Abstract
Background and Aims
Wilson’s disease (WD) is a genetic disease with systemic accumulation of copper that leads to symptoms from the liver and brain. However, the underlying defects in copper transport kinetics are only partly understood. We sought to quantify hepatic copper turnover in patients with WD compared with heterozygote and control subjects using PET with copper‐64 (64Cu) as a tracer. Furthermore, we assessed the diagnostic potential of the method.
Approach and Results
Nine patients with WD, 5 healthy heterozygote subjects, and 8 healthy controls were injected with an i.v. bolus of 64Cu followed by a 90‐min dynamic PET scan of the liver and static whole‐body PET/CT scans after 1.5, 6, and 20 h. Blood 64Cu concentrations were measured in parallel. Hepatic copper retention and redistribution were evaluated by standardized uptake values (SUVs). At 90 min, hepatic SUVs were similar in the three groups. In contrast, at 20 h postinjection, the SUV in WD patients (mean ± SEM, 31 ± 4) was higher than in heterozygotes (24 ± 3) and controls (21 ± 4; p < 0.001). An SUV‐ratio of hepatic 64Cu concentration at 20 and 1.5 h completely discriminated between WD patients and control groups (p < 0.0001; ANOVA). By Patlak analysis of the initial 90 min of the PET scan, the steady‐state hepatic clearance of 64Cu was estimated to be slightly lower in patients with WD than in controls (p = 0.04).
Conclusions
64Cu PET imaging enables visualization and quantification of the hepatic copper retention characteristic for WD patients. This method represents a valuable tool for future studies of WD pathophysiology, and may assist the development of therapies, and accurate diagnosis.
Abbreviations
- ATP7B
ATPase copper‐transporting beta
- SUV
standard uptake value
- VOI
volume of interest
- WD
Wilson’s disease
INTRODUCTION
Wilson’s disease (WD) is a rare autosomal recessive disease caused by mutations in the ATPase copper‐transporting beta (ATP7B) gene leading to impaired function of the copper‐transporting protein, ATP7B. Upon uptake of dietary copper in the liver, the ATP7B protein mediates hepatic clearance by excreting copper into the bile or incorporating copper into the ferroxidase, ceruloplasmin, which is released back into the systemic circulation.[ 1 , 2 , 3 ] Hepatobiliary excretion constitutes the most important route for removal of excess copper from the body whereas urinary excretion is negligible in healthy persons. In WD, dysfunction of the ATP7B protein leads to systemic copper accumulation in multiple organs, most notably in the liver and brain, resulting in hepatic, neurological, and psychiatric symptoms.[ 2 , 4 , 5 , 6 ] Early diagnosis of WD is crucial, given that timely targeted treatment can prevent or reverse clinical progression of the disease, which is lethal when untreated.[ 4 , 5 ] However, diagnosing WD can be challenging given that no clinical symptoms or laboratory tests (including genetic testing) can be used to exclude or diagnose WD with sufficient accuracy.[ 7 , 8 , 9 , 10 ] Accordingly, noninvasive methods to study the hepatic handling of copper are warranted to improve our understanding of the pathophysiology behind WD and assist the development of drugs and diagnostic tools.
Radioactive copper isotopes have been used in the past to study whole‐body copper balance, and blood‐radioactivity–based methods have been evaluated as diagnostic tools in WD.[ 11 , 12 , 13 , 14 , 15 ] For diagnosis, the technique exploits the dysfunctional incorporation of 64Cu into ceruloplasmin characteristic to WD, resulting in a distinct blood radioactivity profile.[ 12 , 15 ] However, this method does not evaluate the fundamental disease‐causing disturbance in WD, namely the inadequate hepatic excretion of excess copper into the bile with consequential retention of copper in the liver.
PET is a promising tool for in vivo assessment of hepatic copper handling, using 64Cu as a tracer. 64Cu PET is characterized by excellent spatial and temporal resolution with a radioactive half‐life of 12.7 h, making it suitable to trace copper transfer between blood, liver, bile, and other compartments with slow turnover.[ 16 ]
Previous human 64Cu PET studies have focused on cancer detection, with only a few preclinical studies focusing on hepatic copper turnover.[ 17 , 18 , 19 , 20 , 21 , 22 ] One 64Cu PET study in ATP7B knockout mice showed markedly reduced clearance of 64Cu from the liver.[ 23 , 24 ] As a preamble to clinical studies, we recently published a PET/CT study of 64Cu biodistribution and dosimetry in healthy subjects, demonstrating that 64Cu PET is a suitable and safe method for the assessment of hepatic copper handling.[ 16 ]
The aim of the present study was to examine the ability of 64Cu PET to quantify the hepatic copper handling characteristic for WD and examine the potential of this method as a diagnostic tool in WD. Thus, we compared the PET‐determined hepatic copper kinetics in patients with WD with that of heterozygotes and healthy subjects.
MATERIALS AND METHODS
Study design
The study included three groups of participants: patients with WD; healthy heterozygote parents or siblings to known WD cases; and healthy subjects. The three groups underwent identical study and scan procedures (Figure 1). Participants fasted overnight for at least 8 h before the first scan, but were allowed to drink tap water. A dynamic PET scan (continuous time‐activity data acquisition, liver only) of 1.5 h was performed immediately after i.v. administration of 64Cu followed by three consecutive static PET (one time point, whole body) scans at 1.5, 6, and 20 h. Patients with WD discontinued zinc or chelator treatment 3 days before the scans and resumed treatment after the final scan. Baseline blood biochemistry was analyzed the day before the first PET/CT scan in all participants (Table 1).
