Abstract
Vitamin D has received much interest during the COVID-19 pandemic as a potential prophylactic or therapeutic agent — but do the available data support its use?
Subject terms: Immunology, SARS-CoV-2, Infection
Early in the COVID-19 pandemic, a lack of vaccines and therapies prompted interest in a potential role for vitamin D in reducing risk or severity of disease. This was based on the recognized effects of vitamin D on host responses to other respiratory viruses, and evidence from randomized controlled trials (RCTs) showing the efficacy of vitamin D supplementation for the prevention of other acute respiratory infections. Since then, effective drugs and vaccines have been developed for COVID-19, with major effects on mortality. Numerous reports associating low vitamin D status with adverse outcomes in COVID-19 have also emerged, together with results of some RCTs. Here, we briefly review the accumulated evidence in this field.
Vitamin D belongs to a group of fat-soluble vitamins with vitamin D3 being the major form in humans. The primary source is via cutaneous synthesis in response to sunlight, but it can also be ingested in the diet. The active vitamin D metabolite, 1,25-dihydroxyvitamin D (1,25(OH)2D), induces innate antiviral effector mechanisms and regulates inflammation in response to infection with rhinovirus, respiratory syncytial virus and influenza viruses (Fig. 1). The relevance of these actions for the host response to SARS-CoV-2 is supported by two lines of evidence. First, the vitamin D-inducible antimicrobial peptide cathelicidin LL-37 has been shown to inhibit SARS-CoV-2 attachment to host cells by blocking both the receptor-binding domain of the S1 subunit of the viral spike protein and the ligand-binding domain of the host-expressed viral entry receptor ACE2 (ref.1). Second, a study in transgenic mice expressing human ACE2 has shown that SARS-CoV-2 induces the expression of CYP27B1, which encodes the 1α-hydroxylase enzyme that catalyses the conversion of the major circulating metabolite 25-hydroxyvitamin D (25(OH)D) to 1,25(OH)2D (ref.2). Prophylactic administration of high-dose vitamin D3 increased the expression of IFNB and reduced inflammation in the lungs after SARS-CoV-2 infection, although this was not associated with a survival benefit2. The relevance of these findings for human disease is supported by the observation that CD4+ T cells in the bronchoalveolar lavage fluid of patients with COVID-19 exhibit increased expression of vitamin D-repressible genes that encode pro-inflammatory cytokines3. However, a case study of COVID-19 arising in a family lacking functioning vitamin D receptor reported a mild disease course and normal development of antigen-specific cellular and humoral immune responses to SARS-CoV-2 (ref.4). This suggests that vitamin D-mediated signalling may have a role in regulating SARS-CoV-2-induced inflammation, but it may not be a pre-requisite for averting severe outcomes or mounting adaptive immune responses to this pathogen.
Fig. 1. Vitamin D in viral infection.
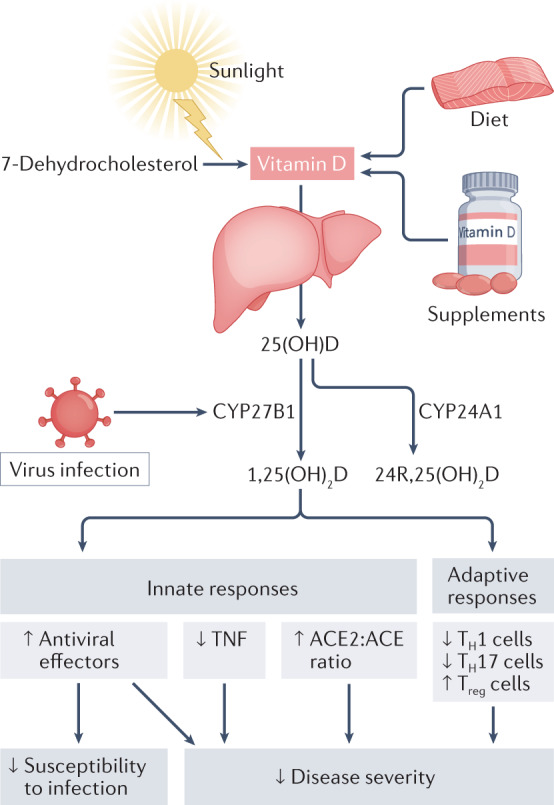
Vitamin D from cutaneous synthesis or oral intake is converted in the liver to 25-hydroxyvitamin D (25(OH)D), the major circulating vitamin D metabolite. In pulmonary epithelium and in leukocytes, respiratory viruses induce the expression of the 25(OH)D hydroxylase CYP27B1, which converts 25(OH)D to the active vitamin D metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D). This supports antiviral effector mechanisms and regulates inflammation by reducing TNF expression, increasing the ACE2:ACE ratio, inhibiting the development of TH1 and TH17 cells, and promoting the development of Treg cells. These anti-inflammatory actions may reduce disease severity associated with cytokine storms. CYP24A1 can catabolize 25(OH)D to the relatively inactive metabolite 24R,25-dihydroxyvitamin D (24R,25(OH)2D).
Observational studies have associated low circulating 25(OH)D concentrations with increased severity of COVID-19 (ref.5). In cross-sectional and case–control studies, in which vitamin D status and clinical outcomes are assessed contemporaneously, such associations may be explained by reverse causality, as SARS-CoV-2 induces the expression of CYP24A1 (ref.2), which results in 25(OH)D catabolism (Fig. 1). Prospective studies circumvent this problem by measuring vitamin D status before outcomes, but they are still potentially open to confounding by factors associated with both low circulating 25(OH)D concentrations and increased COVID-19 severity. Mendelian randomization studies are less open to the effects of reverse causation and confounding, and these have consistently reported no associations between genetically predicted 25(OH)D concentrations and COVID-19 susceptibility or severity6. Ultimately, however, RCTs are needed to determine whether vitamin D supplements may have a role in the treatment or prevention of COVID-19.
