Abstract
Background
Maternal overnutrition during pregnancy predisposes the offspring to cardiometabolic diseases.
Objectives
This systematic review and meta‐analysis aimed to investigate the association between maternal overnutrition and offspring's blood pressure (BP) and the effect of offspring's obesity on this association.
Data sources
PubMed, EMBASE, Clinicaltrials.gov, CENTRAL.
Study selection and data extraction
Human studies published in English before October 2021 were identified that presented quantitative estimates of association between maternal overnutrition just before or during pregnancy and the offspring's BP.
Synthesis
Random‐effect model with the DerSimonian and Laird weighting method was used to analyse regression coefficients or mean differences.
Results
After selection, 17 observational studies (140,517 mother‐offspring pairs) were included. Prepregnancy body mass index (ppBMI) showed positive correlation with BP in offspring (regression coefficient for systolic: 0.38 mmHg per kg/m2, 95% confidence interval (CI) 0.17, 0.58; diastolic: 0.10 mmHg per kg/m2, 95% CI 0.05, 0.14). These indicate 1.9 mmHg increase in systolic and 0.5 mmHg increase in diastolic BP of offspring with every 5 kg/m2 gain in maternal ppBMI. Results on coefficients adjusted for offspring's BMI also showed association (systolic: 0.08 mmHg per kg/m2, 95% CI 0.04, 0.11; diastolic: 0.03 mmHg per kg/m2, 95% CI 0.01, 0.04). Independent from ppBMI, gestational weight gain (GWG) showed positive correlation with systolic BP (systolic BP: 0.05 mmHg per kg, 95% CI 0.01, 0.09), but not after adjustment for offspring's BMI. Mean systolic BP was higher in children of mothers with excessive GWG than in those of mothers with optimal GWG (difference: 0.65 mmHg, 95% CI 0.25, 1.05).
Conclusions
Independent from offspring's BMI, higher prepregnancy BMI may increase the risk for hypertension in offspring. The positive association between GWG and offspring's systolic BP is indirect via offspring's obesity. Reduction in maternal obesity and treatment of obesity in children of obese mothers are needed to prevent hypertension.
Keywords: blood pressure, developmental programming, gestational weight gain, hypertension, maternal obesity
Synopsis
Study question
We aimed to clarify the effect of the maternal overnutrition just before and during pregnancy separately on offspring's blood pressure in a meta‐analysis. In addition, we investigated the mediating effect of offspring's obesity on these associations.
What is already known?
Increasing evidence suggests that maternal overnutrition during pregnancy influences the developmental programming leading to cardiometabolic diseases including obesity and hypertension in the offspring.
What this study adds?
Our meta‐analysis provided evidence that higher maternal prepregnancy body mass index is associated with higher blood pressure even in lean offspring. We found that excessive gestational weight gain is also associated with higher systolic blood pressure in the offspring, but it may act indirectly via offspring's adiposity.
1. BACKGROUND
The global obesity challenge is growing dramatically in reproductive‐aged women, 1 increasing their prepregnancy body mass index (ppBMI). In addition, based on the US Institute of Medicine (IOM) guidelines, about 40% of women show an excessive gestational weight gain (GWG) according to their ppBMI in Western countries. 2 Maternal obesity may influence foetal and neonatal growth by developmental programming and predispose offspring to obesity and cardiovascular diseases including hypertension. 3 This programmed elevation of blood pressure (BP) tracks from early life periods into adulthood resulting in a global burden of cardiovascular mortality. 1 , 4 Modulation of foetal gene expression by maternal overnutrition seems to be more important than genetic factors in determining cardiometabolic health in later life. 5 , 6 There is a stronger association of offspring's BMI with maternal ppBMI compared to paternal BMI. 7 , 8 Thus, the evaluation of early life factors contributing to elevated BP is highly relevant.
Most studies confirm a link between high ppBMI or excessive GWG (used as proxies for early nutritional environment just before and during pregnancy) and offspring's obesity independent from socio‐demographic and lifestyle‐related factors. 7 , 8 , 9 However, the data about association between maternal overnutrition and offspring's BP remain controversial. 10 , 11 , 12 , 13 , 14 Only one systematic review has described a relationship between ppBMI and offspring's systolic BP (SBP), which the authors suggested may be mediated via offspring's obesity, with hypertension developing as a complication of the child's obesity. 15 However, recent studies suggest direct association between ppBMI and offspring's BP independent from offspring's actual nutritional state. 16 , 17 These controversies could be resolved by a meta‐analysis.
Research on the impact of GWG (independent from ppBMI) on offspring's BP is also conflicting. 12 , 18 It is yet to be clarified whether the assumed effect of maternal overnutrition during pregnancy on offspring's BP is mediated by offspring's obesity or the intrauterine environment acts also directly on BP. 17 , 18
Therefore, we aimed to systematically review the literature and perform a meta‐analysis on the impact of maternal overnutrition (1) just before or (2) during pregnancy on offspring's BP. We also aimed to analyse the potential influence of offspring's obesity on these associations and to assess the impact of maternal overnutrition in different trimesters of the pregnancy. We hypothesised that independent from offspring's obesity, a positive correlation exists between maternal overnutrition just before and during pregnancy and offspring's BP.
2. METHODS
2.1. Data sources and search strategies
Our search was conducted in MEDLINE, EMBASE, Clinicaltrials.gov and CENTRAL databases until 20th October 2021 independently by two investigators. This meta‐analysis was conducted according to a preregistered protocol, which we registered on PROSPERO (CRD42018102622). The completed Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist 19 can be found in Table S1. Disagreements were resolved by a third investigator. All relevant studies were identified using predefined search terms. The search strategy and the data extraction procedures are in the Text S1.
2.2. Study selection
The included studies had to provide data about maternal overnutrition just before or during singleton pregnancy in healthy mothers along with offspring BP data. We excluded studies that provided data only of mothers with preeclampsia/eclampsia and did not give relevant data of healthy mothers. However, we included articles which published data of mixed populations that applied proper adjustment for these pathological conditions. Further exclusion criteria were as follows: animal experiments, non‐English studies, studies providing maternal data only after birth and twin pregnancies. We did not use restriction on offspring's age or study design.
