Abstract
Devices for continuous in-vivo testing (CIVT) can detect target substances in real time, thus providing a valuable window into a patient's condition, their response to therapeutics, metabolic activities, and neurotransmitter transmission in the brain. Therefore, CIVT devices have received increased attention because they are expected to greatly assist disease diagnosis and treatment and research on human pathogenesis. However, CIVT has been achieved for only a few markers, and it remains challenging to detect many key markers. Therefore, it is important to summarize the key technologies and methodologies of CIVT, and to examine the direction of future development of CIVT. We review recent progress in the development of CIVT devices, with consideration of the structure of these devices, principles governing continuous detection, and nanomaterials used for electrode modification. This detailed and comprehensive review of CIVT devices serves three purposes: (1) to summarize the advantages and disadvantages of existing devices, (2) to provide a reference for development of CIVT equipment to detect additional important markers, and (3) to discuss future prospects with emphasis on problems that must be overcome for further development of CIVT equipment. This review aims to promote progress in research on CIVT devices and contribute to future innovation in personalized medical treatments.
Keywords: Nanomaterial, In-vivo testing, Microfluidic chip, Continuous detection equipment, Aptamer
Graphical abstract
Highlights
-
•
A detailed and comprehensive review of continuous in vivo testing device.
-
•
The nanomaterials, delicate structures and detection principles of the works are discussed.
-
•
The achievements and shortcomings of the existing devices are summarized.
-
•
The problems that should be solved in the further development of the devices and the future prospects are put forward.
1. Introduction
Personalized medicine customizes medical treatment to individual patients. Therefore, as demand for effective medical treatment increases, personalized medicine has received increasing attention [[1], [2], [3]], with individualized detection biosensors being of vital importance to personalized medical care [4]. In the past few decades, significant progress has been made in development of biosensors, and sensitive and specific detection of various small molecules, nucleic acids, and proteins has been achieved [[5], [6], [7]]. These innovative studies have greatly stimulated development of personalized medical treatment [8]. However, most of these sensors are capable of only a single detection, which limits their application to continuous monitoring of biomarkers in vivo [9].
The CIVT devices have great potential in the detection and treatment of many serious diseases, as well as monitoring multiple biochemical indicators [10,11]. For example, real-time detection of blood glucose makes it possible to control changes in its concentration in diabetics, which is important for guiding their activities, exercise, diet, drug use and other aspects of their lifestyle [12]. This disease requires frequent monitoring of blood glucose, and CIVT can avoid the inconvenience of repeated single measurements and the risk associated with fluctuations between tests [13]. Another example is the concentration of estradiol in the body, which is directly related to various diseases such as ovarian tumors and liver cirrhosis [14]. At the same time, in order to understand changes in ovarian function or track the effects of drug treatment, it is necessary to dynamically monitor estradiol content in patients over a period of time [15]. It is inefficient to test the level of estradiol at random intervals [16], which can be both physically and mentally traumatic for patients. CIVT of estradiol is an ideal solution to these problems [17]. Cytokines are essential indicators of body function and play a key role in controlling cell growth and function [18], and CIVT of cytokines has been shown to be of diagnostic value during medical examinations.
Compared with in vitro testing devices, there are more requirements for CIVT devices. Generally speaking, the first thing to consider for in vitro detection equipment is the sensitivity and specificity. Researchers can improve the performance of the equipment by optimizing the structure and the biometric layer. The biocompatibility and environmental friendliness of the equipment also need to be taken into account. But there is much more to the ongoing development of CIVT devices. First of all, it will be more difficult to achieve CIVT [19]. To prepare a biosensor for CIVT of specific biomarkers, the following problems must be addressed: (1) The internal environment of human body is extremely complex and there are many sources of artifact, which the biosensor must avoid; (2) The equipment must not require external reagents and additional separations, washing, labeling or other steps during detection, meaning it must be an integrated system that can carry out all necessary functions; and (3) The signal produced by individual sensors must be reversible so that they continuously report on biomarkers over time and at different physiological concentrations. Also, since the device needs to be implanted in the human body, the safety of the device also needs to be considered. It cannot cause infection and serious trauma to the human body. However, despite so many requirements and limitations in the development of continuous in-vivo detection equipment, its research is still extremely important. In vitro detection can provide a convenient detection method for various disease markers. On this basis, CIVT devices can provide real-time and continuous detection methods for various markers and human physiological indicators. Obviously, CIVT devices can play a significant role in the evaluation of drug efficacy and disease monitoring. But its role is far more than that. In fact, CIVT devices can even be used as normal health management monitoring tools similar to mobile phones to realize the personalized protection of human health through the real-time monitoring of various relevant markers. CIVT devices are expected to completely change the way of people's health monitoring to a great extent.
Early on in the development of CIVT technology, only a few substances, such as oxygen, lactose and glucose, could be continuously detected in vivo [20]. This is because these analytes could undergo analyte-specific reactions to generate signals (e.g., glucose oxidase can catalyze the reaction of glucose). As the development of biosensors progressed, many new nanomaterials and probes were identified, synthesized and applied to their preparation [21]. Some CIVT equipment has been developed for cytokines, hormones and other markers, and there has been innovative development of real-time monitoring equipment for human healthcare [22]. It is expected that development of CIVT equipment for detection of other markers will have a significant impact on treatment of the corresponding disease [23].
In this review, we comprehensively discuss recent progress and perspectives of CIVT sensors, and point out the advantages and disadvantages of existing systems. It is worth noting that since the main target of this review is implantable sensors, the conduction methods are all based on electrochemical methods. These CIVT devices are classified according to the type of probe they use. Significant attention is given to the structural design of the CIVT device, the method of achieving continuous detection, and the nanomaterials used for modification of electrodes. Finally, we summarize the state of the field and provide insight into future prospects and directions. The overall framework of this paper is shown in Scheme 1, which refers to previous work [24].
SCHEME 1.
The overall framework of this review.
2. Continuous in-vivo testing device
2.1. Difficulties in continuous testing
For a few analytes that can participate in analyte-specific reactions to generate signals, continuous detection is not difficult [25]. The only requirement is to obtain the concentration of the object to be detected by monitoring changes in electrical parameters generated at a specific voltage [26]. But for other analytes that do not undergo analyte-specific reactions, the difficulty of achieving continuous detection is much greater [27]. In those cases, there are several challenges to be overcome in achieving continuous detection [28]. First, the device needs to automatically detect analytes without manual interventions such as sample processing and addition of reagents. Second, since there might be false positives, especially in a complex bodily fluid [29], equipment needs to be highly sensitive and specific. Moreover, body fluids, such as whole blood, tissue fluid or urine may contaminate and corrode the sensor [30]. Therefore, the equipment needs to be able to resist contamination from body fluids to remain functional for long periods of time. Therefore, these three requirements need to be taken into consideration when developing CIVT equipment for the detection of most target substances [31,32].
2.2. Applications of nanomaterials
Nanomaterials are now an indispensable part of the development of various testing equipment and play important roles in the realization of CIVT because of their excellent physical and chemical properties [[33], [34], [35]].
In general, nanomaterials mainly play the following three roles in equipment [36]. First of all, nanomaterials can greatly improve the electrical properties of electrodes [[37], [38], [39]]. Whether it is silicon-based electrodes fabricated by micro-electro-mechanical system, gold electrodes or glassy carbon electrodes, their electrical conductivity and chemical stability generally cannot meet the needs of extremely sensitive biomarker detection [40]. In this case, it is a very effective way to modify the electrode through nanomaterials to improve its properties [41,42]. Secondly, nanomaterials also play a role in generating and amplifying signals in the device [43]. In fact, except for a few analytes that can produce signals from analyte-specific reactions, the detection of most markers requires the presence of substances capable of redox reactions in nanomaterials to generate signals. And when it comes to the CIVT, the need for materials that can generate signals on their own is even more urgent. Because there is no addition of foreign substances after implantation. Finally, various functional groups can be easily modified on nanomaterials, such as amino groups, sulfhydryl groups, and so on. This makes it easier to modify the electrode and more flexible to fix the detection probe [44].
2.3. The development of CIVT devices
The development of CIVT equipment mainly lies in the following aspects: The first is the structural design of the equipment [45,46]. CIVT requires equipment to be able to spontaneously perform testing after being implanted in the human body. So the equipment must integrate all the functional components required for testing [47,48]. The second is the modification of the electrode. This involves the screening and synthesis of nanomaterials [49]. Researchers need to consider the ability of nanomaterials to modify electrodes, the ability to generate and amplify signals, and the ability to fix identification probes, and carefully synthesize nanomaterials to achieve the best performance [50]. The device thus prepared can provide high-sensitivity and high-stability CIVT [51]. Of course, CIVT equipment also has some disadvantages. For example, many devices need to be standardized and calibrated before testing to overcome the interference of different bodily fluid environments. In addition, since the device will be implanted into the human body, the safety of the equipment should be taken into account at the beginning of design. The most intuitive injury comes from the wound injury when the device is implanted into the human body. Therefore, the part of the sensor implanted into the human body should be as small and regular as possible. Fortunately, at present, many CIVT devices implanted in human body will only cause extremely slight injury. In addition, it should be noted that the implanted part of the human body can not cause severe inflammatory reaction. This requires that the equipment must have excellent biocompatibility. Neither the base material of the equipment nor the modified biometric layer should have an impact on the human body. Moreover, because CIVT devices will exist in the human body for a period of time, the device should be stable. When the equipment is taken out of the human body after testing, no residue shall be left in the human body. If it is inevitable to leave a certain amount of residues, only substances that are easy to be discharged by the human body can have a small amount of residues. Therefore, the development of CIVT equipment should not only consider how to achieve detection, but also consider the safety of the equipment. Both are equally important.
