Key Points
Question
How is the clinical outcome of icotinib in treatment-naive patients with clinically diagnosed advanced lung cancer with unknown pathological status and positive epidermal growth factor receptor (EGFR) variants assessed by circulating tumor DNA (ctDNA)?
Findings
In this phase 2 nonrandomized clinical trial including 116 patients with clinically diagnosed advanced lung cancer with unknown pathological status and positive plasma EGFR variants, the objective response rate of first-line icotinib was 52.6%, and the median progression-free survival was 10.3 months.
Meaning
For patients with clinically diagnosed advanced lung cancer who were unable to provide tissues for pathological diagnosis, ctDNA-based EGFR genotyping could help decision-making in particular clinical situations.
This phase 2 nonrandomized clinical trial evaluates the clinical outcome of first-line icotinib in patients with clinically diagnosed advanced lung cancer with unknown pathological status and positive EGFR-sensitizing variants assessed by circulating tumor DNA.
Abstract
Importance
The inability to obtain a pathological diagnosis in a certain proportion of patients with clinically diagnosed advanced lung cancer impedes precision treatment in clinical practice.
Objective
To evaluate the clinical outcome of first-line icotinib in patients with clinically diagnosed advanced lung cancer with unknown pathological status and positive epidermal growth factor receptor (EGFR)–sensitizing variants assessed by circulating tumor DNA (ctDNA).
Design, Setting, and Participants
The Efficiency of Icotinib in Plasma ctDNA EGFR Mutation-Positive Patients Diagnosed With Lung Cancer (CHALLENGE) trial is a prospective, multicentered, open-label, single-arm phase 2 nonrandomized clinical trial conducted between July 1, 2017, and July 31, 2019. Patients with systemic treatment-naive, clinically diagnosed advanced peripheral lung cancer, unknown pathological status, and positive pretreatment plasma EGFR-sensitizing variants were eligible. A total of 391 potentially eligible Chinese patients from 19 centers in China were screened for ctDNA EGFR variants by 3 independent detection platforms (Super amplification refractory mutation system [SuperARMS] polymerase chain reaction, droplet digital polymerase chain reaction, and next-generation sequencing), and those with EGFR variants tested by any platform were included. Analyses were conducted from September 9 to December 31, 2021.
Interventions
Enrolled patients were treated with oral icotinib tablets (125 mg 3 times daily) until disease progression, death, or treatment discontinuation due to various reasons, such as toxic effects and withdrawing consent.
Main Outcomes and Measures
The primary end point was objective response rate (ORR). The secondary end points included progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and the concordance among the 3 detection platforms.
Results
Of 116 included patients, 76 (65.5%) were female, and the median (range) age was 64 (37-85) years. The median (IQR) follow-up duration was 36.3 (30.2-40.7) months. The ORR was 52.6% (95% CI, 43.1%-61.9%). The median PFS and OS were 10.3 months (95% CI, 8.3-12.2) and 23.2 months (95% CI, 17.7-28.0), respectively, and the DCR was 84.5% (95% CI, 76.6%-90.5%). The concordance rate among the 3 detection platforms was 80.1% (313 of 391), and the clinical outcomes in patients identified as positive by any platform were comparable.
Conclusions and Relevance
This prospective phase 2 nonrandomized clinical trial suggests that for patients with clinically diagnosed advanced lung cancer with unknown pathological status, ctDNA-based EGFR genotyping could help decision-making in particular clinical situations, while still warranting future larger-scaled real-world exploration.
Trial Registration
ClinicalTrials.gov Identifier: NCT03346811
Introduction
Histopathological diagnosis and molecular genotyping have been the cornerstones of precision medicine in cancer treatment, especially in non–small-cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) variants. However, there always exist a certain proportion of patients who cannot provide tissues for pathological diagnosis, let alone tissue-based molecular testing.1,2 Even in well-designed prospective trials, approximately one-third of patients failed to provide sufficient tissues for molecular detection.3 Therefore, tissue-based diagnosis and treatment remains challenging in a certain subset of patients.