2.3. Quality assessment
Risk of bias was assessed by the Quality in Prognosis Studies (QUIPS). 20 Two independent investigators performed the quality assessment separately. Disagreements were resolved by a third author. QUIPS covers six main domains: Study Participation, Study Attrition, Prognostic Factor Measurement, Outcome Measurement, Study Confounding, Statistical Analysis and Reporting. For each item of the domains, ‘yes’, ‘no’,’partly’ or ‘unclear’ was used. An overall rating for each domain was assigned as carrying ‘low’, ‘moderate’ or ‘high’ risk of bias defined in the footnote of Table S2.
2.4. Statistical analysis
We used the random effect models with the DerSimonian and Laird weighting method in meta‐analysis to give a summary point estimate along with a 95% confidence interval (95% CI). With regard to the association between maternal nutritional state and offspring's parameters, we analysed either regression coefficients or weighted differences in means (WMD) of offspring's parameters by categories of maternal overnutrition.
If maternal nutritional state was defined as ppBMI or GWG and the articles provided BP increase for other than 1 unit of these parameters, we converted regression coefficients for 1 unit. When possible, we converted standardised (beta) and unstandardised (B) regression coefficients into one another as described earlier. 21 Since corresponding standard deviations were not always available for this conversion, we performed analyses with both B and beta coefficients.
To test whether the association is at least partly the direct effect of maternal overnutrition or only indirect via offspring's actual nutritional state (eg BMI, weight, fat mass), we analysed regression coefficients or WMDs in two models: data adjusted for offspring's actual nutritional state (M2) and without this adjustment (M1).
Several cofactors could influence the association between maternal overnutrition and offspring's BP: maternal cofactors (age, 22 , 23 , 24 , 25 smoking during pregnancy, 26 , 27 , 28 gestational age, 29 , 30 , 31 breast feeding, 32 socioeconomic status /SES/ 33 ) or offspring's covariate (birthweight, 29 , 34 age, sex 30 , 35 ). If the available data allowed, we performed the sensitivity analysis for these major cofactors by excluding those studies, in which the regression coefficients were not adjusted for these important cofactors (Text S1). Preeclampsia/eclampsia have been shown to increase the BP in the offspring 36 ; thus, it could influence the association between maternal overnutrition and offspring's BP. Therefore, we considered it carefully during the literature selection process.
We carried out meta‐analysis if data from at least three studies were available for a given association. If duplicate studies were found in the same population, the larger population was selected for the given data set. To assess heterogeneity, I‐squared statistics were calculated. I‐squared statistics represents the percentage of effect size heterogeneity that cannot be explained by random chance. Heterogeneity could be interpreted as moderate (30%–60%), substantial (50%–90%) or considerable (above 75%). 37 To test the presence of publication bias (small‐study effect), we assessed the symmetry of the funnel plots visually. All statistical analyses were performed with Comprehensive Meta‐analysis Software Version 3 and Stata 11 SE.
3. RESULTS
3.1. Search, selection and characteristics of the studies
Our systematic search produced 7833 records. After careful screening, 30 observational studies were eligible for qualitative synthesis. Thirteen articles 11 , 16 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 were not appropriate for meta‐analysis (flow chart, Figure 1). The reason of their exclusion can be found in Table S3. The remaining 17 articles were analysed 10 , 12 , 13 , 14 , 17 , 18 , 22 , 23 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 (Table 1). Fifteen of them reported ppBMI 12 , 14 , 18 , 22 , 23 , 49 , 52 , 53 , 54 , 55 , 57 or GWG (total 10 , 12 , 14 , 17 , 51 , 56 , 57 or maximum 13 , 18 , 49 , 50 , 52 ) and offspring's BP as continuous variables and provided regression coefficients for describing their links. Ten of these provided B coefficients and the remaining 5 articles beta standardised coefficients (Table S4). Adjustments of the coefficients for different maternal or offspring's cofactors (indicated in Table S4) allowed sensitivity analyses. Five studies excluded mothers with pathological pregnancies (maternal hypertension or diabetes mellitus), and 4 applied proper adjustment for these conditions (see Table S4).
FIGURE 1.
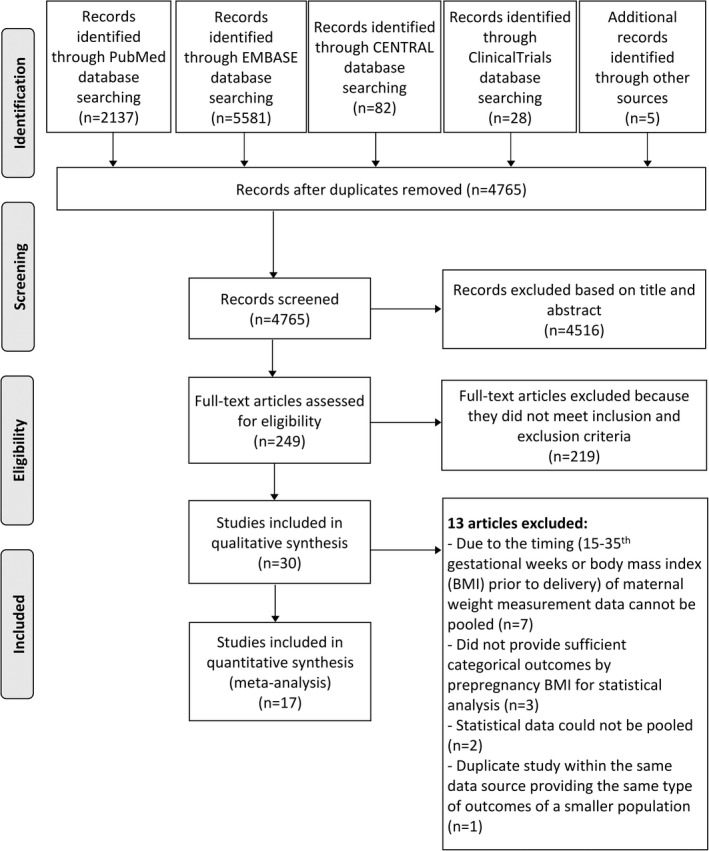
Flow chart of the study selection procedure
TABLE 1.