Some application methods and future application prospects of continuous in-body detection equipment are shown in Scheme 2. With the increase of personal medical demand, the demand for CIVT equipment is also increasing [52]. It is considered to be a powerful device for realizing the diagnosis, treatment and monitoring of disease and providing the basis for basic pathological research [53]. Therefore, the continuous development of in-vivo detection equipment has become a hot and difficult point in the field of biosensors.
SCHEME 2.
A schematic of application and Prospect of Equipment. (1) Neuroprobe that can be easily bent [81]. (2) The carbon fiber microelectrode for the detection of PSA in vivo [61]. (3) A neural recording probe that can also detect cocaine in vivo [85]. (4) The microneedle sensor platform for dual-marker HB/GL detection. The lower part of the figure is the further applications of the device in the future [59].
3. Two types of continuous in-vivo testing devices
3.1. Devices based on analyte-specific reaction
CIVT devices based on analyte-specific reactions can achieve high specificity and sensitivity because signals are generated from the target analytes. These signals arise in various ways, including, for example, enzyme catalysis and reactions in the presence of an external voltage.
Development of CIVT for detection of analytes that can participate in analyte-specific reactions has yielded significant results. In the case of CIVT of glucose in blood, a blood glucose meter has been on the market for many years and is considered to be personal healthcare equipment. Enzymes are well-known detection probes and have already been used in commercialized products for detecting many markers owing to their specific catalytic effect on the target substance. Hence, enzymes have naturally become the focus of research on fabrication of CIVT devices. Due to the ability of enzymes to specifically catalyze the reaction of a target substance, they do not require additional materials to produce chemical reactions. They can also avoid problems of false positives that may result from use of other recognition probes. Therefore, enzymes have attracted a lot of attention as probes and their use is at the forefront of CIVT development.
3.1.1. Devices based on enzyme
Enzyme is a well-known detection probe and has already been applied in the production of commercialized products for detecting many markers. This is owing to its original specific catalytic effect on the target substance. And when it comes to the fabrication of CIVT devices, enzymes have naturally become the focus of research. Due to the ability of the enzyme to specifically catalyze the reaction of the target substance, it does not need to add additional materials that can produce chemical reactions. And at the same time, it can also avoid the false positive problems that may exist in other recognition probes. Therefore, enzyme has attracted a lot of attention in the production of CIVT equipment, and has become a recognition probe at the forefront of development and application.
Among these enzyme-based devices, the most common application is detection of glucose [54]. About five percent of people in the world are diabetic, and as a consequence, no analyte is tested more frequently than glucose. Although glucose can also undergo redox reactions without enzymes, this type of detection has specific requirements for the environment and electrodes in which the reaction occurs, so it cannot meet the needs of general continuous physical examination [55]. The use of glucose oxidase catalytic enzyme can get rid of these needs and has become the first choice for the development of CIVT equipment [56]. Although there have been thousands of articles describing continuous detection of various substances similar to glucose [57], it is impossible to comprehensively review all of them. In view of the extensive development of many CIVT sensors, in this review we will comment and summarize representative examples in order to provide readers with a specific and clear understanding of their development [58].
Hazhir Teymourian and coworkers [59] proposed a continuous ketone bodies monitoring microneedle sensor array for the real-time detection of β-hydroxybutyrate (HB) alongside glucose (GL) in interstitial fluid. The device is shown in Fig. 1(A). Detection of β-hydroxybutyrate (HB, a proxy for ketone bodies) and glucose used a three-electrode system. In addition to a working electrode, a microneedle with integrated Ag/AgCl wire (500 μm) acted as a reference electrode, and a graphite powder/mineral oil-filled microneedle acted as a counter electrode. When the microneedle was implanted in the human body and contacted tissue fluid, a polyvinyl chloride layer and a chitosan layer on two working electrodes were respectively used as selectively permeable and semi-permeable outer membranes to minimize biological contamination of the electrode surface and prevent impurities from affecting the signal. HB analysis was carried out using an electrochemically mediated enzymatic method that relied on HB dehydrogenase (HBD) to catalyze oxidation of HB to acetoacetic acid and at the same time reduce nicotinamide adenine dinucleotide (NAD+, oxidized form) to nicotinamide adenine dinucleotide (NADH, reduced form). The NADH produced was oxidized back to NAD + by 1,10-phenanthroline-5,6-dione. The use of 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide-based carbon paste (CP) electrodes minimized surface contamination from NADH oxidation products and prevented the signal current from being affected. The HBD/NAD + mixture was immobilized by adsorption into the pores of the CP electrode, and cross-linked by glutaraldehyde, allowing the components to contact each other relatively freely. The principle of the working electrode for glucose detection is similar. Experiments have shown that the detection limit of the device for HB can be as low as 50 μM, and for glucose as low as 0.5 mM, indicating extremely high sensitivity. Their device can be used continuously for about 380 min.
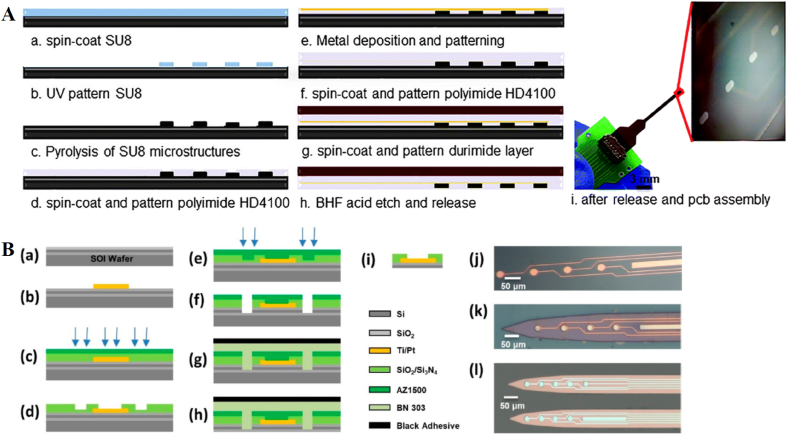
Fig. 1.
Schematic illustration of the CIVT devices. (A) The microneedle sensor platform that can simultaneously detect HB and GL in interstitial fluid. Reprinted with permission from { [59]}. Copyright {2020} American Chemical Society. (B) The detailed preparation process of the CIVT sensor [60]. (C) The sensor that is produced by twisting the component layer of carbon nanotube and helical fiber bundle [61].
Another method to detect glucose content through interstitial fluid was proposed by Muamer and his colleagues [60]. The device consists of three electrodes of the same shape. The substrate of the electrode is formed by 3D printing, a silicon electrode is made on the substrate, and then a gold film is formed by sputtering deposition. Then, the reference electrode needs to be coated with a layer of Ag/AgCl ink, and the working electrode forms Au–Si-microneedle array/ferrocenecored poly(amidoamine) dendrimers/glucose oxidase electrode after a series of functional treatments (the specific treatment steps are clearly presented in the original contribution). As shown in Fig. 1(B), the electrode can penetrate the skin and contact the interstitial fluid to directly form a three electrode system for detection. The enzyme on the working electrode can catalyze the glucose in the interstitial fluid to produce a current, and higher glucose concentration will obviously lead to greater measured current. The experimental results prove that the device has good selectivity, linearity between 1 and 9 mM, and the detection limit of 0.66 mM can be reached. And the device has been successfully applied to the detection of glucose in mice, demonstrating its potential as a continuous in-vivo detection device. However, it is worth noting that this work did not test the continuous detection time of the device in the body. Considering that there is no protective measure against potential contamination on the working electrode, the continuous detection time may not be particularly long.
In order to meet the challenges of complex physical dynamics and biochemical environments in vivo, a fiber-based implantable electrochemical sensor that imitates the layered and spiral assembly of natural soft tissue was proposed by Wang and colleagues [61]. Briefly, the sensor was produced by twisting the component layer of carbon nanotubes (CNTs) and a helical fiber bundle. After further modification, it could be applied to detection of various markers. Twisting multiple fiber bundles together could achieve simultaneous detection of multiple markers. The fiber sensor could be intravenously injected into the body via a syringe, and the other end of the fiber left outside for easy access. This fiber sensor has amazing potential and can be used for detection of a variety of markers. Taking the enzyme-based glucose sensor as an example, as shown in Fig. 1(C), a signal conversion layer composed of polyaniline and Pt nanoparticles was first electrochemically deposited onto the CNT fiber electrode. Pt nanoparticles can catalyze dissociation of intermediate H2O2 and produce a signal current. Then, a response layer formed by glucose oxidase on the linear polysaccharide chitosan and a microporous layer formed by Nafion and glutaraldehyde were modified. The experimental results showed that the working range of the sensor was 2.5–7.0 mM and the detection limit was 50 μM. Moreover, the sensor had a continuous detection time of up to 28 days, which is due to the robust microporous layer formed by Nafion and glutaraldehyde and the small size of the sensor. It is worth mentioning that it was shown that the optical fiber sensor can stably exist in the blood during blood flushing and animal movements, and does not elicit an abnormal or chronic immune response after implantation. This work was extremely complete and excellent, and showed that the device has a very long continuous detection time with minimal trauma while achieving detection of multiple substances.
