Abstract
Purpose
We present the case of a 56-year-old man with stage IV sarcoidosis on veno-venous extracorporeal membrane oxygenation (VV-ECMO) support for the management of respiratory failure receiving treatment with isavuconazole for invasive aspergillosis.
Summary
VV-ECMO is an increasingly utilized life support therapy for patients with cardiac and/or respiratory failure, but its impact on medication dosing is poorly understood. In our patient with invasive Aspergillus infection receiving VV-ECMO, because of difficulty achieving therapeutic serum concentrations of voriconazole, we administered isavuconazole 372 mg intravenously (IV) every 8 hours for 6 doses followed by 372 mg IV once daily. Isavuconazole has a favorable pharmacokinetic and safety profile compared to other azole antifungal agents, but its high protein binding and lipophilicity raise concerns about drug sequestration in the VV-ECMO circuit. To optimize the efficacy and safety of this treatment, the isavuconazole trough concentration was measured at days 5 and 17, at which time it was 1.7 and 0.7 μg/mL, respectively. The dose was subsequently increased to 744 mg IV once daily, and serum trough concentrations were measured 5 and 8 days after dose adjustment, corresponding to 3.7 and 2.9 μg/mL, respectively. To our knowledge, this is the third report to describe inadequate isavuconazole trough concentrations during VV-ECMO support when utilizing standard doses.
Conclusion
In the case described here, standard-dose isavuconazole (372 mg every 8 hours for 6 doses followed by 372 mg daily) did not achieve target trough concentrations in a patient receiving concomitant ECMO support.
Keywords: critical care, extracorporeal membrane oxygenation, infectious diseases, isavuconazole, pharmacokinetics
Key Points
Drug sequestration, increases in volume of distribution, and altered metabolism of medications occur with extracorporeal membrane oxygenation (ECMO) support and may lead to unpredictable serum concentrations of antimicrobial agents.
Isavuconazole’s high lipophilicity and high protein binding are predictors of drug sequestration in patients receiving concomitant ECMO support.
Isavuconazole does not routinely require therapeutic drug monitoring; however, the effect of ECMO on its pharmacokinetics has not been established. Therefore, therapeutic drug monitoring may assist in optimizing pharmacokinetic and pharmacodynamic targets.
Extracorporeal membrane oxygenation (ECMO) is an increasingly utilized life support therapy for patients with cardiac and/or respiratory failure. Despite increasing use, the impact of ECMO on medication dosing remains poorly understood. Critically ill patients receiving ECMO support are subject to significant alterations in pharmacokinetics, including increases in the volume of distribution (Vd), changes to drug clearance, and drug sequestration within the ECMO circuit and components.1 Moreover, critical illness may induce additional pharmacokinetic changes, including altered protein binding and impaired organ function, leading to further complications in patient care. The cumulative impact of both ECMO and critical illness on pharmacokinetics in individual patients makes optimization of drug dosing in this setting extremely challenging.
Isavuconazole is a second-generation triazole antifungal, approved for patients 18 years and older for the treatment of invasive aspergillosis and invasive mucormycosis. Compared to alternative azole antifungal agents, isavuconazole has more stable pharmacokinetics and an improved safety profile, with minimal incidence of adverse events. Important pharmacokinetic characteristics of isavuconazole include its high bioavailability (98%), high protein binding (>99%), and high lipophilicity (logP = 3.6), resulting in extensive drug distribution to tissues (Vd = 450 L).2,3 Isavuconazole is supplied as isavuconazonium sulfate, a highly water-soluble prodrug that is converted to the lipophilic isavuconazole in the systemic circulation. Concerns about drug sequestration within the ECMO circuit arise when using medications with high protein binding and lipophilicity; therefore, this concept warrants further investigation with regard to isavuconazole. Previous data on isavuconazole treatment in patients receiving ECMO support consist of 2 reports describing difficulty reaching therapeutic concentrations, potentially as a result of drug sequestration.4,5 The current case report adds additional information supporting the use of therapeutic drug monitoring in a patient on veno-venous ECMO (VV-ECMO) being treated for an aspergilloma with isavuconazole.
