Abstract
Background
Given differences in buttock versus thigh cellulite, collagenase clostridium histolyticum‐aaes (CCH‐aaes) injection technique may impact treatment effects at these sites.
Aim
To evaluate efficacy and safety of 5 CCH‐aaes injection techniques.
Methods
A phase 2A, open‐label trial enrolled women with mild‐to‐severe cellulite (Clinician Reported Photonumeric Cellulite Severity Scale) on both buttocks or thighs. CCH‐aaes 0.84 mg was administered as 12 injections in each of two buttock or two thigh treatment areas (total dose, 1.68 mg) during three treatment sessions (Days 1, 22, 43). On Day 1, women were sequentially assigned to: Technique A = shallow injection/3 aliquots; Technique B = shallow injection/1 aliquot; Technique C = deep injection/1 aliquot; Technique D = deep and shallow injections/5 aliquots; or Technique E = shallow injection/4 aliquots. Change from baseline in Hexsel Cellulite Severity Scale (CSS) depression depth (range, 0 [no depressions] to 3 [deep depressions]) was assessed at Day 71. Safety was evaluated via adverse events.
Results
Sixty‐three women with buttock (n = 31) or thigh (n = 32) cellulite received ≥1 CCH‐aaes dose. For buttock cellulite, CCH‐aaes injection Technique A resulted in the greatest baseline‐adjusted improvement in CSS score on Day 71 (least‐squares mean, 1.17‐point improvement). For thigh cellulite, CSS score improvement was greatest with Technique D (least‐squares mean, 1.40‐point improvement). CCH injection Techniques A, D, and E were associated with more favorable safety profiles than Techniques B and C.
Conclusion
Different CCH‐aaes injection techniques are required with buttock (Technique A) versus thigh (Technique D) cellulite to optimize treatment outcomes.
Keywords: cellulite, injections, microbial collagenase, Qwo, thigh
1. INTRODUCTION
Collagenase clostridium histolyticum‐aaes (CCH‐aaes; Qwo®) is indicated in the United States for the treatment of moderate‐to‐severe cellulite in the buttocks of adult women, 1 with demonstrated efficacy and safety in phase 2 and phase 3 trials. 2 , 3 , 4 CCH‐aaes treatment for thigh cellulite is currently investigational. The mechanism of action of CCH‐aaes is attributed to Enzymatic Subcision and Remodeling™ (ESR™). In areas with cellulite‐related contour alterations, injection of CCH‐aaes promotes the release of pathogenic collagen‐rich septae, which resolves cellulite‐associated depressions and smooths the skin surface. 2 Histology data support the mechanism of ESR with CCH‐aaes; studies of human abdominal and porcine tissue have shown that CCH‐aaes both lyses mature collagen‐rich septae and stimulates neocollagenesis (i.e., remodeling) of subcutaneous adipose tissue and reorganization into smaller and more homogenous fat lobules. 5
Given the anatomical and histopathological differences in buttock versus thigh cellulite, 6 , 7 injection technique may impact CCH‐aaes treatment effects. The aim of this trial was to evaluate 5 CCH‐aaes injection techniques for the treatment of cellulite of the buttocks and thighs.
2. METHODS
This phase 2a, open‐label trial (ClinicalTrials.gov identifier: NCT03632993) enrolled women aged ≥18 years with mild‐to‐severe cellulite, defined as a rating of 2, 3, or 4 on the Clinician Reported Photonumeric Cellulite Severity Scale. 8 Women were required to have cellulite on bilateral treatment areas (i.e., both buttocks or posterolateral thighs). The protocol was approved by an institutional review board (Advarra Institutional Review Board, Columbia, MD) and the trial was conducted in accordance with the guidelines for Good Clinical Practice of the International Conference on Harmonisation and the ethical principles of the Declaration of Helsinki. All patients provided written informed consent.