FIGURE 1.
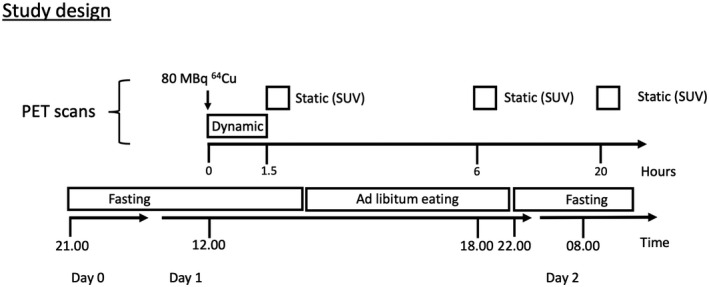
Participants were studied after an overnight fast. The 64Cu tracer was injected intravenously at t = 0 min followed by a 90‐min dynamic PET scan of the liver with arterial blood sampling to determine blood‐to‐liver clearance. The dynamic PET scan was followed by three static PET scans performed 1.5, 6, and 20 h after administration of tracer to determine the hepatic excretion and retention of 64Cu. Participants were allowed to eat and drink freely between the 1.5‐ and 6‐h scan, but fasted again before the 20‐h scan
TABLE 1.
Median (IQR)
| Healthy Controls | Heterozygotes | WD Patients | |
|---|---|---|---|
| Sex: F/M | 5/3 | 2/3 | 2/7 |
| Age (years) | 25 (29) | 34 (19) | 36 (34) |
| Weight (kg) | 84 (23) | 74 (9) | 76 (13) |
| Height (cm) | 173 (17) | 180 (13) | 183 (18) |
| BMI | 27 (6) | 23 (1) | 23 (3) |
| ALT (U/L) | 25 (14) | 18 (12) | 56 (44)* |
| Sodium (mmol/L) | 141 (2) | 141 (2) | 141 (2) |
| Bilirubin (μmol/L) | 7.5 (5) | 8 (5) | 10 (11) |
| ALP (U/L) | 70 (26) | 56 (14) | 81 (18) |
| Hemoglobin (mmol/L) | 8.5 (1) | 8.4 (1) | 9 (1) |
| Urea (mmol/L) | 4.4 (2) | 3.75 (1) | 5.3 (3) |
| INR | 1.0 (0.1) | 1.0 (0.0) | 1.2 (0.1)* |
| Albumin (g/L) | 38 (6) | 40 (3) | 38 (4) |
| C‐reactive protein (mg/L) | 0.9 (4) | 1 (2) | 0.65 (1) |
| Creatinin (µmol/L) | 69.5 (27) | 67 (17) | 69 (21) |
| APTT (seconds) | 24.5 (1) | 25 (1) | 28 (10)* |
| Platelets (×109/L) | 268.5 (54) | 287 (32) | 193 (53) |
| P‐Copper (µmol/L) | 13.8 (14) | 14 (4) | 7 (18) |
| CuEXC (µmol/L) | 0.9 (0.3) | 0.9 (0.1) | 1.3 (0.5) |
| Ferroxidase (g/L) | 0.29 (0.1) | 0.26 (0.1) | 0.09 (0.1)* |
Abbreviations: APTT, activated partial thromboplastin time; BMI, body mass index; CuEXC, exchangeable copper; INR, international normalized ratio; IQR, interquartile range.
p < 0.05 versus healthy.
Participants
We recruited WD patients and heterozygote participants from the outpatient clinic at the Department of Hepatology, Aarhus University Hospital. Healthy persons were included after responding to an advertisement in a local newspaper.
The diagnosis of WD was made according to the American Association for the Study of Liver Diseases and European Association for the Study of the Liver guidelines, where a Leipzig score of ≥4 is considered diagnostic.[ 4 , 5 , 25 ] Eligible participants were ≥18 years. A negative pregnancy test and use of safe contraception (for females) were required. We excluded WD patients with decompensated cirrhosis, a Model for End‐Stage Liver Disease score >11, or a modified Nazer score (revised King’s score) >6.[ 26 ] Heterozygotes and healthy controls had no history of liver disease. Overall exclusion criteria were known hypersensitivity to 64Cu or other ingredients in the formula, malignancy, breastfeeding, or desire to become pregnant before the end of the study.
Catheterizations
Before the 64Cu PET scann, a catheter (Venflon; Becton Dickinson, Franklin Lakes, NJ) was placed in a cubital vein for the administration of 64Cu tracer. A catheter (Artflon; Beckton Dickinson) was placed in a radial artery for blood sampling.
Radiochemistry
The 64Cu radioisotope was obtained from a commercial source (Hevesy Laboratory, DTU Nutech, Risø, Roskilde, Denmark), and the 64Cu tracer solution (a sterile acetate‐buffered solution of 64CuCl2) was subsequently prepared and quality controlled at our center, as earlier described.[ 16 ] Dosimetry data are provided in the Supplementary Methods of the Supporting Information.
64Cu PET imaging
Participants were placed in a supine position in a Siemens Biograph 64 TruePoint PET/CT camera with the liver within the 21.6‐cm axial field of view. A low‐dose CT scan (50 effective mAs with CARE Dose4D, 120 kV, pitch of 0.8 mm, and slice thickness 5.0 mm) was performed before each PET scan for definition of anatomical structures and attenuation correction of PET recordings. The 64Cu solution was administered as an i.v. bolus injection over the course of 10 s (median dose, 72.9 MBq; range, 53–77), followed by a 90‐min dynamic PET scan of the liver, recorded in list mode; time‐frame structure was 12 × 5, 8 × 15, 7 × 60, and 16 × 300 s. Then, three consecutive whole‐body PET/CT scans (top of skull to mid‐thigh; six bed positions) were performed at 1.5, 6, and 20 h after tracer administration (duration, 6, 6, and 10 min per bed position). PET images were reconstructed using three‐dimensional ordered‐subset expectation maximization with four iterations and 21 subsets, a 4‐mm Gauss filter, and 168 × 168 matrix with voxel size 4 × 4 × 5 mm3.
