral unit increased the likelihood of the formation of a new syndesmophyte in the same location 2 years later, but most new syndesmophytes appeared in vertebral units without signs of inflammation (12). These data highlight the relevance of inflammation in driving structural progression but also suggest that other mechanisms may play a role.
Biology, however, cannot fully explain the failure of antiinflammatory drugs in modifying the effect of inflammation on structural damage. The lack of sensitivity to change of the outcome measures has also been proposed previously as a likely explanation (38). If an intervention truly prevents further damage by reducing inflammation (or by any other means), low sensitivity to change of the outcome measure may prevent that such effect becomes evident (e.g., no significant difference between active drug and placebo). Thus far, progression of structural damage has been measured mostly using conventional radiographs, with the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) and the mNY grading system as the outcome measures used most often in the spine and SI joints, respectively. However, both the mSASSS and the mNY have low sensitivity to change, and assessing radiographic progression with the latter is further challenged by its poor reliability (3, 14, 15, 39). It remains to be proven that structural lesions detected on MRI are more sensitive to change than those on radiographs. However, our study suggests that different lesions may yield different results. For instance, compared with erosions or bony spurs, fatty lesions were more prevalent in our population of patients with early axial SpA, especially in the SI joints, leading to more precise estimates. Thus, our data may inform future research aiming at clarifying whether MRI is a valid alternative to conventional radiography in detecting structural treatment effects in patients with axial SpA.
Our study is not without limitations. First, inflammatory and structural lesions, per patient, were read together by the same reader, which obviously may result in overestimating the association between both. This contrasts with other studies in which inflammation and damage were blindly measured using different imaging modalities. However, it should be stressed that readers were still blinded to time order. That is, they did not know if a certain lesion (e.g., BME) pertained to a baseline or to a follow‐up image. Thus, causality by reading, although not impossible, is unlikely to fully explain the impressive associations found in our study. Second, the lack of an association between vertebral corner inflammation on MRI‐spine and erosions and bone spurs should be interpreted with caution. Even though a true lack of association cannot be ruled out, as mentioned above, this also may be due to low statistical power driven by a low number of these lesions in the spine. The role of inflammation on sites other than vertebral corners for the progression of spinal damage should be addressed in future studies.
In conclusion, we have shown that local inflammation is associated with development of structural damage (e.g., fatty lesions), both measured with MRI, over 5 years in the SI joints and spine in early axial SpA. This association is detected with more precision on the SI joints, where structural damage prevails, compared to the spine in early disease. These findings support the concept that MRI is a valid alternative to conventional radiographs in detecting the structural consequences of axial inflammation in patients with early axial SpA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Sepriano had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Sepriano, Ramiro, Landewé, van der Heijde.
Acquisition of data
Moltó, Claudepierre, Wendling, Dougados.
Analysis and interpretation of data
Sepriano, Ramiro, Landewé, Moltó, Claudepierre, Wendling, Dougados, van der Heijde.
Supporting information
Supplementary Table 1 Effect of ≥5 BME lesions on MRI‐Spine on MRI‐spine structural outcomes
ACKNOWLEDGMENTS
The authors thank the following at the different regional participating centers: Maxime Dougados (Paris‐Cochin B), Andre Kahan (Paris‐Cochin A), Philippe Dieudé (Paris‐Bichat), Bruno Fautrel (Paris‐La Pitie‐Salpetriere), Francis Berenbaum (Paris‐Saint‐Antoine), Pascal Claudepierre (Creteil), Maxime Breban (Boulogne‐Billancourt), Bernadette Saint‐Marcoux (Aulnay‐sous‐Bois), Philippe Goupille (Tours), Jean Francis Maillefert (Dijon), Emmanuelle Dernis (Le Mans), Daniel Wendling (Besancon), Bernard Combe (Montpellier), Liana Euller‐Ziegler (Nice), Pascal Richette (ParisLariboisiere), Pierre Lafforgue (Marseille), Patrick Boumier (Amiens), Martin Soubrier (ClermontFerrand), Nadia Mehsen (Bordeaux), Damien Loeuille (Nancy), Rene‐Marc Flipo (Lille), Alain Saraux (Brest), Xavier Mariette (LeKremlin‐Bicetre), Alain Cantagrel (Toulouse), and Olivier Vittecoq (Rouen). They also thank the research nurses, the staff members of the Clinical Research Unit of Paris Centre, the staff members of the Biological Resource Center of Bichat Hospital, the staff members of the Department of Statistics of Nîmes, and all the investigators, and in particular Jerome Allain, Emmanuelle Dernis, Salah Ferkal, Clement Prati, Marie‐Agnes Timsit, and Eric Toussirot, for active patient recruitment and monitoring. The authors are grateful to Miranda van Lunteren for her work in preparing the database used in this study, and to all DESIR readers, without whom this study would not be possible.