Characteristics of the included studies
| Author, year, reference number, country | Sample size | Maternal characteristics | Mean value of the nutritional state (±SD) | Offspring's characteristics | ||
|---|---|---|---|---|---|---|
| Age a (year) | Prevalence of overnutrition (%) | Age a (year) | Male (%) | |||
| Andersson, 2015, 50 Sweden | 89,829 | 23–33 |
GWG: 14 ± 4.2 kg |
18.3 | 100.0 | |
| Daraki, 2015, 51 Greece | 438 | 29–30 |
ppBMI>25: 33.8 |
4 | 52.0 | |
| Dello Russo, 2013, 56 Germany, Hungary, Italy, Cyprus, Spain, Estonia, Sweden, Belgium | 4005 | 28.4 (28.2–28.6) |
excessive GWG: 58.3 |
2–9 | 52.8 | |
| Derraik, 2015, 54 New Zealand | 70 | 17–42 | ppBMI>25: 29.6 | 4–11 | 61.0 | |
| Fraser, 2010, 13 UK | 5154 | 29.2 ± 4.5 |
ppBMI>25: 23.4 excessive GWG: 27.2 |
9 | 49.5 | |
| Gademan, 2010, 55 The Netherlands | 3074 | 25–37 | ppBMI>25: 21.5 | 5–6 | 50.0 | |
| Gaillard, 2015, 49 The Netherlands |
5735 (ppBMI) 3118 (GWG) |
30.3 ± 5.1 |
ppBMI: 23.6 ± 4.3 kg/m2 GWG: 14.9 ± 5.8 kg |
5.6–8 | 49.9 | |
| Gaillard, 2016, 12 Australia | 1392 | 29 ± 5.8 |
ppBMI: 21.3 (17.2–34.4) kg/m2 GWG: 0.4 kg/wk |
16.7–17.7 | 50.7 | |
| Harville, 2019, 57 USA |
200 (pp BMI) 219 (GWG) |
14.3–33.7 | ppBMI: 23.0 ± 5.6 kg/m2 | 27 ± 8.4 | 0.0 | |
| Hochner, 2012, 18 Israel | 1248 | 28.4 ± 5.5 |
ppBMI: 23.9 ± 3.7 kg/m2 GWG: 11.0 ± 4.6 kg |
32 ± 0 | 49.5 | |
| Karachaliou, 2015, 51 Greece | 518 | 29.9 ± 5.0 | excessive GWG: 45.0 | 4 | 50.0 | |
| Laor, 1997, 52 Israel | 10,533 | 20–31 | ND | ND | 17 | 61.4 |
| Lawlor, 2004, 22 Australia | 8458 | 25 ± 5.1 | ppBMI: 22.0 ± 4.1 kg/m2 | 5 | 51.0 | |
| Oken, 2007, 10 USA | 970 | 15–44 | excessive GWG: 51.0 | 3 | 51.0 | |
| Perng, 2014, 14 USA |
1073 (adjusted model) 837 (unadjusted model) |
15–44 |
ppBMI>25: 35.3 excessive GWG: 57.7 |
7.7 (6.6–10.9) | 49.7 | |
| Roberts, 2005, 23 UK |
7430 (systolic) 7428 (diastolic) |
29 ± 4.6 | ppBMI>25: 10.5 | 7.5 | 50.8 | |
| Tam, 2018, 17 China | 371 | 31.3 ± 4.6 | excessive GWG: 41.0 | 7 ± 0 | 52.0 | |
Abbreviations: GWG, gestational weight gain; ND, no data; ppBMI, prepregnancy body mass index.
Age is expressed in mean ± standard deviation or range or median (interquartile range).
Four articles (2 of them exclusively) provided data on offspring's BP by GWG categories based on the 2009 IOM guidelines. 13 , 14 , 17 , 56 In the majority of the studies, prepregnancy body weight was self‐reported for the assessment of ppBMI. 12 , 14 , 18 , 23 , 51 , 52 , 53 , 54 , 55 , 56 , 57 Three studies investigated the impact of GWG in different trimesters on offspring's BP. 12 , 49 , 51 For the measurement of offspring's BP, standardised methods were applied in all included studies. SBP was analysed in all studies, diastolic BP (DBP) only in 14. 12 , 13 , 17 , 18 , 23 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 The number of studies reporting mean arterial pressure or the presence of hypertension alone or as part of metabolic syndrome was not sufficient for statistics. Adjustment for offspring's actual nutritional state (BMI, body weight and fat mass index) in 13 studies (Table S4) allowed the separate analysis of this variable as a potential mediating factor (M2).
The included 17 articles were published from 1997 to 2019. Data of 140,517 mother‐offspring pairs were involved in our analysis. The number of participants (mother‐offspring pairs) ranged 70‐89829 per study. Caucasians were the dominant ethnic group, mostly from Europe and the United States. Twelve articles were prospective, 10 , 12 , 13 , 14 , 17 , 22 , 23 , 49 , 51 , 52 , 53 , 55 and only 5 articles were retrospective cohort studies. 18 , 50 , 54 , 56 , 57 All studies reported women who had full‐term pregnancies. Three studies focussed exclusively on adults (18–32 years). 18 , 50 , 57 Some studies reported data of the same population at different time points (Project Viva and the Jerusalem Perinatal Study). 10 , 14 , 18 , 52 When both studies were applicable, the larger population was selected for the analysis of the given data set. The majority of the studies contained pooled data of males and females.
3.2. Study quality
Results of the risk of bias assessment are found in Table S2. ‘Study participation’ and ‘Statistical analysis and reporting’ domains were evaluated as low or moderate risk of bias in all studies. The domains ‘Prognostic factor measurement’ (maternal nutritional state) and ‘Outcome measurement’ (offspring's BP) were judged to carry low risk of bias in all studies. In contrast, in 66.0% of the prospective studies, the high dropout rate caused selection bias (‘Study attrition’). Only 29.0% of the studies showed a high risk of bias in the domain ‘Study confounding’, since they did not report how the important cofactors were accounted for in the analysis. One study showed the highest risk of bias because it was rated as high risk in two domains (‘Study Attrition’ and ‘Study Confounding’). 23 The study with the lowest risk of bias was rated in all domains as low risk. 56
3.3. Linear type of association between ppBMI and offspring's BP
The analysis of studies that investigated the linear type of association between ppBMI and offspring's BP showed strong positive correlation: regression coefficient for systolic: 0.38 mmHg per kg/m2, 95% CI 0.17, 0.58; diastolic: 0.10 mmHg per kg/m2, 95% CI 0.05, 0.14 (Figure 2, unadjusted model M1). These results indicate 1.9 mmHg increase in SBP and 0.5 mmHg increase in DBP of offspring with every 5 kg/m2 gain in maternal ppBMI. Concerning SBP sensitivity, analyses of the most important covariates provided similar results. Adjustment for breast feeding abolished the associations with DBP. Similar results were obtained when we analysed beta (standardised) regression coefficients (Table S5).