Blood radioactivity concentration
Arterial blood samples were drawn during the 90‐min dynamic PET scan at time points corresponding with the PET frame structure at 12 × 5, 8 × 15, 7 × 60, and 16 × 300 s. In addition, blood samples were collected from a peripheral vein during the initial dynamic PET scan and before each of the subsequent static scans (1.5, 6, and 20 h). All concentration measurements were cross‐calibrated with the PET camera and corrected for radioactive decay.
Image analysis
Fused PET/CT images were analyzed using the PMOD software (version 3.7; PMOD Technologies Ltd., Zürich, Switzerland). Radioactivity concentration in the liver was measured in a 4‐ml spherical volume of interest (VOI) placed in the right liver lobe. VOIs were drawn to include a representative volume of liver tissue while avoiding large intrahepatic blood vessels and bile ducts.
To ensure reproducibility, four different VOIs were drawn and averaged for each scan sampling ~3% of the total liver volume (Figure 2). This approach was chosen instead of measuring mean radioactivity concentration in the entire organ, given that diaphragmatic movements during the scan (breathing) result in noise and large scan‐to‐scan variation. Copper was evenly distributed in the liver, and in accordance the variation coefficient among the four to five VOIs was <5%. For kinetic analysis, the time course of the radioactivity concentration of 64Cu in the liver was generated for the 90‐min dynamic PET scan. The Gjedde‐Patlak linearization was used to calculate the clearance of 64Cu from blood into liver tissue (K; ml blood/min/ml liver tissue) with or without the inclusion of a potential reversible loss‐rate constant (k loss; min−1), representing the loss of tracer from the hepatocytes into bile or blood.[ 27 ] The kinetic model was applied to data 30–90 min after tracer administration to ensure quasi‐steady‐state using arterial plasma radioactivity concentration as the input function. Kinetic model parameters were estimated using software developed in‐house (iFit, v. 0.82; www.liver.dk/ifit.html). For correction of injected dose and body weight between participants, radioactivity concentrations in liver tissue are given as standard uptake value (SUV), .
FIGURE 2.
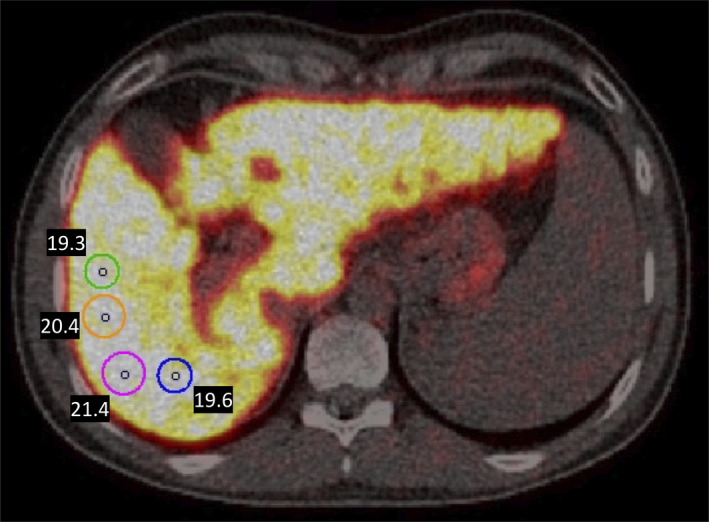
Four VOIs were drawn and averaged for each scan sampling ~3% of total liver volume. Sphere volume is 4 ml for each VOI, but the circles appear unequal in size because of different two‐dimensional slicing
To adjust for variation in hepatic uptake during the initial 90 min and assess solely the hepatobiliary excretion of copper, we calculated the ratio between hepatic 64Cu concentration at 90 min and 20 h postinjection as a measure of hepatic copper retention, SUV‐R = SUV(20 h)/SUV(90 min).
Statistical analysis
Normality was assessed using QQ‐plots. For normally distributed data, we used the ANOVA test followed by the Student t test to examine differences between groups. For non‐normal distributed data, we used the Kruskal‐Wallis test followed by the Mann‐Whitney U test. The time courses of radioactivity concentrations in liver tissue were analyzed by a mixed‐model repeated‐measures ANOVA. All data are expressed as mean (SD), unless otherwise specified. A p value < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using STATA software (version 16; StataCorp LP, College Station, TX) or GraphPad Prism software (version 8; GraphPad Software Inc., La Jolla, CA).
RESULTS
Participant characteristics
Baseline characteristics of the participants are shown in Table 1. In total, 9 WD patients, 5 heterozygotes, and 8 controls were studied. All patients with WD were clinically stable, with no change in treatment for at least 1 year up until the studies; 6 patients were treated with d‐penicillamine, 1 with trientine, and 2 with zinc. Patients had been diagnosed with WD for a median of 18 years (range, 1–49) at time of study entry. All patients primarily had the hepatological phenotype of WD, and at study entry, 2 patients had cirrhosis with portal hypertension, based on liver histology assessed ≤1 year before study entry and clinical examination. Overall, heterozygotes and control participants did not differ in biochemistry, whereas WD patients had slightly elevated mean alanine aminotransferase (ALT), alkaline phosphatase (ALP), a lower platelet count, and a higher INR, reflecting their hepatic phenotype. Genetic information is provided in Table 2.
