Abstract
Background and Objectives
To assess the association of preoperative aerobic fitness and body composition variables with a patient's resilience to the development and impact of postoperative complications after elective colorectal cancer (CRC) surgery.
Methods
Preoperative aerobic fitness was assessed by steep ramp test performance. Preoperative body composition was assessed by muscle mass and density determined from preoperative computed tomography scan analysis at the L3‐level. Complication development and severity was graded according to Clavien‐Dindo. Complication impact was assessed by the time to recovery of physical functioning after complications. Multivariable logistic regression analyses adjusted for age, sex, comorbidities and tumour location was performed.
Results
Of 238 included patients, 96 (40.3%) developed postoperative complications. Better preoperative aerobic fitness decreased the likelihood to develop complications, independent of muscle mass (odds ratio [OR]: 0.55, 95% confidence interval [CI]: 0.35−0.85) or muscle density (OR: 0.57, 95% CI: 0.36−0.89). A prolonged time to recovery following complications was associated with lower preoperative muscle density (OR: 4.14, 95% CI: 1.28−13.41), independent of aerobic fitness.
Conclusions
Lower aerobic fitness increases the risk of complication development, while low muscle density seems associated with a prolonged recovery from complications. Aerobic fitness and muscle density could be valuable additives to preoperative risk assessment.
Keywords: aerobic fitness, colorectal cancer surgery, complication development and subsequent recovery, muscle density, preoperative risk assessment
Abbreviations
- ANOVA
one‐way analysis of variance
- ASA
American Association of Anaesthesiologists
- BMI
body mass index
- CCI
Charlson Comorbidity Index
- CD
Clavien−Dindo
- CI
confidence interval
- CRC
colorectal cancer
- CT
computed tomography
- ERAS
enhanced recovery after surgery
- HU
Hounsfield unit
- IQR
interquartile range
- L3
third lumbar vertebra
- mILAS
modified Iowa level of assistance scale
- MUMC+
Maastricht University Medical Centre
- OR
odds ratio
- SD
standard deviation
- SM‐index
skeletal muscle index
- SM‐RA
skeletal muscle radiation attenuation
- SRT
steep ramp test
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology
- WRpeak
work rate at peak exercise
1. INTRODUCTION
Surgery is the main curative treatment for colorectal cancer (CRC). Surgery inherently exposes patients to multiple perioperative stressors, leading to a physiological stress response including hormonal, metabolic, haematological and immunological changes. 1 , 2 Patients with a reduced capacity to meet these increased physiological demands appear to be more vulnerable to the impact of surgery and have an increased risk for developing postoperative complications. 3
Complications after CRC surgery remain highly prevalent. 4 Traditional predictors of postoperative complications, such as age, sex and comorbidities are insufficiently accurate in estimating a patient's ability to cope with perioperative stressors. Furthermore, these predictors do not anticipate the impact of surgery and potential complications on the recovery of physical functioning. 5 Several physical fitness‐related variables, especially preoperative aerobic fitness and body composition variables, are gaining interest as modifiable risk factors that better reflect a patient's preoperative reserve capacity.
Aerobic fitness reflects the maximal capacity of the pulmonary and cardiovascular systems to take in and deliver oxygen to metabolically active tissues, and the ability of these tissues to extract and use oxygen in response to metabolic needs. 1 Low preoperative aerobic fitness might lead to insufficient oxygen delivery to meet the increased postoperative metabolic demand, 1 resulting in increased complication risk. 6 , 7 In addition to aerobic fitness, preoperative body composition analysis provides information about skeletal muscle mass and muscle density. Myopenia, a condition characterized by low skeletal muscle mass, and myosteatosis, a condition characterized by low muscle density due to increased fat and fluid infiltration in the muscle, reflect reduced energy reserves, malnutrition, and are related to chronic systemic inflammation. 1 , 8 , 9 Preoperative aerobic fitness and body composition variables have been associated with postoperative outcomes in patients with CRC. 6 , 7 , 10 However, both factors have only been studied separately regarding their relationship to postoperative morbidity in CRC surgery. Because preoperative aerobic fitness and body composition differently contribute to a patient's reserve capacity, different associations with postoperative complications and recovery of physical functioning following complications might be expected. The primary aim of this study was to assess how these preoperative physical fitness variables interact and influence each other's relation regarding the development and severity of postoperative complications after elective CRC surgery. The secondary aim was to assess how these physical fitness variables interact and affect the potential individual relation with the recovery of physical functioning in patients with postoperative complications following CRC surgery.
2. MATERIALS AND METHODS
2.1. Study design and population
The study was performed at the Maastricht University Medical Centre (MUMC+) and reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement. 11 The Medical Ethical Committee of the MUMC+/Maastricht University approved the study (15‐4‐234). Between January 2016 and March 2020, patients diagnosed with CRC and scheduled for elective resection were referred to the outpatient physical therapy department for a preoperative physical fitness assessment as part of usual care. Data from all consecutive patients who signed informed consent to use their usual care data for research purposes were prospectively recorded. Baseline characteristics, preoperative aerobic fitness and preoperative abdominal computed tomography (CT) scans were collected. American Society of Anaesthesiologists (ASA) classification 12 and Charlson comorbidity index (CCI) 13 were used to classify comorbidities. Patients without an available CT scan or poor CT scan quality due to radiation artefacts or missing muscle parts on the ventral, dorsal or lateral edges were excluded. Other exclusion criteria were pelvic exenteration, preoperative assessment of physical fitness before neoadjuvant chemoradiotherapy or >2 months before surgery, participation in a prehabilitation programme, no bowel resection performed due to peritoneal metastases and postoperative air‐fluidized sand bed therapy.