Currently, circulating tumor DNA (ctDNA) has been established as an important surrogate of tissues for molecular testing.4,5,6,7 Several studies have exhibited the pivotal role of ctDNA to prospectively predict clinical trajectories,8,9 and our previous prospective trial also proved the feasibility of using ctDNA-based EGFR variation to guide gefitinib in treatment-naive patients with advanced lung adenocarcinoma.10,11 However, the patients in these studies all have definitive pathological diagnoses. For patients with unknown pathologic status, the potential role of ctDNA remains unclear.
Therefore, we initiated this prospective multicentered clinical trial to evaluate the potential of ctDNA to instruct coping strategy in this specific subset of real-world patients.
Methods
The Efficiency of Icotinib in Plasma ctDNA EGFR Mutation-Positive Patients Diagnosed With Lung Cancer (CHALLENGE) trial is a prospective, multicentered, open-label, single-arm phase 2 nonrandomized clinical trial aiming to evaluate the clinical outcome of icotinib in treatment-naive patients with clinically diagnosed advanced peripheral lung cancer with unknown pathological status and positive plasma EGFR-sensitizing variants (EGFR Exon 19 deletion and/or EGFR L858R). The reasons for unobtained pathological diagnosis were prospectively categorized and documented. All potentially eligible patients were screened by 3 independent platforms: Super amplification refractory mutation system (SuperARMS) polymerase chain reaction (PCR), droplet digital PCR (ddPCR), or next-generation sequencing (NGS), and patients with plasma EGFR variants tested by any platform were eligible. Enrolled patients were treated with icotinib tablets (125 mg 3 times daily; Betta Pharmaceuticals) until disease progression, death, or treatment discontinuation due to various reasons, such as toxic effects and withdrawing consent. This study was approved by the Ethics Committee of Cancer Hospital Chinese Academy of Medical Sciences and independent local ethics committees and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants. The trial protocol can be found in Supplement 1. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The primary end point was the objective response rate (ORR). The secondary end points included progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and the concordance among the SuperARMS PCR, ddPCR, and NGS platforms. The justification of sample size, the statistical methods we used to analyze the end points, and the calculation of concordance can be found in the eMethods in Supplement 2. All analyses were conducted using SAS version 9.4 (SAS Institute).
Results
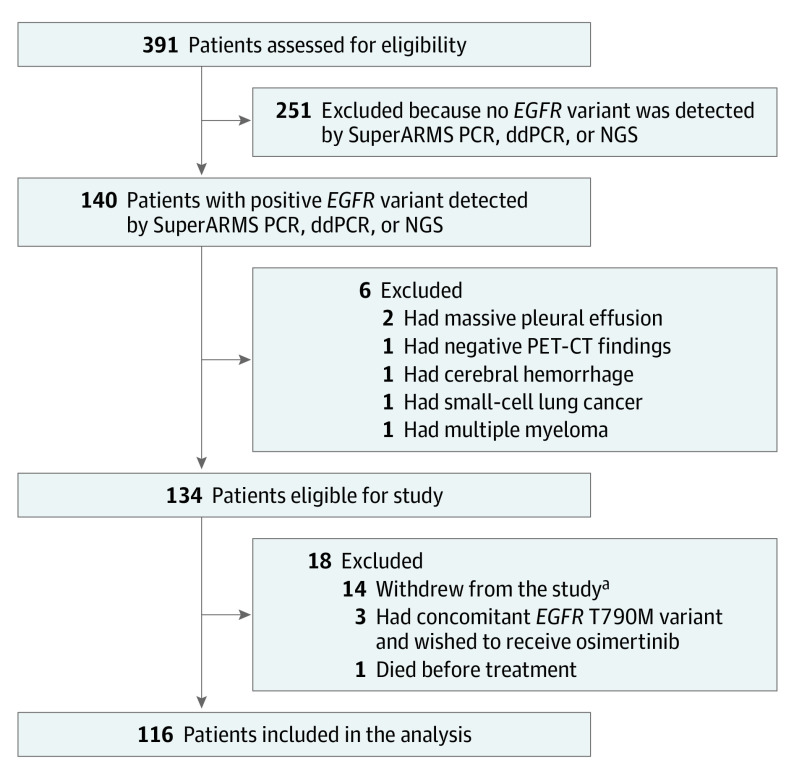
Between July 1, 2017, and July 31, 2019, 391 Chinese patients from 19 centers in China were screened; 134 patients were eligible for enrollment, and a total of 116 patients were included in the trial (Figure 1). Of 116 included patients, 76 (65.5%) were female, and the median (range) age was 64 (37-85) years. The median (IQR) follow-up duration was 36.3 (30.2-40.7) months. The reasons for unobtained pathological diagnosis included advanced age (32 [27.6%]), violation of cultural traditions (31 [26.7%]), and failure of repeating previous biopsies (23 [19.8%]) (Table). After enrollment or medication, 18 patients (15.6%) subsequently provided pathological diagnoses, including 16 lung adenocarcinomas, 1 lung squamous cell carcinoma, and 1 lung adenosquamous carcinoma.
