Abstract
Aim
Currently, it is unknown which patient‐reported outcomes are important for patients with autosomal inherited bleeding disorders. Therefore, the purpose of this study is to systematically review the available literature assessing patient‐reported outcomes and their measurement methods in autosomal inherited bleeding disorders.
Methods
The Embase, Medline ALL, Web of Science Core Collection, Cochrane Central Register of Controlled Trails and Google Scholar databases were searched from inception until 14 August 2020. Studies on patient‐reported outcomes in patients with von Willebrand disease, inherited platelet function disorders and coagulation factor deficiencies were included.
Results
Twenty‐one articles met the inclusion criteria. Three studies were assessed as having poor quality, and therefore a high risk of bias. Nineteen studies had fair quality rating. Different measurements methods were used, ranging from predefined to self‐developed questionnaires. The majority of included studies focused on von Willebrand disease. Patients with von Willebrand disease reported lower health‐related quality of life compared to the general population. Overall, this trend was especially visible in the following domains: vitality, physical and social functioning and pain. Women with inherited bleeding disorders scored lower on health‐related quality of life compared to men, especially women with heavy menstrual bleeding. Patients with joint bleeds or heavy menstrual bleeding reported an increased level of pain.
Conclusion
Patients with autosomal inherited bleeding disorders report lower health related quality of life, especially those with joint bleeds or heavy menstrual bleeding. Numerous measurement methods are used in patients with autosomal inherited bleeding disorders, highlighting the need for studies using established, standardized measurement methods.
Keywords: blood coagulation disorders, blood coagulation disorders, blood platelet disorders, inherited, patient reported outcome measures, quality of life, systematic review, von willebrand diseases
1. INTRODUCTION
The trend towards a more value‐based healthcare system has led to an increasing emphasis on outcome measurement. Especially in the last few years, the interest to incorporate patient‐reported outcomes (PROs) in medical practice and research has grown exponentially. 1 , 2 , 3 PROs are defined as ‘any report coming directly from patients, without interpretation by physicians or persons, about how the patient functions or feels in relation to a health condition and its treatment 4 ’. There are several types of PROs with the most common being self‐reported symptoms, self‐reported functioning, and health‐related quality of life (HRQoL). 5 HRQoL is a broad multidimensional concept that incorporates various domains (e.g., physical, psychologic and social functioning) related to the health status of an individual. 6
PROs are often assessed using questionnaires, otherwise known as patient‐reported outcomes measures (PROMs). 7 PROMs can be classified as either generic or as disease specific. Generic PROMs, such as Euro‐QoL EQ‐5D, consist of questions relevant to multiple disease groups or a healthy population. In contrary, disease specific PROMs focus on particular patient groups and consist of questions that are only related to a given disease, disability or surgery. 7 , 8
Incorporating PROs in clinical practice enables (1) the evaluation of the effectiveness of a healthcare intervention, (2) the assessment of the quality of care and the needs of different populations, (3) the improvement of clinical decision making, and (4) a better understanding and causes of variations in health. 7 , 9 In addition, monitoring PROs may enhance patient engagement and shared decision making, which subsequently leads to a higher quality of care and more patient‐centred care. 8 , 10 , 11 For example, studies in oncology have shown that the systematic collection of PROs result in better symptom control, fewer hospitalizations and better quality of life. 12
The increased attention on more patient‐centred approaches in healthcare has led to more studies examining PROs and PROMs in a variety of diseases, including in patients with inherited bleeding disorders. Inherited bleeding disorders consist of a heterogeneous group of diseases affecting the primary and secondary haemostasis that include abnormalities or deficiencies of platelets or coagulation proteins. As haemophilia A and B are X‐linked, the autosomal inherited bleeding disorders include von Willebrand disease (VWD), inherited platelet function disorders and various coagulation factor deficiencies. Severe disorders have a low prevalence and usually present in childhood, but milder forms are relatively more common and may not be clinically apparent until later in life when patients have haemostatic challenges (e.g., menstruation, dental procedures, surgery or trauma). 13
The clinical presentation of different types of bleeding disorders tends to overlap and symptoms range from more common features such as easy bruising, mucocutaneous bleedings and heavy menstrual bleeding to more severe and uncommon symptoms such as joint bleeds, gastrointestinal‐ and intracranial bleedings. 14 Patients with similar diagnoses and comparable laboratory results do not always present with the same bleeding tendency. 15 , 16 , 17 This inter‐individual variability may complicate identifying the individual patient needs. Moreover, the commonly used measures such as bleeding assessment tools do not always reflect the impact of the disease on a patient's daily life. Incorporating PROs in clinical practice, may support the physician to focus on patient's (unidentified) needs, to identify the burden of disease for each individual patient and to monitor the treatment effect. Therefore, the implementation of PROs may lead to more personalized treatment in patients with autosomal inherited bleeding disorder.
Until now, research on PROs and PROMs in inherited bleeding disorders has mainly focused on haemophilia. 18 , 19 It is unknown which PROs are important for patients with autosomal inherited bleeding disorders and which PROMs are commonly used to measure PROs in this patient population. This systematic literature review aims to summarize the available literature assessing PROs and their measurement methods to identify which patient‐reported outcomes could be important for patients with autosomal inherited bleeding disorders.
2. METHODS
2.1. Article retrieval
This systematic review was registered in PROSPERO (registration number CRD42020199444) and followed the PRISMA methodology for systematic reviews and meta‐analysis and the COSMIN methodology for systematic reviews of Patient‐Reported Outcomes Measures. 20 An information specialist experienced in systematic literature reviews co‐designed and conducted the search strategy. The initial search was designed in Embase using a combination of Emtree and non‐registered index terms and translated into other databases’ syntax (Supplement 1). Key terms include congenital blood clotting disorders, blood clotting deficiency, patient‐reported outcome and quality of life. The literature search was performed in Embase, Medline ALL, Web of Science Core Collection, Cochrane Central Register of Controlled Trails and Google Scholar. All databases were searched from inception until 14 August 2020.
2.2. Study selection
Two reviewers (E.S.H. and M.E.H.) independently screened the articles on potential eligibility. Disagreements between the reviewers were discussed until uniform consensus was reached. English articles reporting on all different types of patient‐reported outcomes in both adult and paediatric patients with autosomal inherited bleeding disorders including von Willebrand disease, inherited platelet function disorders and coagulation factor deficiencies, were included. Articles solemnly focusing on bleeding symptoms or bleeding assessment tools were not included in this study, since bleeding symptoms are not necessary patient‐reported; they can also be reported by someone other than the patient himself/herself, for example in case of grading of bleeding after surgery. Case studies, commentaries, editorials, conference abstracts, economic evaluations and articles about acquired bleeding disorders were excluded. According to the COSMIN methodology, articles were excluded if less than 80 percent of the research population consisted of patients with autosomal inherited bleeding disorders. The reference lists of included studies identified by the search were checked for further relevant studies.
2.3. Data assessment
For each included study, the following information was collected: study design, characteristics of the patient population, mean or median age at study inclusion, used PROMs and measured PROs. In case the psychometric properties of a PROM were assessed, including its acceptability, internal consistency, reliability, validity and responsiveness this was also reported. Two reviewers (E.S.H and M.E.H.) read and abstracted each article; a third reviewer (W.A.) checked table entries for accuracy with regard to the original articles. Data were reviewed descriptively.
2.4. Risk of bias assessment
A single reviewer from the team (E.S.H) assessed risk of bias of included publications using the National Institutes of Health (NIH) quality assessment tool for observation and cross‐sectional studies. 21 Two other reviewers (M.E.H. and W.A.) evaluated the assessment accuracy. Quality rating (good, fair or poor) was used to assess certainty of evidence. Any discrepancies were discussed with all reviewers (E.S.H., M.E.H. and W.A.) until consensus was reached. Studies were not excluded based on risk of bias assessment.