Case report
A 56-year-old Hispanic man (58 kg; body mass index, 20.3 kg/m2) with a past medical history of anxiety and stage IV sarcoidosis receiving chronic steroid therapy underwent upper lobectomy of the right lung 3 years before admission for the management of symptomatic aspergilloma despite chronic voriconazole therapy. The surgery was complicated by a bronchopleural fistula that required surgical management (bronchoplasty with a muscle flap and endobronchial stent) 2 months later. Unfortunately, the bronchopleural fistula recurred approximately 3 months before admission, and the patient was brought to our hospital for an Eloesser flap procedure to manage his persistent bronchopleural fistula and right pneumothorax. Following the procedure, bronchial and pleural fluid cultures grew Aspergillus fumigatus, for which voriconazole 240 mg (4 mg/kg) intravenously (IV) every 12 hours was initiated. Because of his progressively worsening hypoxia and increased work to breathe despite the use of high-flow nasal cannula with an oxygen rate of 40 L/min, the patient was intubated on postoperative day 7. Arterial blood gases were assessed following intubation with ventilator settings of 100% for the fraction of inspired oxygen (FiO2) and 7.5 cm H2O for positive end expiratory pressure together with inhaled epoprostenol therapy. This assessment revealed a pH of 7.47, a partial oxygen pressure of 54 mm Hg, a partial carbon dioxide pressure of 51 mm Hg, and oxygen saturation of 80%. Additional strategies to optimize ventilator support were limited due to persistent pneumothorax. Given these limitations and the rapid progression of respiratory decompensation, the decision was made to place the patient on VV-ECMO for additional lung support several hours after intubation.
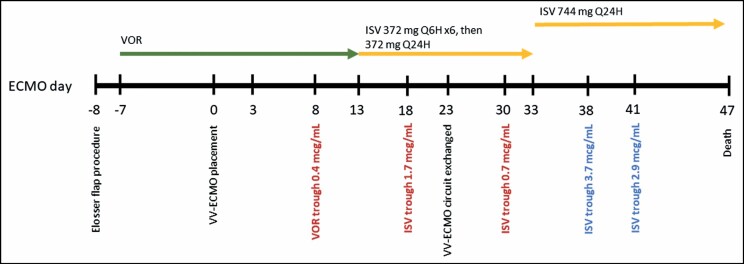
The VV-ECMO circuit comprised P.h.i.s.i.o.-coated polyvinylchloride perfusion tubing (LivaNova USA, Inc., Arvada, CO) and a Rotaflow centrifugal pump with a Quadrox-iD adult oxygenator (Maquet Getinge Group, Rastatt, Germany). Initial VV-ECMO settings included the following: pump flow rate, 4 L/min; pump speed, 3,000 rotations/min; blender FiO2, 100%; and sweep, 2 L/min. No additional pertinent therapies, including renal replacement therapies and/or CytoSorbents technologies (CytoSorbents, Monmouth Junction, NJ), were utilized during the admission. Following VV-ECMO initiation, the steady-state voriconazole trough concentration was assessed on day 14 of treatment, corresponding to 0.4 μg/mL (Figure 1). Because of difficulties achieving therapeutic serum concentrations of voriconazole, therapy was changed to isavuconazole 372 mg IV every 8 hours for 6 doses followed by 372 mg IV once daily. Isavuconazole was chosen over amphotericin B because of its more favorable adverse event profile in the treatment of invasive infections with long durations of therapy. Given the patient’s severity of illness, the likelihood of VV-ECMO circuit sequestration of isavuconazole based on its physicochemical properties, and the intent to optimize isavuconazole’s concentration-dependent antifungal activity, therapeutic drug monitoring of isavuconazole was performed. All samples were drawn from a central venous catheter in a consistent manner and were sent to a reference laboratory (Eurofins Viracor, Inc., Lee’s Summit, MO) and analyzed using liquid chromatography followed by tandem mass spectrometry.
