Abstract
Non-invasive brain stimulation (NIBS) has been seen more common in rehabilitation settings. It can be used for the treatment of stroke, spinal cord injury, traumatic brain injury and multiple sclerosis, as well as for some diagnostic neurophysiological measurements. Two major modalities of NIBS are transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). As an add-on therapy to conventional rehabilitative treatments, the main goal of NIBS is to create neuromodulation by inhibiting or activating neural activity in the targeted cortical region. Indications for therapeutic NIBS in neurorehabilitation are motor recovery, aphasia, neglect, dysphagia, cognitive disorders, spasticity, and central pain. The NIBS can be regarded a safe technique with appropriate patient selection and defined treatment parameters. This review provides an overview on NIBS modalities, specifically TMS and tDCS, the working mechanisms, the stimulation techniques, areas of use, neuronavigation systems and safety considerations.
Keywords: Non-invasive brain stimulation, rehabilitation, transcranial direct current stimulation, transcranial magnetic stimulation
Non-invasive brain stimulation (NIBS) has recently attracted great interest in both a societal and scientific sense. In the relevant literature, it has been used for approximately 30 years since the first definition of transcranial magnetic stimulation (TMS) by Anthony Barker at Sheffield University, UK, in 1985, and the number of scientific publications has been increasing.[1,2] The areas of use are constantly expanding, and the use of NIBS modalities in assisting rehabilitation has seen greater acceptance associated with the level of increasing evidence.
The complex structure of the human nerve system can currently be examined in more detail with new technologies. Diffusion spectrum imaging, which is a new magnetic resonance imaging (MRI) technique, is able to analyze the movement of water molecules along nerve fibers and, thus, the major neuron pathways in the brain can be visualized. It allows the opportunity to understand the central nervous system more comprehensively, and for a more targeted, controlled intervention to be made. Examination of the integrity and dynamics of a specific neural pathway with NIBS allows treatment by modulating this pathway. In this respect, NIBS seems to be non-pharmacological, safe and attractive treatment, which do not require surgery.
BASIC PRINCIPLES
The NIBS consists of neurostimulation in a target area of the brain by applying an electrical current directly or by creating an electrical field with magnetic induction on the scalp. Measurable outputs (motor evoked potential, central transmission time, cortical silent period, intracortical inhibition, intracortical facilitation) are obtained with neurostimulation. In this way, it can be used for diagnostic purposes. In repeated applications, neuromodulation is made by increasing or decreasing cortical excitability. Repeated application of NIBS is used for therapeutic purposes.
The modalities used for NIBS are as follows:
TMS
Transcranial direct current stimulation (tDCS)