TABLE 2.
Genetics
| WD | Mutation 1 | Mutation 2 | |
|---|---|---|---|
| 1 | c.2828G>A | c.2828G>A | Homozygote |
| 2 | c.3818C>T | c.2304insC | Compound heterozygote |
| 3 | c.3207C>A | c.3207C>A | Homozygote |
| 4 | c.3207C>A | c.3473G>T | Compound heterozygote |
| 5 | c.51+4A>T | c.3350_3353delAGCG | Compound heterozygote |
| 6 | c.3062T>A | c.3659C>T | Compound heterozygote |
| 7 | c.3818C>T | c.4126‐2AG | Compound heterozygote |
| 8 | c.51+1G>A | c.2009_2015delATATGCT | Compound heterozygote |
| 9 | c.1772G>A | c.1847G>A | Compound heterozygote |
| Heterozygotes | Mutation 1 | ||
| 1 | c.3207C>A | Heterozygote | |
| 2 | c.3062T>A | or c.3659C>T a | Heterozygote |
| 3 | c.3818C>T | Heterozygote | |
| 4 | c.3207C>A | or c.4088C>T a | Heterozygote |
| 5 | c.2305A>G | Heterozygote |
Genetic analysis only available from related WD patients (parents) and thus the two possible mutations presented.
Blood 64Cu concentration and hepatic uptake
After i.v. administration of 64Cu, arterial blood radioactivity concentration increased rapidly in all three groups, peaking after ~30 s (Figure 3). After an initial decline attributable to immediate biodistribution of 64Cu into the tissue, radioactivity concentration in the blood slowly declined until 90 min, where the lowest concentration was reached. From 90 min to end of study at 1200 min (20 h), a steady increase in blood radioactivity concentration was observed. Time courses of blood radioactivity were not statistically significantly different among the three groups of participants (Figure 3).
FIGURE 3.
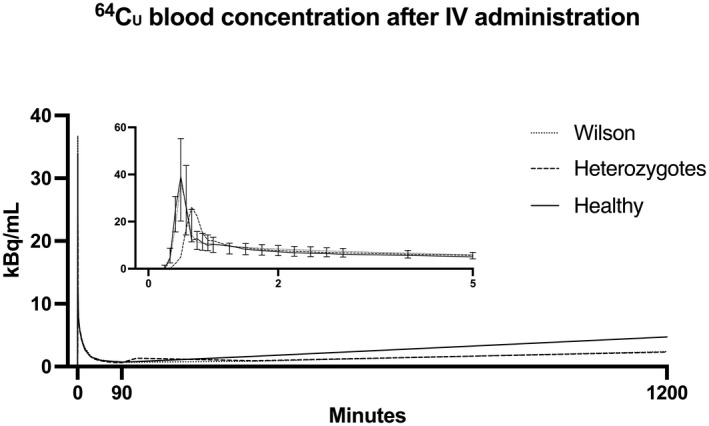
Time course of 64Cu concentration in blood. Following administration of 64Cu, average whole‐blood radioactivity peaked within 30 s, followed by an initial steep decline and a secondary slower decrease. Blood radioactivity reached a nadir at ~90 min, followed by a gradual and significant (ANOVA for repeated‐measurements time, p < 0.001) increase over the subsequent 18 h, most pronounced in healthy subjects, although this was not statistically significant (ANOVA for repeated‐measurements time × group interaction, p = 0.99). n = 7 WD; 4 heterozygotes; 8 healthy subjects. Curves are median for all subjects; error bars (IQR) only for WD patients (min 0–5) otherwise omitted to facilitate reading. Abbreviation: IQR, interquartile range
PET images displayed a rapid and substantial uptake of 64Cu in the liver immediately after administration of the radiotracer in all subjects (Figure 4A). Over the initial 90‐min dynamic scan period, the time course of hepatic radioactivity was similar in the three groups; however, a trend of reduced hepatic uptake in WD patients was observed (Figure 4B). Hepatic steady‐state clearance of 64Cu from blood into liver tissue (K; mL blood/min/ml liver tissue) is a measure of the efficacy of the hepatic removal of 64Cu from the blood and was estimated by kinetic analysis of the 90‐min dynamic PET scans using the Patlak linearization. The Patlak analysis was performed both with and without the inclusion of a rate constant, k loss (min−1), that describes the loss of 64Cu from the liver (Figure S1; Table S1). When k loss was included in the model to account for incorporation in ceruloplasmin and biliary excretion of 64Cu, K (mean ± SD) was 0.064 ± 0.010 ml of blood/min/ml liver tissue in WD patients, which was significantly lower than in both heterozygotes (0.091 ± 0.015 ml of blood/min/ml liver tissue) and controls (0.081 ± 0.018 ml of blood/min/ml liver tissue; p = 0.04), although there was considerable overlap between the groups (Figure S1). The k loss parameter, based on the initial 90 min after administration of 64Cu, was not significantly different between the groups (Table S1). Interestingy, K did not correlate with hepatic copper retention as measured by SUV at 90 min. Because of the complex study setup, complete data sets for analysis of hepatic removal kinetics (i.e., dynamic PET scan and arterial blood samples) were only available for 6 of 9 patients with WD, 3 of 5 heterozygotes, and for all 8 controls.
FIGURE 4.