FIGURE 2.
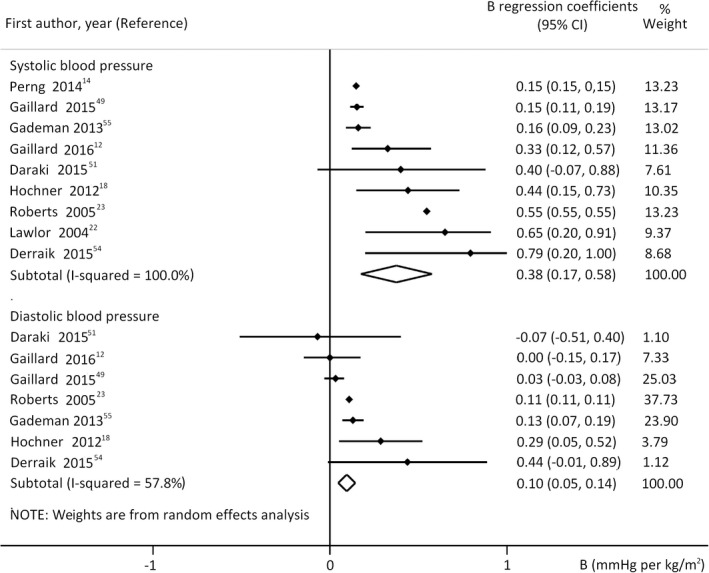
Regression coefficients (B) describing the association between prepregnancy body mass index and offspring's systolic (upper panel n = 28,682 mother‐offspring pairs) or diastolic blood pressure (lower panel n = 19,385 mother‐offspring pairs). The coefficients are not adjusted for offspring's actual nutritional state (model 1). Horizontal bars indicate 95% confidence intervals (95% CI). Diamonds show the overall point estimate with 95% CI
Analysis of B coefficients adjusted for the offspring's actual nutritional state (adjusted model M2) also showed positive associations (Figure 3). All covariates except for breast feeding were taken into consideration in this analysis of DBP. The sensitivity analysis for the important cofactors (except for breast feeding) and analysis of beta coefficients also confirmed positive association (Table S5).
FIGURE 3.
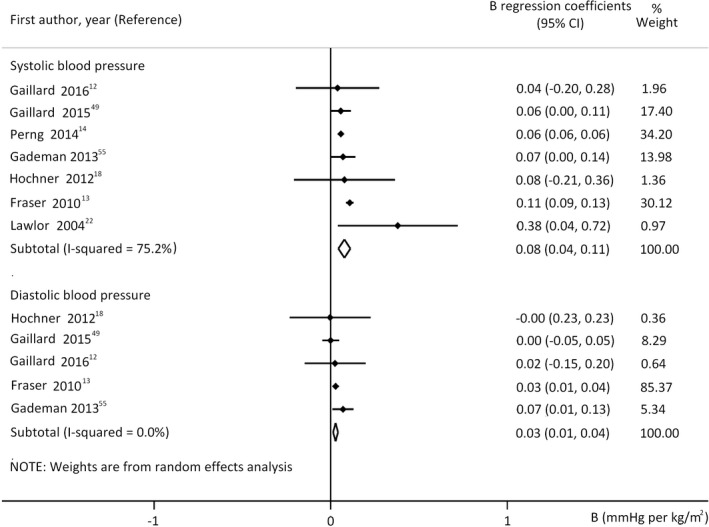
Regression coefficients (B) adjusted for offspring's actual nutritional state (model 2) describing the association between prepregnancy body mass index and offspring's systolic (upper panel n = 26134 mother‐offspring pairs) or diastolic blood pressure (lower panel n = 16603 mother‐offspring pairs). Horizontal bars indicate 95% confidence intervals (95% CI). Diamonds show the overall point estimate with 95% CI
3.4. Linear type of association between GWG and offspring's BP
Positive linear correlation of GWG (in all cases adjusted for ppBMI) was detected with offspring's SBP, but not with DBP (Figure 4, M1). This result indicates 0.05 mmHg increase in offspring's SBP with every 1 kg GWG (independent from ppBMI). All studies in this analysis considered maternal hypertension and diabetes (applying either exclusion or proper adjustments for these conditions). Similar results were obtained with analysis of beta coefficients. However, sensitivity analysis for all cofactors except for birthweight and SES showed weak association between GWG and SBP (Table S5).
FIGURE 4.
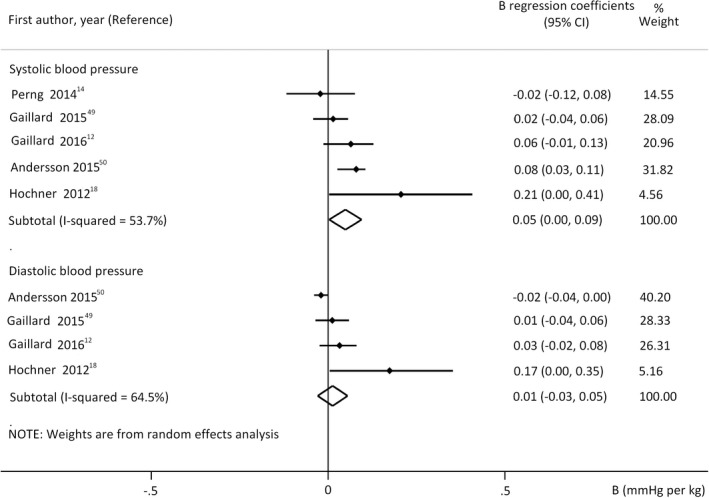
Regression coefficients (B) describing the association between gestational weight gain and offspring's systolic (upper panel n = 96424 mother‐offspring pairs) or diastolic blood pressure (lower panel n = 95587 mother‐offspring pairs). The coefficients are not adjusted for offspring's actual nutritional state (model 1). Horizontal bars indicate 95% confidence intervals (95% CI). Diamonds show the overall point estimate with 95% CI
In model 2, analyses of B coefficients for GWG and BP demonstrated only weak association (Figure 5, M2). These findings suggest that maternal overnutrition during pregnancy may act indirectly via the offspring's actual nutritional state on SBP. Additional analyses of cofactors and analysis of beta coefficients did not change these results (Table S5).