The DESIR cohort is financially supported by an unrestricted grant from Pfizer and is conducted under the control of Assistance publique Hopitaux de Paris via the Clinical Research Unit Paris Centre and under the umbrella of the French Society of Rheumatology and Institut national de la sante et de la recherche medicale (INSERM). Database management is performed within the Department of Epidemiology and Biostatistics. Dr. Sepriano's work was supported by the Fundação para a Ciência e Tecnologia (doctoral grant SFRH/BD/108246/2015).
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Ramiro S, Stolwijk C, van Tubergen A, van der Heijde D, Dougados M, van den Bosch F, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow‐up of the OASIS study. Ann Rheum Dis 2015;74:52–9. [DOI] [PubMed] [Google Scholar]
- 2. Poddubnyy D, Brandt H, Vahldiek J, Spiller I, Song IH, Rudwaleit M, et al. The frequency of non‐radiographic axial spondyloarthritis in relation to symptom duration in patients referred because of chronic back pain: results from the Berlin early spondyloarthritis clinic. Ann Rheum Dis 2012;71:1998–2001. [DOI] [PubMed] [Google Scholar]
- 3. Dougados M, Sepriano A, Molto A, van Lunteren M, Ramiro S, de Hooge M, et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5‐year data of the DESIR cohort. Ann Rheum Dis 2017;76:1823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 5. Baraliakos X, Listing J, Rudwaleit M, Sieper J, Braun J. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther 2008;10:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poddubnyy D, Protopopov M, Haibel H, Braun J, Rudwaleit M, Sieper J. High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 2016;75:2114–8. [DOI] [PubMed] [Google Scholar]
- 7. Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Märker‐Hermann E, Zeidler H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:1369–74. [DOI] [PubMed] [Google Scholar]
- 8. Ramiro S, van der Heijde D, van Tubergen A, Stolwijk C, Dougados M, van den Bosch F, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12‐year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. [DOI] [PubMed] [Google Scholar]
- 9. Machado PM, Baraliakos X, van der Heijde D, Braun J, Landewé R. MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2016;75:1486–93. [DOI] [PubMed] [Google Scholar]
- 10. Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 2009;60:93–102. [DOI] [PubMed] [Google Scholar]
- 11. Baraliakos X, Heldmann F, Callhoff J, Listing J, Appelboom T, Brandt J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti‐TNF agents? A long‐term observational study using MRI and conventional radiography. Ann Rheum Dis 2014;73:1819–25. [DOI] [PubMed] [Google Scholar]
- 12. Van der Heijde D, Machado P, Braun J, Hermann KG, Baraliakos X, Hsu B, et al. MRI inflammation at the vertebral unit only marginally predicts new syndesmophyte formation: a multilevel analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:369–73. [DOI] [PubMed] [Google Scholar]
- 13. Sepriano A, Rudwaleit M, Sieper J, van den Berg R, Landewé R, van der Heijde D. Five‐year follow‐up of radiographic sacroiliitis: progression as well as improvement? Ann Rheum Dis 2016;75:1262–3. [DOI] [PubMed] [Google Scholar]
- 14. Van den Berg R, Lenczner G, Feydy A, van der Heijde D, Reijnierse M, Saraux A, et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs: results from the DESIR cohort. Arthritis Rheumatol 2014;66:2403–11. [DOI] [PubMed] [Google Scholar]
- 15. Van Tubergen A, Heuft‐Dorenbosch L, Schulpen G, Landewé R, Wijers R, van der Heijde D, et al. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann Rheum Dis 2003;62:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ostergaard M, Maksymowych WP, Pedersen SJ, Chiowchanwisawakit P, Lambert RG. Structural lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis: definitions, assessment system, and reference image set. J Rheumatol 2009;36 Suppl 84:18–34. [Google Scholar]
- 17. Krabbe S, Sørensen IJ, Jensen B, Møller JM, Balding L, Madsen OR, et al. Inflammatory and structural changes in vertebral bodies and posterior elements of the spine in axial spondyloarthritis: construct validity, responsiveness and discriminatory ability of the anatomy‐based CANDEN scoring system in a randomised placebo‐controlled trial. RMD Open 2018;4:e000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber U, Lambert RG, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty‐seven subjects. Arthritis Rheum 2010;62:3048–58. [DOI] [PubMed] [Google Scholar]