2.2. Preoperative aerobic fitness assessment
Aerobic fitness was estimated using the steep ramp test (SRT), a short‐time maximal cycle ergometer exercise test (Lode Corival Rehab, Lode BV), 14 and expressed as the attained work rate at peak exercise (WRpeak) adjusted for body mass (SRT WRpeak in W/kg). After 2 min of unloaded cycling, the work rate was increased by 10 W/10 s in a ramp‐like manner until voluntary exhaustion. Patients were asked to maintain a pedalling frequency between 70 and 80 revolutions/min throughout the test. When pedalling frequency dropped definitely <60 revolutions/min (peak exercise), despite verbal encouragement, the test was ended.
2.3. Preoperative body composition analysis
Preoperative skeletal muscle mass and muscle density were analysed using preoperative contrast‐enhanced abdominal CT scans performed as part of usual care. In the case of multiple CT scans, the scan closest to surgery was selected. In the case of neoadjuvant treatment, the CT scan performed after neoadjuvant treatment was used. CT scans were analysed anonymously by a single trained investigator (A. C. M. C.) using slicOmatic 5.0 (TomoVision) software for Microsoft Windows®. During analysis, the investigator was blinded for all patient characteristics. Surface area measurements were performed using transverse slides at the third lumbar vertebra (L3) level where both transverse processes were visible. 15 Total cross‐sectional areas of skeletal muscle were determined using predefined Hounsfield unit (HU) thresholds (−29 to 150 HU) and corrected for the patient's body height to obtain the skeletal muscle index (SM‐index, cm2/m2). Skeletal muscle radiation attenuation (SM‐RA), a measure reflecting muscle radiodensity, was determined using the average HU value of the total skeletal muscle area within the predefined HU ranges. Low muscle mass was defined using sex and body mass index (BMI) adjusted cut‐off values for SM‐index (males: < 43 cm2/m2 when BMI < 25.0 kg/m2, < 53 cm2/m2 when BMI ≥ 25.0 kg/m2; females: < 41 cm2/m2 regardless of BMI). 16 Low muscle density was defined using BMI‐adjusted cut‐off values for SM‐RA (HU < 41 when BMI < 25 kg/m2, and HU < 33 when BMI ≥ 25 kg/m2). 16
2.4. Postoperative outcomes
All patients received similar postoperative care according to the enhanced recovery after surgery (ERAS) protocol. 17 Furthermore, all patients received physical therapy from postoperative day one onwards, which included transfers, airway‐clearing exercises, walking, stair climbing (when needed), muscle function exercises and aerobic fitness exercises. Recovery of physical functioning was assessed daily by the physical therapist using the modified Iowa level of assistance scale (mILAS). 18 This scale assesses the ability to perform five daily activities (supine‐to‐sit, sit‐to‐supine, sit‐to‐stand, walking and stair‐climbing [when‐needed]), which are scored from 0 to 6 for the amount of needed assistance. The mILAS score is calculated by the sum of the five individual scores and ranges from 0 to 30. Higher scores indicate more assistance. Time to recovery of physical functioning was defined as the number of days between surgery and the day when a mILAS score of 0 (mILAS = 0) was reached.
Primary outcome measures were the development and severity of postoperative complications, and were scored using the Clavien−Dindo (CD) classification. 19 Development of any postoperative complication was defined as a CD‐grade ≥ I. Complication severity was graded as minor (CD‐grade I or II) or major (CD‐grade ≥ IIIa). Secondary outcome measure was time to recovery of physical functioning in patients with postoperative complications. The impact of postoperative complications on the time to recovery of physical functioning was defined based on the median time to mILAS = 0 in patients with postoperative complications. Patients with any postoperative complication and a time to recovery of physical functioning shorter than or equal to the median time to mILAS = 0 were classified as having a complicated course with low impact, whereas patients who needed a longer recovery time than the median time to mILAS = 0 were classified as having a complicated course with high impact.
2.5. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp.). Normality was tested using histograms and Kolmogorov−Smirnov tests. According to normality, continuous variables are displayed as mean ± standard deviation (SD) or median and interquartile range [IQR]. Frequencies are presented as absolute numbers and percentages. Independent samples t tests and one‐way analysis of variance (ANOVA) were used for normally distributed variables. Mann−Whitney U tests and Kruskal−Wallis tests were used for nonparametric variables. χ 2 tests were used for categorical values. Correlations were assessed using Pearson correlation coefficients and regression plots. Associations of preoperative aerobic fitness, muscle mass and muscle density with postoperative outcomes were assessed using binary and multinomial logistic regression analyses, adjusted for relevant confounders including age, sex, CCI and tumour location (colon or rectum). Aerobic fitness and body composition variables were also adjusted for each other to assess independent associations. Statistical interactions between preoperative aerobic fitness and body composition variables were checked to determine whether potential adverse effects of poor aerobic fitness and poor body composition might reinforce each other in case of co‐occurrence. Results are presented as odds ratios (OR) with 95% confidence intervals (CI). Two‐tailed p values < 0.05 were considered statistically significant.