For each individual, a CCH‐aaes dose of 0.84 mg was administered as 12 subcutaneous injections in each of the two buttock or two thigh treatment areas for a total dose of CCH‐aaes of 1.68 mg and 24 injections. CCH‐aaes was administered at each of three treatment visits (Days 1, 22, and 43). On Day 1, prior to the first treatment administration, women were sequentially assigned to receive CCH‐aaes via 1 of 5 injection techniques: Technique A = shallow injection/3 aliquots; Technique B = shallow injection/1 aliquot; Technique C = deep injection/1 aliquot; Technique D = deep and shallow injections/5 aliquots; or Technique E = shallow injection/4 aliquots (Figure 1). Efficacy was assessed using the Hexsel Cellulite Severity Scale depression depth (domain B; 4‐point scale scored from 0 [“no depressions”] to 3 [“deep” depressions]) graded by the investigator. 9 In addition, photographs of each treatment area were rated on an exploratory 5‐point Likert scale score of esthetic appearance (−1 [“worse”]; 0 [“same”]; +1 [“improved”]; +2 [“much improved”]; and +3 [“very much improved”]). A central assessor was blinded to treatment and was provided baseline and Day 71 images simultaneously. A single posterior view image was taken for the buttock treatment area, whereas for the thighs, 3 view images (lateral, oblique, posterior) per treatment area were taken and a single score was recorded for the entire thigh. Treatment‐emergent adverse events (AEs) were monitored throughout the study.
FIGURE 1.
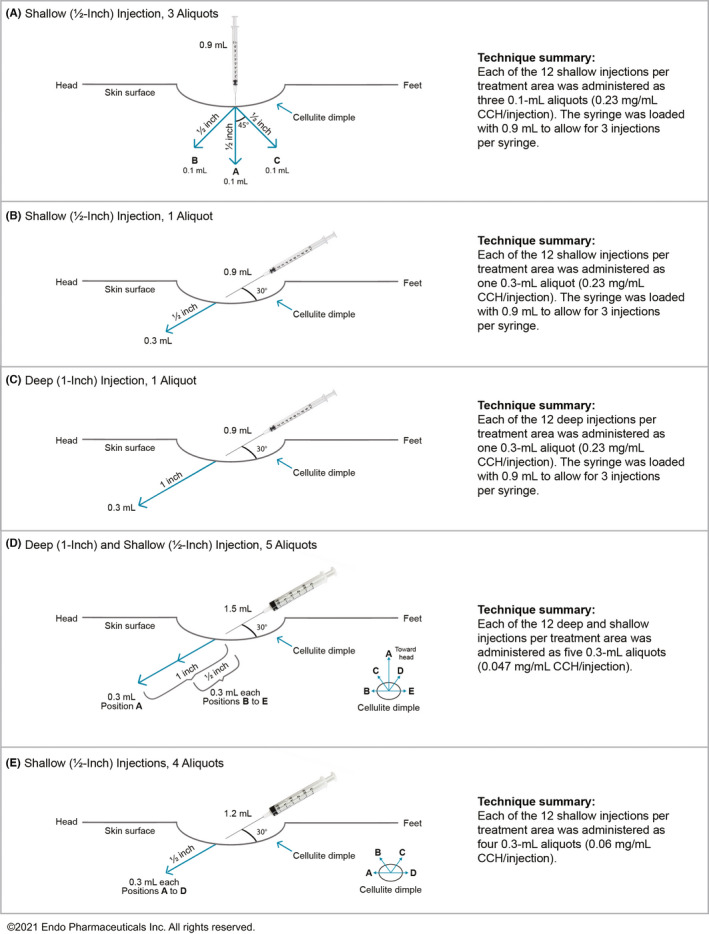
Five CCH‐aaes injection techniques evaluated. CCH‐aaes, collagenase clostridium histolyticum‐aaes
Changes from baseline in dimple depression depth (Hexsel Cellulite Severity Scale score) and esthetic appearance (5‐point Likert scale score) were analyzed using linear mixed models with treatment arm, study visit, and interaction of treatment arm and study visit as fixed effects. Least‐squares mean change from baseline at Day 71 ± 3 days was determined for each treatment area (buttocks or thighs).
3. RESULTS
Sixty‐three women with buttock (n = 31) or thigh (n = 32) cellulite received ≥1 CCH‐aaes injection (Table 1), of whom 60 were evaluable for efficacy (had ≥1 post‐baseline Likert scale assessment for esthetic appearance). At Day 71, mean dimple depression depth improved from baseline in the buttocks and thighs in all injection technique groups (average of left and right buttocks or thighs; Table 2). In the buttock, statistically significant improvement from baseline in dimple depression depth at Day 71 was observed for Techniques A, C, and D; Technique A resulted in the largest improvement, followed by Technique D. In the thigh, Technique D resulted in the largest improvement in dimple depression depth at Day 71; change from baseline was statistically significant for Techniques C, D, and E (Table 2). For esthetic appearance of cellulite, mean numeric improvements from baseline at Day 71 were observed in the buttocks and thighs for all injection technique groups (average of left and right buttocks or thighs; Table 2). At Day 71, statistically significant improvement from baseline in esthetic appearance was observed in the buttock with the use of Techniques A, B, C, and E, and in the thigh with the use of Technique A.