Figure 1.
Clinical time course. ECMO indicates extracorporeal membrane oxygenation; ISV, isavuconazole; VV-ECMO, veno-venous extracorporeal membrane oxygenation.
On the basis of previous data, a goal trough concentration of 2 to 4 μg/mL was established.6-8 Before and during isavuconazole treatment, hepatic function remained within normal limits. The baseline serum creatinine level was 0.6 mg/dL, and the creatinine level remained stable for the duration of treatment with isavuconazole. Additional pertinent laboratory values included a serum albumin concentration that was consistently less than 1.5 mg/dL. Throughout treatment with isavuconazole, no known or potential drug interactions were identified, including interactions affecting cytochrome P-450 and/or p-glycoprotein. On day 5 of isavuconazole therapy, a serum trough concentration was obtained with a measure of 1.7 μg/mL. No dose adjustment was made following the initial measurement. A repeat isavuconazole trough concentration measurement was performed 12 days after determination of the initial level with a result of 0.7 μg/mL. Notably, the VV-ECMO circuit was exchanged approximately 7 days before the follow-up measurement of serum concentration due to the presence of blood clots within the circuit and a subsequent decline in the function of the membrane oxygenator. In response to the subtherapeutic concentration, the isavuconazole dose was increased to 744 mg IV once daily, and a serum trough concentration was measured 5 days after dose adjustment. Following the dose increase, the serum trough concentration increased to 3.7 μg/mL. Another serum trough level was obtained 8 days after the dose increase, with the concentration remaining therapeutic at 2.9 μg/mL. For the remainder of treatment with isavuconazole, there were no observed adverse effects associated with the dose increase, including gastrointestinal effects or shortened QTc interval. A complete timeline outlining dosing and therapeutic drug monitoring results is shown in Figure 1.
Despite advanced surgical and medical therapies, the patient continued to worsen, with hypoxic respiratory failure in the setting of maximal support with mechanical ventilation through a tracheostomy site and VV-ECMO. He developed several complications of therapy, including septic shock, thrombocytopenia, and ischemic limbs. Supportive care measures including VV-ECMO were continued until a decision was made by the patient’s family to pursue comfort-directed measures. Fifty-three days following admission, the patient died from complications of respiratory failure.
Discussion
To our knowledge, this is the third report describing altered isavuconazole serum concentrations during ECMO support and subsequent dose adjustments. Despite isavuconazole’s improved pharmacokinetic profile compared to alternate antifungal agents, we found a significant decrease in isavuconazole serum concentrations during VV-ECMO therapy. Utilizing the standard loading and maintenance dose (372 mg IV every 8 hours for 6 doses, followed by 372 mg IV once daily), we were unable to achieve adequate serum trough concentrations. Although data specific to isavuconazole in VV-ECMO are minimal, reports examining similar azole antifungal agents are consistent with our findings. Previous studies examining the impact of ECMO on voriconazole have found significant drug loss due to sequestration, with an estimated 45% to 70% lost depending on circuit size.9,10 The effect of ECMO on posaconazole is poorly understood, as ex vivo studies have shown drug loss of up to 63% over 24 hours, while case series and reports have identified more variable effects ranging from no to slight alterations in serum trough values.11
It is well understood that high protein binding and lipophilicity are important physiochemical properties that predict drug loss within an ECMO circuit.12,13 As with voriconazole and posaconazole, key pharmacokinetic properties of isavuconazole include its high protein binding and high lipophilicity. Compared to voriconazole, isavuconazole has a higher protein-binding capacity (58% vs 99%) and greater lipophilicity (logP = 2.1 vs 3.6).4 On the basis of these properties, particularly in comparison to agents known to be impacted by ECMO therapy, the potential for sequestration of isavuconazole and subsequent reduction in serum drug concentrations is high. In our case, standard dosing of isavuconazole