Figure 1. Framework of the Study.
ddPCR indicates droplet digital polymerase chain reaction; EGFR, epidermal growth factor receptor; NGS, next-generation sequencing; PCR, polymerase chain reaction; PET-CT, positron emission tomography with computed tomography; SuperARMS, Super amplification refractory mutation system.
aIncluding wishing to receive other treatments, wishing to receive treatment back in their hometown, and declining to receive routine tumor evaluation and follow-up.
Table. Clinicopathological Characteristics of 116 Patients.
| Characteristic | Patients, No. (%) |
|---|---|
| Total, No. | 116 |
| Age, y | |
| Median (range) | 64.4 (37.0-85.0) |
| <70 | 72 (62.1) |
| ≥70 | 44 (37.9) |
| Sex | |
| Male | 40 (34.5) |
| Female | 76 (65.5) |
| Smoking status | |
| Smoker | 29 (25.0) |
| Nonsmoker | 87 (75.0) |
| Brain metastasis | |
| Yes | 37 (31.9) |
| No | 79 (68.1) |
| ECOG score | |
| 0-1 | 105 (90.5) |
| 2-4 | 11 (9.5) |
| EGFR variant subtype | |
| Exon 19 deletion | 44 (37.9) |
| Exon 21 L858R | 65 (56.0) |
| Exon 19 deletion + Exon 21 L858R | 3 (2.6) |
| Exon 19 deletion + Exon 20 T790M | 1 (0.9) |
| Exon 21 L858R + Exon 20 T790M | 3 (2.6) |
| Reasons for unobtained pathological diagnosis | |
| Advanced age | 32 (27.6) |
| Violation of cultural tradition | 31 (26.7) |
| Failure of repeating preceding biopsies | 23 (19.8) |
| Technical obstacles | 19 (16.4) |
| Tiny and diffuse nodules | 14 (12.1) |
| Intolerant to biopsya | 5 (4.3) |
| Poor physical condition | 11 (9.5) |
| ECOG score of 2 combined with severe cardiopulmonary comorbidities | 5 (4.3) |
| ECOG score of 3 | 5 (4.3) |
| ECOG score of 4 | 1 (0.9) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
Including severe cardiopulmonary comorbidities and history of severe anesthetic allergy.
By the data cutoff (September 9, 2021), 28 of 116 patients (24.1%) were alive, and 6 (5.2%) were still receiving treatment. A total of 12 patients (10.3%) were lost to follow-up. In the 116 enrolled patients, the ORR was 52.6% (95% CI, 43.1%-61.9%). The median PFS and OS was 10.3 months (95% CI, 8.3-12.2) and 23.2 months (95% CI, 17.7-28.0), respectively, and the DCR was 84.5% (95% CI, 76.6%-90.5%) (Figure 2; eTable 1 in Supplement 2). A total of 89 patients (76.7%) experienced at least 1 treatment-related adverse event (TRAE), and the most common TRAEs were rash (60 [51.7%]) and fatigue (58 [50.0%]). Eight patients (6.9%) experienced grade 3 to 4 TRAEs, and the most common grade 3 to 4 TRAE was fatigue (3 [2.6%]) (eTable 2 in Supplement 2). There were no newly reported TRAEs, and none of the patients discontinued the treatment due to toxic effects.
Figure 2. Clinical Response and Survival of 116 Patients.