TABLE 1.
Demographic and baseline characteristics
| Parameter | CCH‐aaes injection technique | ||||
|---|---|---|---|---|---|
| A (n = 12) | B (n = 13) | C (n = 12) | D (n = 13) | E (n = 13) | |
| Age, years, mean (SD) | 47.4 (9.1) | 44.5 (9.8) | 50.4 (14.0) | 50.2 (11.9) | 44.5 (10.7) |
| Range, years | 32–60 | 30–60 | 20–66 | 36–73 | 30–59 |
| Race, n (%) | |||||
| Black | 3 (25.0) | 3 (23.1) | 1 (8.3) | 3 (23.1) | 4 (30.8) |
| White | 9 (75.0) | 10 (76.9) | 11 (91.7) | 9 (69.2) | 9 (69.2) |
| Other | 0 | 0 | 0 | 1 (7.7) | 0 |
| BMI, kg/m2, mean (SD) | 33.4 (10.2) | 32.3 (7.6) | 28.6 (5.3) | 25.6 (6.2) | 31.8 (5.0) |
| Range | 24.0–60.9 | 21.7–46.9 | 20.2–37.6 | 14.9–34.5 | 25.1–39.5 |
| Fitzpatrick scale category, n (%) | |||||
| I/II (pale white/fair) | 3 (25.0) | 1 (7.7) | 3 (25.0) | 4 (30.8) | 2 (15.4) |
| III (darker white) | 2 (16.7) | 0 | 5 (41.7) | 3 (23.1) | 1 (7.7) |
| IV (light brown) | 4 (33.3) | 9 (69.2) | 3 (25.0) | 1 (7.7) | 6 (46.2) |
| V (brown) | 0 | 2 (15.4) | 0 | 2 (15.4) | 3 (23.1) |
| VI (dark brown/black) | 3 (25.0) | 1 (7.7) | 1 (8.3) | 3 (23.1) | 1 (7.7) |
| Area treated, n | |||||
| Buttocks | 6 | 6 | 6 | 6 | 7 |
| Posterolateral thighs | 6 | 7 | 6 | 7 | 6 |
| Cellulite severity, mean (SD) a | |||||
| Left buttock | 3.0 (0.6) | 2.5 (0.6) | 3.2 (0.8) | 3.5 (0.8) | 3.0 (0.6) |
| Right buttock | 2.8 (0.8) | 2.3 (0.5) | 3.2 (0.8) | 3.5 (0.8) | 3.1 (0.7) |
| Left thigh | 2.5 (0.6) | 3.4 (0.5) | 3.5 (0.6) | 3.0 (0.6) | 3.3 (1.0) |
| Right thigh | 2.7 (0.5) | 3.4 (0.8) | 3.5 (0.6) | 3.0 (0.6) | 3.5 (0.8) |
Abbreviations: BMI, body mass index; CCH‐aaes, collagenase clostridium histolyticum‐aaes; SD, standard deviation.
Assessed using the Clinician Reported Photonumeric Cellulite Severity Scale. 2
TABLE 2.
Change from baseline to Day 71 in Hexsel Cellulite Severity Scale dimple depth depression domain and esthetic appearance
| Parameter a | CCH‐aaes injection technique | ||||
|---|---|---|---|---|---|
| A (n = 12) | B (n = 13) | C (n = 11) | D (n = 12) | E (n = 12) | |
| Dimple depth depression b | |||||
| Buttock, n | 6 | 6 | 6 | 6 | 7 |
| LSM (95% CI) | −1.17 (−1.61, −0.72) | −0.08 (−0.53, +0.36) | −0.67 (−1.11, −0.22) | −0.83 (−1.28, −0.39) | −0.36 (−0.77, +0.06) |
| Thigh, n | 6 | 7 | 4 c | 5 c | 5 c |
| LSM (95% CI) | −0.33 (−0.83, +0.16) | −0.29 (−0.74, +0.17) | −0.75 (−1.36, −0.14) | −1.40 (−1.94, −0.86) | −0.80 (−1.34, −0.26) |
| Esthetic appearance d | |||||
| Buttock, n | 6 | 6 | 6 | 6 | 7 |
| LSM (95% CI) | 1.08 (0.51, 1.65) | 1.08 (0.51, 1.65) | 1.00 (0.43, 1.57) | 0.50 (−0.07, +1.07) | 1.29 (0.76, 1.81) |
| Thigh, n | 6 | 7 | 4 c | 5 c | 5 c |
| LSM (95% CI) | 0.92 (0.34, 1.50) | 0.43 (−0.11,+ 0.97) | 0.12 (−0.59, +0.84) | 0.30 (−0.34, +0.94) | 0.60 (−0.04, +1.24) |
Abbreviation: CCH‐aaes, collagenase clostridium histolyticum‐aaes; CI, confidence interval; LSM, least‐squares mean.
