Abstract
Aim
To assess the diagnostic accuracy of temporal artery ultrasound compared with temporal artery biopsy and clinical diagnosis in patients with suspected giant cell arteritis (GCA) over 10 years in an Australian center.
Method
Patients presenting to Westmead Hospital with possible GCA from March 2011 to December 2020 were retrospectively identified. The following parameters were obtained from the medical record: clinical presentation, inflammatory markers, temporal artery ultrasound findings, and temporal artery biopsy report. Data were assembled in a 2 × 2 table; sensitivity and specificity of temporal artery ultrasound compared with temporal artery biopsy and clinical diagnosis were calculated.
Results
Over the 10‐year study period, 65 temporal artery ultrasounds were performed in 63 patients (n = 65; 61.9% female) with a mean ± standard deviation age of 69.6 ± 12.3 years. Thirteen out of 65 (20%) temporal artery ultrasounds had findings suggestive of GCA. Twenty patients (31.7%) had a clinical diagnosis of GCA irrespective of sonographic or biopsy findings. Compared with temporal artery biopsy, temporal artery ultrasound had a sensitivity of 71.4% and specificity of 93.3%. Compared with clinical diagnosis made by the treating rheumatologist, temporal artery ultrasound had a sensitivity of 55% and specificity of 95.3%.
Conclusion
Temporal artery ultrasound is a useful non‐invasive investigation in the assessment of suspected GCA. If positive in the setting of a suggestive clinical presentation, a temporal artery ultrasound probably avoids the need for a temporal artery biopsy. Temporal artery ultrasound could be more widely used in the clinical management of GCA.
Keywords: giant cell arteritis, temporal artery, temporal artery biopsy, temporal artery ultrasound
1. INTRODUCTION
Giant cell arteritis (GCA) is the commonest large‐vessel vasculitis, mainly affecting elderly people of Caucasian ancestry, and has an age‐adjusted incidence rate of approximately 2.2/10 000 person‐years. 1 It is less common in Asian countries. A nationwide Japanese study identified an age‐adjusted prevalence of 1.47/100 000. 2 Visual symptoms are reported in up to 26% of patients, with the dreaded complication of permanent visual loss in 15% of such patients—mainly due to anterior ischemic optic neuropathy. 3 Early diagnosis and commencement of corticosteroids are crucial in preventing blindness. Temporal artery biopsy has traditionally been considered the reference standard for diagnosis of GCA.4, 5, 6 However, there is often a delay in performing a temporal artery biopsy for logistical reasons and biopsy may miss the site of pathology because of “skip” lesions. 7 In Australia, a temporal artery biopsy usually requires a hospital admission with consequent increased healthcare costs and potential perioperative complications.
The use of ultrasound to diagnose GCA was first reported over 25 years ago. 8 The complexity and invasiveness of a temporal artery biopsy contrasts with the ease, non‐invasive nature, and immediate result of a temporal artery ultrasound. As such, the European Alliance of Associations for Rheumatology, formerly the European League Against Rheumatism (EULAR), recommends that in centers with readily available high‐quality imaging, ultrasound of the temporal artery, with or without the axillary arteries, should be the first investigation in those with suspected GCA, rather than a temporal artery biopsy. 6 The Outcome Measures in Rheumatology (OMERACT) Large‐Vessel Vasculitis Ultrasound Working Group recommended using the “halo” sign (vessel wall edema and inflammation), and the “compression” sign (inability to ablate the vessel lumen because of wall inflammation) to identify GCA on temporal artery ultrasound. 9 A recent study of 430 patients with suspected GCA found moderate agreement between sonographers (intra‐class correlation coefficient 0.61, 95% confidence interval [CI] 0.48‐0.75), similar to pathologists reporting temporal artery biopsies (0.62, 95% CI 0.49‐0.76). 10 Fast‐track ultrasound clinics for GCA assessment in Europe, and more recently in northern America, have demonstrated promising results.11, 12
Although point‐of‐care ultrasound is widely used in rheumatology for the assessment of inflammatory arthritis and in guiding joint aspiration and injections,13, 14, 15 its use for GCA diagnosis has been limited in Australia. Ultrasound has the potential to lower healthcare costs associated with temporal artery biopsy, because the procedure usually requires day‐admission in Australian hospitals where temporal artery biopsies are rarely performed in surgical rooms alone. This also avoids the morbidity potentially associated with a temporal artery biopsy, for example wound infection, scalp necrosis, and facial nerve injury. Temporal artery ultrasound could also reduce the delay in diagnosing GCA.