Transcranial alternative current stimulation (tACS)
Transcranial random noise stimulation (tRNS)
Laser, ultrasound, virus and other vectors Of these, TMS and tDCS are the most widely used and studied modalities.
Transcranial magnetic stimulation
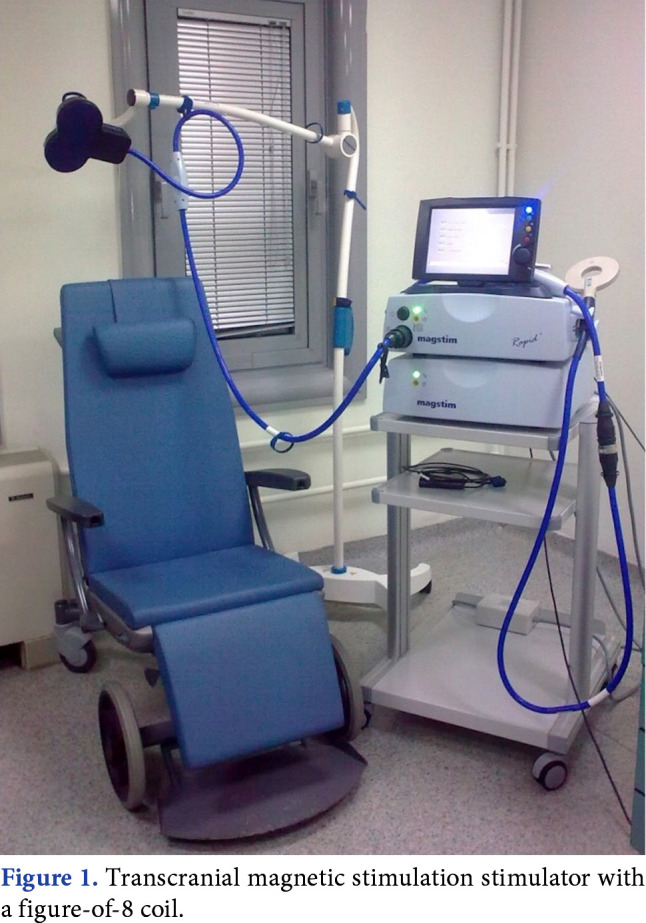
For TMS, a coil is placed on the scalp. When the electric current passes through this, an electromagnetic field is formed perpendicular to the coil. This magnetic field passes without being affected by the electrical resistance of the scalp and skull. In accordance with the Faraday law of electromagnetic induction, an electrical field is formed in the cortical area adjacent to the coil and this induces the electric current. This induced current is a reverse current parallel to the coil.[3] The circular and figure-of-8 (butterfly) coils are most used for TMS. The circular coil stimulates a wider parenchymal area. With the figure-of-8 coil, which is formed by the joining together of two circular coils, focus is on the central mid-point and stimulation is obtained at greater strength (Figure 1). In addition, there is a double-cone coil which is effective at a greater depth compared to the figure-of-8 coil and can be defined as an angulated figure-of-8 coil. The H-coil is used for deep brain stimulation.
Figure 1. Transcranial magnetic stimulation stimulator with a figure-of-8 coil.
Transcranial direct current stimulation
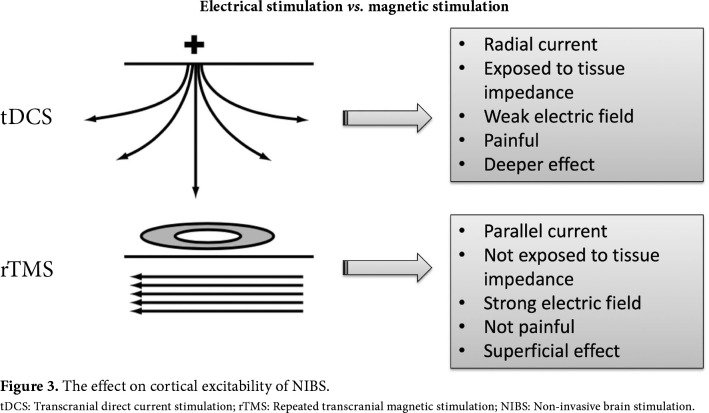
In tDCS, the electric current is applied to the brain using electrode pads placed directly on the scalp. Two types of electrodes are used: anode and cathode. One of the electrodes is placed on the scalp to be over the targeted cortex region and the other is generally placed over the contralateral supraorbital region (Figure 2). The direction of the electric current is from anode to cathode. As the electric current is exposed to impedance of the scalp and skull, the stimulation provided may not be as strong as with TMS, and it may create a feeling of discomfort in the skin. However, the current penetration is higher compared to TMS.[4] Comparison of electrical stimulation and magnetic stimulation is presented in Figure 3.
Figure 2. Transcranial direct current stimulation stimulator and the placement of electrodes.
Figure 3. The effect on cortical excitability of NIBS. tDCS: Transcranial direct current stimulation; rTMS: Repeated transcranial magnetic stimulation; NIBS: Non-invasive brain stimulation.
In both TMS and tDCS, the electric current advances toward the cell membrane that can be stimulated and changes the transmembrane potential. This results in membrane depolarization and axon potential starts, which progresses along the neural pathway with the normal nerve transmission mechanisms.[5] Ultimately, the effects of stimulation can be seen as stimulated neural activity on electroencephalography, as changes in blood flow and metabolism on positron emission tomography (PET), functional MRI, and single-photon emission computed tomography (SPECT), and as muscle contraction and motor evoked potential (MEP) on electromyography.