- 19. Maksymowych WP, Lambert RG, Østergaard M, Pedersen SJ, Machado PM, Weber U, et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis 2019;78:1550–8. [DOI] [PubMed] [Google Scholar]
- 20. Lukas C, Cyteval C, Dougados M, Weber U. MRI for diagnosis of axial spondyloarthritis: major advance with critical limitations ‘Not everything that glisters is gold (standard)’. RMD Open 2018;4:e000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramiro S, Claudepierre P, Sepriano A, van Lunteren M, Molto A, Feydy A, et al. Which scoring method depicts spinal radiographic damage in early axial spondyloarthritis best? Five‐year results from the DESIR cohort. Rheumatology (Oxford) 2018;57:1991–2000. [DOI] [PubMed] [Google Scholar]
- 22. Dougados M, Etcheto A, Molto A, Alonso A, Bouvet S, Daurès JP, et al. Clinical presentation of patients suffering from recent onset chronic inflammatory back pain suggestive of spondyloarthritis: the DESIR cohort. Joint Bone Spine 2015;82:345–51. [DOI] [PubMed] [Google Scholar]
- 23. De Hooge M, Pialat JB, Reijnierse M, van der Heijde D, Claudepierre P, Saraux A, et al. Assessment of typical SpA lesions on MRI of the spine: do local readers and central readers agree in the DESIR‐cohort at baseline? Clin Rheumatol 2017;36:1551–9. [DOI] [PubMed] [Google Scholar]
- 24. Jacquemin C, Rubio Vargas R, van den Berg R, Thévenin F, Lenczner G, Reijnierse M, et al. What is the reliability of non‐trained investigators in recognising structural MRI lesions of sacroiliac joints in patients with recent inflammatory back pain? Results of the DESIR cohort. RMD Open 2016;2:e000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Hooge M, van den Berg R, Navarro‐Compan V, Reijnierse M, van Gaalen F, Fagerliet K, al. Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann Rheum Dis 2016;75:1308–14. [DOI] [PubMed] [Google Scholar]
- 26. Bennett AN, Rehman A, Hensor EM, Marzo‐Ortega H, Emery P, McGonagle D. The fatty Romanus lesion: a non‐inflammatory spinal MRI lesion specific for axial spondyloarthropathy. Ann Rheum Dis 2010;69:891–4. [DOI] [PubMed] [Google Scholar]
- 27. Lambert RG, Bakker PA, van der Heijde D, Weber U, Rudwaleit M, Hermann KG, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958–63. [DOI] [PubMed] [Google Scholar]
- 28. Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:703–9. [DOI] [PubMed] [Google Scholar]
- 29. Rudwaleit M, Jurik AG, Hermann KG, Landewé R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. [DOI] [PubMed] [Google Scholar]
- 30. Hermann KG, Baraliakos X, van der Heijde DM, Jurik AG, Landewé R, Marzo‐Ortega H, et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis 2012;71:1278–88. [DOI] [PubMed] [Google Scholar]
- 31. Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Krishnananthan R, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9. [DOI] [PubMed] [Google Scholar]
- 32. Madari Q, Sepriano A, Ramiro S, Molto A, Claudepierre P, Wendling D, et al. 5‐year follow‐up of spinal and sacroiliac MRI abnormalities in early axial spondyloarthritis: data from the DESIR cohort. RMD Open 2020;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sepriano A, Ramiro S, Landewé R, Dougados M, van der Heijde D. Percentage of progressors in imaging: can we ignore regressors? RMD Open 2019;5:e000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker‐Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 35. Wanders AJ, Landewé RB, Spoorenberg A, Dougados M, van der Linden S, Mielants H, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum 2004;50:2622–32. [DOI] [PubMed] [Google Scholar]
- 36. Van Calster B, Steyerberg EW, Collins GS, Smits T. Consequences of relying on statistical significance: some illustrations. Eur J Clin Invest 2018;48:e12912. [DOI] [PubMed] [Google Scholar]
- 37. Van der Heijde D, Landewé R. Inhibition of spinal bone formation in AS: 10 years after comparing adalimumab to OASIS. Arthritis Res Ther 2019;21:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maksymowych WP. The role of imaging in the diagnosis and management of axial spondyloarthritis. Nat Rev Rheumatol 2019;15:657–72. [DOI] [PubMed] [Google Scholar]
- 39. Ramiro S, van der Heijde D, Sepriano A, van Lunteren M, Moltó A, Feydy A, et al. Spinal radiographic progression in early axial spondyloarthritis: five‐year results from the DESIR cohort. Arthritis Care Res (Hoboken) 2019;71:1678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Effect of ≥5 BME lesions on MRI‐Spine on MRI‐spine structural outcomes