FIGURE 5.
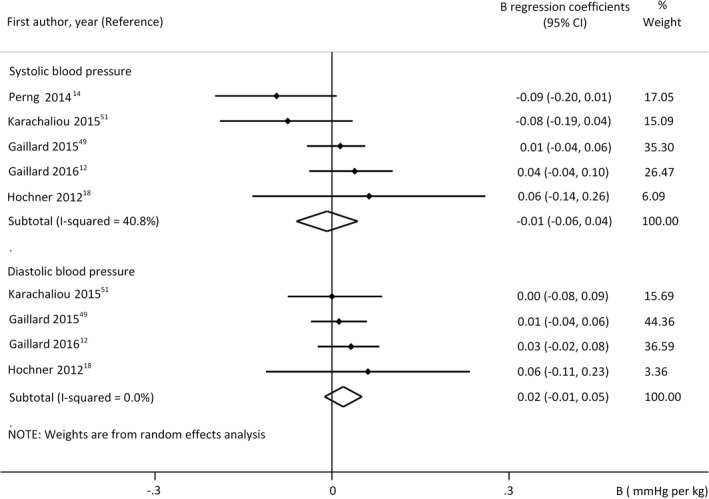
Regression coefficients (B) adjusted for offspring's actual nutritional state (model 2) describing the association between gestational weight gain and offspring's systolic (upper panel n = 7349 mother‐offspring pairs) or diastolic blood pressure (lower panel n = 6276 mother‐offspring pairs). Horizontal bars indicate 95% confidence intervals (95% CI). Diamonds show the overall point estimate with 95% CI
Although, analyses on GWG involved studies of different age groups, the studies on the youngest and oldest offspring presented similar data. Therefore, age does not appear to influence our results. 10 , 18
The number of available studies allowed the analysis of the first trimester GWG only in M2. We did not find relevant association (B for SBP: 0.02 mmHg per kg, 95% CI −0.01, 0.04, I 2 = 0%; DBP: 0.02 mmHg per kg, 95% CI −0.03, 0.07; I 2 = 42.2%). 12 , 49 , 51
3.5. Association between GWG (according to IOM categories) and offspring's BP
Our categorisation‐based analysis excluded offspring of mothers with suboptimal GWG. In model M1, our meta‐analysis indicated 0.65 mmHg higher mean SBP in children of mothers with excessive GWG compared with those who gained weight within the recommended range (Figure 6). In model M2, the analysis showed similar difference with smaller reliability. These findings are in accordance with those of the analyses of the previous linear models. Here, we could analyse DBP only in M2 and did not find relevant difference (0.23 mmHg, 95% CI −0.21, 0.675). 13 , 17 , 56 Data of these analyses were adjusted only for offspring's age and sex.
FIGURE 6.
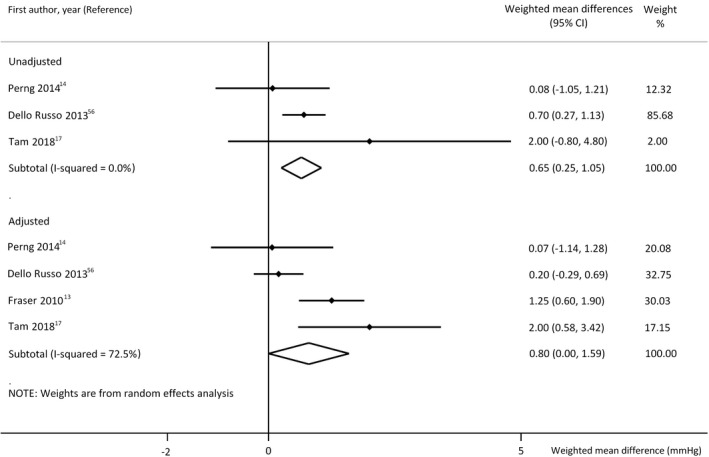
Differences in mean systolic blood pressure values without (model 1, upper panel n = 5213 mother‐offspring pairs) and with adjustment for offspring's actual nutritional state (model 2, lower panel n = 10,603 mother‐offspring pairs) in offspring of mothers with excessive gestational weight gain (GWG) compared with those of adequate GWG. Horizontal bars indicate 95% confidence intervals (95% CI). Diamonds show the overall point estimate with 95% CI
3.6. Publication bias
Visual inspection of the funnel plots suggested no small‐study effect except for studies of Figure 2, but analysis of beta instead of B showed similar results. Thus, the results of our analyses appear not to be influenced by small‐study effect.
4. COMMENT
4.1. Principal findings
Our meta‐analysis demonstrated early‐onset positive linear association between ppBMI and offspring's SBP even in children independent from the most important covariates: maternal age, SES, smoking during pregnancy, gestational age, breast feeding, birthweight, offspring's age and sex. Our results on coefficients adjusted for offspring's nutritional state indicate an, at least partly, direct effect of ppBMI on SBP and DBP independent from offspring's adiposity.
Our meta‐analysis showed positive correlation between GWG and offspring's SBP, independent from ppBMI and important cofactors. We also found higher SBP in offspring of mothers with excessive GWG as compared with those of adequate GWG. The relationship was attenuated by additional adjustment for offspring's nutritional state; therefore, the association between GWG and offspring's SBP appears to be indirect, mediated via offspring's adiposity.
4.2. Strengths of the study
Our meta‐analysis is the first to demonstrate the impact of ppBMI and GWG separately on offspring's BP. Further strengths include the separate investigation of direct versus indirect influence of offspring's nutritional state, the high number of participants and exclusion of twin pregnancies and preeclampsia/eclampsia. Low heterogeneity and thorough analysis of the important covariates showed reliability of conclusive results.
4.3. Limitations of the data
Limitations include the involvement of retrospective studies, the lack of investigation of sex differences and self‐reported prepregnancy weight in most of the studies. However, self‐reported and measured maternal weight have been reported to be strongly correlated. 58 Offspring of diabetic or hypertensive mothers have been shown to have higher BP, 59 , 60 but eight studies did not completely exclude mothers with hypertension or diabetes mellitus and did not apply adjustment for these conditions. Thus, maternal hypertension and diabetes may have biased our analyses of ppBMI. However, these conditions did not bias our conclusive results on GWG. Although the offspring's mean age range was wide, we took it into consideration in the interpretation of our data.