STIMULATION TECHNIQUES
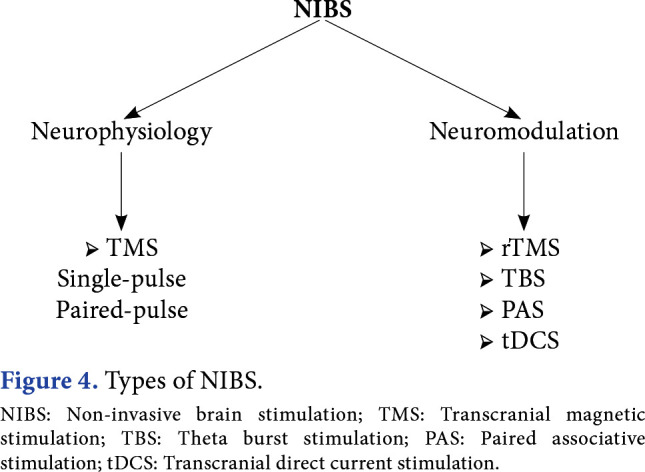
When TMS are used for diagnostic purpose, some neurophysiological measurements are taken, and for this, single-pulse and paired-pulse TMS is used. With single-pulse TMS, cortical excitability, motor threshold, pyramidal path transmission, silent period, motor cortex mapping, and cortical plasticity can be studied. With double-pulse TMS, intracortical inhibition and intracortical facilitation can be examined.[6] Following central nervous system damage, the prognosis can be estimated with these neurophysiological measurements, or the response to therapeutic interventions can be monitored. When NIBS is used for therapeutic purpose, the aim is to create neuromodulation by inhibiting or activating neural activity in the targeted cortical region. Repeated TMS (rTMS), tDCS, theta burst stimulation (TBS), and paired associative stimulation (PAS) are used for neuromodulation (Figure 4).
Figure 4. Types of NIBS. NIBS: Non-invasive brain stimulation; TMS: Transcranial magnetic stimulation; TBS: Theta burst stimulation; PAS: Paired associative stimulation; tDCS: Transcranial direct current stimulation.
The rTMS is applied as stimulations at the same dose at a specific frequency in regular sequences over the target cortical region.[7] Different parameters (intensity and duration) of frequency and pattern of the stimulus lead to changes in the cortical excitability. One pulse per sec, 1 Hz, is defined as “low-frequency rTMS”, and above this as “high-frequency rTMS”. In a general sense, continuous administration of low- frequency rTMS reduces cortical excitability, whereas rTMS given as bursts (sudden sequences) at high- frequency of ≥5 Hz increases cortical excitability. This inhibition and facilitation in cortical excitability can be seen as a decrease or increase in peripherally recorded MEP amplitude. The rTMS treatment is given in sessions lasting an average of 20 to 30 min.
The neuromodulation effect in tDCS varies according to the placement of the electrodes.[8] The current is from anode to cathode. In anodal stimulation, the anode electrode is placed on the motor cortex to be stimulated or on another cortical target region, and the cathode electrode is placed over the contralateral supraorbital region. Anodal stimulation has a facilitation effect on cortical activity. In cathodal stimulation, the electrode placement and neuromodulation effect are reversed. It is applied as low currents of 1 to 2 mA for durations of 10 to 30 min.
The TBS is a new TMS application formed from a series of stimulations given in a specific pattern.[9] It was originally developed in an animal model to create modulation in cortical activity by mimicking the tetanic stimulus used in the stimulation of synaptic plasticity. The TBS is formed of three 50 Hz bursts of stimulation repeated every 200 msec. Continuous TBS is applied as a total of 600 pulses without interruption in 40 sec and it has an inhibitor effect on cortical excitability. Intermittent TBS is an application lasting for a total of 190 sec, applied as a series of 2 sec every 10 sec, formed of a total of 600 pulses, and it has a facilitator effect. The main advantage of TBS compared to rTMS is that it allows more pulses in a shorter time.
The PAS is formed of repeated peripheral and central stimulus pairs.[10] There is electrical stimulation of the median nerve followed by TMS stimulus of the contralateral motor cortex after a certain time. The time between the two stimuli determines the modulation. The PAS-10, applied at intervals of 10 msec has an inhibitor effect, and PAS-25 at intervals of 25 msec, a facilitator effect. A 30-min protocol can be applied formed of 180 paired stimuli applied every 10 sec.