The aim of the study was to assess the diagnostic accuracy of temporal artery ultrasound compared with the reference standard of temporal artery biopsy in patients presenting to our center with suspected GCA over 10 years.
2. MATERIALS AND METHODS
This was a single‐center, retrospective study conducted at Westmead Hospital, a tertiary referral teaching hospital in Sydney, Australia. Patients with suspected GCA who underwent a temporal artery ultrasound between March 2011 and December 2020 were identified from the Ultrasound Department's database. Temporal artery ultrasounds performed for GCA diagnosis were included in the study, those performed for other purposes were excluded. Scans were performed on Philips iU22 machines between 2011 and 2013. Between 2014 and 2017, Philips iU22 machines were gradually phased out and replaced by Toshiba/Canon Aplio 500 machines. Ultrasounds from 2018 onwards were all performed on Aplio 500 machines. Equipment settings on the Philips iU22 machines were as follows: hockey stick probe L15‐7 MHz, depth of field 1.5 cm, color Doppler scale 9.5 cm/s. Settings for the Aplio 500 machines were as follows: hockey stick L14‐7 MHz or high‐resolution linear array transducer L18‐7 MHz, depth of field 1.5 cm, color Doppler scale 10 cm/s, superb microvascular imaging (SMI) 2.4. Ultrasound stand‐off pads were used, as required.
Ultrasounds were performed by qualified sonographers registered with the Australian Sonographer Accreditation Registry. A total of 12 general sonographers with variable experience performed temporal artery ultrasounds over the study period. Ultrasounds were reported by two experienced radiologists (co‐authors DF and SA) and were considered positive for GCA based on interpretation of sonographic findings, provided as a concluding statement for each ultrasound. Sonographers and radiologists were unaware of biopsy results (usually unavailable at time of ultrasound), but were not blinded to clinical information. Evaluation of arteries other than the temporal artery was at sonographer discretion.
Corresponding clinical medical records were reviewed to retrieve temporal artery biopsy findings, patient demographics, and levels of pre‐treatment serum inflammatory markers (erythrocyte sediment rate [mm/h] and C‐reactive protein [mg/L]). Temporal artery biopsy reports were used to identify biopsy‐proven GCA. Diagnosis made by the treating rheumatologist at the time of hospital discharge was used as the definition of clinical GCA diagnosis. Rheumatologists were not blinded to temporal artery ultrasound nor to biopsy results.
2.1. Statistical analysis
These were performed using STATA (Stata V.17, StataCorp, College Station, TX, USA). Summary statistics were expressed as means and standard deviations. The strength of association between variables of interest was assessed using Spearman correlation coefficients (r‐value). The threshold for statistical significance was set at a P‐value below 0.05 (two‐tailed).
2.2. Ethics
This study was approved as a quality assurance project by the Westmead Scientific Advisory Quality Assurance Committee (No. 2001‐07 QA).
3. RESULTS
A total of 65 temporal artery ultrasounds were performed in 63 patients with suspected GCA over the 10‐year study period (Figure 1). Patient characteristics and presenting clinical features are outlined in Table 1. Nine patients (14%) had pre‐existing systemic rheumatic disease (n = 5 polymyalgia rheumatica, n = 3 rheumatoid arthritis, n = 1 medium‐vessel vasculitis). Eight of these were on immunosuppressives (n = 5 on prednisone, n = 2 on methotrexate, n = 1 on both) at the time of suspected GCA presentation.
FIGURE 1.
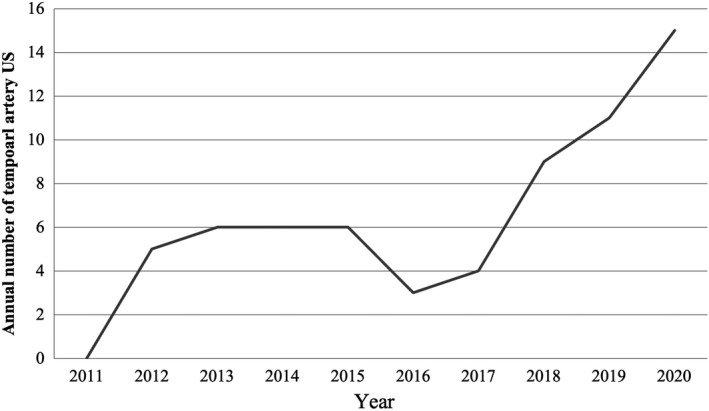
Annual number of temporal artery ultrasounds (US) performed per year between 2011 and 2020 at Westmead Hospital, Sydney, Australia
TABLE 1.