3. RESULTS
Of 505 patients undergoing elective CRC surgery, 304 patients underwent preoperative physical fitness assessment followed by elective surgical tumour resection. Reasons for not receiving preoperative physical fitness assessment are listed in Figure 1. Sixty‐six patients were excluded and 238 patients were included in the study (Figure 1). Baseline characteristics are shown in Table 1. Median time between preoperative physical fitness assessment and surgery was 10.5 days [6.0; 17.0]. Median time between preoperative CT scan and surgery was 25.0 days [19.0; 35.5]. Preoperative aerobic fitness was not correlated with muscle mass (r: 0.081; p = 0.215), whereas a moderately positive correlation was observed between preoperative aerobic fitness and muscle density (r: 0.597; p < 0.001) (Figure 2).
Figure 1.
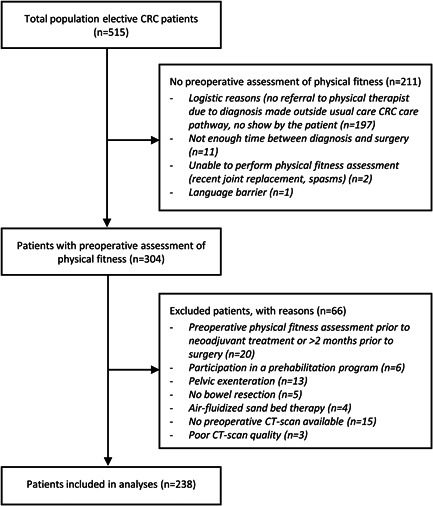
Patient flow chart
Table 1.
Baseline characteristics and postoperative outcome according to complication severity
| Overall (n = 238) | No (n = 142) | Minor (CD I−II) (n = 57) | Major (CD ≥ IIIa) (n = 39) | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 69.3 (±9.9) | 68.8 (±10.2) | 70.5 (±8.6) | 69.0 (±10.8) | 0.578 | ||||
| Sex | 0.281 | ||||||||
| Male | 134 (56.3%) | 74 (52.1%) | 36 (63.2%) | 24 (61.5%) | |||||
| Female | 104 (43.7%) | 68 (47.9%) | 21 (36.8%) | 15 (38.5%) | |||||
| BMI (kg/m2) | 27.0 (±5.01) | 26.6 (±5.0) | 27.5 (±4.7) | 27.3 (±5.6) | 0.435 | ||||
| Haemoglobin (g/dl) | 12.8 (±2.0) | 12.9 (±2.8) | 12.9 (±3.0) | 12.5 (±1.8) | 0.605 | ||||
| Smoking (yes) | 30 (12.6%) | 18 (12.7%) | 2 (3.5%) | 10 (25.6%) | 0.006 | ||||
| CCI | 3.00 [3.00; 5.00] | 3.00 [2.00; 5.00] | 3.00 [3.00; 5.00] | 5.00 [3.00; 6.00] | 0.008 | ||||
| ASA | <0.001 | ||||||||
| I | 20 (8.4%) | 17 (12.0%) | 3 (5.3%) | 0 (0.0%) | |||||
| II | 153 (64.3%) | 97 (68.3%) | 39 (68.4%) | 17 (43.6%) | |||||
| III | 65 (27.3%) | 28 (19.7%) | 15 (26.3%) | 22 (56.4%) | |||||
| Neoadjuvant therapy | 51 (21.4%) | 23 (16.2%) | 17 (29.8%) | 11 (28.2%) | 0.056 | ||||
| Tumour location | 0.035 | ||||||||
| Colon | 159 (66.8%) | 103 (72.5%) | 36 (63.2%) | 20 (51.3%) | |||||
| Rectum | 79 (33.2%) | 39 (27.5%) | 21 (36.8%) | 19 (48.7%) | |||||
| Surgical approach | 0.110 | ||||||||
| Laparoscopy/robot (assisted) | 214 (89.9%) | 132 (93.0%) | 50 (87.7%) | 32 (82.1%) | |||||
| Laparotomy | 24 (10.1%) | 10 (7.0%) | 7 (12.3%) | 7 (17.9%) | |||||
| Preoperative physical fitness | |||||||||
| SRT WRpeak (W/kg) | 2.151 (±0.806) | 2.260 (±0.827) | 2.022 (±0.697) | 1.942 (±0.824) | 0.035 | ||||
| Body composition | |||||||||
| SM‐index (cm2/m2) | 45.12 (±8.71) | 44.75 (±8.38) | 46.70 (±9.16) | 44.18 (±9.13) | 0.274 | ||||
| SM‐RA (HU) | 37.62 ± 9.41 | 38.53 (±9.17) | 37.22 (±8.37) | 35.25 (±11.27) | 1.142 | ||||
| Low muscle mass (yes) | 142 (59.7%) | 87 (40.1%) | 27 (47.4%) | 28 (71.8%) | 0.047 | ||||
| Low muscle density (yes) | 98 (41.2%) | 51 (35.9%) | 24 (42.1%) | 23 (59.0%) | 0.034 | ||||
| Postoperative outcomes | |||||||||
| Conversion | 32 (13.4%) | 10 (7.0%) | 14 (24.6%) | 8 (20.5%) | 0.001 | ||||
| Time to mILAS=0 (days) | 4.00 [3.0; 7.0] | 3.00 [2. 0; 4.0] | 6.00 [4.5; 10.0] | 17.00 [7.0; 25.0] | <0.001 | ||||
| LOS (days) | 5.00 [4.0; 10.0] | 4.00 [3.0; 5.0] | 9.00 [6.0; 14.5] | 22.00 [14.0; 31.0] | <0.001 | ||||
| Readmission | 25 (10.5%) | 2 (0.7%) | 10 (17.5%) | 13 (33.3%) | <0.001 | ||||
Note: Data displayed as absolute number (%), mean ± SD, or median [IQR]. Statistically significant values are displayed in italic and bold.