3. RESULTS
3.1. Data retrieval
The systematic literature search yielded a total of 1959 non‐duplicate references that were screened using predetermined inclusion‐ and exclusion criteria. The flow chart (Figure 1) shows the process of article selection from initial search to final inclusion or exclusion. A total of 21 articles met the inclusion criteria for this systematic review. All studies were published between 2000 and 2020 (Figure 2). Seven studies were conducted in the Netherlands, 22 , 23 , 24 , 25 , 26 , 27 , 28 five in the United Kingdom, 29 , 30 , 31 , 32 , 33 four in the United States, 34 , 35 , 36 , 37 three in Canada, 38 , 39 , 40 one in Sweden 41 and one in Iran. 42
FIGURE 1.
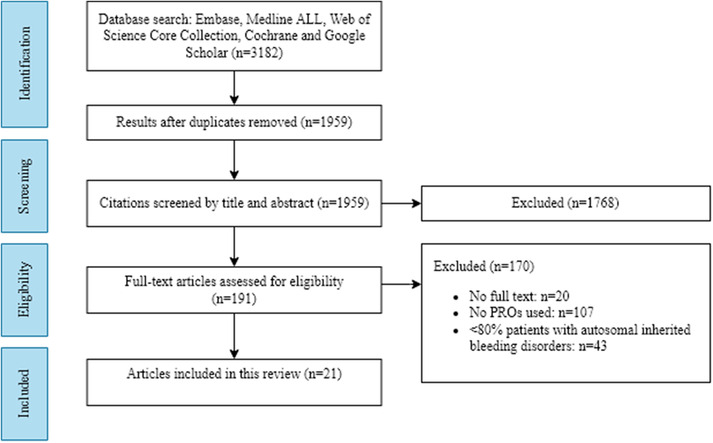
PRISMA flow diagram for study identification.
Abbreviation: PROs; patient‐reported outcomes
FIGURE 2.
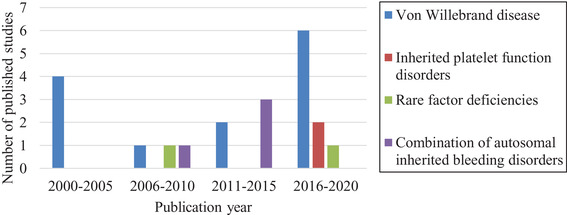
Publication year of included studies
Figure 3 shows the risk of bias assessment of the included studies. Three studies were assessed as having poor quality and therefore a high risk of bias. The remaining articles had a fair quality rating. The most prevalent limitations were found in items related to sample size justification, exposure assessment prior to outcome measurements and statistical analyses.
FIGURE 3.
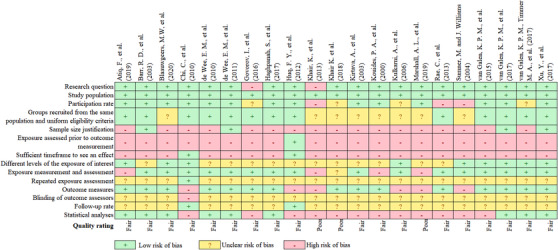
Risk of bias assessment using the National Institute of Health quality assessment tool for observational cohort and cross‐sectional studies
3.2. Diseases studied
The majority of included articles focused solely on von Willebrand disease (VWD) (n = 13). 22 , 24 , 25 , 26 , 27 , 28 , 34 , 35 , 37 , 38 , 39 , 40 , 41 Other studies included patients with inherited platelet function disorders (n = 2), 23 , 33 coagulation factor deficiencies (n = 2), 32 , 42 or a combination of different autosomal inherited bleeding disorders (n = 4). 29 , 30 , 31 , 39
3.3. Measurement methods
Studies used different measurement methods including predefined, routinely assessed PROMs (n = 11, 54%), self‐developed PROMs (n = 5, 23%), or a combination of both (n = 2, 9%). Three studies used semi‐structured questionnaires assessing PROs in an interview format (n = 3, 14%). The most frequently used predefined PROMs were the Short‐Form 36 (SF‐36) and the Health Utilities Index (HUI). Of the 21 included studies, two studies validated the applied PROMs specifically in patients with an inherited bleeding disorder. 26 , 28 Assessment of the development and psychometric properties of each PROM are summarized in Table 1.
TABLE 1.
Patient‐reported outcome measures used in patients with autosomal inherited bleeding disorders
| Name of instrument | Type | Aim | Dimensions/ domains | Items and scoring | Studies (n), references | Psychometric qualities a |
|---|---|---|---|---|---|---|
| B‐IPQ | Generic | To assess the cognitive and emotional representations of illness | Consequences, timeline, personal control, treatment control, identity, concern, understanding, emotional response, and causal factors | Nine items rated on a linear scale from 0 to 10; higher scores indicate negative illness perception expect for the dimensions personal control, treatment control and understanding | 1 23 | |
| CES‐D | Generic | To assess severity of depressive symptoms | Depression | 20 items rated on 5‐point Likert scale; higher scores indicate greater symptoms | 1 34 | |
| CHQ‐CF87 | Generic | To assess HRQoL in children | General health perceptions, physical functioning, role/social physical functioning, bodily pain, role/social emotional functioning, role/social behavioural functioning, parent impact‐time, parent impact‐emotional, self‐esteem, mental health, behaviour, family activities, family cohesion, and change in health | 87 items rated on a 4‐ to 6‐point Likert scale; higher scores indicate better or more positive health states | 1 24 | |
| CHQ‐ PF50 | Generic | To assess caregiver's perceptions of their child's (ages 5–18) HRQoL | General health perceptions, physical functioning, role/social physical functioning, bodily pain, role/social emotional functioning, role/social behavioural functioning, parent impact‐time, parent impact‐emotional, self‐esteem, mental health, behaviour, family activities, family cohesion, and change in health | 50 items rated on a 4‐ to 6‐point Likert scale; higher scores indicate better or more positive health states | 1 24 | |
| D‐AIMS2affect | Disease‐ specific | To measure changes in global health, pain, mobility and social function in patients with arthritis | Mobility, physical activity, dexterity, household activity, social activities, activities of daily living, pain, depression, anxiety, arm function, social support, and work | 77 items; higher scores indicate better function | 1 26 | |
| Haemo‐QOL | Disease‐specific | To assess HRQoL in children with haemophilia | Physical health, feeling, view of yourself, family, friends, other people, nursery school/kindergarten, treatment, perceived support, coping, relationships, and future | 21, 64 or 77 items on a 5‐point Likert scale; higher scores indicate more impairment and poorer quality of life | 1 42 | |
| HUI23S4.15Q | Generic | To assess health status and HRQoL |
Sensation, mobility, self‐care, emotion, cognition, and pain (HUI2). Vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain (HUI3) |
14 items with different levels (from 3 to 6); higher scores indicate better HRQoL | 1 38 | |
| HUI3 | Generic | To assess health status | Vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain | Eight items with different levels (5 or 6); higher score indicate better HRQoL | 1 38 , 39 | |
| HAL | Disease‐specific | To determine how bleeding episodes and progressive joint impairments impact self‐perceived functional abilities | Upper extremity activities, basic lower extremity activities, and complex lower extremity activities | 42 items across 7 domains rated on a 6‐point Likert scale; higher scores indicate greater functional status | 2 26 , 28 |
Construct validity HAL/HJHS : rs = ‐.71 HAL/SF‐36 physical functioning: rs = .65 HAL/SF‐36 physical component summary: rs = .62 HAL/IPAQ : rs = .69 |
| IPAQ | Generic | To assess the level of community participation | Autonomy indoors, autonomy outdoors, family roles, social relationships, paid work and education | 39 items rated on a 4‐point Likert scale; higher scores indicate greater restriction in participation or greater perception of problems in participation | 1 26 | |
| ITQOL | Generic | To assess caregiver's perceptions of their child's (ages 0–5) HRQoL | Physical functioning, growth and development, bodily pain, temperament and moods, general behaviour, getting along, general health perceptions, parental impact‐emotional, parental impact‐time, family cohesion, and change in health | 97 items rated on a 4‐ to 6‐ point Likert scale; higher scores indicate better HRQoL | 1 24 | |
| Kids’ ITP Tool | Disease‐specific | To assess HRQoL in children with ITP | No separate domains | 26 items on a 6‐point Likert scale: higher scores indicate greater impact of disease and poorer quality of life | 1 33 |
The Kids’ ITP Tool is not a valuable outcome measure for children with platelet disorders. The majority of the questions scored ‘not applicable’ or ‘never/rarely applicable’ |
| MPQ | Generic | To assess the different qualities of the subjective pain experience | The sensory, affective and evaluative aspects of pain | 20 items rated on a 5‐point Likert scale; higher scores indicate higher levels of pain | 1 26 | |
| SF‐36 | Generic | To assess the physical component and the mental component of HRQoL | Physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health | 36 items; higher scores indicate lower levels of disability | 7 22 , 23 , 25 , 27 , 28 , 40 , 41 | |
| VAS | Generic | To assess joint pain | Pain | Symptoms are recorded with a handwritten mark placed at one point along the length of a 10‐cm line; higher scores indicate higher levels of pain | 1 26 |
Abbreviations: B‐IPQ, Brief Illness Perception Questionnaire; CES‐D, Centre for Epidemiological Studies Depression Scale; CHQ‐CF87, Child Health Questionnaire Child Form 87; CHQ‐PF50, Child Health Questionnaire Parent Form 50; D‐AIMS2affect, Dutch Arthritis Impact Measurement Scales‐2, Haemo‐QOL, Haemophilia Quality of Life Questionnaire for Children; HAL, Health Utilities Index Mark 2; HUI23S4.15Q, Health Utilities Index Mark 2/3 15‐item Questionnaire; HUI3, Health Utilities Index Mark 3; HUI2, Haemophilia Activity List score; HJHS, Haemophilia Joint Health Score; HRQoL, Health‐Related Quality of Life; ITP, immune thrombocytopenia; IPAQ, Impact on Participation and Autonomy Questionnaire; IYQOL, Infant and Toddler Quality of Life Questionnaire; IPFD, Inherited platelet function disorders; MPQ, McGill Pain Questionnaire, QoL; Quality of Life, SF‐36; Short‐Form 36, VAS; Visual Analog Scale.