Characteristics of patients with suspected giant cell arteritis
| Patient characteristics (n = 63) | |
|---|---|
| Age (years), mean ± SD | 69.6 ± 12.3 |
| Gender (female:male, n [%]) | 39 (62%): 24 (38%) |
| Pre‐existing rheumatic disease, n (%) | n (%) |
| Polymyalgia rheumatica | 5 (8%) |
| Rheumatoid arthritis | 3 (5%) |
| Medium‐vessel vasculitis | 1 (2%) |
| Presenting symptoms, n (%) | n (%) |
| Headache | 45 (71%) |
| Visual changes | 19 (30%) |
| Jaw claudication | 11 (17%) |
| Limb girdle symptoms | 6 (10%) |
| Fatigue | 8 (13%) |
| Fever | 4 (6%) |
| Weight loss | 4 (6%) |
| Retro‐orbital pain | 3 (5%) |
| Generalized ache | 4 (6%) |
| Neck pain | 3 (5%) |
| Raised inflammatory markers at presentation | n (%) |
| ESR (>20 mm/h), n (%) | 52 (83%) |
| ESR (mm/h), mean ± SD | 63.7 ± 39.6 |
| CRP (>5 mg/L), n (%) | 38 (60%) |
| CRP (mg/L), mean ± SD | 64.4 ± 76.1 |
| Examination findings reported at presentation, n (%) | n (%) |
| Temporal artery tenderness | 15 (24%) |
| Temporal artery pulse absent | 6 (10%) |
| Temporal artery thickened | 7 (11%) |
| Anterior ischemic optic neuropathy/optic disk swelling | 5 (8%) |
Abbreviations: CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; SD, standard deviation.
Thirteen (20%) patients had a positive ultrasound for GCA, with either a “halo sign” or hypoechoic wall thickening documented on ultrasound reports. Ultrasound reporting was not standardized and included additional comments on temporal artery patency, stenosis/occlusion, or flow velocity. The “compression” sign was not measured in any of the reports. Figure 2 demonstrates representative examples of positive and negative temporal artery ultrasounds from our study sample.
FIGURE 2.
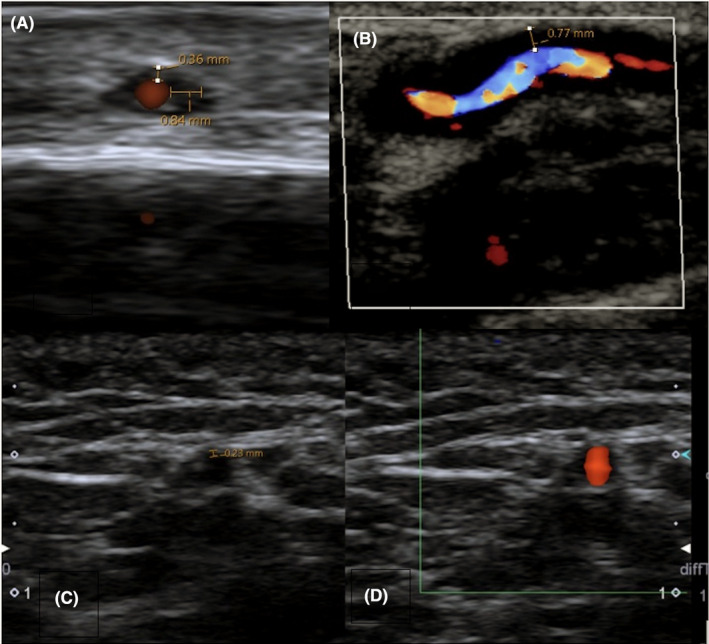
Positive and negative temporal artery ultrasounds. Transverse (A) and longitudinal (B) images of a positive temporal artery Doppler ultrasound with “halo” sign. Note thickened edematous wall of 0.84 mm in (A) and 0.77 mm in (B). (C) and (D) show transverse sonographic images of a normal temporal artery in gray‐scale (C) and with the Doppler setting (D). Images captured using Aplio 500 US machine (hockey stick L14‐7MHz transducer)
Forty‐five (69%) ultrasounds assessed both temporal arteries. One study examined the ipsilateral axillary artery, whereas another examined both carotid arteries looking for evidence of vasculitis. Two patients had a repeat temporal artery ultrasound of the contralateral side with the same result—which was negative for vasculitis.