4.4. Interpretation
In accord with earlier observations, our results suggest that pregnancy overnutrition is related to higher risk for hypertension, cardiovascular events and mortality in later life. 48 , 61 , 62 We observed weaker association of maternal ppBMI with offspring's DBP than SBP. This association was independent from the most important cofactors except for breast feeding. Since in model 2 we could test all cofactors except breast feeding, we cannot exclude its potential influence. Interestingly, the effect of ppBMI in model 2 appears to be attenuated in young adults indicating the increasing influence of offspring's adiposity with age. 12 , 18 This could explain in part the controversies in the literature.
Independent from ppBMI, higher GWG is also associated with offspring's obesity. This observation highlights the justification of the separate analysis of GWG and the importance of avoiding excessive weight gain during pregnancy even in normal‐weight women. 7 , 10 We found positive correlation of GWG and offspring's SBP. However, the association was weaker if the coefficients were adjusted for offspring's birthweight or actual nutritional state. That means that offspring's adiposity plays a crucial role in the development of high SBP. Maternal GWG has been shown to be associated with high birthweight which also predicts later adiposity. Thus, birthweight is on the pathway between GWG and offspring's BP in the same way as offspring's BMI. 63 We found an elevation of SBP in offspring of mothers with excessive GWG; thus, GWG may have an additional impact on the risk of hypertension, as suggested qualitatively by the trends in previous studies on adult offspring. 11 , 50
Therefore, reduction of obesity in childhood could help to prevent the development of hypertension and complications arising from early life exposures. Since the first trimester GWG reflects the maternal fat deposition and it is the main period of the organogenesis, its separate analysis is especially important. 49 Although some studies suggest its positive association with offspring's BP, 49 , 51 we could not confirm it due to the lack of available data without adjustment for offspring's nutritional state. In our model 2, the first trimester GWG did not correlate with offspring's BP in accordance with the results of total GWG. However, offspring of mothers with excessive GWG tended to show higher SBP even after adjustment for offspring's actual adiposity. Since these data were not adjusted for birthweight, its mediating role is possible.
In contrast to earlier reviews, 8 , 15 which suggested only indirect relationship between ppBMI and offspring's BP, our meta‐analysis demonstrated, at least partly, a direct association. However, our results suggest indirect effect of GWG on offspring's SBP via offspring's obesity. Our results could be explained by different mechanisms of intrauterine programming modified by ppBMI and GWG. In addition to genetic predisposition, maternal prepregnancy obesity reflects maternal fat accumulation and low‐grade inflammation, while GWG also reflects fluid expansion and growth of the foetus, placenta and uterus. 2
Investigation of the underlying mechanisms in animal experiments revealed an additive effect of maternal obesity and gestational maternal high‐fat diet (HFD) on BP regulation. 64 As a direct consequence of maternal obesity, hypertension develops in juvenile offspring of obese dams even before the onset of their own adiposity (before hyperleptinemia and insulin resistance). It is attributed to selective resistance to leptin, a key hypothalamic regulator of metabolic and cardiovascular systems leading to sympathetic nervous system overactivity to stress and increased renal norepinephrine concentration and renin expression. 65 , 66 Since the anorexigenic leptin effects are suppressed in offspring of obese mothers, they become obese and hyperleptinemic further inducing obesity and hypertension. Activation of central melanocortins via leptin‐mediated or independent pathways could also worsen their hypertension (ie indirect programming of hypertension through greater adiposity in offspring of obese dams). 67 Gestational maternal HFD or high‐saturated‐fat diet can induce a hypothalamic proinflammatory state and impair central leptin and insulin signalling in the offspring. 68 Prenatal hypothalamic inflammation contributes to renin‐angiotensin system overactivity resulting in prenatally programmed hypertension in offspring. 64 In addition, high glucose and amino acid load due to maternal overnutrition contributes to permanent hyperinsulinemia and increased risk for obesity and obesity‐related hypertension in the offspring. 69 Thus, maternal overnutrition is shown to exhibit transgenerational effects probably through epigenetic modifications and cardiometabolic changes in the offspring. 70
5. CONCLUSIONS
In conclusion, our meta‐analysis demonstrates the determining role of both ppBMI and excessive GWG (independent of ppBMI) on offspring's BP. Furthermore, it highlights the need of effective preventive strategies such as nutritional corrections before conception and during pregnancy. Our findings support the role of offspring's adiposity in the association between GWG and offspring's BP. This finding emphasises the importance of prevention of obesity especially in children of obese otherwise healthy mothers to prevent high BP. Further long‐term studies are necessary to confirm the impact of maternal overnutrition on the development of hypertension in adult offspring and to analyse the trimester‐specific effect of GWG.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
Text S1
ACKNOWLEDGEMENT
None.