3.4. Patient‐reported outcomes
The included studies focused on several different PROs, such as HRQoL, physical functioning and pain. Table 2 provides an overview of PROs of included studies.
TABLE 2.
Overview of study characteristics, the used patient‐reported outcome measures and findings on patient‐reported outcomes of the included studies on autosomal inherited bleeding disorders
| Author, (year), country | Study design and characteristics of population (n; gender; disease type; age) | PROMs | Measured PROs | PROs and conclusions a |
|---|---|---|---|---|
| Atiq, F., et al. (2019). The Netherlands 22 | Prospective, multicentre789 patients with VWD (61% female)
|
|
|
|
| Barr, R. D., et al. (2003). Canada 38 | Prospective, single centre28 patients with VWD (64% female)
|
|
|
|
| Blaauwgeers, M.W, et al. (2020). The Netherlands 23 |
Prospective, multicentre 156 confirmed or suspected patients with CPD (81% female) |
|
|
|
| Chi, C., et al. (2010). UK 29 | Retrospective, single centre42 patients with different inherited bleeding disorders and HMB (all female)
|
|
|
Before treatment, around half of patients had low HRQoL, especially on the domain of general health and daily activity and after treatment; almost no patient had low HRQoL. Before treatment, 88% of patients had dysmenorrhea and after treatment, 17% of patients had dysmenorrhea. Before treatment, almost half of patients reported missing school because of their menstruation, and after treatment, none of the patients reported missing school. |
| de Wee, E. M., et al. (2010). The Netherlands 25 |
Prospective, multicentre 509 patients with VWD (62% female) Type 1: 282 Type 2: 196 Type 3: 21 Unspecified: 10 Age (years): range: 16–87 |
SF‐36 |
HRQoL Physical activity General health Pain |
Women have lower HRQoL compared to normative data, especially on the domains of general health, vitality and physical component summary (P < .001). Men have a lower HRQoL compared to normative data, especially on the domains pain and vitality (P < .005). Higher bleeding scores have a negative impact on physical domains of HRQoL (P < .001). Patients with VWD type 3 score lower in the domains physical functioning, pain and physical component summary, compared to patients with VWD type 1 and 2 (P < .05). |
| de Wee, E. M., et al. (2011). The Netherlands 24 |
Prospective, multicentre 133 patients with VWD (41% females) Type 1: 69 Type 2: 49 Type 3: 15 Age (years) range: .3‐17 |
CHQ‐CF87 CHQ‐PF50 ITQOL |
HRQoL Physical functioning General health Psychosocial functioning Pain |
Preschool children have lower HRQoL scores compared to normative data, especially in the domains of general health and parental time impact (P≤.05) and higher scores in the domain change in health (P≤.05). Schoolchildren have lower HRQoL compared to normative data, especially in the domains of physical functioning, role functioning – emotional/behavioural, general health and physical summary (P≤.05). There is no difference in HRQoL between boys and girls (preschool and schoolchildren). Schoolchildren with VWD type 3 have lower HRQoL compared to patients with VWD type 1 and 2 and normative data, especially in the domains of pain, general health, parental impact‐emotional, family activities and physical summary (P≤.05). Schoolchildren with severe bleeding phenotype have lower HRQoL scores in the social, emotional and physical domains Pp≤.05). |
| Govorov, I., et al. (2016). Sweden 41 |
Prospective, single centre 30 patients with VWD (all female) Type 1: 15 Type 2: 11 Type 3: 4 Age (years; SD) mean: 35.1 (8.1) range: 19–51 |
SF‐36 |
HRQoL Impact of menstruation on overall life activities |
Menstruation affects patients on mood (90.9%), family life (72.7%), vacations (68.2%), and the ability to go to work (45.5%). Patients have similar levels of HRQoL compared to normative data (P > .10). Patients with VWD type 1, 2 and 3 have similar scores of HRQoL (P > .10). Patients with HMB have more bodily pain compared to normative data (P < .10). |
| Haghpanah, S., et al. (2017). Iran 42 |
Prospective, single centre 52 patients with rare factor deficiencies (46% female) FI def.: 9 FV def.: 2 FVII def.: 15 FX def.: 7 FXI def.: 8 FXIII def.: 8 FV & FVIII def.: 3 Age (years; SD) mean: 13.96 (4.50) range: 4–18 |
Haemo‐QOL |
|
|
| Huq, F. Y., et al. (2012). UK 30 |
Retrospective, single centre 12 patients with different inherited bleeding disorders (all female) and HMB VWD: 3 VWD & HA carrier: 2 IPFD: 2 FXI def.: 2 FX def.: 1 HA carrier: 2 Age (years) mean: 44.3 range: 34–51 |
Ad hoc questionnaire |
HRQoL General health Physical activity Dysmenorrhoea |
After treatment HRQoL scores improve (P < .0001), especially scores on general health and health and daily activity (P < .01). Before treatment 58% of patients reported moderate to severe dysmenorrhoea and after treatment 16.6% patients had dysmenorrhoea (P < .01). Before treatment more than half of patients reported moderately or more interference of dysmenorrhoea with daily work and after treatment 8.3% patients had (P < .01). |
| Khair, K., et al. (2013). UK 31 |
Prospective, single centre 45 patients with different inherited bleeding disorders (all female) VWD: 23 HA carrier: 9 FXIII def.: 3 IPFD: 4 FXI def.: 2 FX def.: 1 FVII def.: 1 FV def.: 1 GT: 2 Unknown: 1 Age (years, SD) mean: 19.8 (5.21) range: 9–34 |
Ad hoc questionnaire Focus group |
HRQoL Sport participation |
Almost all patients (90%) are not adversely affected by their disease. 80% of patients is limited in sport participation due to their bleeding disorder. Around half of patients have concerns about fertility. A subgroup of patients experience stigmatisation, isolation and bullying. |
| Khair K. et al. (2018). UK 33 |
Prospective, single centre 16 patients with CPD (50% female) Age (years) mean: 11 range: 6–17 |
Kids’ ITP Tool Focus group |
General health Psychosocial functioning |
Most patients have good general health. More than half of children are more often than not tired. Most patients have good psychosocial functioning. |
| Kirtava, A., et al. (2003). USA 34 |
Prospective, multicentre 102 patients with VWD (all female) Age (years; SD) mean: 39 (11.9) range: 18–70 Control group: Individuals without VWD (n = 88) |
Ad hoc questionnaire CES‐D |
Daily activities and lifestyle Depression Health perception |
Patients have similar prevalence of depression compared to controls (P > .2). Patients were more negatively affected in activity (routine work and social activities) and lifestyle by their menstruation compared to controls (P = .004). Patient more often reported negative perception of health (fair or poor) compared to controls (P = .004). |
| Kouides, P. A., et al. (2000). USA 35 |
Prospective, multicentre 81 patients with VWD (all female) Type 1: 81 Age (years; SD) mean: 31.0 (10.7) range: 11–49 Control group: women with menstrual cycles and no VWD (n = 150) |
Ad hoc questionnaire |
HRQoL Psychosocial functioning |