Twenty (31.7%) patients were given a clinical diagnosis of GCA by the treating rheumatologist at the end of their hospital admission. Of these, 15 patients had a positive temporal artery biopsy, including nine patients who also had a positive temporal artery ultrasound. One patient was diagnosed with GCA based on a positive ultrasound alone and did not proceed to biopsy. One patient had a normal ultrasound and biopsy but had evidence of large‐vessel vasculitis on positron emission tomography‐computed tomography. Three patients with a negative biopsy and ultrasound were treated as GCA based on overall clinical picture.
Figure 3 shows the patients who underwent temporal artery ultrasound and temporal artery biopsies with corresponding results. Nineteen patients did not undergo a temporal artery biopsy following a temporal artery ultrasound. Within this group, one had a positive temporal artery ultrasound—this was deemed sufficient by the treating clinician to commence GCA treatment. The remaining 18 patients had a negative temporal artery ultrasound and were deemed unlikely to have GCA based on overall clinical assessment. Of these, only six patients were given an alternative diagnosis on discharge (infection, n = 4; stroke, n = 1; and inflammatory arthritis, n = 1). One patient’s symptoms and raised inflammatory markers resolved without intervention.
FIGURE 3.
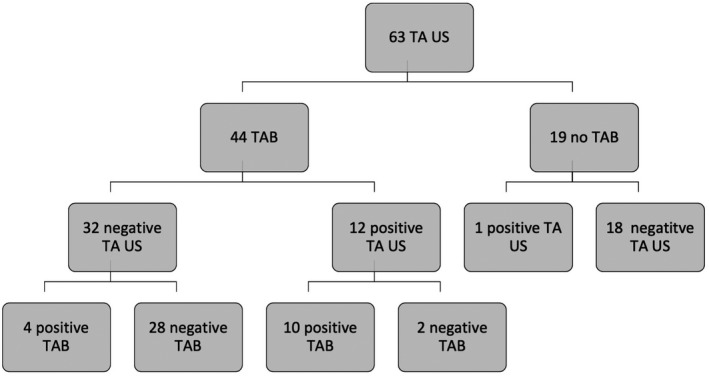
Flowchart of patients who underwent temporal artery ultrasound (TA US) and temporal artery biopsy (TAB)
Compared with temporal artery biopsy, temporal artery ultrasound had a sensitivity of 71.4%, specificity of 93.3%, positive predictive value of 83.3%, and negative predictive value of 87.5% (Table 2A). There was a moderately strong correlation between temporal artery ultrasound findings and the results of temporal artery biopsy (r‐value 0.67, P < 0.001). Six patients had discordant ultrasound and temporal artery biopsy results (Table 3). Of these, two patients had positive temporal artery ultrasound but negative biopsy: one was diagnosed with osteomyelitis of the right ear, and the second was diagnosed with temporomandibular disorder with raised erythrocyte sediment rate and C‐reactive protein attributed to underlying multiple myeloma. Compared with clinical diagnosis, temporal artery ultrasound had a sensitivity of 55%, specificity of 95.3%, positive predictive value of 84.6%, and negative predictive value of 83% (Table 2B).
TABLE 2.
2 × 2 table comparing (A) temporal artery ultrasound and temporal artery biopsy, and (B) temporal artery ultrasound and clinical diagnosis
| A. | TAB positive | TAB negative | |
|---|---|---|---|
| US positive | 10 | 2 | PPV 83.3% |
| US negative | 4 | 28 | NPV 87.5% |
| Sensitivity 71.4% | Specificity 93.3% |
| B. | Clinical diagnosis positive | Clinical diagnosis negative | |
|---|---|---|---|
| US positive | 11 | 2 | PPV 84.6% |
| US negative | 9 | 41 | NPV 83% |
| Sensitivity 55% | Specificity 95.3% |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; TAB, temporal artery biopsy; US, ultrasound.