Efficacy evaluable population (all women who completed screening procedures, received ≥1 CCH‐aaes injection, and had ≥1 post‐baseline assessment for esthetic appearance); data averaged for left and right anatomy for women with both baseline and Day 71 visits.
Hexsel Cellulite Severity Scale depression depth (domain B; 4‐point scale scored from 0 [“no depressions”] to 3 [“deep” depressions]).
Some patients did not have a depression depth or esthetic assessment at both baseline and Day 71 visit.
5‐point Likert scale score ranging from −1 (“worse”) to +3 (“very much improved”). Positive change indicated improvement.
Across treatment areas, Techniques A, D, and E were associated with more favorable overall safety profiles than Techniques B and C (Table 3). Injection‐site nodule was most frequently reported for Techniques B and C. Injection‐site bruising and injection‐site discoloration were most frequently reported for Techniques B and C, respectively.
TABLE 3.
Adverse events
| Women with an AE, n (%) a | CCH‐aaes injection technique | ||||
|---|---|---|---|---|---|
| A (n = 12) | B (n = 13) | C (n = 12) | D (n = 13) | E (n = 13) | |
| ≥1 AE | 12 (100) | 11 (84.6) | 12 (100) | 13 (100) | 13 (100) |
| ≥1 AE leading to discontinuation | 0 | 0 | 1 (8.3) | 1 (7.7) | 0 |
| Most common AEs (≥3 women in any group) | |||||
| Injection‐site pain | 12 (100) | 9 (69.2) | 12 (100) | 13 (100) | 9 (69.2) |
| Injection‐site bruising | 9 (75.0) | 11 (84.6) | 12 (100) | 6 (46.2) | 11 (84.6) |
| Injection‐site nodule | 5 (41.7) | 10 (76.9) | 10 (83.3) | 3 (23.1) | 8 (61.5) |
| Injection‐site warmth | 6 (50.0) | 5 (38.5) | 2 (16.7) | 2 (15.4) | 4 (30.8) |
| Injection‐site pruritus | 6 (50.0) | 4 (30.8) | 2 (16.7) | 3 (23.1) | 2 (15.4) |
| Injection‐site hemorrhage b | 3 (25.0) | 0 | 1 (8.3) | 7 (53.8) | 2 (15.4) |
| Injection‐site discoloration | 2 (16.7) | 6 (46.2) | 1 (8.3) | 0 | 1 (7.7) |
| Injection‐site swelling | 1 (8.3) | 1 (7.7) | 1 (8.3) | 3 (23.1) | 2 (15.4) |
| Injection‐site induration | 2 (16.7) | 1 (7.7) | 0 | 3 (23.1) | 1 (7.7) |
Abbreviations: AE, adverse event; CCH‐aaes, collagenase clostridium histolyticum‐aaes.
Safety population (all women who received ≥1 CCH‐aaes injection).
All AEs classified as injection‐site hemorrhage (the preferred term) for reporting purposes were identified with the term “injection‐site ecchymosis” by investigators during the trial.
4. DISCUSSION
The primary objective of this study was to assess the treatment effects of CCH‐aaes when administered by different injection techniques in women with buttock and thigh cellulite. These techniques differed in various ways, such as the CCH‐aaes concentration and volume per injection, needle angle, and injection depth. Despite these differences, CCH‐aaes was efficacious and improved the appearance of cellulite in the buttocks and thighs using any of the 5 injection techniques. However, Techniques B and C had the least favorable safety profiles; and therefore, these 1‐aliqout (bolus) techniques may not be optimal for treatment of buttock or thigh cellulite, regardless of efficacy. Overall and consistent with known anatomical differences between buttock and thigh cellulite, these efficacy and safety results showed that different CCH‐aaes injection techniques are needed for buttock cellulite (Technique A [shallow, half‐inch injections; 3 aliquots]) versus thigh cellulite (Technique D [deep, 1‐inch, and shallow, half‐inch, injections; 5 aliquots]) to optimize treatment outcomes. It is reassuring that the results with Technique A for buttock cellulite are consistent with the technique described in US prescribing information for CCH‐aaes and the efficacy and safety profiles observed in two randomized, phase 3 trials. 4 Technique D was evaluated in an open‐label, phase 3b trial of CCH‐aaes for the treatment of thigh cellulite in women (NCT04170296).