Patients were more negatively affected by their menstruation compared to controls in the domains general activity, ability to work or go to school, family activities, ability to enjoy life, sleep, mood and overall HRQoL (P < .001). Approximately half (46%) of patients report losing time from work or school due to HMB. |
|
Kulkarni, A., et al. (2006). UK 32 |
Prospective, single centre 14 patients with FVII deficiency (all female) Age (years) median: 35 range: 15–50 Control group: Female staff at Royal Free Hospital (n = 23) |
Ad hoc questionnaire |
HRQoL General health Dysmenorrhoea |
Patients have lower HRQoL compared to controls, especially on the domain of general health (P < .005) and daily activities (P = .0002). Patients have more dysmenorrhoea (P = .019) and interference of dysmenorrhoea with daily work (P = .001) compared to controls. During menstruation patients have lower HRQoL compared to controls (P = .03). |
|
Marshall, A. L., et al. (2019). USA 36 |
Prospective, single centre 82 VWD (all female) Type 1: 58 Type 2: 22 Type 3: 3 Age (years) median: 54 |
Ad hoc questionnaire |
Reproductive health experience Activities and lifestyle |
Patients primarily use negative adjectives to describe HMB and reported a negative impact on their academic, home and sexual life. Patients primarily use negative adjectives to describe their reproductive experiences. |
| Rae, C., et al. (2013). Canada 39 |
Prospective, single centre 417 patients with different inherited bleeding disorders (75.5% female) VWD: 359 Other bleeding disorders (all female): 56 Age (years) range: 12–84 |
HUI3 |
HRQoL Daily activities |
Patients with different type or severity of disease have similar HRQoL (P = .914). Women with VWD have lower HRQoL compared to women with other inherited bleeding disorders (P = .017) and men with VWD (P = .039). Women aged from 20 to 84 years have lower HRQoL compared to women with other inherited bleeding disorder and normative data (P < .05). Women with VWD and HMB have similar HRQoL compared to women with other inherited bleeding disorder and HMB (P = .100). Women with HMB have lower HRQoL compared to women with no HMB (P < .001), especially on the domains of cognition (P < .001) and pain (P < .002). Women with HMB have more interference of HMB with daily activities compared to women with no HMB (P < .001). Women with iron deficiency have similar HRQoL compared to women with no iron deficiency (P = .459). |
| Sumner, M. and J. Williams (2004). USA 37 |
Prospective, multicentre 79 patients with VWD (58% female) Type 3: 79 Age (years) mean: 24.2 range: 1–60 |
Ad hoc questionnaire | Participation | Approximately 40% of patients lost days from work or school, the majority caused by bleeding or other VWD related complications. |
| van Galen, K. P. M., et al. (2017). The Netherlands 26 |
Prospective, multicentre 48 patient with VWD (40% female), history of joint bleeds Type 1: 8 Type 2: 21 Type 3: 19 Age (years) range: 18–78 Control group: Patients with VWD and no history of joint bleeds (n = 48) |
D‐AIMS2affect HAL IPAQ MPQ VAS pain |
Functional limitations Participation and autonomy Anxiety Depression (Joint) pain |
Patients with joint bleeds have more functional limitations compared to controls (P < .01). Patients with joint bleeds have similar scores on participation and autonomy compared to controls (P = .14). Patients with joints bleeds have similar levels of joint pain (P = .22), anxiety (P = .33) and mood (P = .87) compared to controls. Patients with joint bleeds and arthropathy have more functional limitations compared to patients without arthropathy (P < .01). Patients with joint bleeds and arthropathy have higher joint pain levels compared to patients without arthropathy (P < .01). Patients with joint bleeds and arthropathy have lower scores on participation and autonomy compared to patients without arthropathy (P < .1). Patients with joints bleeds and arthropathy have similar levels of anxiety ( P = .90) and mood (P = .14) compared to patients without arthropathy. |
| van Galen, K. P. M., et al. (2015). Netherlands 27 |
Prospective, multicentre 184 patients with VWD and a history of joint bleeds Age (years) range: 16–83 Control group: Patients with VWD and no history of joint bleeds (n = 620) |
SF‐36 |
HRQoL (Joint) pain |
Patients with self‐reported joint bleeds have lower HRQoL compared to patients without self‐reported joint bleeds, especially in the domains vitality, pain, general health, mental health, social functioning and role emotional. Patients with self‐reported joints bleeds and without self‐reported joints bleeds have both high scores on the domain physical functioning. (Self‐reported) joint damage seems to be largely responsible for the association between joint bleeds and lower HRQoL. Patients with a history of joint bleeds have more pain compared to controls (P = .008). |
| Van Galen, K. P. M., et al. (2017). The Netherlands 28 |
Prospective, multicentre 96 patients with VWD (40% female) Type 1: 28 Type 2: 46 Type 3: 22 Age (years) mean: 46 range: 18–80 |
HAL | Functional limitations | Patients with > 5 joint bleeds have more functional limitations compared to those with ≤ 5 joint bleeds (P < .01). |
| Xu, Y., et al. (2017). Canada 40 |
Prospective, single centre 102 patients with VWD (78% female) Type 1: 82 Type 2: 16 Type 3: 4 Age (years; SD) mean: 37.5 (14.8) |
SF‐36 |
HRQoL Vitality General health Social functioning Physical activities |
Patients have lower HRQoL compared to normative data in all SF‐36 domains (P < .05), except in the domain role emotional. The largest difference was found in the domains general health, vitality and social functioning (P < .5). Patients with different VWD severity have similar scores on HRQoL (P≥.05). Patients with VWD type 1, 2 and 3 have similar scores of HRQoL ( P ≥.05). Patients with VWD type 3 have lower scores on physical component summary compared to patients with VWD type 2 (P = .04). |
Abbreviations: B‐IPQ, Brief Illness Perception Questionnaire; BMI, Body Mass Index; CES‐D, Center for Epidemiological Studies Depression Scale; CHQ‐CF87, Child Health Questionnaire Child Form 87; CHQ‐PF50, Child Health Questionnaire Parent Form 50; CPD, Congenital blood platelet disorder; D‐AIMS2affect, Dutch Arthritis Impact Measurement Scales‐2; def, deficiency; GT, Glanzmann's thrombocytopenia; HA, haemophilia; Haemo‐QOL, Haemophilia Quality of Life Questionnaire for Children; HAL, Haemophilia Activity List score; HMB, heavy menstrual bleeding; HUI23S4.15Q, Health Utilities Index Mark 2/3 15‐item Questionnaire; HUI3, Health Utilities Index Mark 3; HRQoL, Health‐Related Quality of Life; IPAQ, Impact on Participation and Autonomy Questionnaire; IFPD, Inherited platelet function disorder; ITP, immune thrombocytopenia; ITQOL, Infant and Toddler Quality of Life Questionnaire; m, meter; MPQ, McGill Pain Questionnaire; PROMs, Patient‐reported outcome measures; PROs, Patient‐reported outcomes; SD, standard deviation; SF‐36, Short‐Form 36; UK, United Kingdom; USA, United States of America; VAS, Visual Analog Scale; VWD, Von Willebrand disease.