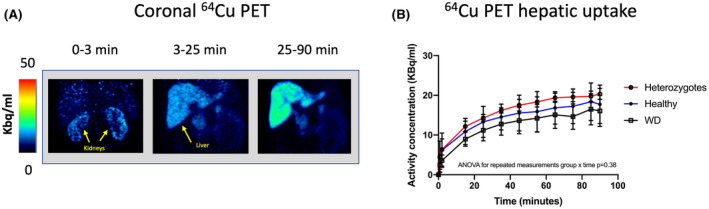
Hepatic 64Cu uptake from blood. (A) Summated coronal PET images in a representative healthy subject showing avid renal uptake of 64Cu, followed by subsequent 64Cu accumulation in the liver. (B) Average hepatic time‐activity curves in all subjects demonstrating comparable hepatic uptake of copper from the blood during the initial 90 min following administration of tracer (ANOVA for repeated‐measurements group × time, p = 0.38)
Hepatic excretion and retention of 64Cu
By visual examination of PET images, notable amounts of 64Cu were observed in gallbladder contents after 1.5 h in 6 of the 8 healthy participants, whereas none was visible in patients with WD or heterozygous participants (Figure 5A). Diffuse 64Cu uptake in the intestinal wall was observed in all participants, but tracer was present in the colonic lumen only in healthy participants (Figure 5A). Robust kinetic analysis for quantification of the hepatobiliary excretion of 64Cu was not possible.
FIGURE 5.
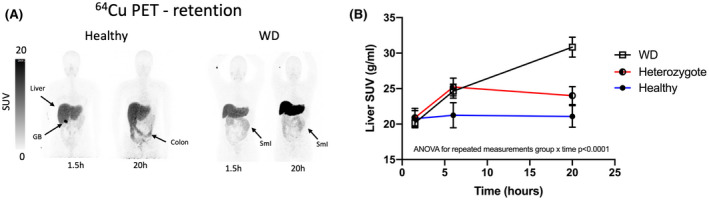
Hepatic excretion and retention of 64Cu. (A) Static 64Cu PET scans at 1.5 and 20 h postinjection in a healthy subject and a patient with WD. In the healthy subject, early excretion into the gallbladder (GB) is clearly visible 1.5 h after radiotracer injection, and excreted tracer is evident in the colonic lumen after 20 h. In contrast, there is no tracer in the GB of the WD patient, and the activity in the small intestines (SmI) represents minor uptake in the intestinal wall, which occurred immediately after radiotracer injection. (B) Mean hepatic concentration of 64Cu (SUV) over 20 h after tracer administration, measured regionally in the liver. In WD patients, hepatic SUV values increased throughout the 20‐h study period, whereas a plateau was reached in healthy and heterozygote subjects
In heterozygote and control participants, the mean hepatic concentration of 64Cu (SUV) peaked at 6 h and then slowly decreased, whereas in patients with WD, 64Cu in the liver more than doubled from 6 to 20 h (Figure 5B; p < 0.001; repeated‐measures ANOVA group × time interaction). Thus, the mean SUV at 20 h was significantly higher (p < 0.0001, t test) in WD patients (31 ± 4) than in both the heterozygote participants (24 ± 3) and healthy controls (21 ± 4). The mean SUV was nearly 15% higher in heterozygotes than in control participants, but this difference was not statistically significant. Hepatic retention of 64Cu after 20 h was pronounced in patients with WD, and moderate retention was observed in heterozygotes when compared with controls (Figure 5B).
Evaluation of hepatic 64Cu SUV ratios for identification of WD
In order to improve the discrimination between groups, we calculated SUV‐R as the ratio between hepatic radioactivity concentrations at 20 and 1.5 h. Using the SUV‐R, 64Cu PET accurately identified patients with WD, given that there was no overlap between individual values from the WD group and the heterozygote and control groups when applying a separating threshold of SUV‐R = 1.3 (Figure 6C). The SUV‐R could not be used to discriminate between the heterozygote controls and healthy controls, given that there was considerable overlap between the two groups (Figure 6C).
FIGURE 6.
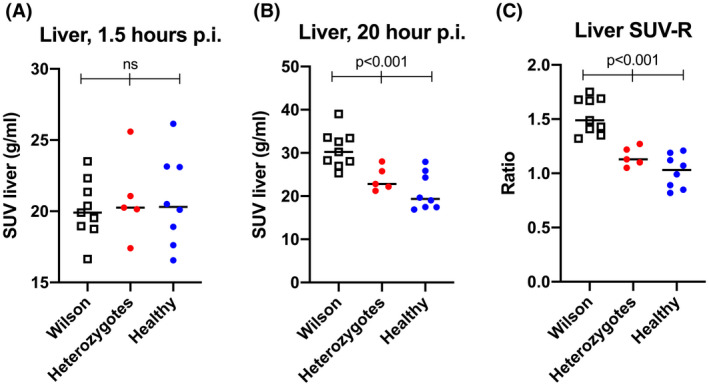
An SUV ratio of hepatic 64Cu accurately identifies patients with WD. (A,B) Individual values of hepatic concentration of 64Cu (SUV) at 1.5 and 20 h postinjection (p.i.) in patients with WD, heterozygotes, and healthy controls. Groups were similar 90 min p.i., but differed significantly after 20 h. (C) When a ratio of 20‐h/1.5‐h hepatic SUV values (SUV‐R) was used, WD patients had significantly higher levels than those of healthy and heterozygote subjects, and there was no overlap between patients with WD and heterozygotes. Horizontal lines represent group means
DISCUSSION
The main finding of this human 64Cu PET study of patients with WD, heterozygotes, and controls was that the method was able to visualize and quantify the pathophysiological hepatic copper retention characteristic of WD. Second, we found that a simple ratio of retained hepatic 64Cu at 20 and 1.5 h postinjection was able to clearly discriminate between patients with WD, heterozygotes, and healthy controls, thus reflecting the severely impaired hepatobiliary excretion of copper in WD, but also demonstrating a moderate impairment in heterozygous persons.