Abbreviations: ASA, American Society of Anaesthesiologists physical status classification; BMI, body mass index; CCI, Charlson comorbidity index; CD, Clavien−Dindo; HU, Hounsfield Unit; LOS, length of hospital stay; mILAS, modified Iowa level of assistance scale; SM, skeletal muscle; SM‐RA, skeletal muscle radiation attenuation; SRT, steep ramp test; WRpeak, peak work rate.
Figure 2.
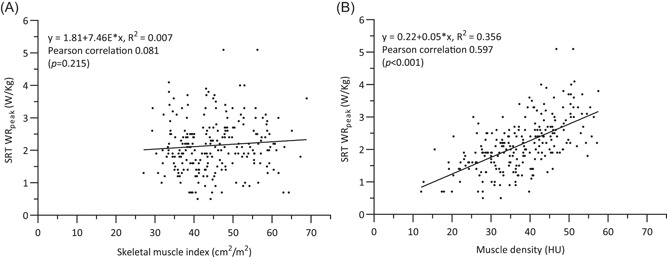
Correlations and regression plots of (A) muscle mass, (B) muscle density and preoperative aerobic fitness
3.1. Preoperative aerobic fitness, body composition and postoperative complications
Significant differences in aerobic fitness were observed when comparing groups based on complication severity, with the lowest SRT WRpeak (W/kg) measured in patients with the highest complication severity (p = 0.035). Low muscle mass and low muscle density were significantly more prevalent in patients with a higher complication severity (p = 0.047 and p = 0.034 respectively; Table 1).
Multivariable logistic regression analysis (Table 2) showed that a better preoperative aerobic fitness level was significantly associated with a lower likelihood of postoperative complications (OR: 0.54, 95% CI: 0.35−0.85). Associations remained significant after additional adjustment for low muscle mass (OR: 0.55, 95% CI: 0.35−0.85) or low muscle density (OR: 0.57, 95% CI: 0.36−0.89). When adjusted for aerobic fitness, low muscle mass was not associated with postoperative complications (OR: 0.92, 95% CI: 0.52−1.62). Low muscle density was statistically significantly associated with complications in the unadjusted analysis (OR: 1.71, 95% CI: 1.01−2.90), but the association was attenuated in the multivariable analysis (OR: 1.33, 95% CI: 0.69−2.54; Table 3). There was no statistical interaction between the level of aerobic fitness and low muscle density (p = 0.991) or low muscle mass (p = 0.710).
Table 2.
Logistic regression analyses for development of postoperative complications and impact of complications on recovery time
| Postoperative complications (CD ≥ I) (n = 238)a | Time to mILAS = 0 > 8 days (n = 96)b | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| SRT WRpeak(W/kg) | ||||||
| Unadjusted | 0.66 | (0.46–0.91) | 0.012 | 0.90 | (0.52–1.55) | 0.700 |
| Adjusted | 0.54 | (0.35–0.85) | 0.007 | 0.76 | (0.34–1.69) | 0.492 |
| Adjusted, including muscle massc | 0.55 | (0.35–0.85) | 0.008 | 0.65 | (0.31–1.38) | 0.259 |
| Adjusted, including muscle densityd | 0.57 | (0.36–0.89) | 0.013 | 0.95 | (0.40–2.25) | 0.910 |
| Muscle masse | ||||||
| Unadjusted | 0.85 | (0.50–1.44) | 0.540 | 1.62 | (0.71–3.68) | 0.249 |
| Adjusted | 0.85 | (0.49–1.50) | 0.578 | 1.19 | (0.44–3.22) | 0.734 |
| Adjusted, including aerobic fitnessf | 0.92 | (0.52–1.62) | 0.763 | 1.27 | (0.46–3.51) | 0.642 |
| Muscle densityg | ||||||
| Unadjusted | 1.71 | (1.01–2.90) | 0.046 | 2.54 | (1.11–5.80) | 0.027 |
| Adjusted | 1.57 | (0.83–2.94) | 0.163 | 4.20 | (1.33–13.25) | 0.014 |
| Adjusted, including aerobic fitness f | 1.33 | (0.69–2.54) | 0.396 | 4.14 | (1.28–13.41) | 0.018 |
Note: Statistically significant values are displayed in italic and bold.
Abbreviations: BMI, body mass index; CD, Clavien−Dindo; CI, confidence interval; mILAS, modified Iowa level of assistance scale; OR, odds ratio; SRT, steep ramp test; WRpeak, peak work rate.
Multivariable analysis in total population (n = 238), adjusted for age, sex, Charlson comorbidity index, and tumour location.