So far, 11 RCTs investigating the therapeutic effects of vitamin D in COVID-19 have reported. They are diverse with respect to study design (5 are placebo-controlled, 6 are open-label or single-blind), sample size (ranging from 30 to 543 participants), the nature of the intervention (8 investigate vitamin D3, 2 investigate 25(OH)D3 and 1 investigates 1,25(OH)2D3) and primary outcomes (mortality, duration of hospital stay, intensive care requirement, resolution of symptoms and viral clearance). Their findings are also heterogeneous: seven trials report null results, whereas four report favourable effects of the intervention on a primary or co-primary outcome. The most striking positive result comes from an open-label RCT of oral 25(OH)D3 administration conducted in 76 adults hospitalized with COVID-19 in Spain: this trial reported that just 2% of participants randomized to intervention were admitted to intensive care, as compared with 50% of those randomized to control7. However, this study is at high risk of bias, owing to imbalance in baseline characteristics and its open-label design, as knowledge of allocation could have influenced physicians’ decision to admit participants to intensive care. Moreover, background therapy (azithromycin and hydroxychloroquine) was unconventional, compromising the generalizability of results to patients receiving current standards of care. Other well-conducted RCTs (such as ref.8) have not demonstrated sustained or consistent benefits of vitamin D on mortality, intensive care requirement or duration of hospital stay.
Two RCTs investigating prophylactic vitamin D have also reported contrasting results. A phase 2 placebo-controlled RCT in 321 healthcare workers in Mexico, conducted before roll-out of SARS-CoV-2 vaccination, reported a strong protective effect of daily oral administration of 4,000 IU vitamin D3 for one month against incident SARS-CoV-2 infection9. This finding surprised many, given that the duration of the intervention (1 month) was insufficient for participants in the intervention arm to experience a large increase in circulating 25(OH)D concentrations. By contrast, an open-label phase 3 RCT in the UK involving 6,200 adults and conducted during the SARS-CoV-2 vaccine roll-out showed no effect of implementing a test-and-treat approach to the correction of sub-optimal vitamin D status via daily oral administration of either 800 or 3,200 IU vitamin D3 over 6 months10. The interpretation of this result is complicated by the pragmatic nature of this trial, which allowed for the consumption of vitamin D supplements among participants randomized to its control arm; however, a sensitivity analysis excluding data from control arm participants who took off-trial supplements also yielded a null finding. Results from placebo-controlled phase 3 trials of prophylactic vitamin D and cod liver oil (ClinicalTrials.gov: NCT04609423, NCT04483635 and NCT04536298) are pending, and these should clarify whether prophylactic administration of vitamin D supplements can influence the risk or severity of COVID-19. However, the lack of a consistent protective signal from RCTs reporting so far is reflected by the absence of any recommendation relating to prophylactic or therapeutic use of vitamin D for COVID-19 in guidelines from national or international bodies.
If vitamin D does have favourable immunomodulatory effects in COVID-19, then demonstrating a meaningful clinical benefit of supplementation over existing standards of care is likely to become increasingly challenging, as ever more effective pharmacological therapies and vaccines emerge.
Competing interests
A.R.M. declares receipt of funding, reagents or consultancy or speaker fees from companies who manufacture or sell vitamin D supplements (Pharma Nord Ltd, DSM Nutritional Products Ltd, Thornton & Ross Ltd, Hyphens Pharma Ltd, Synergy Biologics Ltd, Cytoplan Ltd; Abiogen Pharma Ltd); receipt of a speaker fee from the Linus Pauling Institute; and participation on Data and Safety Monitoring Boards for vitamin D trials (Pan African Clinical Trials Registry ref PACTR20200989766029, ClinicalTrials.gov: NCT04641195). A.R.M. and M.T.C. declare unpaid work as programme committee members for the Vitamin D Workshop.
References
- 1.Wang C, et al. Human cathelicidin inhibits SARS-CoV-2 infection: killing two birds with one stone. ACS Infect. Dis. 2021;7:1545–1554. doi: 10.1021/acsinfecdis.1c00096. [DOI] [PubMed] [Google Scholar]
- 2.Arora J, et al. Vitamin D and the ability to produce 1,25(OH)2D are critical for protection from viral infection of the lungs. BioRxiv. 2022 doi: 10.1101/2022.06.29.498158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauss D, et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022;23:62–74. doi: 10.1038/s41590-021-01080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kongsbak-Wismann M, et al. Normal T and B cell responses against SARS-CoV-2 in a family with a non-functional vitamin D receptor: a case report. Front. Immunol. 2021;12:758154. doi: 10.3389/fimmu.2021.758154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dissanayake HA, et al. Prognostic and therapeutic role of vitamin D in COVID-19: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2021;107:1484–1502. doi: 10.1210/clinem/dgab892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler-Laporte G, et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 host genetics initiative: a Mendelian randomization study. PLoS Med. 2021;18:e1003605. doi: 10.1371/journal.pmed.1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Entrenas Castillo M, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murai IH, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villasis-Keever MA, et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch. Med. Res. 2022;53:423–30. doi: 10.1016/j.arcmed.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolliffe DA, et al. Vitamin D supplements for prevention of Covid-19 or other acute respiratory infections: a phase 3 randomized controlled trial (CORONAVIT) MedRxiv. 2022 doi: 10.1101/2022.03.22.22271707. [DOI] [Google Scholar]