If provided in the article, the P‐values will be shown in the table.
3.4.1. Patient‐reported outcomes in von Willebrand disease
3.4.1.1. Health‐related quality of life
Three studies assessed HRQoL in adult patients with VWD 25 , 38 , 40 (Table 2). Compared to the general population, patients with VWD reported lower HRQoL, with the largest measured effects in the domains vitality, social functioning and pain. 38 , 40 Barr et al. found that female patients scored lower compared to men in the domains emotion, cognition and pain. 38 Two studies found a difference between the type of VWD and HRQoL measured. 25 , 40 De Wee et al. found that patients with VWD type 3 scored significantly lower on the physical part of HRQoL and higher on pain levels compared to patients with VWD type 1 and 2. 25 The study by Xu et al. supports this finding and also found that patients with VWD type 3 scored significantly lower on the physical domain of HRQoL compared to patients with VWD type 2. 40 In contrast, two other studies found no difference in HRQoL between patients with VWD type 1, 2 and 3. 38 , 39
One study 24 assessed HRQoL in children with VWD. They found that both preschool and school children had lower HRQoL compared to the general population in the domain general health perception. No difference was observed in HRQoL reported by parents of preschool children across the three types of VWD. In school children, parents of patients with VWD type 3 reported significantly lower HRQoL compared to the general population and patients with VWD type 1 and 2. No significant difference was found in HRQoL between boys and girls in both preschool and school children. 24
3.4.1.2. Self‐perceived physical functioning
Four studies 26 , 27 , 28 , 37 measured the influence of arthropathy and joint bleeds on self‐perceived physical functioning in adult patients with VWD. Patients with a history of joint bleeds scored lower on functional abilities. 26 , 28 , 37 The Von Willebrand in the Netherlands (WiN)‐study found that patients with joint bleeds reported a lower overall HRQoL, especially with regard to social participation and physical limitation. In addition, they experienced more pain compared to patients without joint bleeds. 26 , 27
The WiN study also assessed sports participation and physical activity in adult patients with VWD. 22 Almost 70% of patients with VWD participated in various types of sports. No difference in sports participation among the three types of VWD was reported. Lack of time (47.4%), lack of motivation (27.4%), physical limitations (26.4%) and fear of bleeding (6.9%) and other reasons (8.6%) were reported as reasons why patients did not participate in sports. Factors that were independently associated with physical limitations included age, BMI and VWD type 3. 22 Patients with VWD type 3 participated significantly less in sports due to fear of bleeding.
3.4.2. Patient‐reported outcomes in platelet function disorders
Two studies 23 , 33 assessed PROs in inherited platelet function disorders. Patients with inherited platelet function disorders show decreased HRQoL compared to the general population. Significant differences were found in the following domains: physical functioning, limitations in daily activities, limitations in social activities, energy levels and fatigue, pain and general health status. 23
3.4.3. Patient‐reported outcomes in coagulation factor deficiencies
Two studies 32 , 42 assessed PROs in coagulation factor deficiencies. Children with factor VII, X, XI, XIII and fibrinogen deficiencies reported an impaired HRQoL in the domains family and friends, indicating that the bleeding disorder had a negative impact on the relationships with their family and friends. Lower health status and being affected by the disease were significantly associated with HRQoL. No differences in HRQoL scores were reported between men and women, the different factor deficiencies and disease severity levels. 42
3.4.4. Patient‐reported outcomes specifically in women with autosomal inherited bleeding disorders
Ten articles specifically focused on HRQoL in women because they face reproduction related haemostatic challenges including the menstrual cycle and child delivery. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 39 , 41
In women with inherited bleeding disorders, patients with heavy menstrual bleeding reported significantly lower HRQoL compared to those without heavy menstrual bleeding. 30 , 34 , 39 Only Govorov et al. found no significant difference in HRQoL score between women with and without heavy menstrual bleeding. 41 Menstruation and especially heavy menstrual bleeding were reported as debilitating with regard to daily activities, 34 , 35 , 36 , 39 , 41 social relations, 31 , 41 sport activities 22 , 33 and the ability to work or go to school. 29 , 32 , 35 , 36 , 41
Four studies reported a high prevalence of dysmenorrhea in women with heavy menstrual bleeding, 29 , 30 , 32 , 39 and two studies evaluated the influence of treatment for heavy menstrual bleeding and dysmenorrhea on HRQoL. 29 , 30 The study by Chi et al. used a combination of therapies including tranexamic acid, oral contraceptive pill, desmopressin nasal spray and factor concentrates, 29 whereas Hug et al. used an ablative procedure as treatment for heavy menstrual bleeding. 30 Both studies found that treatment improved general health, daily activity, dysmenorrhea, interference of dysmenorrhea with daily work and overall HRQoL 29 , 30 (Table 3).
TABLE 3.
Influence of treatment for heavy menstrual bleeding on health‐related quality of life
| Chi et al. (2010)[29] | Huq et al. (2012)[30] | |||
|---|---|---|---|---|
| Before treatment a (n = 24) | After treatmenta (n = 24) | Before treatmenta (n = 12) | After treatment a (n = 12) | |
| General health | ||||
| Poor | 16 (67%) | 1 (4%) | 8 (67%) | 0 (0%) |
| Fair | 5 (21%) | 4 (17%) | 1 (8%) | 1 (8%) |
| Good | 2 (8%) | 7 (29%) | 2 (17%) | 3 (25%) |
| Very good | 1 (4%) | 10 (42%) | 1 (8%) | 0 (0%) |
| Excellent | 0 (0%) | 2 (8%) | 0 (0%) | 8 (67%) |
| Health and daily activity score [median (range)] | ||||
| A b | 10 (3–17) | 2 (0–15) | 15 (10–20) | 0 (0–0) |
| B c | 4 (2–4) | 2 (0–4) | 3.5 (1–4) | 0 (0–0) |
| Dysmenorrhoea | ||||
| None | 0 (0%) | 3 (13%) | 2 (17%) | 10 (83%) |
| Very mild | 0 (0%) | 12 (50%) | 1 (8%) | 1 (8%) |
| Mild | 3 (13%) | 5 (21%) | 0 (0%) | 0 (0%) |
| Moderate | 5 (21%) | 3 (13%) | 2 (17%) | 0 (0%) |
| Severe | 11 (46%) | 1 (4%) | 3 (25%) | 1 (8%) |
| Very severe | 5 (21%) | 0 (0%) | 4 (33%) | 0 (0%) |
| Interference of dysmenorrhoea with daily work | ||||
| Not at all | 0 (0%) | 7 (29%) | 2 (17%) | 11 (92%) |
| A little bit | 0 (0%) | 10 (42%) | 3 (25%) | 0 (0%) |
| Moderately | 3 (13%) | 5 (21%) | 1 (8%) | 1 (8%) |
| Quite a bit | 13 (54%) | 2 (8%) | 2 (17%) | 0 (0%) |
| Extremely | 8 (33%) | 0 (0%) | 4 (33%) | 0 (0%) |
| QoL score | ||||
| Median (range) | 26 (15–43) | 44 (24–54) | 17 (10–27) | 54 (52–56) |
Abbreviation: QoL; Quality of Life.
Comparison in all categories between pre‐ and post‐treatment were statistically significant (P < .01).
Health and daily activity score A includes a list of 10 activities varying in intensities, for example, running, climbing flights of stairs, lifting or carrying groceries and walking, each scored 2 (menstruation limited the activity a lot), 1 (a little), 0 (not at all).
Health and daily activity score B includes four questions on whether they had to cut down on the amount of activity, accomplished less, felt limited or had difficulties in performing work during menstruation. A score of 0–4 was assigned depending on the number of positive responses.