3.1.2. Devices with no recognition probe
There are some special target substances. For their detection, there is no need to identify the probe. They produce chemical reactions and signals themselves in the presence of specific voltages. For detection of this class of target material, nanomaterials are used to target a specific substance and amplify the signal. This kind of continuous physical examination sensor is usually used to detect substances in the brain to monitor nerve signaling.
As an example, Castagnola and coworkers [62] proposed simultaneous detection of multiple neurotransmitters (Fig. 2(A)). Glassy carbon microelectrode arrays were prepared on flexible substrates based on a carbon microelectromechanical system (C-MEMS) microfabrication process. There are four exposed detection pads in the electrode. This sensor detects current generated by dopamine and serotonin at the appropriate voltage and calculates their concentration. During fabrication, the electrode surface becomes rich in functional groups such as carboxyl, carbonyl and hydroxyl, which further enhance adsorption of dopamine and serotonin and greatly improve detection sensitivity. This device enables simultaneous detection of serotonin and dopamine in the range of 10–200 nM with a detection limit of 10 nM. The device can continuously test neurotransmitters for 8 h in vivo without significant loss of function.
Fig. 2.
The fabrication process of the microelectrode arrays based on Micro-Electro-Mechanical System (MEMS). (A) The glassy carbon microelectrode arrays for the detection of multiple neurotransmitters simultaneously [62]. (B) Silicon substrate based microelectrode arrays for the detection of dopamine in the brain. Reprinted with permission from { [63]}. Copyright {2019} American Chemical Society.
There is another device that was fabricated on a silicon substrate based on a MEMS microfabrication process [63]. The aim of that device was to use real-time detection of the concentration of dopamine in the brain to study the response to treatment for Alzheimer's disease. As shown in Fig. 2(B), through three-step lithography, nine recording microelectrode arrays, connecting wires and pads were fabricated on a silicon substrate. By forming a Ti/Pt metal layer on the silicon substrate, nine recording microelectrode arrays were fabricated to ensure that multiple channels could be detected at the same time, which increases sensitivity to the weak signal in the complex environment of a body fluid. The device's pad has a position to connect with an external device and transmit the signal. The CIVT equipment could detect at concentrations between 50 nM and 16.3 μM, with a detection limit of 50 nM. The device's performance can fully meet the specific requirements of dopamine detection in vivo. This is a typical example of using continuous in-vivo detection equipment to research and treat a specific disease. It also shows the importance of research on continuous in-vivo detection equipment. We compare the performance of these representative CIVT devices without aptamer probes in Table 1. It is worth noting that after the detection, the sensor can be reused by cleaning and modifying the nano materials. Until there is a significant decline in performance, we will recycle the sensor, the metal on the sensor will be treated and reused, and other materials will also undergo professional harmless treatment to prevent damage to the environment.
Table 1.
A list of the representative CIVT sensors without aptamer.
| Probe | Device/Nanomaterial | Linear Range | LOD | Detection time | Targeted analytes | Reference |
|---|---|---|---|---|---|---|
| Enzyme | Microneedle/Carbon paste | – | 50 μM | 380 min | Glucose and β-hydroxybutyrate | [59] |
| Enzyme | Microneedle/Ferrocenecored poly(amidoamine) dendrimers | 1–9 mM | 0.66 mM | – | Glucose | [60] |
| Enzyme | Fiber bundle/Carbon nanotube | 2.5–7.0 mM | 50 μ M | 28 days | Glucose | [61] |
| No probe | Glassy carbon microelectrode arrays | 10 nM–200 nM | 10 nM | 8 h | Serotonin and dopamine | [62] |
| No probe | Silicon based microelectrode arrays/Ti/Pt | 50 nM-16.3 μM | 50 nM | – | Dopamine | [63] |
3.2. Devices based on the specific recognition of aptamers
Although there has been extensive development of CIVT devices based on spontaneous reactions of analytes, research on other continuous in-vivo detection devices based on aptamers is ongoing. This is because devices based on the spontaneous reaction of analytes can only detect a small number of substances that can themselves react at a specific voltage. Moreover, there are only a few target substances that rely on enzymes to catalyze reactions since many key markers do not have corresponding enzymes. Obviously, this limits medical applications.
Aptamers have recently been proposed as recognition probes that can be synthesized by systematic evolution of ligands using exponential enrichment [64]. Compared with traditional recognition probes, aptamers are highly suited to continuous in-vivo detection. First, production of aptamers is simpler and relatively inexpensive. Second, aptamers are more amenable to the chemical modifications needed to provide them with desirable properties. The last and most important point is that they are highly stable over a wide range of temperatures and relatively resistant to acids and bases. These characteristics provide aptamers with great potential for development of CIVT technology.
Because of the strong application potential of aptamers, in this review they will be divided into traditional electrochemical aptamer-based biosensors (EAB), further improved EAB, competitive EAB and EAB with specific reaction of analytes.
3.2.1. Electrochemical aptamer-based devices
Most EABs proposed in recent years are based on easy modification of various groups at the ends of the aptamer. One end the aptamer is covalently bound (e.g., by thiolation) to the surface of the electrode, and the other modified with a redox reporter molecule (methylene blue, ferrocene, etc). Upon binding to the corresponding target, the distance between the redox reporter and electrode changes, which alters electron transfer kinetics between the redox reporter and electrode, resulting in a measurable change in current that can be used to calculate changes in concentration of the target substance. This approach requires not only detection of conformational changes in the aptamer but also prevention of contamination of the equipment by body fluids.
Using the properties of aptamers, which are easily modified by various functional groups, highly sensitive CIVT biosensors can easily be fabricated. A representative study was carried out by Idili and co-authors in which a CIVT device was used in real time (seconds) to monitor small molecules characteristic of metabolism [65]. In their study, the corresponding aptamer was modified with a six-carbon thiol for attachment to a gold electrode on its 5′ end and a methylene blue (MB) molecule on its 3’ end for a redox reaction to produce electrons used for the signal. When the aptamer bound to its target, it folded into a stem ring structure so that the MB molecule was closer to the electrode. This device is shown in Fig. 3(A). Three electrodes of diameter 75 μm, namely a working (gold) electrode, a reference (Ag/AgCl) electrode and counter (platinum) electrode, were bound together to form a three electrode system. The working electrode was cleaned and its surface roughened prior to binding of the aptamer. The sensor was inserted into the jugular vein of a rat via a 22-gauge urinary catheter to detect metabolites in the blood. The CIVT equipment could detect phenylalanine in the range of 30 μM to 1 mM with a temporal resolution of 12 s. Measurements could be made continuously for more than 70 min.
Fig. 3.
Principles of representative aptamer-based CIVT sensors. (A) A gold wire based CIVT device for the monitoring of small molecules [65]. (B) The aptamer-based CIVT sensors with a polysulfone membrane protect the electrode from contamination by blood cells [66]. (C) The aptamer-based CIVT sensors with FC as the redox reporter for target drug delivery [67].
This type of CIVT sensor can be easily applied to a variety of target substances. Netzahualcóyotl and coworkers [66] developed a CIVT device to detect four drugs in the blood for several hours with the aim of providing guidance for individualized medical treatment and opening up new avenues of personalized medicine. The aptamer in this biosensor was also modified at both ends, one to fix the aptamer to the electrode, and the other modified with MB molecules. The electron transfer rate of the folded aptamer bound to the target substance was higher than when unbound, thus generating a stronger signal. It is worth noting that this sensor used a microporous (0.2 μm) polysulfone membrane to protect the electrode from contamination by blood cells. Experimental results show that this protective film played a key protective role in continuous in-vivo testing. The proposed sensor is shown in Fig. 3(B). This sensor could be inserted into a rat jugular vein through an 18-gauge catheter, and perform continuous detection for up to 4 h. This sensor detected kanamycin concentration in the range of 34–400 μM.
The method used for continuous in-vivo detection can also be used for targeted drug delivery. Cao and colleagues [67] developed a novel and interesting continuous in-vivo device based on a sophisticated aptamer design to detect interferon-γ (IFN-γ) and deliver drugs based on its concentration in the body. The design is shown schematically in Fig. 3(C). Inflammation leads to production of IFN-γ in rats, and the fixed aptamer in this design contained aspirin that was released when the aptamer bound to IFN-γ. In this way, higher IFN-γ concentration associated with greater inflammation leads to increased release of aspirin. Therefore, local delivery of appropriate drugs to target tissues on demand was achieved while adverse responses to systemic administration were avoided. The working electrode of this device was based on a glass carbon (GC) rod. After cleaning and modification, the GC electrode was electrochemically reduced and modified with 4-carboxyphenyl. After cleaning and drying, the carboxyl groups on the electrode surface were activated and streptavidin was added. An aptamer probe modified with biotin at one end was fixed to the electrode surface via the streptavidin. The other end of the aptamer was modified with ferrocene (FC) and aspirin was bound inside the aptamer ring. When the aptamer bound to IFN-γ, aspirin was released and the FC moved farther away on average from the electrode surface, thus reducing current between it and the electrode, which allowed calculation of the concentration of IFN-γ. This device had a detection limit as low as 10 pg/mL over a range of 10 pg/mL to 500 pg/mL. Acceptable detection sensitivity was maintained for 24 h use in vivo. Most importantly, this work demonstrated a clever method of targeted drug delivery through the ability of aptamers to bind to target substances.