Multivariable analysis in subgroup with postoperative complications (n = 96), adjusted for age, sex, Charlson comorbidity index, tumour location, and complication severity (minor [CD I−II] vs. major [CD ≥ IIIa] complication).
Also adjusted for muscle mass.
Also adjusted for muscle density.
Dichotomized by sex and BMI adjusted cut‐off values (low muscle mass in males: <43 cm2/m2 when BMI <25.0 kg/m2, <53 cm2/m2 when BMI ≥ 25.0 kg/m2; low muscle mass in females: <41 cm2/m2 regardless of BMI). 16
Also adjusted for aerobic fitness.
Dichotomized by BMI adjusted cut‐off values (low muscle density: HU < 41 when BMI < 25 kg/m2, and HU < 33 when BMI ≥ 25 kg/m2. 16
Table 3.
Multinomial logistic regression analyses for complication severity a
| No vs. minor (ref = no) | No vs. major (ref = no) | Minor versus major (ref = minor) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | |
| SRT WRpeak(W/kg) | |||||||||
| Unadjusted | 0.68 | (0.46–1.00) | 0.062 | 0.59 | (0.37–0.96) | 0.032 | 0.87 | (0.50–1.50) | 0.609 |
| Adjusted | 0.57 | (0.34–0.95) | 0.030 | 0.50 | (0.27–0.93) | 0.028 | 0.88 | (0.44–1.75) | 0.712 |
| Adjusted, including muscle massb | 0.59 | (0.36–0.99) | 0.044 | 0.46 | (0.24–0.88) | 0.018 | 0.77 | (0.38–1.58) | 0.483 |
| Adjusted, including muscle densityc | 0.57 | (0.34–0.96) | 0.035 | 0.55 | (0.29–1.03) | 0.063 | 0.97 | (0.48–1.96) | 0.932 |
| Muscle massd | |||||||||
| Unadjusted | 0.57 | (0.31–1.06) | 0.075 | 1.61 | (0.74–3.49) | 0.229 | 2.83 | (1.19–6.75) | 0.019 |
| Adjusted | 0.57 | (0.30–1.09) | 0.087 | 1.77 | (0.76–4.12) | 0.182 | 3.13 | (1.24–7.93) | 0.016 |
| Adjusted, including aerobic fitnesse | 0.60 | (0.31–1.16) | 0.132 | 1.99 | (0.84–4.73) | 0.117 | 3.30 | (1.29–8.47) | 0.013 |
| Muscle densityf | |||||||||
| Unadjusted | 1.30 | (0.69–2.43) | 0.416 | 2.57 | (1.24–5.29) | 0.011 | 1.98 | (0.87–4.52) | 0.106 |
| Adjusted | 1.25 | (0.60–2.58) | 0.603 | 2.25 | (0.92–5.58) | 0.077 | 1.81 | (0.67–4.90) | 0.246 |
| Adjusted, including aerobic fitnesse | 1.07 | (0.51–2.25) | 0.869 | 1.87 | (0.74–4.69) | 0.184 | 1.75 | (0.64–4.83) | 0.277 |
Note: Statistically significant values are displayed in italic and bold.
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; SRT, steep ramp test; WRpeak, peak work rate.
Multivariable analysis in total population (n = 238), adjusted for age, sex, Charlson comorbidity index and tumour location. Complication severity: no complications (CD 0), minor complications (CD I‐II), major complications (CD ≥ IIIa).
Also adjusted for muscle mass.
Also adjusted for muscle density.
Dichotomized by sex and BMI adjusted cut‐off values (low muscle mass in males: <43 cm2/m2 when BMI <25.0 kg/m2, <53 cm2/m2 when BMI ≥ 25.0 kg/m2; low muscle mass in females: <41 cm2/m2 regardless of BMI). 16
Also adjusted for aerobic fitness.
Dichotomized by BMI adjusted cut‐off values (low muscle density: HU < 41 when BMI < 25 kg/m2, and HU < 33 when BMI ≥ 25 kg/m2. 16
3.2. Preoperative aerobic fitness, body composition and postoperative complication severity
Multinomial logistic regression analysis (Table 3) showed that preoperative aerobic fitness was inversely associated with minor complications (OR: 0.57, 95% CI: 0.34−0.95) and major complications (OR: 0.50, 95% CI: 0.27−0.93), when adjusted for confounders. The association with minor complications also remained statistically significant when adjusted for low muscle mass (OR: 0.59, 95% CI: 0.36−0.99) or low muscle density (OR: 0.57, 95% CI: 0.34−0.96). Aerobic fitness remained significantly associated with major complications when adjusted for low muscle mass (OR: 0.46, 95% CI: 0.24−0.88), but the association attenuated when adjusted for low muscle density (OR: 0.55, 95% CI: 0.29−1.03).
In the case of complications, patients with low muscle mass were more likely to develop major complications than minor complications (OR: 3.30, 95% CI: 1.29−8.47), despite the fact that no significant associations were found between low muscle mass and the development of minor or major postoperative complications. Low muscle density was not associated with minor complications but significantly associated with the development of major complications (OR: 2.57, 95% CI: 1.24−5.29) in the unadjusted analysis. The association with major complications lost significance in the multivariable analysis (OR: 1.87, 95% CI: 0.74−4.69).