Eitmann S, Mátrai P, Németh D, et al. Maternal overnutrition elevates offspring’s blood pressure—A systematic review and meta‐analysis. Paediatr Perinat Epidemiol. 2022;36:276–287. doi: 10.1111/ppe.12859
Funding information
This study was funded by University of Pécs (KA‐2019‐44) and Grant of the National Research, Development and Innovation Office, Hungary (K138452)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. WHO . Noncommunicable diseases country profiles 2018. Geneva, Switzerland 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/274512/9789241514620‐eng.pdf?sequence=1&isAllowed=y (accessed 9 June 2021)
- 2. Institute of Medicine . Weight gain during pregnancy: reexamining the guidelines. The National Academies Press; 2009. [PubMed] [Google Scholar]
- 3. Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, Dolinsky VW. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci. 2018;55(2):71‐101. [DOI] [PubMed] [Google Scholar]
- 4. Regnault N, Kleinman KP, Rifas‐Shiman SL, Langenberg C, Lipshultz SE, Gillman MW. Components of height and blood pressure in childhood. Int J Epidemiol. 2014;43(1):149‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obregon MJ. Maternal obesity results in offspring prone to metabolic syndrome. Endocrinology. 2010;151(8):3475‐3476. [DOI] [PubMed] [Google Scholar]
- 6. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early‐life conditions on adult health and disease. N Engl J Med. 2008;359(1):61‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voerman E, Santos S, Patro Golab B, et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta‐analysis. PLoS Med. 2019;16(2):e1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol. 2015;30(11):1141‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heslehurst N, Vieira R, Akhter Z, et al. The association between maternal body mass index and child obesity: a systematic review and meta‐analysis. PLoS Med. 2019;16(6):e1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oken E, Taveras EM, Kleinman KP, Rich‐Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322.e1‐322.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119(13):1720‐1727. [DOI] [PubMed] [Google Scholar]
- 12. Gaillard R, Welten M, Oddy WH, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio‐metabolic risk factors in adolescent offspring: a prospective cohort study. BJOG Int J Obstet Gynaecol. 2016;123(2):207‐216. [DOI] [PubMed] [Google Scholar]
- 13. Fraser A, Tilling K, Macdonald‐Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121(23):2557‐2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol. 2014;24(11):793‐800.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ludwig‐Walz H, Schmidt M, Günther ALB, Kroke A. Maternal prepregnancy BMI or weight and offspring's blood pressure: Systematic review. Matern Child Nutr. 2017;14(2):e12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaseva N, Vääräsmäki M, Sundvall J, et al. Gestational diabetes but not prepregnancy overweight predicts for cardiometabolic markers in offspring twenty years later. J Clin Endocrinol Metab. 2019;104(7):2785‐2795. [DOI] [PubMed] [Google Scholar]
- 17. Tam CHT, Ma RCW, Yuen LY, et al. The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia. 2018;61(12):2539‐2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hochner H, Friedlander Y, Calderon‐Margalit R, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow‐up Study. Circulation. 2012;125(11):1381‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 20. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280‐286. [DOI] [PubMed] [Google Scholar]
- 21. Eitmann S, Németh D, Hegyi P, et al. Maternal overnutrition impairs offspring's insulin sensitivity: a systematic review and meta‐analysis. Matern Child Nutr. 2020;16(4):e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawlor DA, Najman JM, Sterne J, Williams GM, Ebrahim S, Davey SG. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater‐University study of pregnancy and its outcomes. Circulation. 2004;110(16):2417‐2423. [DOI] [PubMed] [Google Scholar]
- 23. Roberts RJ, Leary SD, Smith GD, Ness AR; ALSPAC Study Team . Maternal age in pregnancy and offspring blood pressure in childhood in the Avon Longitudinal Study of Parents and Children (ALSPAC). J Hum Hypertens. 2005;19(11):893‐900. [DOI] [PubMed] [Google Scholar]
- 24. Whincup PH, Cook DG, Shaper AG. Early influences on blood pressure: a study of children aged 5–7 years. BMJ. 1989;299(6699):587‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verroken C, Zmierczak HG, Goemaere S, Kaufman JM, Lapauw B. Maternal age at childbirth is associated with offspring insulin sensitivity: a cross‐sectional study in adult male siblings. Clin Endocrinol. 2017;86(1):52‐59. [DOI] [PubMed] [Google Scholar]
- 26. Rogers JM. Smoking and pregnancy: epigenetics and developmental origins of the metabolic syndrome. Birth Defects Res. 2019;111(17):1259‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falkner B. Maternal and gestational influences on childhood blood pressure. Pediatr Nephrol. 2020;35(8):1409‐1418. [DOI] [PubMed] [Google Scholar]
- 28. Li L, Peters H, Gama A, et al. Maternal smoking in pregnancy association with childhood adiposity and blood pressure. Pediatr Obes. 2016;11(3):202‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta‐analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59(2):226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertagnolli M, Luu TM, Lewandowski AJ, Leeson P, Nuyt AM. Preterm birth and hypertension: is there a link? Curr Hypertens Rep. 2016;18(4):28. [DOI] [PubMed] [Google Scholar]
- 31. Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1941‐R1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin RM, Gunnell D, Smith GD. Breastfeeding in infancy and blood pressure in later life: systematic review and meta‐analysis. Am J Epidemiol. 2005;161(1):15‐26. [DOI] [PubMed] [Google Scholar]
- 33. van den Berg G, van Eijsden M, Galindo‐Garre F, Vrijkotte TG, Gemke RJ. Explaining socioeconomic inequalities in childhood blood pressure and prehypertension: the ABCD study. Hypertension. 2013;61(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Li H, Liu S‐J, et al. The associations of high birth weight with blood pressure and hypertension in later life: a systematic review and meta‐analysis. Hypertens Res. 2013;36(8):725‐735. [DOI] [PubMed] [Google Scholar]
- 35. Talbot CPJ, Dolinsky VW. Sex differences in the developmental origins of cardiometabolic disease following exposure to maternal obesity and gestational diabetes (1). Appl Physiol Nutr Metab. 2019;44(7):687‐695. [DOI] [PubMed] [Google Scholar]
- 36. Hoodbhoy Z, Mohammed N, Nathani KR, et al. The impact of maternal preeclampsia and hyperglycemia on the cardiovascular health of the offspring: a systematic review and meta‐analysis. Am J Perinatol. 2021. 10.1055/s-0041-1728823. Epub ahead of print. PMID: 33940650. [DOI] [PubMed] [Google Scholar]
- 37. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- 38. Eriksson JG, Sandboge S, Salonen M, Kajantie E, Osmond C. Maternal weight in pregnancy and offspring body composition in late adulthood: findings from the Helsinki Birth Cohort Study (HBCS). Ann Med. 2015;47(2):94‐99. [DOI] [PubMed] [Google Scholar]