3.2.2. Optimization works in EAB
A large number of CIVT devices have been proposed for detection of various biomarkers, demonstrating the broad potential of this method. However, there are still many factors that limit the performance of CIVT aptamer sensors (Sec. 3.1). For example, nucleases in blood can lead to the degradation of aptamers, aptamer sensors may have serious baseline drift in blood, and are inherently limited by single point binding. In view of these problems and limitations of aptamer sensors, many studies have proposed alternative approaches.
In principle, aptamer degradation caused by nucleases is the most serious concern because it results in the loss of the detection probe. Therefore, researchers have sought ways to modify the aptamer to protect it from nucleases. Rowe and colleagues [68] showed that RNA-based aptamers are unsuitable for use in the manufacture of CIVT equipment as their performance deteriorates rapidly. DNA-based aptamers perform much better and are stable in body fluids for up to a week. Therefore, DNA-based aptamers are the preferred choice for continuous in-vivo detection equipment. However, the period over which most DNA aptamer-based devices remained sensitive did not meet expectations. Shaver and coworkers [69] found that the continuous detection time of an aptamer-based sensor in body fluid was no more than 12 h, which may have been the result of degradation by nucleases and desorption of the self-assembled monolayer. Introduction of aptamer anti-nuclease enantiomers and development of more stable self-assembled monolayers may improve the method.
Aptamers can be used as extremely stable and specific recognition probes, which simplifies development of CIVT devices. However, as previously discussed, these devices need to rely on active algorithms to correct for a drifting baseline, otherwise the result will be very biased and the accuracy of the equipment will be reduced. In response to this problem, Hui and colleagues [70] developed a cell-membrane-mimicking phosphatidylcholine (PC)-terminated monolayer to reduce baseline drift. Starting with traditional aptamer-based CIVT equipment [71], the authors introduced a phosphatidylcholine (PC)-terminated self-assembled monolayer onto the sensor, which significantly increased monitoring time and reduced baseline drift. Therefore, their approach eliminated dependence on an algorithm to correct for baseline drift, which obviously increased the accuracy and reliability of the results. The sensor diagram with the added self-assembled layer is shown in Fig. 4(A). Obviously, on the basis of the traditional EAB, this work added a PC-terminated self-assembled monolayer between the surface of the electrode and the aptamer. The biomimetic monolayer containing the phosphatidylcholine head group (orange spheres in Fig. 4(A)) largely eliminated the signal drift that occurs when these sensors were used in whole blood. The experimental results showed that the sensor could make measurements for up to 12 h.
Fig. 4.
Improvement work in EAB. (A) The CIVT device with a cell-membrane-mimicking phosphatidylcholine (PC)-terminated monolayers to reduce baseline drift [70]. (B) The CIVT device with generating stronger conformational changes through engineered aptamers to obtain stronger signal response. Reprinted with permission from { [73]}. Copyright {2014} American Chemical Society.
Variations on the detection method have also been reported. Like common EAB sensors, square wave voltammetry is used to detect current. Although this method is convenient and fast, changes in the detected peak current are derived from changes in the electron transfer rate, and the transfer kinetics are indirectly reported through changes in peak current. As a result, significant drift occurs when the sensor is used in vivo. One way to avoid drift is to use chrono¬amperometry to directly measure changes in electron transfer kinetics caused by aptamer binding to the target. In this way, the lifetime of the chronoamperometric decay depends only on the relative amounts of bound and unbound aptamers, yielding an EAB with excellent in-vivo drift resistance [72]. Its fabrication and use are very similar to those of the previously described sensors, and will not be described in detail here. However, its in-vivo anti-drift ability was greatly enhanced, while still possessing a detection range for tobramycin of 1 μM to 1 mM and a detection limit of 1 μM.
The essence of traditional electrochemical aptamer equipment used for detection is that the binding of the aptamer to the target substance causes a conformational change in the aptamer, so that the redox reporter at the end of the aptamer moves closer to or farther away from the electrode surface. Therefore, starting from this essential principle, methods have been developed to increase the degree of conformational change in the aptamer to cause greater signal changes and therefore provide for detection of extremely low concentrations of target substances [73]. This work incorporated rational design and modification of aptamer sequences, as shown schematically in Fig. 4(B). The modified aptamer exhibited significantly stronger affinity, and its introduction into the sensor yielded encouraging results. The authors succeeded in increasing sensitivity without affecting specificity and selectivity of the sensor. Transforming the aptamer is an idea very worth pursuing further.
Another problem is that, generally speaking, the affinity between the aptamer and the target substance is fixed, which means that for a given EAB the detection range is usually fixed (of course, there may be some differences over a very small range). However, the traditional EAB described above may not provide for the detection range of the sensor required for personalized medical treatment. Especially for monitoring and treatment using specific drugs, the detection range of the EAB sensor should be adjustable. In order to address this requirement, remote site mutations and allosteric inhibition were used to adjust the dynamic range of an EAB sensor to change target affinity by about three orders of magnitude, which was obviously significant [74]. An adjustable detection range is very helpful for clinical applications as it increases accuracy and reduces error rates. If this technology can be extended to other target substances, and the detection range of the target substance can be adjusted to meet the requirements of a given treatment, it will greatly improve drug delivery, targeted cancer treatment, and other therapies.
Although traditional EAB sensors generally use gold electrodes as a substrate for the fixed end of the aptamer, other materials have been explored. Asai and colleagues [75] used boron-doped diamond (BDD) instead of a gold electrode as a substrate, and adriamycin could be detected at concentrations as low as 49 nM and as high as 2.3 μM. Compared with traditional EABs based on gold electrodes, BDD does not need any special consideration in this review, and because the aptamer can be adsorbed onto the surface of BDD, the sensor can be prepared by immersing the BDD electrode in aptamer solution for 30 min. Obviously, the preparation process is much simpler.
3.2.3. Competitive mechanism-based CIVT sensors
The use of aptamers is not limited to relying on conformational changes to produce signals. In fact, nucleotide-based aptamers can not only bind to the target substance, but also to a complementary nucleotide sequence. And even if the aptamer is already bound to the complementary DNA sequence, and the aptamer-target conjugate is more stable than the aptamer-DNA duplex, the aptamer will detach from the complementary DNA and bind to the target. This creates a competitive mechanism.
Based on this principle, Zhang and colleagues [76]proposed a competitive CIVT sensor based on aptamers. A schematic diagram of this device is shown in Fig. 5(A). The working electrode was electrochemically coated with nanogold so that it exhibited excellent conductivity. The surface of the electrode was first fixed with ssDNA1 via a gold sulfhydryl bond, and ssDNA2 then bound to the ssDNA1. After adding the target substance, the ssDNA2 bound to the target substance and detached from the ssDNA1 bound to the electrode surface, and the electrical signal generated by redox of MB decreased accordingly. This sensor enabled detection of adenosine concentration in the body over a range of 0.1 nM–1 mM with a detection limit of 0.1 nM for at least 3 h. Furthermore, if this competitive method causes more redox reporters to dissociate due to binding of the aptamer to the target substance, it can produce greater signal changes and thereby further enhance sensitivity. Jiang and coworkers [77] proposed an aptasensor with an aptamer superstructure designed to detect ATP. The superstructure is illustrated in Fig. 5(B). Firstly, an oligonucleotide was fixed onto a gold electrode, and then an aptamer probe with bound MB and another oligonucleotide with a complementary sequence bound to the aptamer. In this way, the aptamer superstructure with a large number of aptamers and MB was formed on the surface of the electrode through partial length DNA complementarity. When the target substance was added, there was significant aptamer and corresponding MB detachment. This sensor also enabled detection of adenosine triphosphate concentration in the range of 0.1 nM–1 mM with a limit detection of 0.1 nM.
Fig. 5.
The CIVT sensor based on the competitive mechanism. (A) The CIVT device for the detection of adenosine [76]. (B) The CIVT device with an aptamer superstructure for the detection of ATP [77].
There have also been many other designs proposed for competitive aptamer-based sensors. For example, an aptamer can bind to a target substance to restore the electrocatalytic performance of platinum nanoparticles wrapped by the aptamer, and therefore restore the signal [78]. Substances that can form a strong hybrid structure with the aptamer can also be used to prevent the aptamer from binding to the target substance, and the aptamer can be released by removing the strong hybrid structure. Although some advanced proposals have not been used in the development of CIVT equipment, we believe that they have great potential and are worth investigating.