In line with our a priori expectations and as inferred from previous genetic, histological, and 64Cu blood radioactivity studies, we observed a marked hepatic retention of copper in patients with WD, as compared with healthy controls.[ 23 , 24 ] The high hepatic 64Cu concentrations 20 h after administration in WD patients when compared with heterozygotes and healthy participants were caused by a lack of hepatobiliary excretion, as supported by the absence of 64Cu in the gallbladder and colon of patients. According to the Patlak analysis, steady‐state clearance of copper from blood to liver tissue was slightly lower in patients with WD than in the two other groups. This difference could not be observed when looking only at the 90‐min hepatic 64Cu activity, demonstrating that a longer observation period is necessary for discrimination of the groups. One possibility is that a backflux of non‐ceruloplasmin‐bound Cu from liver to plasma was higher in WD patients, and, in fact, the k‐loss rate constant was higher in WD patients (Table S1), although this did not reach statistical significance. Another possibility is that the increased hepatic Cu load somehow inhibited hepatic uptake of Cu from the blood. Given that ATP7B is not expressed at the sinusoidal membrane of the hepatocyte and therefore not involved in the hepatocellular uptake of copper, the dysfunction of ATP7B in WD cannot explain this finding. Instead, the decreased hepatic uptake could reflect a homeostatic defense mechanism, counteracting the pathologically increased hepatic copper content in WD patients; a similar mechanism has been hypothesized in cholestatic liver diseases, such as primary biliary cholangitis, where hepatic transporters responsible for uptake of bile acids are down‐regulated in liver tissue.[ 28 , 29 ]
Interestingly, also the asymptomatic heterozygotes displayed some degree of hepatic retention of 64Cu compared with healthy controls, most pronounced at 6 h postinjection. At 20 h, the course of the hepatic 64Cu concentration in heterozygotes stagnated as opposed to the WD group, but was still higher than control values. This observation demonstrates that one normal ATP7B gene is enough to maintain a near‐normal copper homeostasis in accordance with the observation that heterozygotes remain asymptomatic.[ 6 , 14 ] Concurrently, it illustrates that heterozygotes do not have normal copper metabolism, which raises the question of whether heterozygotes may be prone to symptomatic copper accumulation if exposed to high copper loads. This finding also has implications for future developments of gene therapy in WD and suggests that total normalization of the hepatic handling of copper may not be necessary to obtain clinically sufficient results. The data allow for a preliminary understanding of the whole‐body copper kinetics. In all groups, the initial dose of 64Cu was taken up rapidly (10–15 min) from the blood and presumably distributed into a number of tissues, including the liver (Figure 3).[ 16 ] In all groups, the liver then continued to accumulate 64Cu, even though the blood concentration was near constant (Figure 4B). This indicated redistribution from other tissues to the liver through the bloodstream. In healthy controls, hepatic 64Cu accumulation became steady after 90 min, reflecting equilibrium between hepatic uptake and biliary excretion (Figure 5B). In WD patients, the accumulation of 64Cu continued until 24 h postdose, reflecting continued redistribution of 64Cu from other tissues to the liver with no biliary excretion (Figure 5B). WD patients never reached steady state. Heterozygotes reached a steady state at 6 h, but were otherwise similar to healthy controls.
We considered a number of possible biases. Uneven sex distribution among groups was a theoretical bias because women more often develop acute hepatic failure, and estrogen is known to affect biliary excretion of other substances.[ 10 ] The sample was too small to exclude an effect of sex, but in the data (Table S2), there was no obvious trend in that direction. The same was true when women on estrogen replacement were compared to those without (Table S2). Two of the WD patients had cirrhosis of the liver, and portosystemic shunting may affect hepatic removal kinetics. However, with K values below 10% of the hepatic blood flow, portosystemic shunting will have only minor effects. Again, the sample size is too small for statistical evaluation, but the findings in patients with cirrhosis were quite similar to those without (Table S2). In a similar way, current treatment did not clearly affect the findings. Also, our study was not powered to explore the relation between individual disease‐causing mutations (Table 2) and parameters of copper kinetics. The method rather quantifies the fundamental pathophysiology in WD: the inability to excrete excess copper to the bile.
The most important limitation of the present study was that patients were not treatment naïve, which excluded the possibility for meaningful comparison with other routine diagnostic tests for copper metabolism (serum ceruloplasmin, total serum copper concentration, 24‐h urinary copper excretion, and exchangeable copper). The rate of incorporation of 64Cu into ceruloplasmin would be of interest, but was not measured by the 64Cu PET method. P‐ceruloplasmin concentrations and SUV at 20 h correlated when analyzed in all groups combined, but not within the individual group (Figure S2).
Radiocopper was used earlier in WD research and clinical workup.[ 13 , 14 , 15 , 30 ] As such, Czlonkowska et al. have used i.v. 64Cu and blood radioactivity curves as an adjunct diagnostic tool for WD since the 1970s. Their experience with 71 patients, homozygote for WD mutations, and 21 heterozygote siblings was recently published.[ 15 ] Here, the 48‐h/1.5‐h blood radioactivity ratio yielded almost perfect diagnostic accuracy (98.6% sensitivity, 100% specificity) to discriminate between WD and heterozygote siblings. Whereas this method primarily assesses the ability of the liver to incorporate copper into ceruloplasmin, 64Cu PET adds a dimension to radiocopper studies by providing whole‐body functional imaging with detailed and dynamic information about the hepatic uptake and retention of copper over time.