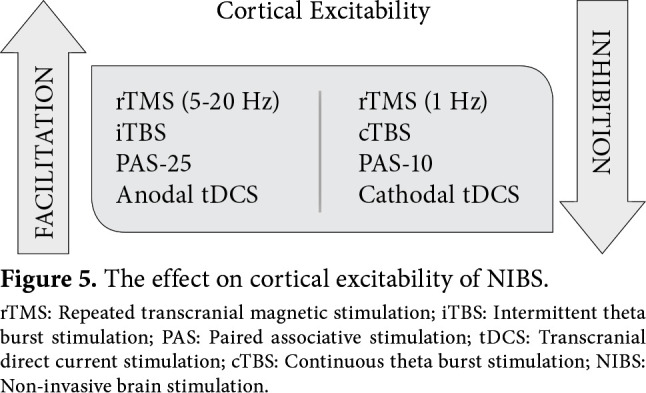
To summarize, high-frequency rTMS, intermittent TBS, PAS-25, and anodal tDCS have a facilitation effect on cortical excitability, and low-frequency rTMS, continuous TBS, PAS-10, and cathodal tDCS have an inhibition effect on cortical excitability (Figure 5). The effect of rTMS on cortical excitability has been shown to continue after the end of stimulation, which is called as “after effect”.[11] The observation of this phenomenon has been associated with functional reorganization and plasticity. It has been suggested that a series of mechanisms occur at the cellular, synaptic, and regional level for this “after effect”. The most focused on subject is the long-term potentiation and depression-like effect, which is one of the important arguments of synaptic plasticity.
Figure 5. The effect on cortical excitability of NIBS. rTMS: Repeated transcranial magnetic stimulation; iTBS: Intermittent theta burst stimulation; PAS: Paired associative stimulation; tDCS: Transcranial direct current stimulation; cTBS: Continuous theta burst stimulation; NIBS: Non-invasive brain stimulation.
NON-INVASIVE BRAIN STIMULATION INDICATIONS
The indications for therapeutic NIBS can be gathered under four main headings as shown in Table 1. One of the most important areas of use is for neurorehabilitation. For each disease, different cortical region is targeted for stimulation. Also, the stimulation protocol is determined according to target cortical region. When stroke is considered, the basic mechanism of motor dysfunction is a decrease in cortical excitability in the affected hemisphere and a compensatory increase in the unaffected hemisphere. This increased activity in the healthy hemisphere increases transcallosal inhibition and further suppresses activity in the affected hemisphere. When this is taken into consideration, treatment in respect of neuromodulation may be in two forms. These are to increase excitability in the affected hemisphere with techniques providing facilitation and to decrease excitability in the healthy hemisphere with inhibitor techniques.
Stroke is the most studied condition in which NIBS modalities are used for neurorehabilitation. Positive results have been reported in motor functional recovery. An increase in grip strength has been reported with low-frequency rTMS applied to the healthy hemisphere after stroke.[12] Similarly, rTMS has been shown to be useful in lower extremity motor recovery and to provide improvements in walking speed and the Fugl-Meyer assessment scale.[13,14] Following motor recovery in stroke, aphasia is the second most studied area. In a recent meta-analysis, low-frequency rTMS applied combined with speech therapy was shown to be of benefit in naming, recall, understanding, written language, and functional communication.[15]
The treatment of neglect after stroke is another condition where rTMS is effective. With rTMS applied adjuvant to conventional treatments, decrease in neglect and improved daily living activities have been determined.[16,17] Those NIBS modalities, particularly rTMS, are useful in the treatment of dysphagia after stroke according to meta-analyses.[18,19] There has also been an increase in studies of the effect of rTMS on cognitive function disorder in patients with traumatic brain injuries. However, the number of studies in this area is limited and a definitive effect has not been shown.[20]
It is thought that rTMS can be effective in spinal cord injury (SCI) using the corticospinal pathway. The rTMS studies after SCI have mostly been conducted on spasticity, pain, and motor recovery. In studies investigating the role of NIBS in spasticity, no benefit has been seen on the spasticity occurring after stroke.[21,22] However, it has been reported that rTMS is effective in the spasticity seen associated with lesions at the level of the spinal cord and the brain stem (SCI, multiple sclerosis).[23] In the treatment of resistant central pain seen after SCI, rTMS has been shown to have pain-relieving effect in the short term.[24,25] In studies on motor recovery,[26,27] high-frequency rTMS lead to an increase in lower extremity motor scores and functional improvements in patients with incomplete SCI.