3.4.5. Association of bleeding scores and health‐related quality of life
Five publications assessed the correlation between bleeding assessment tools scores and HRQoL. 23 , 24 , 25 , 40 , 42 Bleeding scores were determined using validated tools including the Tosetto Bleeding Score, 24 , 25 , 42 the International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH‐BAT) 23 , 40 and the self‐administered Bleeding Assessment Tool (Self‐BAT). 40 Three studies found that a more severe bleeding phenotype (i.e., higher bleeding scores) was associated with lower HRQoL, 24 , 25 , 42 two studies did not find such an association. 23 , 40 Higher bleeding scores were associated with lower scores on physical and social functioning, bodily pain and general health in both children and adult patients with VWD. 24 , 25 One study adjusted for age, gender, comorbidity, employment and educational status and still found significantly lower HRQoL scores in patients with higher bleeding scores. 25
4. DISCUSSION
The aim of this systematic review was to summarize the available literature assessing PROMs and PROs in patients with autosomal inherited bleeding disorders. Integrating the patients’ perspective has increasingly gained attention in both clinical research as well as in clinical practice, especially since objective measures such as laboratory values or bleeding assessment tools do not always reflect the impact of an inherited bleeding disorder and its treatment on patient's daily life. To the best of our knowledge, this is the first systematic review assessing PROs in autosomal inherited bleeding disorders.
This systematic review shows that overall, patients with autosomal inherited bleeding disorders have lower HRQoL compared to the general population. This trend is especially visible in the following domains: vitality, physical and social functioning, pain and health in general. The relationship between bleeding scores and HRQoL was unclear, with some studies finding positive and others negative associations. 23 , 24 , 25 , 40 , 42 This may be related to the use of different assessment tools. However, it should be noted that bleeding scores reflect bleeding symptoms that have occurred earlier during a patient's lifetime. Therefore, bleeding assessment tools are less able to convey changes in bleeding phenotype over time, for example after a specific intervention or treatment. 23 , 43 Most included studies focused on patients with VWD, the most common inherited bleeding disorder, and on women with bleeding disorders. Women generally scored lower on several HRQoL domains compared to men, due to their specific haemostatic challenges.
Heavy menstrual bleeding is the most common symptom that women with bleeding disorders experience. 44 Prevalence rates range from 32% to 100% in a large review on women with VWD. 44 Heavy menstrual bleeding impacts daily activities, 34 , 35 , 36 , 39 , 41 social relations, 31 , 41 sport activities 22 , 33 and the ability to work or go to school. 29 , 32 , 35 , 36 , 41 Women with inherited bleeding disorders without heavy menstrual bleeding report better HRQoL. 30 , 34 , 39 Three studies suggested that lower HRQoL in women may be specifically associated with iron deficiency anaemia secondary to heavy menstrual bleeding. 39 , 40 , 45 Treatment for heavy menstrual bleeding such as oral contraceptive therapy or endometrial ablation was found to significantly improve quality of life in girls and women with autosomal inherited bleeding disorders. 29 , 30
The increased level of pain in patients with autosomal inherited bleeding disorders is likely to be related to dysmenorrhea and joint bleeds. The latter is, however, rare in autosomal bleeding disorders and only occurs in patients with a severe bleeding phenotype, such as in patients with VWD type 3. 46 , 47 , 48 Joint bleeds often lead to joint impairment and can hamper daily activities. Dysmenorrhea seems to be more frequent in women with heavy menstrual bleeding. 29 , 30 , 32 , 39 Although some studies in the general population also describe an association between heavy menstrual bleeding and dysmenorrhea, no clear correlation has yet been identified. 49 , 50 , 51 , 52 It has been hypothesized that heavy menstrual bleeding leads to retrograde bleeding, which is the reflux of menstrual blood out of the uterine cavity and may result in endometriosis with pelvic pain. 53 , 54 Another explanation is that heavy menstrual bleeding leads to increased uterus contractions accompanied with more pain.
Four studies focused specifically on other autosomal inherited bleeding disorders than VWD. 23 , 32 , 33 , 42 It is difficult to draw clear conclusions with regard to the impact of these disorders on PROs, as the disorders evaluated were heterogeneous and patient numbers were small. Generally, similar HRQoL domains were affected compared to patients with VWD.
In contrast to research in patients with autosomal inherited bleeding disorders, quality of life research in patients with haemophilia has been conducted for several decades. Generally, patients with haemophilia have a higher burden of disease compared to most other inherited bleeding disorders. Patients need regular prophylactic treatment with coagulation factor concentrates and may suffer from hemarthrosis and synovitis with joint destruction. The HRQoL of patients with haemophilia is especially affected in the domains of general health, physical functioning and pain. 55 , 56 , 57 This review found that in patients with VWD similar domains were affected, however this was most likely influenced by different disease symptoms. Heavy menstrual bleeding was reported as a main influence on HRQoL, which is in contrast to patients with haemophilia as this predominantly affects males. In both diseases; however, the severity of disease is strongly related to the HRQoL of patients. In patients with VWD type 3, the most severe type of VWD, HRQoL is comparable to that observed in severe haemophilia, specifically in the domains physical functioning, general health and physical component, although patients with haemophilia scored lower. 25
Our study has several limitations. Firstly, a variety of PROMs are used in autosomal inherited bleeding disorders. This heterogeneity in combination with the use of non‐standardized PROMs makes it difficult to assess and quantify PROs. Therefore, reported results and conclusions about the quality of life of patients with autosomal inherited bleeding disorders should be interpret with this limitation in mind. Secondly, from all the used PROMs, only one PROM (i.e., the Haemophilia Activity List) has undergone any degree of development and validation in patients with inherited bleeding disorders. 26 , 28 The use of non‐validated or inadequately targeted PROMs in a study that has not considered their psychometric properties may have adverse consequences. This includes ethical concerns surrounding patients having to complete measures that are incapable of capturing the patient's perspective and can therefore have unreliable or biased results. Thirdly, the various autosomal inherited bleedings disorders were not equally represented in this systematic review and most studies focused on patients with VWD. The latter is also partly due to the substantial amount of studies originating from the WiN study group, which might have led to bias as a result of partially overlapping sets of participants. Fourthly, the NIH risk of bias assessment raised some key issues including lack of effect sizes that allow for comparison between study populations and controls. This means, that even if a study finds significant differences in PROs, the relevance of this result might be questionable. Therefore, caution should be observed in the generalization of findings. Additionally, only a minority of studies adjusted for key confounding variables such as demographic data including age and socio‐economic status, while these factors have been previously described as predictors of HRQoL. 58 , 59 , 60 Fifthly, only few studies compared the study population with the general population or patients with and without a certain symptom. This limits the capacity to attribute causality. Lastly, additional unpublished work or studies published in languages other than English were not included in this review, which may have biased the results.
Our systematic review highlights the need for future studies using established standards to analyse PROs in patients with autosomal inherited bleeding disorders. The use of a validated method to evaluate and compare PROs across populations, conditions, research studies and clinical practices is important to improve quality of care for the individual patient. An example of such a method is the Patient‐Reported Outcomes Measurement Information System (PROMIS). However, one should be careful that in diseases that only have a small effect on HRQoL such a generic instrument might not be sensitive enough to measure a clinically meaningful effect. In that case, a disease specific measurement may still be necessary which need to be developed in patients with autosomal inherited bleeding disorders. Furthermore, it is important that future studies focus specifically on woman due to their haemostatic challenges and the association between bleeding scores and PROs. Finally, with the right PROMs, measuring PROs in patients with autosomal inherited bleeding disorders could help with the –sometimes difficult‐ decision to start treatment for specific subgroups of patients, for example, patients with a more severe bleeding phenotype, like VWD type 3, who may benefit from prophylactic or other supportive therapy.
AUTHORS CONTRIBUTIONS
E.S.H and M.E.H. designed the study and conducted the search strategy. E.S.H. and M.E.H. screened studies for eligibility and completed the data extraction. E.S.H., M.E.H. and W.A. assessed the risk of bias. E.S.H, M.E.H. and W.A. interpreted and analysed data. E.S.H., M.E.H. and W.A. wrote the manuscript. F.W.G.L., J.A.H., S.C.G., R.E.G.S, S.E.M.S, H.F.L. and M.H.C. critically revised the manuscript. H.F.L and M.H.C supervised the study. All authors read and approved the final manuscript.