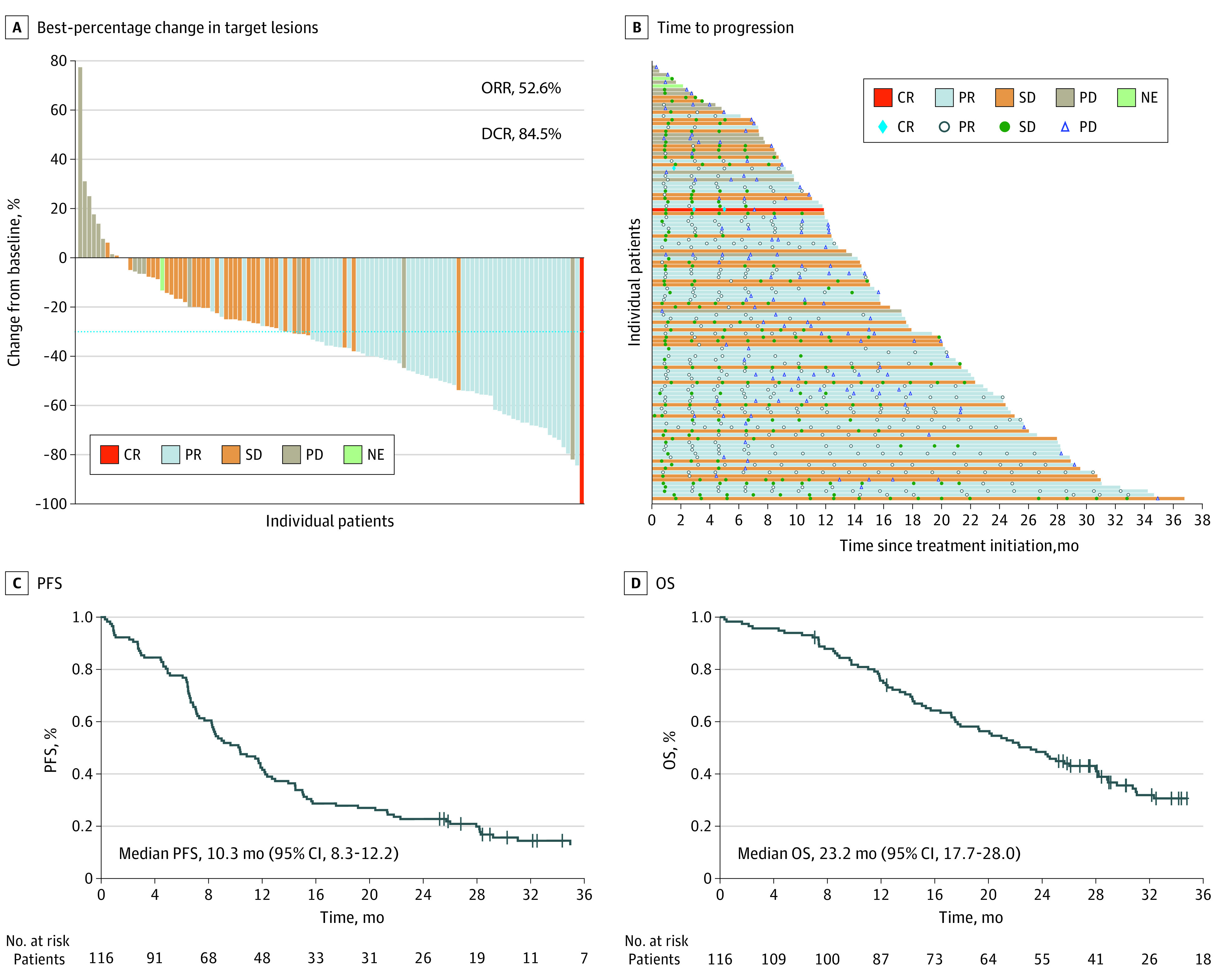
A, Waterfall plot of best-percentage change in target lesions from baseline during icotinib treatment. B, Swimmer plot of time to progression during icotinib treatment. C, Kaplan-Meier estimates of progression-free survival (PFS). D, Kaplan-Meier estimates of overall survival (OS). CR indicates complete response; DCR, disease control rate; NE, not evaluable; ORR, overall response rate; PD, progressed disease; PR, partial response; SD, stable disease.