NEURONAVIGATION
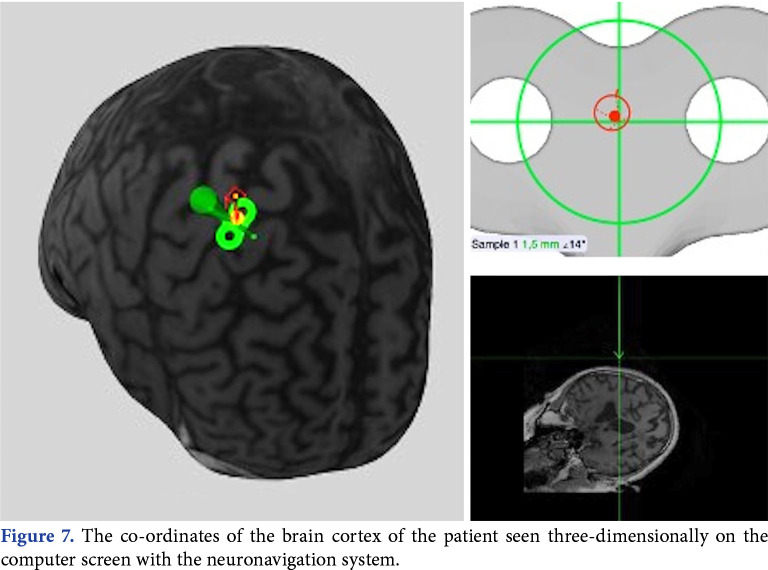
One of the important points to make the procedure effective in TMS is the correct localization of the coil on the target cortex region. Neuronavigation can be of assistance for the correct application. The brain coordinates obtained from MRI slices of the patient are used in the neuronavigation system. The coordinates of the coil and the head coordinates determined using the coil tracer, patient tracer and optic camera of the infrared position sensor are transferred to a common three-dimensional reference system (Figure 6).[28] In this way, target stimulation area of the brain can be monitored on a computer screen during the application (Figure 7).
Figure 6. Neuronavigation system.
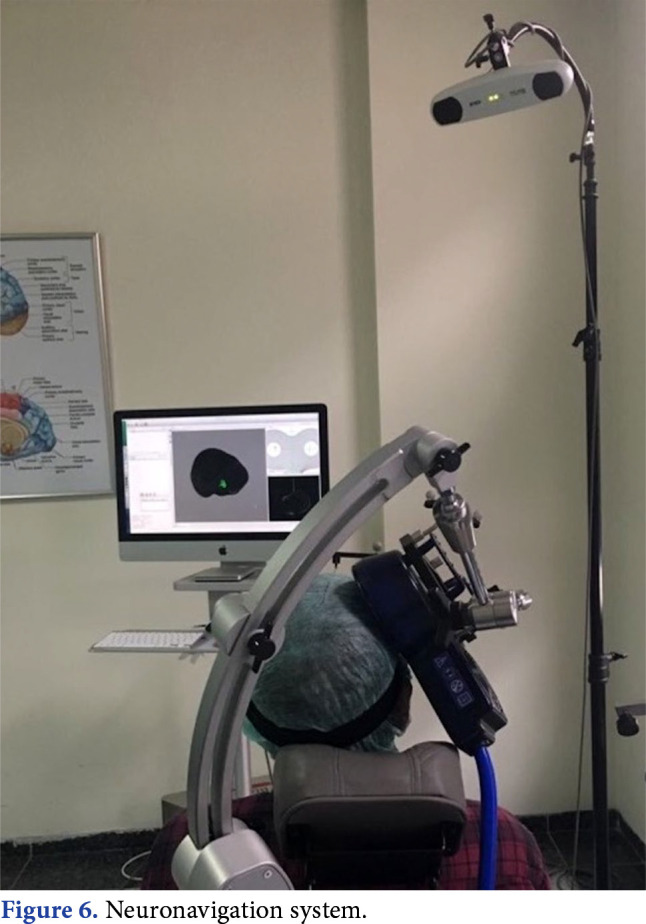
Figure 7. The co-ordinates of the brain cortex of the patient seen three-dimensionally on the computer screen with the neuronavigation system.