FINANCIAL DISCLOSURES AND FUNDING
F.W.G. Leebeek has received unrestricted research grants from CSL Behring, Shire/Takeda, Sobi and uniQure. He is a consultant for CSL Behring, Shire/Takeda, Biomarin and uniQure, of which the fees go to the University. He received travel support from Sobi. He is DSMB member of a study sponsored by Roche. S.C. Gouw received unrestricted research grant from Sobi. R.E.G. Schutgens has received unrestricted research and speaker fees from Bayer, Shire, NovoNordisk, Sobi and Octapharma. S.E.M. Schols has received travel grants from Takeda and Bayer, an unrestricted educational grant from Takeda and has served as a steering board member for Roche and Novo Nordisk. M.H. Cnossen has received investigator‐initiated research‐ and travel grants over the years from the Netherlands Organization for Scientific Research (NWO), the Netherlands Organization for Health Research and Development (ZonMw), the Dutch ‘Innovatiefonds Zorgverzekeraars’, Pfizer, Baxter/Baxalta/Shire, Bayer Schering Pharma, CSL Behring, Sobi, Novo Nordisk, Novartis and Nordic Pharma and has served as a steering board member for Roche and Bayer. All grants, awards and fees go to the institution. The other authors declare no conflicts of interest relevant to the contents of this manuscript.
Supporting information
Supporting information
ACKNOWLEDGEMENTS
The authors wish to thank Dr M.F.M. Engel from the Erasmus MC Medical Library for developing and updating the search strategies. The SYMPHONY consortium, which aims to orchestrate personalized treatment in patients with bleeding disorders, is a unique collaboration between patients, health care professionals and translational and fundamental researchers specialized in inherited bleeding disorders, as well as experts from multiple disciplines. SYMPHONY aims to identify best treatment choice for each individual based on bleeding phenotype. In order to achieve this goal, workpackages have been organized according to three themes, for example, Diagnostics (workpackage 3&4), Treatment (workpackages 5–9) and Fundamental Research (workpackages 10–12). This research received funding from the Netherlands Organization for Scientific Research (NWO) in the framework of the NWA‐ORC Call grant agreement NWA.1160.18.038. Principal investigator: Dr M.H. Cnossen. Beneficiaries of the SYMPHONY consortium: Erasmus MC – Sophia Children's Hospital, University Medical Centre Rotterdam, project leadership and coordination; Sanquin Diagnostics; Sanquin Research; Amsterdam University Medical Centres; University Medical Center Groningen; University Medical Centre Utrecht; Leiden University Medical Centre; Radboud University Medical Centre; Netherlands Society of Haemophilia Patients (NVHP); Netherlands Society for Thrombosis and Haemostasis (NVTH); Bayer B.V., CSL Behring B.V., Swedish Orphan Biovitrum (Belgium) BVBA/SPRL.
van Hoorn ES, Houwing ME, Al Arashi W, et al., Patient‐reported outcomes in autosomal inherited bleeding disorders: a systematic literature review. Haemophilia. 2022;28:197–214. 10.1111/hae.14492
Evelien S. van Hoorn, Maite E. Houwing, Wala Al Arashi contributed equally to the manuscript.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed in this study.
REFERENCES
- 1. Porter ME, Larsson S, Lee TH. Standardizing patient outcomes measurement. N Engl J Med. 2016;374:504‐506. [DOI] [PubMed] [Google Scholar]
- 2. Elf M, Flink M, Nilsson M, Tistad M, von Koch L, Ytterberg C. The case of value‐based healthcare for people living with complex long‐term conditions. BMC Health Services Res. 2017;17:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porter ME, Lee TH. The strategy that will fix health care. Harv Bus Rev. 2013;91:1‐19. [Google Scholar]
- 4. Administration USFaD . Guidance for industry, patient‐reported outcome measures: use in medical product development to support labeling claim. Fed Regist. 2009;74(35):65132‐65133. [Google Scholar]
- 5. Wilson IB, Cleary PD. Linking clinical variables with health‐related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273:59‐65. [PubMed] [Google Scholar]
- 6. Andresen EM, Meyers AR. Health‐related quality of life outcomes measures. Arch Phys Med Rehabil. 2000;81:S30‐S45. [DOI] [PubMed] [Google Scholar]
- 7. Weldring T, Smith SMS. Article commentary: Patient‐Reported Outcomes (PROs) and Patient‐Reported Outcome Measures (PROMs). Health Services Insights. 2013;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marshall S, Haywood K, Fitzpatrick R. Impact of patient‐reported outcome measures on routine practice: a structured review. J Eval Clin Pract. 2006;12:559‐568. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan RM, Bush JW. Health‐related quality of life measurement for evaluation research and policy analysis. Health Psychol. 1982;1:61‐80. [Google Scholar]
- 10. Lavallee DC, Chenok KE, Love RM, et al. Incorporating patient‐reported outcomes into health care to engage patients and enhance care. Health Aff (Millwood). 2016;35:575‐582. [DOI] [PubMed] [Google Scholar]
- 11. Greenhalgh J, Gooding K, Gibbons E, et al. How do patient reported outcome measures (PROMs) support clinician‐patient communication and patient care? A realist synthesis. J Patient Rep Outcomes. 2018;2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bouazza YB, Chiairi I, El Kharbouchi O, et al. Patient‐reported outcome measures (PROMs) in the management of lung cancer: a systematic review. Lung Cancer. 2017;113:140‐151. [DOI] [PubMed] [Google Scholar]
- 13. Boender J, Kruip MJHA, Leebeek FWG. A diagnostic approach to mild bleeding disorders. J Thromb Haemost. 2016;14:1507‐1516. [DOI] [PubMed] [Google Scholar]
- 14. Sharathkumar AA, Pipe SW. Bleeding disorders. Pediatr Rev. 2008;29:121. [DOI] [PubMed] [Google Scholar]
- 15. Acharya SS. Rare bleeding disorders in children: identification and primary care management. Pediatrics. 2013;132:882‐892. [DOI] [PubMed] [Google Scholar]
- 16. Palla R, Peyvandi F, Shapiro AD. Rare bleeding disorders: diagnosis and treatment. Blood. 2015;125:2052‐2061. [DOI] [PubMed] [Google Scholar]
- 17. Leebeek FW, Eikenboom JC, Von Willebrand's disease. N Engl J Med. 2016;375:2067‐2080. [DOI] [PubMed] [Google Scholar]
- 18. Dover S, Blanchette VS, Srivastava A, Fischer K, Abad A, Feldman BM. Clinical outcomes in hemophilia: towards development of a core set of standardized outcome measures for research. Res Pract Thromb Haemost. 2020;00:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Limperg PF, Terwee CB, Young NL, et al. Health‐related quality of life questionnaires in individuals with haemophilia: a systematic review of their measurement properties. Haemophilia. 2017;23:497‐510. [DOI] [PubMed] [Google Scholar]
- 20. Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient‐reported outcome measures. Qual Life Res. 2018;27:1147‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institutes of Health . Quality assessment tool for observational cohort and cross‐sectional studies. National Heart, Lung, and Blood Institute, 2021. [Google Scholar]
- 22. Atiq F, Mauser‐Bunschoten EP, Eikenboom J, et al. Sports participation and physical activity in patients with von Willebrand disease. Haemophilia. 2019;25:101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blaauwgeers MW, Kruip MJHA, Beckers EAM, et al. Congenital platelet disorders and health status–related quality of life. Res Pract Thromb Haemost. 2020;4:100‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Wee EM, Fijnvandraat K, De Goede‐Bolder A, et al. Impact of von Willebrand disease on health‐related quality of life in a pediatric population. J Thromb Haemost. 2011;9:502‐509. [DOI] [PubMed] [Google Scholar]