TABLE 3.
Summary of patients with discordant temporal artery ultrasound and biopsy results
| Age (years) | Gender | Clinical details |
ESR (mm/h) a |
CRP (mg/L) a |
Ultrasound result | Temporal artery biopsy result |
|---|---|---|---|---|---|---|
| 61 | Male | Bilateral headache | 125 | 54 | Positive | Negative |
| Known multiple myeloma | ||||||
| Final diagnosis: TMJ disorder | ||||||
| 54 | Male | Right temporal headache | 76 | 9 | Positive | Negative |
| Final diagnosis: right ear osteomyelitis | ||||||
| 87 | Female | Jaw claudication | 51 | 24 | Negative | Positive |
| Blurred vision | ||||||
| Temporal artery tenderness | ||||||
| 60 | Female | Limb girdle symptoms | 115 | 95 | Negative | Positive |
| 67 | Female | Occipital headache | 83 | 36 | Negative | Positive |
| Blurred vision | ||||||
| 83 | Female | Temporal headache | 75 | 23 | Negative | Positive |
| Blurred vision |
Abbreviations: CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; TMJ, temporomandibular disorder.
ESR and CRP at presentation.
4. DISCUSSION
Although temporal artery ultrasound is recommended by EULAR as the first‐line imaging modality in patients with suspected GCA, it is used less commonly in Australian, Asian, and North American institutions compared with European centers. 16 This difference in practice and experience is reflected in the respective guidelines from EULAR and the American College of Rheumatology, with the American College of Rheumatology preferring unilateral temporal artery biopsy as the initial investigation.6, 17 Similarly, in Australia, temporal artery biopsy remains the reference standard for many rheumatologists despite its attendant morbidity and logistical delays. It is therefore essential to establish the diagnostic accuracy of temporal artery ultrasound in an Australian cohort compared with not only temporal artery biopsy, the traditional reference standard, but also clinical diagnosis.
In our single‐center study, temporal artery ultrasound had a sensitivity of 71.4% and specificity of 93.3% compared with temporal artery biopsy. Our cohort's sensitivity and specificity were comparable to findings from international centers, where the sensitivity ranged between 53% and 78% and the specificity between 79% and 81%.10, 18 A previous South Australian study comparing temporal artery ultrasound to biopsy found a lower sensitivity of 40%. 19 Our study's higher accuracy could be the result of improved ultrasound equipment and sonographer experience. Our study was not powered to compare the accuracy between older and newer ultrasound machines. Of note, the annual number of temporal artery ultrasounds performed at our center has increased over the last 3 years (Figure 1), suggesting a greater awareness of, and confidence in, the utility of this modality in GCA diagnosis.
More than half our patients with a negative temporal artery ultrasound proceeded to temporal artery biopsy. Four of them demonstrated histological features of GCA. This sequential use of negative temporal artery ultrasound followed by temporal artery biopsy is reflective of real‐world practice. A recent prospective study found that temporal artery biopsy following a negative temporal artery ultrasound improved sensitivity from 52.8% to 78.9% compared with temporal artery ultrasound alone. 20 The most cost‐effective strategy was to perform temporal artery ultrasound in all patients with suspected GCA followed by selective use of temporal artery biopsy, compared with a biopsy for all patients with suspected GCA. 10
Our study also compared the sensitivity and specificity of temporal artery ultrasound against clinical diagnosis and found a lower sensitivity of 55% (Table 2B). Furthermore, three patients eventually diagnosed with, and treated for GCA by a rheumatologist had a negative ultrasound and biopsy. Similar findings were demonstrated in other studies,10, 18 highlighting the challenges of GCA diagnosis where clinical assessment plays an important role. Potential explanations for a negative temporal artery ultrasound and biopsy in clinical GCA include extracranial vasculitis proven only on positron emission tomography‐computed tomography and reduced test accuracy because of previous high‐dose prednisone.10, 21
One of the major limitations of ultrasound is its user‐dependent nature. Standardization of ultrasound imaging protocols may improve sensitivity. A recent study showed that adding the axillary artery to GCA ultrasound assessment improved sensitivity from 52% to 71%. 22 Standardization of ultrasound reporting, by using the “halo sign” and “compression sign” to identify GCA as recommended by OMERACT, 9 may also improve its reliability and accuracy. In our cohort, 30 (46%) of our temporal artery ultrasounds predated the OMERACT statement. Measures have since been taken to ensure that the scanning protocol at our center includes careful assessment and reporting of these signs.