64Cu PET imaging using the SUV ratio showed good discrimination of patients with WD toward heterozygotes and healthy controls, but could not discriminate between heterozygote controls and healthy controls. Larger studies including more patients of all WD phenotypes will be necessary to evaluate the use of 64Cu PET as a diagnostic tool. Because the retention of 64Cu in the liver is a pathophysiological hallmark in WD, the method has the potential to perform as a diagnostic gold standard, being useful in both presymptomatic and symptomatic cases. The prospect that a single investigation could diagnose or exclude WD is attracting. At the same time, the complex methodology and need to perform two consecutive PET scans on separate days are obvious practical drawbacks, which limit use to selected cases, where the diagnosis is uncertain. The present data do, however, suggest that longer follow‐up using, for instance, a single PET scan 48 h after tracer administration, would allow for complete separation between WD cases and heterozygotes, and this should be evaluated in future studies.
The 64Cu PET method may prove useful to evaluate the effect of both established and promising disease‐modifying interventions in patients with WD. For example, 64Cu PET could serve to evaluate the effect of zinc therapy on intestinal and hepatic copper absorption as shown in our previous PET study with oral administration of 64Cu in healthy subjects.[ 16 ] Moreover, 64Cu PET can be used to evaluate the direct effects of drugs and gene therapy on hepatic copper handling. This may be particularly important because WD is a rare disease, and therefore choosing the right drugs for large trials is paramount. In addition, preclinical 64Cu PET studies in animal models of WD can be used in early drug development, followed by human PET studies to help qualify drugs before initiating randomized clinical trials with clinical outcomes.
In conclusion, this human 64Cu PET/CT study of WD showed that patients with WD exhibit marked retention of copper in the liver as shown by in vivo imaging, which clearly discriminates WD patients from heterozygote and healthy subjects. This is in accordance with the known pathophysiology of the disease. The 64Cu PET method holds potential for detailed mechanistic studies of WD as well as clinical studies of drug efficacy and diagnosis.
CONFLICT OF INTEREST
Dr. Ott received grants from Alexion.
AUTHOR CONTRIBUTIONS
Conception and design of study: Peter Ott, Hendrik Vilstrup, Susanne Keiding, and Thomas Damgaard Sandahl. Generation, collection, assembly: Thomas Damgaard Sandahl, Lars C. Gormsen, and Ditte Emilie Munk. Copper handling and preparation: Dirk Bender, Karina Vase. Statistical analysis and interpretation of data: Ole Lajord Munk, Lars C. Gormsen, Mikkel Vendelbo, Thomas Damgaard Sandahl, and Kristoffer Kjærgaard Drafting: Thomas Damgaard Sandahl and Lars C. Gormsen. Revision of the manuscript: All authors. Approval of the final version of the manuscript: All authors. Thomas Damgaard Sandahl, Lars C. Gormsen, and Kristoffer Kjærgaard. verified the data.
ETHICS
The study was approved by the Central Denmark Region Committees on Health Research Ethics (no.: 1‐10‐72‐196‐16) and the Danish Medicines Agency (EudraCT no.: 2016–001975‐59), conducted in accordance with the Helsinki Declaration, and monitored by the Good Clinical Practice Unit at Aarhus University Hospital. Written informed consent was obtained from all participants. There were no complications to the procedures, except from one case of small local hematoma after catheterization of a radial artery.
CLINICAL TRIAL NUMBER
EudraCT 2016–001975‐59.
Supporting information
Supplementary Material
ACKNOWLEDGMENTs
We are grateful to the patients and their families, without whom this study would not have been possible. We also thank the staff at Department of Hepatology and Gastroenterology, and Department of Nuclear Medicine and PET, Aarhus University Hospital, for competent assistance with procedures involving patients.