resulted in significantly lower trough concentrations than expected. Increasing the dose to twice the standard maintenance dose (744 mg/day) was necessary to achieve trough values of 2 to 4 μg/mL. This finding is consistent with previous reports of isavuconazole therapeutic drug monitoring during concomitant ECMO support. In both reports, use of standard-dose isavuconazole during concomitant ECMO therapy resulted in comparatively lower serum trough values, including potentially subtherapeutic values.4,5,7 In a mixed cohort of patients reported by Zurl and colleagues,5 patients receiving extracorporeal therapies were found to have lower serum trough values than those not receiving extracorporeal therapies. Extracorporeal therapies used in this cohort included ECMO, continuous renal replacement therapy, and CytoSorbents technologies.5 Additionally, in a case report presented by Zhao and colleagues,4 use of standard-dose isavuconazole for treatment of blastomycosis resulted in an initial isavuconazole trough concentration of 1.9 μg/mL. As in our report, the patient required an increased isavuconazole dose and was administered 372 mg twice daily to achieve trough levels of 4.1 μg/mL and 4.7 μg/mL. While the dose adjustment strategy was different in this previous case (doubling the frequency rather than the dose) than in our case, the outcomes were similar in that both regimens achieved a trough concentration within the range supported for therapeutic efficacy. These case reports together highlight the potential importance of therapeutic drug monitoring for isavuconazole in this complex patient population.
Our patient case provides insight into the appropriate timing of therapeutic drug monitoring in patients receiving isavuconazole while on ECMO. In our case, the initial isavuconazole trough concentration was 1.7 μg/mL, which was subtherapeutic based on our established goal of 2 to 4 μg/mL, but within the margin of error that did not warrant a dose adjustment. Following a circuit exchange, the isavuconazole trough concentration declined markedly to 0.7 μg/mL. Adsorption of drug following ECMO circuit exchange has been described previously with voriconazole and may result in reduced serum concentrations until the membrane oxygenator is saturated.14 Following saturation of the membrane oxygenator, significant increases in serum concentration may occur, illustrating the need for follow-up monitoring. In a case described by Spriet and colleagues,15 use of voriconazole during ECMO support led to initial subtherapeutic concentrations followed by supratherapeutic values after presumed saturation of the membrane oxygenator. Given this observation, it may be prudent to evaluate isavuconazole trough values with the initial dose, when the ECMO circuit is exchanged, and following any dose adjustments.15
In previous literature, steady-state trough concentrations are achieved on day 3 following loading doses and day 14 when no loading dose is utilized.15 Therapeutic drug monitoring of isavuconazole to optimize efficacy and safety is not routinely recommended, resulting in a lack of clearly defined goal serum concentrations. In our patient, we targeted a goal serum trough concentration of 2 to 4 μg/mL based on reports from clinical trials and postmarketing experience with isavuconazole. Previous literature suggests that most patients maintain a therapeutic level between 2 and 4 μg/mL,6-8 with an upper limit set at 5 μg/mL to minimize gastrointestinal adverse effects.11 A study examining plasma concentrations in 283 samples from real-world patients receiving isavuconazole within the SECURE trials found mean plasma concentrations of 2.98 and 3.30 μg/mL in phase 1 and phase 3 trials, respectively, although it should be noted that neither study included patients who were unlikely to survive 30 days or those on mechanical ventilation.6,7 Borman and colleagues8 found similar results using data from multiple laboratories in the United Kingdom to assess trough levels in 150 samples. In their study, most patients (41.3%) were reported to have levels of 2 to 4 μg/mL.8 Because of the predictability of isavuconazole pharmacokinetics in both clinical trials and real-world patients, routine monitoring is unlikely to be useful.6-8 However, our report suggests the utility of therapeutic drug monitoring in critically ill patients on ECMO due to considerable pharmacokinetic changes.