A small number of studies showed that sonographers could be readily up‐skilled in temporal artery ultrasound. A Spanish study enrolled 72 rheumatologists with no previous ultrasound expertise into a standardized training program consisting of one theoretical session, six image‐reading videos, and a single hands‐on acquisition training session. It was deemed effective based on high inter‐reader and intra‐reader κ coefficient, sensitivity and specificity compared with clinical diagnosis. 23 A more recent multicenter prospective study also found standardized training (5 hours of theoretical teaching, 10 hours of hands‐on experience) resulted in excellent agreement between trainee and expert sonographers. 24 Both studies suggested that an effective training program can be implemented in clinical practice to improve the reliability of clinician‐performed temporal artery ultrasound.
Our study had several limitations. First, it was performed in a single center. Expanding this to other sites would improve study generalizability. Second, study findings may have been confounded by indication. As cases were collected over 10 years, indications for, and clinician attitudes towards, temporal artery ultrasound may have altered over time. Lastly, our temporal artery ultrasounds were performed using several different types of machines by sonographers with a range of scanning experience. The imaging protocol was also not standardized—some patients underwent unilateral ultrasounds whereas others underwent bilateral imaging. An important outcome from this study has been implementation of a standard ultrasound protocol for GCA diagnosis in our center.
5. CONCLUSION
The sensitivity and specificity of temporal artery ultrasound at our center for the diagnosis of GCA was comparable to international data, confirming its potential use as a non‐invasive investigation in GCA assessment. The high specificity means that a positive temporal artery ultrasound may avoid the need for a temporal artery biopsy with the attendant morbidity, healthcare costs, delay in diagnosis and logistic issues. Given the increasing use of musculoskeletal ultrasound in Australia and Asia with wider availability of ultrasound machines and the relative ease of skill acquisition, temporal artery ultrasound could be more widely used in the management of GCA. Standardization of ultrasound imaging protocols and reporting in GCA and improved sonographer training would be valuable strategies to improve patient outcomes.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGEMENTS
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: A single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447–453. doi: 10.1111/1756-185X.14288
REFERENCES
- 1. Smeeth L, Cook C, Hall AJ. Incidence of diagnosed polymyalgia rheumatica and temporal arteritis in the United Kingdom, 1990–2001. Ann Rheum Dis. 2006;65(8):1093‐1098. doi: 10.1136/ard.2005.046912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ninan J, Lester S, Hill C. Diagnosis and management of giant cell arteritis: an Asian‐Pacific perspective. Int J Rheum Dis. 2019;22:28‐40. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez‐Gay MA, Garcia‐Porrua C, Llorca J, et al. Visual manifestations of giant cell arteritis: trends and clinical spectrum in 161 patients. Medicine. 2000;79(5):283‐292. doi: 10.1097/00005792-200009000-00001 [DOI] [PubMed] [Google Scholar]
- 4. Salvarani C, Cantini F, Boiardi L, et al. Polymyalgia rheumatica and giant‐cell arteritis. N Engl J Med. 2002;347(4):261‐271. doi: 10.1056/NEJMra011913 [DOI] [PubMed] [Google Scholar]
- 5. Ponte C, Martins‐Martinho J, Luqmani R. Diagnosis of giant cell arteritis. Rheumatology. 2020;59(Supplement_3):iii5‐iii16. doi: 10.1093/rheumatology/kez553 [DOI] [PubMed] [Google Scholar]
- 6. Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636‐643. doi: 10.1136/annrheumdis-2017-212649 [DOI] [PubMed] [Google Scholar]
- 7. Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg. 2017;20:1‐5. doi: 10.1016/j.amsu.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt WA, Kraft HE, Vorpahl K, et al. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337(19):1336‐1342. doi: 10.1056/NEJM199711063371902 [DOI] [PubMed] [Google Scholar]