3.3. Preoperative aerobic fitness, body composition and time to recovery of physical functioning in case of postoperative complications
Median time to mILAS = 0 in patients with postoperative complications was 8 days. As such, time to mILAS = 0 was dichotomized as ≤8 (low impact) and >8 days (high impact). A total of 96 patients developed postoperative complications; 52 patients recovered within the median time of 8 days, and 44 patients needed >8 days for their recovery of physical functioning. Low muscle density was significantly more prevalent in patients who needed a longer recovery time compared with patients who recovered within the median recovery time (respectively 61.4% vs. 38.5%; p = 0.025; Table 4). When adjusted for sex, age, CCI, tumour location, aerobic fitness and complication severity (minor vs. major complications), low muscle density was strongly associated with a longer time (>8 days) needed to recover in case of postoperative complications (OR: 4.14, 95% CI: 1.28−13.41; Table 2). No significant associations were observed between aerobic fitness or low muscle mass and time to recovery of physical functioning after postoperative complications. No statistically significant interactions were observed between aerobic fitness and muscle density (p = 0.864) or muscle mass (p = 0.585).
Table 4.
Baseline characteristics of patients with postoperative complications (n = 96)
| Complication and time to mILAS = 0 ≤ 8 days (n = 52) | Complication and time to mILAS = 0 > 8 days (n = 44) | p value | |||
|---|---|---|---|---|---|
| Age (years) | 71.8 [61.8; 75.7] | 70.4 [66.3; 77.0] | 0.988 | ||
| Sex | 0.526 | ||||
| Male | 31 (59.6%) | 29 (65.9%) | |||
| Female | 21 (40.4%) | 15 (34.1%) | |||
| BMI (kg/m2) | 27.2 [24.4; 29.6] | 28.4 [23.6; 30.8] | 0.421 | ||
| CCI | 4.00 [3.00; 5.00] | 4.00 [3.00; 6.00] | 0.849 | ||
| ASA | 0.220 | ||||
| I | 3 (5.8%) | 0 (0.0%) | |||
| II | 31 (59.6%) | 25 (56.8%) | |||
| III | 18 (34.6%) | 19 (43.2%) | |||
| Tumour location | 0.580 | ||||
| Colon | 29 (55.8%) | 27 (61.4%) | |||
| Rectum | 23 (44.2%) | 17 (38.6%) | |||
| Preoperative physical fitness | |||||
| SRT WRpeak (W/kg) | 1.922 [1.488; 2.489] | 1.861 [1.427; 2.328] | 0.487 | ||
| Body composition | |||||
| Low muscle mass (yes) | 27 (51.9%) | 28 (63.6%) | 0.248 | ||
| Low muscle density (yes) | 20 (38.5%) | 27 (61.4%) | 0.025 | ||
| Complication severity | <0.001 | ||||
| Minor complications (CD I−II) | 41 (78.8%) | 16 (36.4%) | |||
| Major complications (CD ≥ IIIa) | 11 (21.2%) | 28 (63.6%) | |||
Note: Data displayed as absolute number (%) or median [IQR]. Statistically significant values are displayed in italic and bold.
Abbreviations: ASA, American Society of Anaesthesiologists physical status classification; BMI, body mass index; CCI, Charlson comorbidity index; CD, Clavien−Dindo; mILAS, modified Iowa level of assistance scale; OR, odds ratio; SRT, steep ramp test; WRpeak, peak work rate.
4. DISCUSSION
This study demonstrated that preoperative aerobic fitness and muscle density are independently and differently associated with postoperative complications. Preoperative aerobic fitness was strongly associated with the incidence of overall postoperative complications, regardless of preoperative muscle density or muscle mass. When addressing the relation of preoperative aerobic fitness with complications in more detail by using complication severity, aerobic fitness was associated with developing both minor and major postoperative complications; however, the association with major complications lost statistical significance when adjusted for preoperative muscle density. Preoperative muscle density was not associated with the incidence of minor postoperative complications but was significantly associated with major complications in the unadjusted analysis. Despite losing statistical significance, the individual associations of preoperative aerobic fitness and muscle density with the development of major complications remained substantial in the multivariable analyses. From these results, it appears that patients with a lower preoperative aerobic fitness have an increased risk for minor postoperative complications, whereas both poor preoperative aerobic fitness and low preoperative muscle density lead to a higher risk for major postoperative complications. Although no overall association of preoperative muscle mass with the development of postoperative complications was observed, patients with low muscle mass who developed complications were more likely to develop major than minor complications. Interestingly, muscle density, and not preoperative aerobic fitness or muscle mass, appeared to be predictive of the impact of complications on postoperative time to recovery of physical functioning.