- 39. Bucci M, Huovinen V, Guzzardi MA, et al. Resistance training improves skeletal muscle insulin sensitivity in elderly offspring of overweight and obese mothers. Diabetologia. 2016;59(1):77‐86. [DOI] [PubMed] [Google Scholar]
- 40. Veena SR, Krishnaveni GV, Karat SC, Osmond C, Fall CH. Testing the fetal overnutrition hypothesis; the relationship of maternal and paternal adiposity to adiposity, insulin resistance and cardiovascular risk factors in Indian children. Public Health Nutrition. 2013;16(9):1656‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clark PM, Atton C, Law CM, Shiell A, Godfrey K, Barker DJ. Weight gain in pregnancy, triceps skinfold thickness, and blood pressure in offspring. Obstet Gynecol. 1998;91(1):103‐107. [DOI] [PubMed] [Google Scholar]
- 42. Godfrey KM, Forrester T, Barker DJP, et al. Maternal nutritional status in pregnancy and blood pressure in childhood. Br J Obstet Gynaecol. 1994;101(5):398‐403. [DOI] [PubMed] [Google Scholar]
- 43. Hrolfsdottir L, Rytter D, Olsen SF, et al. Gestational weight gain in normal weight women and offspring cardio‐metabolic risk factors at 20 years of age. Int J Obes. 2015;39(4):671‐676. [DOI] [PubMed] [Google Scholar]
- 44. Phillips DI, Bennett FI, Wilks R, et al. Maternal body composition, offspring blood pressure and the hypothalamic‐pituitary‐adrenal axis. Paediatr Perinat Epidemiol. 2005;19(4):294‐302. [DOI] [PubMed] [Google Scholar]
- 45. Eisenman JC, Sarzynski MA, Tucker J, Heelan KA. Maternal prepregnancy overweight and offspring fatness and blood pressure: role of physical activity. Pediatr Exerc Sci. 2010;22(3):369‐378. [DOI] [PubMed] [Google Scholar]
- 46. Filler G, Yasin A, Kesarwani P, Garg AX, Lindsay R, Sharma AP. Big mother or small baby: which predicts hypertension? J Clin Hypertens. 2011;13(1):35‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaillard R, Steegers EAP, Duijts L, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension. 2014;63(4):683‐691. [DOI] [PubMed] [Google Scholar]
- 48. Torres CHA, Schultz LF, Veugelers PJ, Mastroeni SSBS, Mastroeni MF. The effect of pre‐pregnancy weight and gestational weight gain on blood pressure in children at 6 years of age. J Public Health. 2021;43(2):e161‐e170. [DOI] [PubMed] [Google Scholar]
- 49. Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW. Maternal weight gain in different periods of pregnancy and childhood cardio‐metabolic outcomes. The generation R study. Int J Obes. 2015;39(4):677‐685. [DOI] [PubMed] [Google Scholar]
- 50. Andersson ES, Tynelius P, Nohr EA, Sørensen TI, Rasmussen F. No association of maternal gestational weight gain with offspring blood pressure and hypertension at age 18 years in male sibling‐pairs: a prospective register‐based cohort study. PLoS One. 2015;10(3):e0121202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karachaliou M, Georgiou V, Roumeliotaki T, et al. Association of trimester‐specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol. 2015;212(4):502.e1‐502.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Laor A, Stevenson DK, Shemer J, Gale R, Seidman DS. Size at birth, maternal nutritional status in pregnancy, and blood pressure at age 17: population based analysis. BMJ. 1997;315(7106):449‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Daraki V, Georgiou V, Papavasiliou S, et al. Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: the Rhea pregnancy cohort Crete, Greece. PLoS One. 2015;10(5):e0126327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Derraik JG, Ayyavoo A, Hofman PL, Biggs JB, Cutfield WS. Increasing maternal prepregnancy body mass index is associated with reduced insulin sensitivity and increased blood pressure in their children. Clin Endocrinol. 2015;83(3):352‐356. [DOI] [PubMed] [Google Scholar]
- 55. Gademan MG, van Eijsden M, Roseboom TJ, van der Post JA, Stronks K, Vrijkotte TG. Maternal prepregnancy body mass index and their children's blood pressure and resting cardiac autonomic balance at age 5 to 6 years. Hypertension. 2013;62(3):641‐647. [DOI] [PubMed] [Google Scholar]
- 56. Dello Russo M, Ahrens W, De Vriendt T, et al. Gestational weight gain and adiposity, fat distribution, metabolic profile, and blood pressure in offspring: the IDEFICS project. Int J Obes. 2013;37(7):914‐919. [DOI] [PubMed] [Google Scholar]
- 57. Harville EW, Apolzan JW, Bazzano LA. Maternal pre‐pregnancy cardiovascular risk factors and offspring and grandoffspring health: Bogalusa daughters. Int J Environ Res Public Health. 2018;16(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mamun AA, Callaway LK, O'Callaghan MJ, et al. Associations of maternal pre‐pregnancy obesity and excess pregnancy weight gains with adverse pregnancy outcomes and length of hospital stay. BMC Pregnancy Childbirth. 2011;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rice MM, Landon MB, Varner MW, et al. Pregnancy‐associated hypertension and offspring cardiometabolic health. Obstet Gynecol. 2018;131(2):313‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aceti A, Santhakumaran S, Logan KM, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta‐analysis. Diabetologia. 2012;55(11):3114‐3127. [DOI] [PubMed] [Google Scholar]
- 61. Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow‐up of 1 323 275 person years. BMJ. 2013;347:f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long‐term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med. 2014;46(6):434‐438. [DOI] [PubMed] [Google Scholar]
- 63. Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within‐family comparison. Lancet. 2010;376(9745):984‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang Y‐P, Huo Y‐L, Fang Z‐Q, et al. Maternal high‐fat diet acts on the brain to induce baroreflex dysfunction and sensitization of angiotensin II‐induced hypertension in adult offspring. Am J Physiol Heart Circ Physiol. 2018;314(5):H1061‐H1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Samuelsson A‐M, Morris A, Igosheva N, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55(1):76‐82. [DOI] [PubMed] [Google Scholar]
- 66. Taylor PD, Samuelsson AM, Poston L. Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol. 2014;210(3):508‐523. [DOI] [PubMed] [Google Scholar]
- 67. Samuelsson A‐M, Mullier A, Maicas N, et al. Central role for melanocortin‐4 receptors in offspring hypertension arising from maternal obesity. Proc Natl Acad Sci USA. 2016;113(43):12298‐12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cesar HC, Pisani LP. Fatty‐acid‐mediated hypothalamic inflammation and epigenetic programming. J Nutr Biochem. 2017;42:1‐6. [DOI] [PubMed] [Google Scholar]
- 69. Plagemann A. Perinatal programming: the state of the art. Walter de Gruyter GmbH & Co.; 2011. [Google Scholar]
- 70. Lee HS. Impact of maternal diet on the epigenome during in utero life and the developmental programming of diseases in childhood and adulthood. Nutrients. 2015;7(11):9492‐9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Text S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