ETHICAL APPROVAL
The protocol was approved by an institutional review board (Advarra Institutional Review Board, Columbia, MD) and the trial was conducted in accordance with the guidelines for Good Clinical Practice of the International Conference on Harmonisation and the ethical principles of the Declaration of Helsinki. All patients provided written informed consent.
CONFLICT OF INTEREST
Joely Kaufman‐Janette reports serving as a clinical investigator and consultant for Endo Pharmaceuticals Inc. Bruce E. Katz reports serving as a clinical investigator and consultant for Endo Pharmaceuticals Inc. Saji Vijayan is an employee of Endo Pharmaceuticals Inc. Qinfang Xiang is an employee of Endo Pharmaceuticals Inc. Michael S. Kaminer reports serving as a clinical investigator and consultant for Endo Pharmaceuticals Inc.
AUTHOR CONTRIBUTIONS
Joely Kaufman‐Janette, Bruce E. Katz, Saji Vijayan, and Michael S. Kaminer were involved in the study design. Joely Kaufman‐Janette, Bruce E. Katz, and Michael S. Kaminer were involved in data acquisition. Qinfang Xiang performed statistical analyses. All authors contributed to data interpretation, writing, critical review, and editing of the manuscript and approved the manuscript for submission.
ACKNOWLEDGMENTS
We would like to thank George Omburo, PhD, for his contributions to the study design. Technical editorial and medical writing assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, Synchrony Medical Communications LLC, West Chester, PA, with funding from Endo Pharmaceuticals Inc.
Kaufman‐Janette J, Katz BE, Vijayan S, Xiang Q, Kaminer MS. Evaluation of five collagenase clostridium histolyticum‐aaes injection techniques for the treatment of cellulite on the buttock or thigh. J Cosmet Dermatol. 2022;21:1448–1453. doi: 10.1111/jocd.14842
Funding information
Endo Pharmaceuticals Inc., Malvern, PA. The sponsor was involved in the study design and the collection, analysis, and interpretation of data
DATA AVAILABILITY STATEMENT
Authors elect not to share the data.
REFERENCES
- 1. QWO® (Collagenase Clostridium Histolyticum‐aaes) for Injection for Subcutaneous Use [package insert]. Endo Aesthetics LLC; 2020. [Google Scholar]
- 2. Sadick NS, Goldman MP, Liu G, et al. Collagenase clostridium histolyticum for the treatment of edematous fibrosclerotic panniculopathy (cellulite): a randomized trial. Dermatol Surg. 2019;45:1047‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaufman‐Janette J, Bass LS, Xiang Q, McLane M, Kirby MT, Vijayan S. Efficacy, safety, and durability of response of collagenase clostridium histolyticum‐aaes for treating cellulite. Plast Reconstr Surg Glob Open. 2020;8:e3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaufman‐Janette J, Joseph JH, Kaminer MS, et al. Collagenase clostridium histolyticum‐aaes for the treatment of cellulite in women: results from two phase 3 randomized, placebo‐controlled trials. Dermatol Surg. 2021;47:649‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shridharani SM. Injectable cellulite treatment—collagenase clostridium histolyticum‐aaes effect on tissue histology. Presented at Vegas Cosmetic Surgery & Aesthetic Dermatology; September 26, 2020; Las Vegas, NV. [DOI] [PMC free article] [PubMed]
- 6. Hexsel DM, Abreu M, Rodrigues TC, Soirefmann M, Do Prado DZ, Gamboa MM. Side‐by‐side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg. 2009;35:1471‐1477. [DOI] [PubMed] [Google Scholar]
- 7. Querleux B, Cornillon C, Jolivet O, Bittoun J. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118‐124. [DOI] [PubMed] [Google Scholar]
- 8. Cohen JL, Sadick NS, Kirby MT, et al. Development and validation clinician and patient reported photonumeric scales to assess buttocks cellulite severity. Dermatol Surg. 2020;46:1628‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hexsel DM, Dal'Forno T, Hexsel CL. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. 2009;23:523‐528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors elect not to share the data.