- 9. Chrysidis A, Duftner C, et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT Large Vessel Vasculitis Ultrasound Woking Group. RMD Open. 2018;4:e000598. doi: 10.1136/rmdopen-2017-000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luqmani R, Lee E, Singh S, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and Treatment of Giant Cell Arteritis (TABUL): a diagnostic accuracy and cost‐effectiveness study. Health Technol Assess. 2016;20(90):1‐238. doi: 10.3310/hta20900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diamantopoulos A, Haugeberg G, Lindland A, Myklebust G. The fast‐track ultrasound clinic for early diagnosis of giant cell arteritis signficiantly reduces permanent visual impairments: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology. 2016;55:66‐70. doi: 10.1093/rheumatology/kev289 [DOI] [PubMed] [Google Scholar]
- 12. Oshinsky C, Bays A, Sacksen I, et al. Vascular ultrasound for giant cell arteritis: establishing a protocol using vascular sonographers in a fast‐tract clinic in the United States. ACR Open Rheumatol. 2021. doi: 10.1002/acr2.11346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mandl P, Aletaha D. The role of ultrasound and magnetic resonance imaging for treat to target in rhuematoid arthritis and psoriatic arthritis. Rheumatology. 2019;58:2091‐2098. doi: 10.1093/rheumatology/kez397 [DOI] [PubMed] [Google Scholar]
- 14. Wu T, Dong Y, Song HX, Fu Y, Li JH. Ultrasound‐guided versus landmark in knee arthrocentesis: a systematic review. Semin Arhtritis Rheum. 2015;45(5):627‐632. doi: 10.1016/j.semarthrit.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 15. Joplin S, Van Der Zwan R, Joshua F, Wong P. Medication adherence in patients with rheumatoid arthritis: the effect of patient education, health literacy, and musculoskeletal ultrasound. Biomed Res Int. 2015;2015:1‐10. doi: 10.1155/2015/150658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gribbons K, Ponte C, et al. Diagnostic assessment strategies and disease subsets in giant cell arteritis: data from an international observational cohort. Arthritis Rheumatol. 2020;72(4):667‐676. doi: 10.1002/art.41165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maz M, Chung S, Abril A, et al. 2021 American College of Rheumatology Vasculitis Foundation guideline for the management of giant cell arteritis and Takayasu arteritis. Arthritis Rheumatol. 2021;73(8):1349‐1365. doi: 10.1002/art.41774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rinagel M, Chatelus E, et al. Diagnostic performance of temporal artery ultrasound for the diagnosis of giant cell arteritis: a systematic review and meta‐analysis of the literature. Autoimmun Rev. 2019;18(1):56‐61. doi: 10.1016/j.autrev.2018.07.0212 [DOI] [PubMed] [Google Scholar]
- 19. Black R, Roach D, et al. The use of temporal artery ultrasound in the diagnosis of giant cell arteritis in routine practice. Int J Rheum Dis. 2013;16(3):352‐357. doi: 10.1111/1756-185X.12108 [DOI] [PubMed] [Google Scholar]
- 20. Conway R, O'Neill L, et al. Performance characteristics and predictors of temporal artery ultrasound for the diagnosis of giant cell arteritis in routine clinical practice in a prospective cohort. Clin Exp Rheumatol. 2019;37:72‐78. [PubMed] [Google Scholar]
- 21. Ponte C, Serafim A, Monti S, et al. Early variation of ultrasound halosign with treatment and relation with clinical features in patients with giant cell arteritis. Rheumatology. 2020;59(12):3717‐3726. doi: 10.1093/rheumatology/keaa196 [DOI] [PubMed] [Google Scholar]
- 22. Hop H, Mulder D, Sandovici M, et al. Diagnostic value of axillary artery ultrasound in patients with suspected giant cell arteritis. Rheumatology. 2020;59:3676‐3684. doi: 10.1093/rheumatology/keaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Miguel E, Castillo C, Rodriguez A, et al. Learning and reliability of colour Doppler ultrasound in giant cell arteritis. Clin Exp Rheumatol. 2009;27(1 Suppl 52):S53‐S58. [PubMed] [Google Scholar]
- 24. Chrysidis S, Terslev L, Christensen R, et al. Vascular ultrasound fo the diagnosis of giant cell arteritis: a reliability and agreement study based on a standardised training programme. RMD Open. 2020;6(3):e001337. doi: 10.1136/rmdopen-2020-001337 [DOI] [PMC free article] [PubMed] [Google Scholar]