3.2.4. Combination of aptamer and analyte-specific reaction
As mentioned above, some substances have redox activity and can be used for direct in-vivo electrochemical analysis (e.g., detection of dopamine). However, the in-vivo environment is extremely complex, and there are many other electrochemically active endogenous compounds, so such probe-free sensors sometimes suffer from low selectivity and false positives. Therefore, researchers have also begun to use aptamers for the detection of these types of substances owing to the surprisingly high selectivity and stability of aptamers. Therefore, these two types of binding can use the high affinity of aptamers to achieve stronger sensitivity. At the same time, because the antigen itself can undergo redox reaction, there is no need to add redox mediators or labeling in the preparation of sensors. For example, the binding of aptamers to dopamine can be detected by surface plasmon resonance. Lower detection limits can also be achieved by using electromechanical chemical transistors and aptamers [79]. Of course, there are also tests for dopamine based on the traditional EAB principle [80]. All these demonstrate that the aptamer-based sensor is a promising device for detection of this class of substances.
An aptamer-based CIVT has been proposed for detection of serotonin (Fig. 6(A)) [81]. The detection probes were fabricated using traditional MEMS technology, and the aptamers were successfully immobilized after thiolation. Experiments showed that the sensor could detect serotonin over a concentration range of 10 fM to 100 μM. It is worth noting that the detection principle is based on a conformational change in the negatively charged aptamer backbone when the aptamer binds to the target, and the resulting surface charge redistribution is detected by voltage-gated semiconductors. This sensing mechanism is independent of the charge or electrochemical properties of the analyte itself, and thus has the potential to be a general method for detection of small molecules.
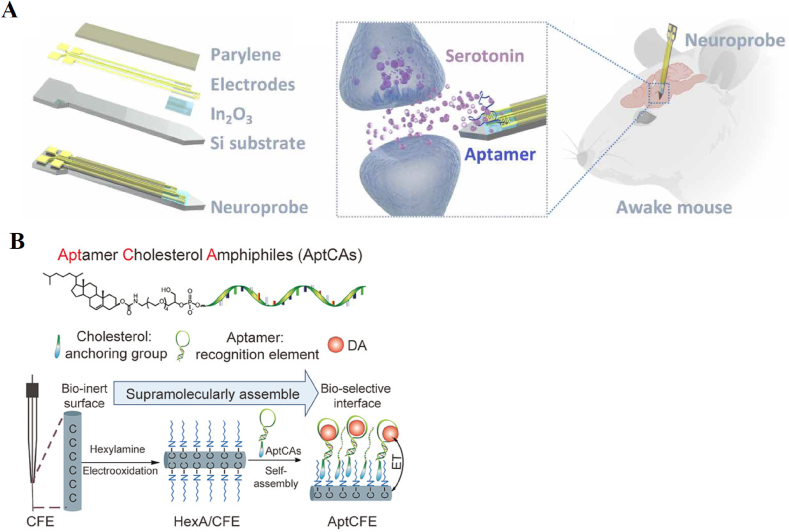
Fig. 6.
The aptamer-based CIVT sensors for the detection of analyte witch have analyte-specific reaction. (A) The MEMS-based CIVT device for the detection of serotonin [81]. (B) The carbon fiber microelectrode based CIVT device [82].
Almost all of the aptamer-based sensors described above use gold-thiol bonds to immobilize the aptamers. This method is simple and effective, but when the equipment is not suitable for binding gold nanoparticles, immobilization of the aptamers can be challenging. For example, carbon fiber microelectrodes (CFEs) are a new type of substrate for continuous in-body testing equipment. Compared with traditional silicon substrates, CFEs can significantly reduce damage to the brain during implantation due to their excellent biocompatibility, extremely small volume and softness. And they have greater stability in vivo and therefore a longer detection time. However, the surface of CFEs is chemically inert, and immobilization of aptamers on CFEs has always been a major challenge. To address this challenge, Hou and colleagues [82] introduced a novel continuous in-vivo detection sensor, which successfully immobilized and detected aptamers on CFEs. A schematic diagram of its assembly is shown in Fig. 6(B). The CFE is first functionalized with an alkyl chain, and then the aptamer is covalently linked to inert tri(ethylene glycol) and cholesterol to form an amphiphilic assembly with one end being hydrophilic and the other hydrophobic. Through a non-covalent lipid-alkyl chain reaction, the assembly can be firmly immobilized on the CFE.
We compare the performance of these representative CIVT devices with aptamer as probes in Table 2.
Table 2.
A list of the representative aptamer-based CIVT sensors.
| Device/Nanomaterial | Linear Range | LOD | Detection time | Targeted analytes | Reference |
|---|---|---|---|---|---|
| Gold wire | 30 μM to 1 mM | 30 μM | 70 min | Phenylalanine | [65] |
| Gold wire | 34 μM–400 μM | 34 μM | 280 min | Kanamycin | [66] |
| Glass carbon rod | 10 pg/mL to 500 pg/mL | 10 pg/mL | 24 h | Interferon- γ | [67] |
| Gold electrode/phosphatidylcholine | – | – | 12 h | Kanamycin and doxorubicin | [70] |
| Gold wire | 1 μM to 1 mM | 1 μM | – | Tobramycin | [72] |
| boron doped diamond | 49 nM to 2.3 uM | 49 nM | – | Adriamycin | [75] |
| Pt electrode/rGO-AUNPs | 0.1 nM to 1 mM | 0.1 nM | 180 min | Adenosine | [76] |
| gold electrode/ | 0.1 nM to 1 mM | 0.1 nM | – | Adenosine triphosphate | [77] |
| MEMs-based electrode/In2O3 | 10 fM to 100 μM | 10 fM | Serotonin | [81] |
4. Conclusion and future perspectives
In terms of basic application, CIVT equipment for target substances can realize the monitoring of diseases, the evaluation of the health status of the human body and the therapeutic effect of drugs. The development of today's equipment makes it possible to monitor many target substances, which further broadens the application scope and potential of CIVT equipment. Further, CIVT equipment also provides huge opportunities for basic scientific research, including research on the pathogenesis of diseases, research on the mechanism of changes in physiological functions, and guidance on targeted drug use.
But there are still a lot of problems with current devices that need to be solved before this new class of devices can actually hit the market and revolutionize every aspect of personalized medicine. Some of our perspectives on the remaining challenges and future directions are as follows:
-
(1)
Recognition probe: The first issue, and the most important, is the specificity of the device when the sensor detects targets in vivo. Due to the complex environment and many interfering substances in the body, non-specific adsorption is an unavoidable problem for many CIVT devices. For targets detected without probes, their presence can be known from redox reactions at specific voltages. However, substances that can react at similar voltages clearly interfere with their detection. For target substances that require probes for detection, the probes will also bind to certain substances similar in structure to them, resulting in false positive reactions. From a granular perspective, this seems inevitable. However, the discovery of aptamers gave researchers some light. Extremely stable chemical properties and super specificity make it more and more popular. This trend is also reflected from the previous introduction of this article: even for targets that do not need probes for detection, researchers have turned to the exploration and use of corresponding aptamers for detection. We deeply believe that the broad adaptability and potential future of aptamers will enable the continuous development and improvement of in vivo detection equipment.
-
(2)
Contamination: Another concern is the contamination of the device in the body. This is reflected in two aspects, the first is the pollution of the equipment to the human body. Continuous in vivo detection equipment needs to be implanted into the human body to detect target substances in body fluids. Therefore, the human body's rejection of the device and infection need to be taken into account. Generally speaking, implantable devices are first designed to minimize harm to the human body, so their shape should be smooth and the size should be minimized without compromising performance. The substrate of the device itself should also be biocompatible, and the current devices including silicon-based, carbon-based, etc. perform well in this regard. In addition, it should be noted that the contamination of the equipment by human body fluids will greatly limit the sustainable detection time of the equipment. Many devices today basically have a continuous detection time of tens of hours to several days, which is usually caused by the contamination of the device by bodily fluids. For example, the physical adsorption of blood cells, red blood cells, etc. in the blood in the device reduces the sensitivity of the device. Degradation of aptamers by nucleases causes the device to lose its detection capability, among other things. This requires researchers to take into account the effects of the body fluid environment in which they are located when developing the device. For example, select nanomaterials with strong chemical stability and are not easy to adsorb impurities, add filter membranes to the equipment, and modify the aptamers to prevent them from being affected by nucleases.
-
(3)
Fabrication: The manufacture of most CIVT devices still requires extremely cumbersome processes. This is because in vivo testing does have higher requirements. For example, sometimes it is necessary to penetrate the skin or skull, so the device needs to have a strong rigidity. The electrical conductivity of the internal environment is very poor, and there are higher requirements for the electrical conductivity of the device, and so on. However, all kinds of cumbersome processes do affect the cost and use threshold of CIVT equipment. Ideally, we hope that the device is easy to fabricated with low cost to meet the needs of the market. There have also been some new attempts, such as the use of carbon fiber microelectrodes, to make the preparation process simple. Or using new substrate materials with better performance and lower cost. This trend is to be encouraged. Further research should also consider this aspect, that is, while having strong performance, the fabrication process should be as simple as possible and the cost should be reduced as much as possible.