- 25. De Wee EM, Mauser‐Bunschoten EP, Van Der Bom JG, et al. Health‐related quality of life among adult patients with moderate and severe von Willebrand disease. J Thromb Haemost. 2010;8:1492‐1499. [DOI] [PubMed] [Google Scholar]
- 26. Van Galen KPM, De Kleijn P, Foppen W, et al. Long‐term impact of joint bleeds in von Willebrand disease: a nested case‐control study. Haematologica. 2017;102:1486‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Galen KPM, Sanders YV, Vojinovic U, et al. Joint bleeds in von Willebrand disease patients have significant impact on quality of life and joint integrity: a cross‐sectional study. Haemophilia. 2015;21:e185‐e92. [DOI] [PubMed] [Google Scholar]
- 28. Van Galen KPM, Timmer MA, De Kleijn P, et al. Joint assessment in von willebrand disease: validation of the haemophilia joint health score and haemophilia activities list. Thromb Haemost. 2017;117:1464‐1470. [DOI] [PubMed] [Google Scholar]
- 29. Chi C, Pollard D, Tuddenham EGD, Kadir RA. Menorrhagia in adolescents with inherited bleeding disorders. J Pediatr Adolesc Gynecol. 2010;23:215‐222. [DOI] [PubMed] [Google Scholar]
- 30. Huq FY, Al‐Haderi M, Kadir RA. The outcome of endometrial ablation in women with inherited bleeding disorders. Haemophilia. 2012;18:413‐420. [DOI] [PubMed] [Google Scholar]
- 31. Khair K, Holland M, Pollard D. The experience of girls and young Women with inherited bleeding disorders. Haemophilia. 2013;19:e276‐e281. [DOI] [PubMed] [Google Scholar]
- 32. Kulkarni A, Lee CA, Griffeon A, Kadir RA. Disorders of menstruation and their effect on the quality of life in women with congenital factor VII deficiency. Haemophilia. 2006;12:248‐252. [DOI] [PubMed] [Google Scholar]
- 33. Khair K, Holland M. The Kids' immune thrombocytopenia Tool is not suitable for assessing quality of life in children with platelet function disorders. Haemophilia. 2018;24:e259‐e261. [DOI] [PubMed] [Google Scholar]
- 34. Kirtava A, Drews C, Lally C, Dilley A, Evatt B. Medical, reproductive and psychosocial experiences of women diagnosed with von Willebrand's disease receiving care in haemophilia treatment centres: a case‐control study. Haemophilia. 2003;9:292‐297. [DOI] [PubMed] [Google Scholar]
- 35. Kouides PA, Phatak PD, Burkart P, et al. Gynaecological and obstetrical morbidity in women with type I von Willebrand disease: results of a patient survey. Haemophilia. 2000;6:643‐648. [DOI] [PubMed] [Google Scholar]
- 36. Marshall AL, Dasari H, Warner ND, Grill DE, Nichols WL, Pruthi RK. Self‐reported reproductive health experiences in women with von Willebrand disease: a qualitative interview‐based study. J Obstet Gynaecol. 2019;39:288‐290. [DOI] [PubMed] [Google Scholar]
- 37. Sumner M, Williams J. Type 3 von Willebrand disease: assessment of complications and approaches to treatment – results of a patient and hemophilia treatment center survey in the United States. Haemophilia. 2004;10:360‐366. [DOI] [PubMed] [Google Scholar]
- 38. Barr RD, Sek J, Horsman J, et al. Health status and health‐related quality of life associated with von Willebrand disease. Am J Hematol. 2003;73:108‐114. [DOI] [PubMed] [Google Scholar]
- 39. Rae C, Furlong W, Horsman J, et al. Bleeding disorders, menorrhagia and iron deficiency: impacts on health‐related quality of life. Haemophilia. 2013;19:385‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu Y, Deforest M, Grabell J, Hopman W, James P. Relative contributions of bleeding scores and iron status on health‐related quality of life in von Willebrand disease: a cross‐sectional study. Haemophilia. 2017;23:115‐121. [DOI] [PubMed] [Google Scholar]
- 41. Govorov I, Ekelund L, Chaireti R, et al. Heavy menstrual bleeding and health‐associated quality of life in women with von willebrand's disease. Exp Ther Med. 2016;11:1923‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haghpanah S, Mohtadi H, Akbari M, Karimi M. Quality of life in children and adolescents with rare bleeding disorders in Southern Iran. Clin Appl Thromb Hemost. 2017;23:652‐656. [DOI] [PubMed] [Google Scholar]
- 43. Rydz N, James PD. The evolution and value of bleeding assessment tools. J Thromb Haemost. 2012;10:2223‐2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. James AH. More than menorrhagia: a review of the obstetric and gynaecological manifestations of bleeding disorders. Haemophilia. 2005;11:295‐307. [DOI] [PubMed] [Google Scholar]
- 45. Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. Randomised study of cognitive effects of iron supplementation in non‐anaemic iron‐deficient adolescent girls. Lancet. 1996;348:992‐996. [DOI] [PubMed] [Google Scholar]
- 46. de Wee EM, Sanders YV, Mauser‐Bunschoten EP, et al. Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108:683‐692. [DOI] [PubMed] [Google Scholar]
- 47. Federici AB. Clinical diagnosis of von Willebrand disease. Haemophilia. 2004;10(Suppl 4):169‐176. [DOI] [PubMed] [Google Scholar]
- 48. van Galen KP, Mauser‐Bunschoten EP, Leebeek FW. Hemophilic arthropathy in patients with von Willebrand disease. Blood Rev. 2012;26:261‐266. [DOI] [PubMed] [Google Scholar]
- 49. Harlow SD, Park M. A longitudinal study of risk factors for the occurrence, duration and severity of menstrual cramps in a cohort of college women. Br J Obstet Gynaecol. 1996;103:1134‐1142. [DOI] [PubMed] [Google Scholar]
- 50. Sundell G, Milsom I, Andersch B. Factors influencing the prevalence and severity of dysmenorrhoea in young women. Br J Obstet Gynaecol. 1990;97:588‐594. [DOI] [PubMed] [Google Scholar]
- 51. Patel V, Tanksale V, Sahasrabhojanee M, Gupte S, Nevrekar P. The burden and determinants of dysmenorrhoea: a population‐based survey of 2262 women in Goa, India. BJOG. 2006;113:453‐463. [DOI] [PubMed] [Google Scholar]
- 52. Hoppenbrouwers K, Roelants M, Meuleman C, et al. Characteristics of the menstrual cycle in 13‐year‐old Flemish girls and the impact of menstrual symptoms on social life. Eur J Pediatr. 2016;175:623‐630. [DOI] [PubMed] [Google Scholar]
- 53. James AH. More than menorrhagia: a review of the obstetric and gynaecological manifestations of von Willebrand disease. Thromb Res. 2007;120:S17‐S20. [DOI] [PubMed] [Google Scholar]
- 54. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235‐258. [DOI] [PubMed] [Google Scholar]
- 55. Barr RD, Saleh M, Furlong W, et al. Health status and health‐related quality of life associated with hemophilia. Am J Hematol. 2002;71:152‐160. [DOI] [PubMed] [Google Scholar]
- 56. Molho P, Rolland N, Lebrun T, et al. Epidemiological survey of the orthopaedic status of severe haemophilia A and B patients in France. The French Study Group. secretariat.haemophiles@cch.ap‐hop‐paris.fr. Haemophilia. 2000;6:23‐32. [DOI] [PubMed] [Google Scholar]
- 57. Trippoli S, Vaiani M, Linari S, Longo G, Morfini M, Messori A. Multivariate analysis of factors influencing quality of life and utility in patients with haemophilia. Haematologica. 2001;86:722‐728. [PubMed] [Google Scholar]
- 58. Hemingway H, Nicholson A, Stafford M, Roberts R, Marmot M. The impact of socioeconomic status on health functioning as assessed by the SF‐36 questionnaire: the Whitehall II Study. Am J Public Health. 1997;87:1484‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pappa E, Kontodimopoulos N, Papadopoulos AA, Niakas D. Assessing the socio‐economic and demographic impact on health‐related quality of life: evidence from Greece. Int J Public Health. 2009;54:241‐249. [DOI] [PubMed] [Google Scholar]
- 60. Ross NA, Garner R, Bernier J, et al. Trajectories of health‐related quality of life by socio‐economic status in a nationally representative Canadian cohort. J Epidemiol Community Health. 2012;66:593‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed in this study.