SAFETY
From a safety point of view, the peak magnetic field strength formed with TMS is <2 Tesla. When it is considered that the MRI devices currently used have electromagnetic strength of ≥3 Tesla, the electromagnetic field that occurs with TMS is at an acceptable level. Moreover, compared to MRI, the magnetic field of TMS is more focal and only affects the targeted brain region. The strength of the magnetic field created by TMS within the tissue decreases exponentially with distance from the source. In other words, tissue at a distance of a few centimeters from the coil is not affected by the magnetic field. In experimental studies, it has been shown that electromagnetic tissue damage can occur with continuous stimulation at 50 Hz for 7 h. The most feared side-effect of TMS is the triggering of epileptic seizures. However, studies with large samples have concluded that, in a population with no risk of epileptic attack, there is no greater risk than normal of the development of a new attack.[29] Other than epilepsy, temporary effects may be seen such as headache (20 to 25%), neck pain (10 to 15%), tinnitus, cognitive effects (temporary concentration and memory disorders), acute mood changes (irritability), and neurocardiogenic syncope. According to the international safety guidelines, with appropriate patient selection and defined treatment parameters, TMS can be a safe technique.[30]
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 2.Liew SL, Santarnecchi E, Buch ER, Cohen LG. Non-invasive brain stimulation in neurorehabilitation: Local and distant effects for motor recovery. Front Hum Neurosci. 2014;8:378–378. doi: 10.3389/fnhum.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 4.Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation. Brain Stimul. 2009;2:241–245. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: Definition, selection, and reporting practices. Brain Stimul. 2012;5:435–453. doi: 10.1016/j.brs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freitas C, Farzan F, Pascual-Leone A. Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: Why, how, and what is the ultimate goal. Front Neurosci. 2013;7:42–42. doi: 10.3389/fnins.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 11.Valero-Cabré A, Pascual-Leone A, Rushmore RJ. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur J Neurosci. 2008;27:765–774. doi: 10.1111/j.1460-9568.2008.06045.x. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Li K, Chen Q, Yin J, Bai D. Repetitive transcranial magnetic stimulation on motor recovery for patients with stroke: A PRISMA compliant systematic review and meta- analysis. Am J Phys Med Rehabil. 2020;99:99–108. doi: 10.1097/PHM.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 13.Tung YC, Lai CH, Liao CD, Huang SW, Liou TH, Chen HC. Repetitive transcranial magnetic stimulation of lower limb motor function in patients with stroke: A systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2019;33:1102–1112. doi: 10.1177/0269215519835889. [DOI] [PubMed] [Google Scholar]
- 14.Ghayour-Najafabadi M, Memari AH, Hosseini L, Shariat A, Cleland JA. Repetitive transcranial magnetic stimulation for the treatment of lower limb dysfunction in patients poststroke: A systematic review with meta-analysis. J Stroke Cerebrovasc Dis. 2019;28:104412–104412. doi: 10.1016/j.jstrokecerebrovasdis.2019.104412. [DOI] [PubMed] [Google Scholar]