The data presented within this case report are not without limitations. First, given the long half-life of isavuconazole (~130 hours), steady-state serum concentrations may not be reached until several weeks after the initiation of therapy. The loading dose of 372 mg every 8 hours for 6 doses provides a way to attain steady state more quickly; however, the exact time at which steady-state equilibrium is reached, particularly during ECMO support, is unknown.2 In this patient, we obtained follow-up serum concentrations at 5 and 8 days after dose adjustment. Given the extremely long half-life of the drug, this may have been too early to capture further accumulation within the patient or further sequestration within the ECMO circuit. Additionally, we did not reload the patient at the time of the dose increase. As in the standard dosing regimen, providing a loading regimen of isavuconazole for the first 48 hours following the dose adjustment may have allowed quicker achievement of steady-state trough values.
Conclusion
In the case described here, standard-dose isavuconazole (372 mg every 8 hours for 6 doses followed by 372 mg daily) did not achieve target trough concentrations in a patient receiving concomitant ECMO support.
Contributor Information
Michelle Miller, Department of Pharmacy, Temple University Hospital, Philadelphia, PA, USA.
Geena Kludjian, Department of Pharmacy, Temple University Hospital, Philadelphia, PA, USA.
Kerry Mohrien, Department of Pharmacy, Temple University Hospital, Philadelphia, PA, USA.
Kazumi Morita, Department of Pharmacy, Temple University Hospital, Philadelphia, PA, USA.
Disclosures
The authors have declared no potential conflicts of interest.
References
- 1. Cheng V, Abdul-Aziz MH, Roberts JA, Shekar K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J Thorac Dis. 2018;10(suppl 5):S629-S641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cresemba (isavuconazole). Package insert. Astellas Pharma US; 2019. [Google Scholar]
- 3. Larger P, Wenzler U, Schmitt Hoffmann AH. Comparison of isavuconazole and other azoles with respect to physicochemical and pharmacokinetic properties affecting tissue penetration. Poster presented at: ESCMID; April 2017; Vienna, Austria. https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=52107 [Google Scholar]
- 4. Zhao Y, Seelhammer TG, Barreto EF, Wilson JW. Altered pharmacokinetics and dosing of liposomal amphotericin B and isavuconazole during extracorporeal membrane oxygenation. Pharmacotherapy. 2020;40(1):89-95. [DOI] [PubMed] [Google Scholar]
- 5. Zurl C, Waller M, Schwameis F, et al. Isavuconazole treatment in a mixed patient cohort with invasive fungal infections: outcome, tolerability and clinical implications of isavuconazole plasma concentrations. J Fungi. 2020;6(2):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desai A, Kovanda L, Kowalski D, et al. Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to aspergillus and other filamentous fungi. Antimicrob Agents Chemother. 2016;60(9):5483-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andes D, Kovanda L, Desai A, et al. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother. 2018;62(7):e00585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borman AM, Hughes JM, Oliver D, et al. Lessons from isavuconazole therapeutic drug monitoring at a United Kingdom reference center. Med Mycol. 2020;58(7):996-999. [DOI] [PubMed] [Google Scholar]
- 9. Mehta NM, Halwick DR, Dodson BL, et al. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33(6):1018-1024. [DOI] [PubMed] [Google Scholar]
- 10. Cies JJ, Moore WS 2nd, Giliam N, et al. Oxygenator impact on voriconazole in extracorporeal membrane oxygenation circuits. Perfusion. 2020;35(6):529-533. [DOI] [PubMed] [Google Scholar]
- 11. Furfaro E, Signori A, Di Grazia C, et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J Antimicrob Chemother. 2019;74(8):2341-2346. [DOI] [PubMed] [Google Scholar]
- 12. Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care. 2012;27(6):741.e9-741.e18. [DOI] [PubMed] [Google Scholar]
- 13. Shekar K, Roberts JA, Barnett AG, et al. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit Care. 2015;19:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winiszewski H, Rougny AC, Lagouttes-Renosi J, et al. The pharmacokinetic challenge of treating invasive aspergillosis complicating severe influenzae assisted by extracorporeal membrane oxygenation. Crit Care. 2018;22(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spriet I, Annaert P, Meersseman P, et al. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J Antimicrob Chemother. 2009;63(4):767-770. [DOI] [PubMed] [Google Scholar]