Sandahl TD, Gormsen LC, Kjærgaard K, Vendelbo MH, Munk DE, Munk OL, et al. The pathophysiology of Wilson’s disease visualized: A human 64Cu PET study. Hepatology. 2022;75:1461–1470. 10.1002/hep.32238
Funding information
Supported by a grant from The Memorial Foundation of Manufacturer Vilhelm Pedersen & Wife. The foundation played no role in the planning or any other phase of the study
REFERENCES
- 1. Scheinberg IH, Sternlieb I. Wilson’s disease. Philadelphia, PA: WB Saunders; 1984. [Google Scholar]
- 2. Gitlin JD. Wilson disease. Gastroenterology. 2003;125:1868–77. [DOI] [PubMed] [Google Scholar]
- 3. Lutsenko S, Petris MJ. Function and regulation of the mammalian copper‐transporting ATPases: insights from biochemical and cell biological approaches. J Membr Biol. 2003;191:1–12. [DOI] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol. 2012;56:671–85. [DOI] [PubMed] [Google Scholar]
- 5. Roberts EA, Schilsky ML; American Association for the Study of Liver Diseases (AASLD) . Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–111. [DOI] [PubMed] [Google Scholar]
- 6. Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, et al. Wilson disease. Nat Rev Dis Primers. 2018;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang N, Sandahl TD, Ott P, Kepp KP. Computing the pathogenicity of Wilson’s disease ATP7B mutations: implications for disease prevalence. J Chem Inf Model. 2019;59:5230–43. [DOI] [PubMed] [Google Scholar]
- 8. Stattermayer AF, Entenmann A, Gschwantler M, Zoller H, Hofer H, Ferenci P. The dilemma to diagnose Wilson disease by genetic testing alone. Eur J Clin Invest. 2019;49:e13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandahl TD, Laursen TL, Munk DE, Vilstrup H, Weiss KH, Ott P. The prevalence of Wilson’s disease: an update. Hepatology. 2020;71:722–32. [DOI] [PubMed] [Google Scholar]
- 10. Ferenci P, Stremmel W, Członkowska A, Szalay F, Viveiros A, Stättermayer AF, et al. Age and sex but not ATP7B genotype effectively influence the clinical phenotype of Wilson disease. Hepatology. 2019;69:1464–76. [DOI] [PubMed] [Google Scholar]
- 11. Hill GM, Brewer GJ, Juni JE, Prasad AS, Dick RD. Treatment of Wilson’s disease with zinc. II. Validation of oral 64copper with copper balance. Am J Med Sci. 1986;292:344–9. [DOI] [PubMed] [Google Scholar]
- 12. Osborn SB, Roberts CN, Walshe JM. Uptake of radiocopper by the liver. A study of patients with Wilson’s disease and various control groups. Clin Sci. 1963;24:13–22. [PubMed] [Google Scholar]
- 13. Biesold D, Günther K. Improved method for investigation of copper metabolism in patients with Wilson’s disease using 64Cu. Clin Chim Acta. 1972;42:353–9. [Google Scholar]
- 14. Czlonkowska A, Galewicz A, Rodo M, Wehr H. Observations on copper metabolism in Wilson’s disease. Acta Univ Carol Med Monogr. 1973;56:175–7. [PubMed] [Google Scholar]
- 15. Czlonkowska A, Rodo M, Wierzchowska‐Ciok A, Smolinski L, Litwin T. Accuracy of the radioactive copper incorporation test in the diagnosis of Wilson disease. Liver Int. 2018;38:1860–6. [DOI] [PubMed] [Google Scholar]
- 16. Kjærgaard K, Sandahl TD, Frisch K, Vase KH, Keiding S, Vilstrup H, et al. Intravenous and oral copper kinetics, biodistribution and dosimetry in healthy humans studied by [(64)Cu]copper PET/CT. EJNMMI Radiopharm Chem. 2020;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avila‐Rodriguez MA, Rios C, Carrasco‐Hernandez J, Manrique‐Arias JC, Martinez‐Hernandez R, García‐Pérez FO, et al. Biodistribution and radiation dosimetry of [(64)Cu]copper dichloride: first‐in‐human study in healthy volunteers. EJNMMI Res. 2017;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capasso E, Durzu S, Piras S, Zandieh S, Knoll P, Haug A, et al. Role of (64)CuCl 2 PET/CT in staging of prostate cancer. Ann Nucl Med. 2015;29:482–8. [DOI] [PubMed] [Google Scholar]
- 19. Piccardo A, Paparo F, Puntoni M, Righi S, Bottoni G, Bacigalupo L, et al. (64)CuCl2 PET/CT in prostate cancer relapse. J Nucl Med. 2018;59:444–51. [DOI] [PubMed] [Google Scholar]
- 20. Panichelli P, Villano C, Cistaro A, Bruno A, Barbato F, Piccardo A, et al. Imaging of brain tumors with copper‐64 chloride: early experience and results. Cancer Biother Radiopharm. 2016;31:159–67. [DOI] [PubMed] [Google Scholar]
- 21. Wachsmann J, Peng F. Molecular imaging and therapy targeting copper metabolism in hepatocellular carcinoma. World J Gastroenterol. 2016;22:221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang F, Jiao P, Qi M, Frezza M, Dou QP, Yan B. Turning tumor‐promoting copper into an anti‐cancer weapon via high‐throughput chemistry. Curr Med Chem. 2010;17:2685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng F, Lutsenko S, Sun X, Muzik O. Imaging copper metabolism imbalance in Atp7b (‐/‐) knockout mouse model of Wilson’s disease with PET‐CT and orally administered 64CuCl2. Mol Imaging Biol. 2012;14:600–7. [DOI] [PubMed] [Google Scholar]
- 24. Peng F, Lutsenko S, Sun X, Muzik O. Positron emission tomography of copper metabolism in the Atp7b‐/‐ knock‐out mouse model of Wilson’s disease. Mol Imaging Biol. 2012;14:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferenci P, Caca K, Loudianos G, Mieli‐Vergani G, Tanner S, Sternlieb I, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23:139–42. [DOI] [PubMed] [Google Scholar]
- 26. Dhawan A, Taylor RM, Cheeseman P, De Silva P, Katsiyiannakis L, Mieli‐Vergani G. Wilson's disease in children: 37‐year experience and revised King's score for liver transplantation. Liver Transpl. 2005;11:441–8. [DOI] [PubMed] [Google Scholar]
- 27. Patlak CS, Blasberg RG. Graphical evaluation of blood‐to‐brain transfer constants from multiple‐time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–90. [DOI] [PubMed] [Google Scholar]
- 28. Kjærgaard K, Frisch K, Sørensen M, Munk OL, Hofmann AF, Horsager J, et al. Obeticholic acid improves hepatic bile acid excretion in patients with primary biliary cholangitis. J Hepatol. 2021;74:58–65. [DOI] [PubMed] [Google Scholar]
- 29. Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, et al. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38:717–27. [DOI] [PubMed] [Google Scholar]
- 30. Osborn SB, Walshe JM. Studies with radiocopper (64Cu) in Wilson’s disease: dynamics of copper transport. Clin Sci. 1965;29:575–81. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