- 15.Yao L, Zhao H, Shen C, Liu F, Qiu L, Fu L. Low-frequency repetitive transcranial magnetic stimulation in patients with poststroke aphasia: Systematic review and meta- analysis of its effect upon communication. J Speech Lang Hear Res. 2020;63:3801–3815. doi: 10.1044/2020_JSLHR-19-00077. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Li Y, Yang Y, Qu Y, Li S. Efficacy of noninvasive brain stimulation on unilateral neglect after stroke: A systematic review and meta-analysis. Am J Phys Med Rehabil. 2018;97:261–269. doi: 10.1097/PHM.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 17.Salazar APS, Vaz PG, Marchese RR, Stein C, Pinto C, Pagnussat AS. Noninvasive brain stimulation improves hemispatial neglect after stroke: A systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99:355–366. doi: 10.1016/j.apmr.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Dong L, Cong X, Luo H, Li W, Meng P, et al. Comparative efficacy of non-invasive neurostimulation therapies for poststroke dysphagia: A systematic review and meta-analysis. Neurophysiol Clin. 2021;51:493–506. doi: 10.1016/j.neucli.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Chiang CF, Lin MT, Hsiao MY, Yeh YC, Liang YC, Wang TG. Comparative efficacy of noninvasive neurostimulation therapies for acute and subacute poststroke dysphagia: A systematic review and network meta-analysis. Arch Phys Med Rehabil. 2019;100:739–750. doi: 10.1016/j.apmr.2018.09.117. [DOI] [PubMed] [Google Scholar]
- 20.Tsai PY, Chen YC, Wang JY, Chung KH, Lai CH. Effect of repetitive transcranial magnetic stimulation on depression and cognition in individuals with traumatic brain injury: A systematic review and meta-analysis. Sci Rep. 2021;11:16940–16940. doi: 10.1038/s41598-021-95838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu P, Huang Y, Wang J, An X, Zhang T, Li Y, et al. Repetitive transcranial magnetic stimulation as an alternative therapy for stroke with spasticity: A systematic review and meta- analysis. J Neurol. 2021;268:4013–4022. doi: 10.1007/s00415-020-10058-4. [DOI] [PubMed] [Google Scholar]
- 22.McIntyre A, Mirkowski M, Thompson S, Burhan AM, Miller T, Teasell R. A systematic review and meta-analysis on the use of repetitive transcranial magnetic stimulation for spasticity poststroke. PM R. 2018;10:293–302. doi: 10.1016/j.pmrj.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Korzhova J, Sinitsyn D, Chervyakov A, Poydasheva A, Zakharova M, Suponeva N, et al. Transcranial and spinal cord magnetic stimulation in treatment of spasticity: A literature review and meta-analysis. Eur J Phys Rehabil Med. 2018;54:75–84. doi: 10.23736/S1973-9087.16.04433-6. [DOI] [PubMed] [Google Scholar]
- 24.Gao F, Chu H, Li J, Yang M, DU L, Li J, et al. Repetitive transcranial magnetic stimulation for pain after spinal cord injury: A systematic review and meta-analysis. J Neurosurg Sci. 2017;61:514–522. doi: 10.23736/S0390-5616.16.03809-1. [DOI] [PubMed] [Google Scholar]
- 25.Yılmaz B, Kesikburun S, Yaşar E, Tan AK. The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. J Spinal Cord Med. 2014;37:397–400. doi: 10.1179/2045772313Y.0000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan R, Qu M, Yuan Y, Lin M, Liu T, Huang W, et al. Clinical benefit of rehabilitation training in spinal cord injury: A systematic review and meta-analysis. E398-E410Spine (Phila Pa 1976) 2021;46 doi: 10.1097/BRS.0000000000003789. [DOI] [PubMed] [Google Scholar]
- 27.Kesikburun S, Uran A, Yasar E, Yilmaz B. The effect of high frequency repetitive transcranial magnetic stimulation on motor recovery and gait parameters in chronic in-complete spinal cord injury; A randomized controlled study. Ann Phys Rehabil Med. 2022 doi: 10.5606/tftrd.2023.11585. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schönfeldt-Lecuona C, Lefaucheur JP, Cardenas-Morales L, Wolf RC, Kammer T, Herwig U. The value of neuronavigated rTMS for the treatment of depression. Neurophysiol Clin. 2010;40:37–43. doi: 10.1016/j.neucli.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Pereira LS, Müller VT, da Mota Gomes M, Rotenberg A, Fregni F. Safety of repetitive transcranial magnetic stimulation in patients with epilepsy: A systematic review. Epilepsy Behav. 2016;57:167–176. doi: 10.1016/j.yebeh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmöller J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol. 2021;132:269–306. doi: 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]