-
(4)
Integration of CIVT devices with portable analysis equipment: The last problem is that many of the current continuous in-body testing equipment is still a frontend testing component. They may be able to perform precise, continuous monitoring of some targets, but they cannot escape their reliance on large chemical analysis stations. This has a huge impact on their industrial application. Limited by large chemical stations, their applications can only be limited to hospitals, research institutes and other places with corresponding instruments, and cannot form a wide range of applications [83]. We believe that continuous in-vivo detection devices can revolutionize and change personalized medicine, but before that, they should be applied to personalized medicine first. Therefore, continuous in-body detection equipment should be combined with portable analysis equipment, so that the real-time monitoring characteristics of continuous in-body detection can be fully utilized. The portable analysis device can send real-time monitoring results to the doctor through the Bluetooth component for analysis. In addition, it can also be turned into a wearable device through a watch strap or other convenient fixing means.
Successful outcomes from these efforts will result in even better performing CIVT devices. Finally, it will become the best choice for residents to monitor their own health status at home and promote the research on disease mechanism, and fundamentally change personalized medical treatment. The ultimate purpose of CIVT is to monitor the existence of any target substance we need to know in real time through a system with less resource consumption and complete functions, and make the research on the mechanism and medication of complex diseases more convenient and accurate. We firmly believe that CIVT device can fulfill all expectations in the future. At present, there are already some commercially available CIVT devices. We refer to the previous literature to list and summarize the commercialized devices [84]. As shown in Table 3, they achieved excellent results in disease monitoring and patient health monitoring. We believe that more and more CIVT devices targeting different markers will make a great contribution to the industry and the sensor market as research continues to deepen.
Table 3.
A list of the commercialized devices.
| Company, product | Body fluid | Detection time | Approval stage | Targets | website |
|---|---|---|---|---|---|
| Freestyle Libre 3, Abbott, Inc. | Interstitial fluid | 14 Days | FDA approved | Glucose | https://www.freestylelibre.us/ |
| Dexcom G6 CGM, Dexcom, Inc. | Interstitial fluid | 10 Days | FDA approved | Glucose | https://www.dexcom.com/ |
| DHD-6000, Donghwa,Inc | Contact with skin | power supply | CFDA approved | heart rate | http://www.donghuayuan.com/ |
| BPBIO 750, Biospace Co., Ltd | Contact with skin | Power supply | CFDA approved | Blood pressure | http://www.inbody.com |
Credit author statement
Tao Ming: Conceptualization, Investigation, Writing – original draft; Jinping Luo: Investigation, Writing-review &editing. Yu Xing: Investigation. Yan Cheng: Investigation. Juntao Liu: Investigation. Shuai Sun: Writing-review &editing. Fanli Kong: Writing-review &editing. Shihong Xu: Writing-review &editing. Yuchuan Dai: Writing-review &editing. Jingyu Xie: Writing-review &editing. Hongyan Jin: Resources, Validation, Writing-review &editing. Xinxia Cai: Project administration, Conceptualization, Writing-review &editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was sponsored by the National Key R&D Program of China (2017YFA0205902), the National Natural Science Foundation of China (No. 62121003,61960206012, 81971348, 62171434, 61775216, 61771452, 61975206, 61971400, 61973292), the Natural Science Foundation of Beijing(4202081) and the Scientific Instrument Developing Project of the Chinese Academy of Sciences (Grant No. GJJSTD20210004).
Contributor Information
Hongyan Jin, Email: maggijhy@163.com.
Xinxia Cai, Email: xxcai@mail.ie.ac.cn.
References
- 1.Roberts T.C., Langer R., Wood M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020;19(10):673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Q., Tang Q., Wang Z.L., Li Z. Self-powered cardiovascular electronic devices and systems. Nat. Rev. Cardiol. 2021;18(1):7–21. doi: 10.1038/s41569-020-0426-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhao L., Wang L., Zheng Y., Zhao S., Wei W., Zhang D., Fu X., Jiang K., Shen G., Han W. Highly-stable polymer-crosslinked 2D MXene-based flexible biocompatible electronic skins for in vivo biomonitoring. Nano Energy. 2021;84 doi: 10.1016/j.nanoen.2021.105921. [DOI] [Google Scholar]
- 4.Hamburg M.A., Collins F.S. The path to personalized medicine. N. Engl. J. Med. 2010;363(4):301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 5.Yager P., Edwards T., Fu E., Helton K., Nelson K., Tam M.R., Weigl B.H. Microfluidic diagnostic technologies for global public health. Nature. 2006;442(7101):412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 6.Sun J., Yang A., Zhao C., Liu F., Li Z. Recent progress of nanogenerators acting as biomedical sensors in vivo. Sci. Bull. 2019;64(18):1336–1347. doi: 10.1016/j.scib.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhong B., Jiang K., Wang L., Shen G. Wearable sweat loss measuring devices: from the role of sweat loss to advanced mechanisms and designs. Adv. Sci. 2022;9(1) doi: 10.1002/advs.202103257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gascoyne P., Mahidol C., Ruchirawat M., Satayavivad J., Watcharasit P., Becker F.F. Microsample preparation by dielectrophoresis: isolation of malaria. Lab Chip. 2002;2(2):70–75. doi: 10.1039/B110990C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavlovic E., Lai R.Y., Wu T.T., Ferguson B.S., Sun R., Plaxco K.W., Soh H.T. Microfluidic device architecture for electrochemical patterning and detection of multiple DNA sequences. Langmuir. 2008;24(3):1102–1107. doi: 10.1021/la702681c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das Thakur M., Salangsang F., Landman A.S., Sellers W.R., Pryer N.K., Levesque M.P., Dummer R., McMahon M., Stuart D.D. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494(7436):251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou Y., Bo L., Li Z. Recent progress in human body energy harvesting for smart bioelectronic system. Fundamental Research. 2021;1(3):364–382. doi: 10.1016/j.fmre.2021.05.002. [DOI] [Google Scholar]
- 12.Gaster R.S., Hall D.A., Nielsen C.H., Osterfeld S.J., Yu H., Mach K.E., Wilson R.J., Murmann B., Liao J.C., Gambhir S.S., Wang S.X. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat. Med. 2009;15(11):1327–1332. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao Y., Lubin A.A., Heeger A.J., Plaxco K.W. Label-free electronic detection of thrombin in blood serum by using an aptamer-Based sensor. Angew. Chem. Int. Ed. 2005;44(34):5456–5459. doi: 10.1002/anie.200500989. [DOI] [PubMed] [Google Scholar]
- 14.Swensen J.S., Xiao Y., Ferguson B.S., Lubin A.A., Lai R.Y., Heeger A.J., Plaxco K.W., Soh H.T. Continuous, real-time monitoring of cocaine in undiluted blood serum via a microfluidic, electrochemical aptamer-Based sensor. J. Am. Chem. Soc. 2009;131(12):4262–4266. doi: 10.1021/ja806531z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbs N.A., Twelves C.J., Gillies H., James C.A., Harper P.G., Rubens R.D. Gender affects doxorubicin pharmacokinetics in patients with normal liver biochemistry. Cancer Chemother. Pharmacol. 1995;36(6):473–476. doi: 10.1007/BF00685796. [DOI] [PubMed] [Google Scholar]
- 16.Bach D.M., Straseski J.A., Clarke W. Therapeutic drug monitoring in cancer chemotherapy. Bioanalysis. 2010;2(5):863–879. doi: 10.4155/bio.10.48. [DOI] [PubMed] [Google Scholar]
- 17.Vallée-Bélisle A., Ricci F., Uzawa T., Xia F., Plaxco K.W. Bioelectrochemical switches for the quantitative detection of antibodies directly in whole blood. J. Am. Chem. Soc. 2012;134(37):15197–15200. doi: 10.1021/ja305720w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermette P., Meagher L. Interactions of phospholipid- and poly(ethylene glycol)-modified surfaces with biological systems: relation to physico-chemical properties and mechanisms. Colloids Surf. B Biointerfaces. 2003;28(2):153–198. doi: 10.1016/S0927-7765(02)00160-1. [DOI] [Google Scholar]