Among the 391 screened patients, 140 (35.8%) had plasma EGFR-sensitizing variants tested by any of the 3 platforms (Figure 1). The EGFR positive rate was the highest through NGS (114 of 140 [81.4%]), followed by ddPCR (103 of 140 [73.5%]) and SuperARMS PCR (100 of 140 [71.4%]), and the concordance rate among these 3 platforms was 80.1% (313 of 391). Notably, the clinical outcomes in patients detected positive by any platform were comparable (eFigure and eTable 3 in Supplement 2).
Discussion
The inability to obtain histopathological diagnosis in patients with lung cancer has deprived some patients of the opportunity to benefit from precision treatment. In this study, we unveiled the potential of ctDNA-based EGFR genotyping to prospectively guide first-line icotinib treatment in patients with clinically diagnosed advanced lung cancer and unknown pathological status. To the best of our knowledge, this is the first prospective study focusing on this specific population to provide evidence for possible coping strategies.
Icotinib, a first-generation EGFR tyrosine kinase inhibitor independently developed in China, exhibited an ORR of 64.8% in treatment-naive patients with NSCLC harboring EGFR variants derived from tissue-based testing from the Icotinib Versus First-line Chemotherapy Plus Maintenance Treatment in EGFR Positive Lung Adenocarcinoma Patients (CONVINCE) trial.12 In a phase IV trial containing 6087 patients with advanced NSCLC treated with icotinib in the real world, the ORR in treatment-naive patients with EGFR-variant NSCLC was 56.3%.13 Our study demonstrated an ORR of 52.6% (95% CI, 43.1%-61.9%), inferior than that in CONVINCE trial,12 which could be mainly explained by the different inclusion criteria between the rigorously designed phase III trial and our study for this specific population. Compared with the CONVINCE trial, we enrolled a higher proportion of patients with older age (median [range] age, 64 [37-85] years vs 56 [35-74] years), Eastern Cooperative Oncology Group performance status 2-4 (11 of 116 [9.5%] vs 7 of 148 [4.7%]), brain metastasis (37 of 116 [31.9%] vs 41 of 148 [27.7%]), and EGFR L858R (65 of 116 [56.0%] vs 68 of 148 [45.9%]). Moreover, the EGFR variants occurred in nonadenocarcinoma14 and the clonal hematopoiesis,15 although very rare in EGFR-sensitizing variants (Exon 19 deletion and L858R) could also lead to an inferior ORR. However, the ORR in our study was similar with the real-world study, further indicating the potential of ctDNA-based EGFR genotyping to guide first-line icotinib in patients with clinically diagnosed lung cancer in the real world.
In addition, the median PFS in our study was comparable to the CONVINCE trial (10.3 months [95% CI, 8.3-12.2] vs 11.2 months). The shorter median OS (23.2 months [95% CI, 17.7-28.0] vs 30.5 months) could be explained by the higher proportion of patients with worse conditions and the lack of following precise therapeutic strategy owing to the uncertain pathological status. Moreover, although NGS could uncover more EGFR-positive patients, the clinical outcomes guided by 3 platforms were similar, suggesting that for this specific subset of patients, the assessment of ctDNA-based EGFR variants via any platform was feasible.
Limitations
This study has limitations. First, this trial was initiated and conducted before osimertinib was approved as first-line treatment for EGFR-variant NSCLC in China. Further studies are needed to explore whether the same findings could be observed with third-generation tyrosine kinase inhibitors. Next, histopathological diagnosis still remains the criterion standard in current oncology practice, and the reasons for unobtained pathological diagnosis in this study might be limited to regional issues in China. However, we indeed provided a promising future solution for this specific subset of patients who do exist worldwide. Additionally, although historical controls were used, the interpretation of our findings should still be cautious because of its single-arm setting.
Conclusions
For patients with clinically diagnosed advanced lung cancer with unknown pathological status, ctDNA-based EGFR genotyping could help decision-making under particular clinical situations, while still warranting future larger-scaled real-world exploration.