Perioperative stressors result in an increasing demand on a patient's cardiovascular and pulmonary systems to increase the intake, transport and utilization of oxygen. 20 On the one hand, patients with poor aerobic fitness levels might be unable to sufficiently increase their cardiopulmonary performance in response to perioperative stress and could become prone to minor complications (such as cardiovascular and pulmonary events), as well as to major complications (such as anastomotic leakages and intra‐abdominal abscess formation). 6 , 20 , 21 On the other hand, suboptimal body composition, expressed as low muscle density and low muscle mass, reflects reduced energy reserves and malnutrition, and is related to chronic systemic inflammation. 1 , 8 , 9 Low muscle density characterizes myosteatosis. Due to increased intramuscular fat depositions, myosteatosis may represent the manifestation of metabolic risk factors, such as insulin resistance and chronically elevated levels of pro‐inflammatory cytokines. 8 , 22 , 23 Low muscle mass, which characterizes myopenia, can also reflect disturbances in energy levels, nutritional status and inflammatory responses. 1 , 8 , 9 A relationship exists between low muscle density, low muscle mass and systemic inflammation; however, it remains unclear whether altered muscle mass and muscle density lead to chronic systemic inflammation or if inflammatory changes cause muscle alterations. 8 , 22 , 24 , 25 , 26 However, the pro‐inflammatory state related to myopenia and myosteatosis might disturb the physiological inflammatory responses to withstand perioperative stressors, impairing wound and anastomotic healing and increasing the risk for postoperative infections. 27 , 28 This might explain why low muscle density is predominantly associated with the risk for major complications, rather than minor complications, 29 , 30 and why patients with low muscle mass who developed complications were more likely to develop major than minor complications. In contrast to previous literature, 31 , 32 no direct associations between muscle mass and complications were found. However, low muscle mass might only reflect a part of malnutrition and other factors, including muscle density, might also be important. 9 , 33
Surprisingly, a better preoperative aerobic fitness does no longer seem to significantly influence postoperative recovery of physical functioning once complications have occurred. However, patients with low muscle density were four times more likely to need a longer time to recover from postoperative complications. Where poor preoperative aerobic fitness increases the risk of postoperative complications, the course of recovery of physical functioning following complications might depend more on other underlying factors, such as a patient's 'immunological reserve capacity'. Patients with an altered inflammatory state might need significantly more time to recover from complications. Unfortunately, no preoperative nutritional scores, preoperative albumin or C‐reactive protein levels were available, limiting this study to further assess the level of malnutrition or preoperative inflammatory state in the included patients. As suggested by the positive correlation observed in this study and by previous literature, 34 poor aerobic fitness and low muscle density may coincide in CRC patients. In this study, however, no significant statistical interaction between preoperative aerobic fitness and low muscle density was observed, indicating that these co‐occurring risk factors do not seem to reinforce each other's negative effects on postoperative complications and recovery. Future studies are needed to further assess the relationship between myopenia, myosteatosis and systemic inflammation, and to gain insight into postoperative changes in aerobic fitness, inflammatory status and muscle metabolism caused by perioperative stress and complications.
Improving preoperative aerobic fitness by interventions like exercise prehabilitation possibly enables patients to better withstand perioperative stressors, and lowers the risk of postoperative morbidity, particularly in patients with poor preoperative aerobic fitness. 35 , 36 Prehabilitation can improve preoperative aerobic fitness, 35 and decrease the perioperative loss of lean body mass. 37 As increased intramuscular fat accumulation is associated with poor physical activity, prehabilitation might also improve muscle mass and muscle density by remodelling intramuscular fat distribution, and induce anti‐inflammatory effects. 38 , 39 , 40 However, it remains unclear if a short period of exercise prehabilitation causes clinically relevant improvements in muscle density. 38 Future research should focus on improving preoperative body composition variables and preoperative aerobic fitness to make patients more resilient to perioperative stressors and the consequences of complications. Future studies should look beyond predicting the development of complications alone and focus on predicting the potential impact of complications on postoperative recovery. This will help to anticipate potential problems and to better inform patients on what someone is likely to expect in the full range of postoperative recovery.
5. CONCLUSIONS
Preoperative aerobic fitness and muscle density seem independent risk factors for postoperative complications and the course of recovery of physical functioning in case of complications. Where poor preoperative aerobic fitness increases the risk of developing both minor and major complications, low muscle density was associated with a prolonged recovery from complications. Both variables could be valuable additives to improve preoperative risk assessment in CRC surgery and to offer patient‐tailored preoperative preventive interventions.
CONFLICT OF INTERESTS
Anne C. M. Cuijpers, Bart C. Bongers, Aniek F. J. M. Heldens, Martijn J. L. Bours, Nico L. U. van Meeteren, Laurents P. S. Stassen and Tim Lubbers declare no conflict of interests. Nico L. U. van Meeteren is professor and executive director of Health~Holland. No staff member of Health~Holland (including the executive director) can ever be involved in the assessment, allocation, and board decisions regarding applications. Health~Holland does not interfere in any way during the implementation of projects. Only after financial and administrative completion of the project, and after delivery of the formal report to Health~Holland, Nico L. U. van Meeteren became involved in the writing and editing of this article. Therefore, none of the authors declare any conflict of (financial) interest.
ETHICS STATEMENT
The Medical Ethical Committee of the MUMC+/Maastricht University approved the study (registration number 15‐4‐234). Therefore, the study has been performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments. Data from all consecutive patients who signed informed consent to use their usual care data for research purposes were prospectively recorded.
AUTHOR CONTRIBUTIONS
Anne C. M. Cuijpers contributed for investigation, methodology, data curation, formal analysis, writing original draft, and visualization. Bart C. Bongers contributed for conceptualization, funding acquisition, methodology, formal analysis, and writing original draft. Aniek F. J. M. Heldens contributed for investigation, data curation, and writing review and editing. Martijn J. L. Bours contributed for methodology, formal analysis, and writing review and editing. Nico L. U. van Meeteren contributed for conceptualization, supervision, and writing review and editing. Laurents P. S. Stassen contributed for conceptualization, supervision, and writing review and editing. Tim Lubbers contributed for conceptualization, methodology and writing review and editing. All authors read and approved the final manuscript.