- 19.Qiu L., Zhang T., Jiang J., Wu C., Zhu G., You M., Chen X., Zhang L., Cui C., Yu R., Tan W. Cell membrane-anchored biosensors for real-time monitoring of the cellular microenvironment. J. Am. Chem. Soc. 2014;136(38):13090–13093. doi: 10.1021/ja5047389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker D.A., Gough D.A. A continuous, implantable lactate sensor. Anal. Chem. 1995;67(9):1536–1540. doi: 10.1021/ac00105a010. [DOI] [Google Scholar]
- 21.Leung K.-H., He B., Yang C., Leung C.-H., Wang H.-M.D., Ma D.-L. Development of an aptamer-based sensing platform for metal ions, proteins, and small molecules through terminal deoxynucleotidyl transferase induced G-Quadruplex formation. ACS Appl. Mater. Interfaces. 2015;7(43):24046–24052. doi: 10.1021/acsami.5b08314. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J., Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov. 2017;16(3):181–202. doi: 10.1038/nrd.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao W., Li B., Yao R., Li Z., Wang X., Dong X., Qu H., Li Q., Li N., Chi H., Zhou B., Xia Z. Intuitive label-free SERS detection of bacteria using aptamer-based in situ silver nanoparticles synthesis. Anal. Chem. 2017;89(18):9836–9842. doi: 10.1021/acs.analchem.7b01813. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J., Guo Y., Bi Z., Huang Z., Yu G., Wang J. Artificial Intelligence Review; 2022. Segmentation of Prostate Ultrasound Images: the State of the Art and the Future Directions of Segmentation Algorithms. [DOI] [Google Scholar]
- 25.Wu D., Gao T., Lei L., Yang D., Mao X., Li G. Colorimetric detection of proteins based on target-induced activation of aptazyme. Anal. Chim. Acta. 2016;942:68–73. doi: 10.1016/j.aca.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Zwang T.J., Hürlimann S., Hill M.G., Barton J.K. Helix-dependent spin filtering through the DNA Duplex. J. Am. Chem. Soc. 2016;138(48):15551–15554. doi: 10.1021/jacs.6b10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen J., Wang H., Li C., Zhao Y., Yu X., Luo X. Label-free electrochemical aptasensor for adenosine detection based on cascade signal amplification strategy. Biosens. Bioelectron. 2017;90:356–362. doi: 10.1016/j.bios.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Wu N., Zhang Y., Ye X., Hu Y., Ding T., Chen S. Sulfation pattern of fucose branches affects the anti-hyperlipidemic activities of fucosylated chondroitin sulfate. Carbohydr. Polym. 2016;147:1–7. doi: 10.1016/j.carbpol.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Le V.S., Jeong J.-E., Kim B., Lee J., Kyhm K., Woo H.Y. Inhibitor effects on molecular beacon-based mercury assays for tuning of detection range. Sensor. Actuator. B Chem. 2017;240:810–817. doi: 10.1016/j.snb.2016.09.034. [DOI] [Google Scholar]
- 30.Pfeiffer F., Rosenthal M., Siegl J., Ewers J., Mayer G. Customised nucleic acid libraries for enhanced aptamer selection and performance. Curr. Opin. Biotechnol. 2017;48:111–118. doi: 10.1016/j.copbio.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Keefe A.D., Pai S., Ellington A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010;9(7):537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiammengo R., Jäschke A. Nucleic acid enzymes. Curr. Opin. Biotechnol. 2005;16(6):614–621. doi: 10.1016/j.copbio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Kosman J., Juskowiak B. Peroxidase-mimicking DNAzymes for biosensing applications: a review. Anal. Chim. Acta. 2011;707(1):7–17. doi: 10.1016/j.aca.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 34.Achenbach J.C., Nutiu R., Li Y. Structure-switching allosteric deoxyribozymes. Anal. Chim. Acta. 2005;534(1):41–51. doi: 10.1016/j.aca.2004.03.080. [DOI] [Google Scholar]
- 35.Nie J., Zhang D.-W., Tie C., Zhou Y.-L., Zhang X.-X. A label-free DNA hairpin biosensor for colorimetric detection of target with suitable functional DNA partners. Biosens. Bioelectron. 2013;49:236–242. doi: 10.1016/j.bios.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Travascio P., Li Y., Sen D. DNA-enhanced peroxidase activity of a DNA aptamer-hemin complex. Chem. Biol. 1998;5(9):505–517. doi: 10.1016/S1074-5521(98)90006-0. [DOI] [PubMed] [Google Scholar]
- 37.Adar F. Academic Press; 1978. 2 - Electronic Absorption Spectra of Hemes and Hemoproteins, the Porphyrins; pp. 167–209. [DOI] [Google Scholar]
- 38.Ma Y., Zheng Q., Liu Y., Shi B., Xue X., Ji W., Liu Z., Jin Y., Zou Y., An Z., Zhang W., Wang X., Jiang W., Xu Z., Wang Z.L., Li Z., Zhang H. Self-powered, one-stop, and multifunctional implantable triboelectric active sensor for real-time biomedical monitoring. Nano Lett. 2016;16(10):6042–6051. doi: 10.1021/acs.nanolett.6b01968. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z., Ma Y., Ouyang H., Shi B., Li N., Jiang D., Xie F., Qu D., Zou Y., Huang Y., Li H., Zhao C., Tan P., Yu M., Fan Y., Zhang H., Wang Z.L., Li Z. Transcatheter self-powered ultrasensitive endocardial pressure sensor. Adv. Funct. Mater. 2019;29(3) doi: 10.1002/adfm.201807560. [DOI] [Google Scholar]
- 40.Gouterman M. The Porphyrins, Academic Press; 1978. 1 - Optical Spectra and Electronic Structure of Porphyrins and Related Rings; pp. 1–165. [DOI] [Google Scholar]
- 41.Clark L.C., Jr., Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962;102(1):29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang D., Wang L., Shen G. Nanofiber/nanowires-based flexible and stretchable sensors. J. Semiconduct. 2020;41(4) doi: 10.1088/1674-4926/41/4/041605. [DOI] [Google Scholar]
- 43.Strickland E.H., Ziegler F.D., Anthony A. Oxygen electrode for measurement of tissue slice respiration. Nature. 1961;191(4792):969–970. doi: 10.1038/191969a0. [DOI] [Google Scholar]
- 44.Nadia Ahmad N.F., Nik Ghazali N.N., Wong Y.H. Wearable patch delivery system for artificial pancreas health diagnostic-therapeutic application: a review. Biosens. Bioelectron. 2021;189 doi: 10.1016/j.bios.2021.113384. [DOI] [PubMed] [Google Scholar]
- 45.Ahmadi F., McLoughlin I.V., Chauhan S., ter-Haar G. Bio-effects and safety of low-intensity, low-frequency ultrasonic exposure. Prog. Biophys. Mol. Biol. 2012;108(3):119–138. doi: 10.1016/j.pbiomolbio.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Bagherifard S., Tamayol A., Mostafalu P., Akbari M., Comotto M., Annabi N., Ghaderi M., Sonkusale S., Dokmeci M.R., Khademhosseini A. Dermal patch with integrated flexible heater for on demand drug delivery. Advanced Healthcare Materials. 2016;5(1):175–184. doi: 10.1002/adhm.201500357. [DOI] [PubMed] [Google Scholar]
- 47.Bian S., Zhu B., Rong G., Sawan M. Towards wearable and implantable continuous drug monitoring: a review. Journal of Pharmaceutical Analysis. 2021;11(1):1–14. doi: 10.1016/j.jpha.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bandodkar A.J., Jia W., Wang J. Tattoo-based wearable electrochemical devices: a review. Electroanalysis. 2015;27(3):562–572. doi: 10.1002/elan.201400537. [DOI] [Google Scholar]
- 49.Berget C., Messer L.H., Forlenza G.P. A clinical overview of insulin pump therapy for the management of diabetes: past, present, and future of intensive therapy. Diabetes Spectr. 2019;32(3):194–204. doi: 10.2337/ds18-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broza Y.Y., Zhou X., Yuan M., Qu D., Zheng Y., Vishinkin R., Khatib M., Wu W., Haick H. Disease detection with molecular biomarkers: from chemistry of body fluids to nature-inspired chemical sensors. Chem. Rev. 2019;119(22):11761–11817. doi: 10.1021/acs.chemrev.9b00437. [DOI] [PubMed] [Google Scholar]
- 51.Christiansen M., Bailey T., Watkins E., Liljenquist D., Price D., Nakamura K., Boock R., Peyser T. A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous-generation system. Diabetes Technol. Therapeut. 2013;15(10):881–888. doi: 10.1089/dia.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cobelli C., Renard E., Kovatchev B. Artificial pancreas: past, present, future. Diabetes. 2011;60(11):2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deshpande S., Pinsker J.E., Zavitsanou S., Shi D., Tompot R., Church M.M., Andre C., Doyle F.J., Dassau E. Design and clinical evaluation of the interoperable artificial pancreas system (iAPS) smartphone app: interoperable components with modular design for progressive artificial pancreas research and development. Diabetes Technol. Therapeut. 2018;21(1):35–43. doi: 10.1089/dia.2018.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heller A., Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008;108(7):2482–2505. doi: 10.1021/cr068069y. [DOI] [PubMed] [Google Scholar]
- 55.Rao J.R., Richter G. Implantable bio-electrochemical power sources. Naturwissenschaften. 1974;61(5):200–206. doi: 10.1007/BF00599917. [DOI] [PubMed] [Google Scholar]