Trial Protocol
eMethods.
eTable 1. The Clinical Outcomes Evaluated by Independent Review Committee (N = 116)
eTable 2. The Treatment-Related Adverse Events of 116 Patients
eTable 3. The Clinical Outcomes of Patients With EGFR Variants Detected Using 3 Independent Platforms
eFigure. Venn Diagram Exhibiting the Distribution and Clinical Outcomes of Patients With EGFR Variants Detected Using 3 Independent Platforms
eReferences.
References
- 1.Fenizia F, De Luca A, Pasquale R, et al. EGFR mutations in lung cancer: from tissue testing to liquid biopsy. Future Oncol. 2015;11(11):1611-1623. doi: 10.2217/fon.15.23 [DOI] [PubMed] [Google Scholar]
- 2.Ayyappan S, Gonzalez C, Yarlagadda R, Zakharia Y, Woodlock TJ. Lung cancer in the very elderly: incidence, presentation, and diagnostic decision-making. a retrospective analysis at a teaching community hospital. J Community Hosp Intern Med Perspect. 2011;1(3):1. doi: 10.3402/jchimp.v1i3.7313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa DB, Kobayashi S, Tenen DG, Huberman MS. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancers. Lung Cancer. 2007;58(1):95-103. doi: 10.1016/j.lungcan.2007.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabon JJ, Simmons AD, Lovejoy AF, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun. 2016;7:11815. doi: 10.1038/ncomms11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loman N, Saal LH. The state of the art in prediction of breast cancer relapse using cell-free circulating tumor DNA liquid biopsies. Ann Transl Med. 2016;4(suppl 1):S68. doi: 10.21037/atm.2016.10.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinert T, Schøler LV, Thomsen R, et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut. 2016;65(4):625-634. doi: 10.1136/gutjnl-2014-308859 [DOI] [PubMed] [Google Scholar]
- 7.Rossi D, Diop F, Spaccarotella E, et al. Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood. 2017;129(14):1947-1957. doi: 10.1182/blood-2016-05-719641 [DOI] [PubMed] [Google Scholar]
- 8.Papadimitrakopoulou VA, Han JY, Ahn MJ, et al. Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer. 2020;126(2):373-380. doi: 10.1002/cncr.32503 [DOI] [PubMed] [Google Scholar]
- 9.Sueoka-Aragane N, Nakashima C, Yoshida H, et al. The role of comprehensive analysis with circulating tumor DNA in advanced non-small cell lung cancer patients considered for osimertinib treatment. Cancer Med. 2021;10(12):3873-3885. doi: 10.1002/cam4.3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Cheng Y, An T, et al. Detection of EGFR mutations in plasma circulating tumour DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicentre clinical trial. Lancet Respir Med. 2018;6(9):681-690. doi: 10.1016/S2213-2600(18)30264-9 [DOI] [PubMed] [Google Scholar]
- 11.Duan J, Xu J, Wang Z, et al. Refined stratification based on baseline concomitant mutations and longitudinal circulating tumor DNA monitoring in advanced EGFR-mutant lung adenocarcinoma under gefitinib treatment. J Thorac Oncol. 2020;15(12):1857-1870. doi: 10.1016/j.jtho.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 12.Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi: 10.1093/annonc/mdx359 [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Han B, Gu A, et al. A single-arm, multicenter, safety-monitoring, phase IV study of icotinib in treating advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2014;86(2):207-212. doi: 10.1016/j.lungcan.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Zhang Y, Jin B, et al. Efficacy of EGFR tyrosine kinase inhibitors for non-adenocarcinoma lung cancer patients harboring EGFR-sensitizing mutations in China. J Cancer Res Clin Oncol. 2016;142(6):1325-1330. doi: 10.1007/s00432-016-2133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filipska M, Rosell R. Mutated circulating tumor DNA as a liquid biopsy in lung cancer detection and treatment. Mol Oncol. 2021;15(6):1667-1682. doi: 10.1002/1878-0261.12983 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods.
eTable 1. The Clinical Outcomes Evaluated by Independent Review Committee (N = 116)
eTable 2. The Treatment-Related Adverse Events of 116 Patients
eTable 3. The Clinical Outcomes of Patients With EGFR Variants Detected Using 3 Independent Platforms
eFigure. Venn Diagram Exhibiting the Distribution and Clinical Outcomes of Patients With EGFR Variants Detected Using 3 Independent Platforms
eReferences.