- 56.Dewanti A.R., Duine J.A. Reconstitution of membrane-integrated quinoprotein glucose dehydrogenase apoenzyme with PQQ and the holoenzyme's mechanism of action. Biochemistry. 1998;37(19):6810–6818. doi: 10.1021/bi9722610. [DOI] [PubMed] [Google Scholar]
- 57.Malmstadt H.V., Pardue H.L. Quantitative analysis by an automatic potentiometric reaction rate method. Specific Enzymatic Determination of Glucose. Anal. Chem. 1961;33(8):1040–1047. doi: 10.1021/ac60176a054. [DOI] [PubMed] [Google Scholar]
- 58.Luo X.-L., Xu J.-J., Zhao W., Chen H.-Y. Glucose biosensor based on ENFET doped with SiO2 nanoparticles. Sensor. Actuator. B Chem. 2004;97(2):249–255. doi: 10.1016/j.snb.2003.08.024. [DOI] [Google Scholar]
- 59.Teymourian H., Moonla C., Tehrani F., Vargas E., Aghavali R., Barfidokht A., Tangkuaram T., Mercier P.P., Dassau E., Wang J. Microneedle-based detection of ketone bodies along with glucose and lactate: toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal. Chem. 2020;92(2):2291–2300. doi: 10.1021/acs.analchem.9b05109. [DOI] [PubMed] [Google Scholar]
- 60.Dervisevic M., Alba M., Yan L., Senel M., Gengenbach T.R., Prieto-Simon B., Voelcker N.H. Transdermal electrochemical monitoring of glucose via high-density silicon microneedle array patch. Adv. Funct. Mater. 2022;32(3) doi: 10.1002/adfm.202009850. [DOI] [Google Scholar]
- 61.Wang L., Xie S., Wang Z., Liu F., Yang Y., Tang C., Wu X., Liu P., Li Y., Saiyin H., Zheng S., Sun X., Xu F., Yu H., Peng H. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nature Biomedical Engineering. 2020;4(2):159–171. doi: 10.1038/s41551-019-0462-8. [DOI] [PubMed] [Google Scholar]
- 62.Castagnola E., Thongpang S., Hirabayashi M., Nava G., Nimbalkar S., Nguyen T., Lara S., Oyawale A., Bunnell J., Moritz C., Kassegne S. Glassy carbon microelectrode arrays enable voltage-peak separated simultaneous detection of dopamine and serotonin using fast scan cyclic voltammetry. Analyst. 2021;146(12):3955–3970. doi: 10.1039/D1AN00425E. [DOI] [PubMed] [Google Scholar]
- 63.Xiao G., Song Y., Zhang Y., Xing Y., Zhao H., Xie J., Xu S., Gao F., Wang M., Xing G., Cai X. Microelectrode arrays modified with nanocomposites for monitoring dopamine and spike firings under deep brain stimulation in rat models of Parkinson's disease. ACS Sens. 2019;4(8):1992–2000. doi: 10.1021/acssensors.9b00182. [DOI] [PubMed] [Google Scholar]
- 64.Ming T., Cheng Y., Xing Y., Luo J., Mao G., Liu J., Sun S., Kong F., Jin H., Cai X. Electrochemical microfluidic paper-based aptasensor platform based on a biotin–streptavidin system for label-free detection of biomarkers. ACS Appl. Mater. Interfaces. 2021;13(39):46317–46324. doi: 10.1021/acsami.1c12716. [DOI] [PubMed] [Google Scholar]
- 65.Idili A., Gerson J., Kippin T., Plaxco K.W. Seconds-Resolved, in situ measurements of plasma phenylalanine disposition kinetics in living rats. Anal. Chem. 2021;93(8):4023–4032. doi: 10.1021/acs.analchem.0c05024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arroyo-Currás N., Somerson J., Vieira P.A., Ploense K.L., Kippin T.E., Plaxco K.W. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl. Acad. Sci. USA. 2017;114(4):645. doi: 10.1073/pnas.1613458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao C., Jin R., Wei H., Liu Z., Ni S., Liu G.-J., Young H.A., Chen X., Liu G. Adaptive in vivo device for theranostics of inflammation: real-time monitoring of interferon-γ and aspirin. Acta Biomater. 2020;101:372–383. doi: 10.1016/j.actbio.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowe A.A., Miller E.A., Plaxco K.W. Reagentless measurement of aminoglycoside antibiotics in blood serum via an electrochemical, ribonucleic acid aptamer-based biosensor. Anal. Chem. 2010;82(17):7090–7095. doi: 10.1021/ac101491d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaver A., Kundu N., Young B.E., Vieira P.A., Sczepanski J.T., Arroyo-Currás N. Nuclease hydrolysis does not drive the rapid signaling decay of DNA aptamer-based electrochemical sensors in biological fluids. Langmuir. 2021;37(17):5213–5221. doi: 10.1021/acs.langmuir.1c00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H., Dauphin-Ducharme P., Arroyo-Currás N., Tran C.H., Vieira P.A., Li S., Shin C., Somerson J., Kippin T.E., Plaxco K.W. A biomimetic phosphatidylcholine-terminated monolayer greatly improves the in vivo performance of electrochemical aptamer-Based sensors. Angew. Chem. Int. Ed. 2017;56(26):7492–7495. doi: 10.1002/anie.201700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H., Li S., Dai J., Li C., Zhu M., Li H., Lou X., Xia F., Plaxco Kevin W. High frequency, calibration-free molecular measurements in situ in the living body. Chem. Sci. 2019;10(47):10843–10848. doi: 10.1039/C9SC04434E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arroyo-Currás N., Dauphin-Ducharme P., Ortega G., Ploense K.L., Kippin T.E., Plaxco K.W. Subsecond-resolved molecular measurements in the living body using chronoamperometrically interrogated aptamer-based sensors. ACS Sens. 2018;3(2):360–366. doi: 10.1021/acssensors.7b00787. [DOI] [PubMed] [Google Scholar]
- 73.Schoukroun-Barnes L.R., Wagan S., White R.J. Enhancing the analytical performance of electrochemical RNA aptamer-based sensors for sensitive detection of aminoglycoside antibiotics. Anal. Chem. 2014;86(2):1131–1137. doi: 10.1021/ac4029054. [DOI] [PubMed] [Google Scholar]
- 74.Li S., Li C., Wang Y., Li H., Xia F. Re-engineering electrochemical aptamer-based biosensors to tune their useful dynamic range via distal-site mutation and allosteric inhibition. Anal. Chem. 2020;92(19):13427–13433. doi: 10.1021/acs.analchem.0c02782. [DOI] [PubMed] [Google Scholar]
- 75.Asai K., Yamamoto T., Nagashima S., Ogata G., Hibino H., Einaga Y. An electrochemical aptamer-based sensor prepared by utilizing the strong interaction between a DNA aptamer and diamond. Analyst. 2020;145(2):544–549. doi: 10.1039/C9AN01976F. [DOI] [PubMed] [Google Scholar]
- 76.Zhang D., Ma J., Meng X., Xu Z., Zhang J., Fang Y., Guo Y. Electrochemical aptamer-based microsensor for real-time monitoring of adenosine in vivo. Anal. Chim. Acta. 2019;1076:55–63. doi: 10.1016/j.aca.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 77.Jiang Y., Ma W., Ji W., Wei H., Mao L. Aptamer superstructure-based electrochemical biosensor for sensitive detection of ATP in rat brain with in vivo microdialysis. Analyst. 2019;144(5):1711–1717. doi: 10.1039/C8AN02077A. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Mao J., Ji W., Feng T., Fu Z., Zhang M., Mao L. Collision of aptamer/pt nanoparticles enables label-free amperometric detection of protein in rat brain. Anal. Chem. 2019;91(9):5654–5659. doi: 10.1021/acs.analchem.8b05457. [DOI] [PubMed] [Google Scholar]
- 79.Liang Y., Guo T., Zhou L., Offenhäusser A., Mayer D. Label-free split aptamer sensor for femtomolar detection of dopamine by means of flexible organic electrochemical transistors. Materials. 2020;13(11) doi: 10.3390/ma13112577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farjami E., Campos R., Nielsen J.S., Gothelf K.V., Kjems J., Ferapontova E.E. RNA aptamer-based electrochemical biosensor for selective and label-free analysis of dopamine. Anal. Chem. 2013;85(1):121–128. doi: 10.1021/ac302134s. [DOI] [PubMed] [Google Scholar]
- 81.C. Zhao, M. Cheung Kevin, I.W. Huang, H. Yang, N. Nakatsuka, W. Liu, Y. Cao, T. Man, S. Weiss Paul, G. Monbouquette Harold, M. Andrews Anne, Implantable aptamer–field-effect transistor neuroprobes for in vivo neurotransmitter monitoring, Sci. Adv. 7(48) eabj7422. 10.1126/sciadv.abj7422. [DOI] [PMC free article] [PubMed]
- 82.Hou H., Jin Y., Wei H., Ji W., Xue Y., Hu J., Zhang M., Jiang Y., Mao L. A generalizable and noncovalent strategy for interfacing aptamers with a microelectrode for the selective sensing of neurotransmitters in vivo. Angew. Chem. Int. Ed. 2020;59(43):18996–19000. doi: 10.1002/anie.202008284. [DOI] [PubMed] [Google Scholar]
- 83.Wang L., Jiang K., Shen G. A perspective on flexible sensors in developing diagnostic devices. Appl. Phys. Lett. 2021;119(15) doi: 10.1063/5.0057020. [DOI] [Google Scholar]
- 84.Adeel M., Rahman M.M., Caligiuri I., Canzonieri V., Rizzolio F., Daniele S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112331. [DOI] [PubMed] [Google Scholar]
- 85.Taylor I.M., Du Z., Bigelow E.T., Eles J.R., Horner A.R., Catt K.A., Weber S.G., Jamieson B.G., Cui X.T. Aptamer-functionalized neural recording electrodes for the direct measurement of cocaine in vivo. J. Mater. Chem. B. 2017;5(13):2445–2458. doi: 10.1039/C7TB00095B. [DOI] [PMC free article] [PubMed] [Google Scholar]