SYNOPSIS
Preoperative aerobic fitness and muscle density seem independent risk factors for respectively the incidence and impact of postoperative complications in patients undergoing elective colorectal cancer surgery. Patients with a lower aerobic fitness are more prone to develop both minor and major complications. Postoperative recovery of physical functioning in case of complications appears to depend on other underlying factors, such as preoperative muscle density.
ACKNOWLEDGEMENTS
This study is part of a larger project, the public−private partnership project (PROCLINA), which is co‐funded by an unconditional research grant from Medical Research Data Management (MRDM), as well as by the Ministry of Economic Affairs by means of a PPP Allowance made available by Health~Holland, Top Sector Life Sciences & Health (LSH M17073). Health~Holland encourages innovative research by financially supporting public−private partnerships in the life sciences and health sector, with the aim of developing sustainable and innovative products and services. The consortium has made agreements about the intellectual property (IP) related to the knowledge and products that will be developed in the project. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Cuijpers ACM, Bongers BC, Heldens AFJM, et al. Aerobic fitness and muscle density play a vital role in postoperative complications in colorectal cancer surgery. J Surg Oncol. 2022;125:1013‐1023. 10.1002/jso.26817
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bongers BC, Dejong CHC, den Dulk M. Enhanced recovery after surgery programmes in older patients undergoing hepatopancreatobiliary surgery: what benefits might prehabilitation have? Eur J Surg Oncol. 2021;47(3, Part A):551‐559. [DOI] [PubMed] [Google Scholar]
- 2. Cusack B, Buggy DJ. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020;20(9):321‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dronkers J, Witteman B, van Meeteren N. Surgery and functional mobility: doing the right thing at the right time. Tech Coloproctol. 2016;20(6):339‐341. [DOI] [PubMed] [Google Scholar]
- 4. Tevis SE, Kennedy GD. Postoperative complications: looking forward to a safer future. Clin Colon Rectal Surg. 2016;29(3):246‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cuijpers ACM, Coolsen MME, Schnabel RM, van Santen S, Olde Damink SWM, van de Poll MCG. Preoperative risk assessment: a poor predictor of outcome in critically ill elderly with sepsis after abdominal surgery. World J Surg. 2020;44(12):4060‐4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. West MA, Asher R, Browning M, et al. Validation of preoperative cardiopulmonary exercise testing‐derived variables to predict in‐hospital morbidity after major colorectal surgery. Br J Surg. 2016;103(6):744‐752. [DOI] [PubMed] [Google Scholar]
- 7. Moran J, Wilson F, Guinan E, McCormick P, Hussey J, Moriarty J. Role of cardiopulmonary exercise testing as a risk‐assessment method in patients undergoing intra‐abdominal surgery: a systematic review. Br J Anaesth. 2016;116(2):177‐191. [DOI] [PubMed] [Google Scholar]
- 8. Deutz NEP, Ashurst I, Ballesteros MD, et al. The underappreciated role of low muscle mass in the management of malnutrition. J Am Med Dir Assoc. 2019;20(1):22‐27. [DOI] [PubMed] [Google Scholar]
- 9. Zamboni M, Gattazzo S, Rossi AP. Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med. 2019;10(1):5‐6. [DOI] [PubMed] [Google Scholar]
- 10. van Vugt JLA, Coebergh van den Braak RRJ, Lalmahomed ZS, et al. Impact of low skeletal muscle mass and density on short and long‐term outcome after resection of stage I‐III colorectal cancer. Eur J Surg Oncol. 2018;44(9):1354‐1360. [DOI] [PubMed] [Google Scholar]
- 11. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217‐222. [DOI] [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 14. Weemaes ATR, Beelen M, Bongers BC, Weijenberg MP, Lenssen AF. Criterion validity and responsiveness of the steep ramp test to evaluate aerobic capacity in cancer survivors participating in a supervised exercise rehabilitation program. Arch Phys Med Rehabil. 2021;102:2150‐2156. 10.1016/j.apmr.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 15. Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol. 2004;97(6):2333‐2338. [DOI] [PubMed] [Google Scholar]
- 16. Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539‐1547. [DOI] [PubMed] [Google Scholar]
- 17. Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations: 2018. World J Surg. 2019;43(3):659‐695. [DOI] [PubMed] [Google Scholar]
- 18. Shields RK, Enloe LJ, Evans RE, Smith KB, Steckel SD. Reliability, validity, and responsiveness of functional tests in patients with total joint replacement. Phys Ther. 1995;75(3):169‐176. [DOI] [PubMed] [Google Scholar]
- 19. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies SJ, Wilson RJ. Preoperative optimization of the high‐risk surgical patient. Br J Anaesth. 2004;93(1):121‐128. [DOI] [PubMed] [Google Scholar]
- 21. Heldens AFJM, Bongers BC, Lenssen AF, Stassen LPS, Buhre WF, van Meeteren NLU. The association between performance parameters of physical fitness and postoperative outcomes in patients undergoing colorectal surgery: an evaluation of care data. Eur J Surg Oncol. 2017;43(11):2084‐2092. [DOI] [PubMed] [Google Scholar]
- 22. Abbass T, Dolan RD, Laird BJ, McMillan DC. The relationship between imaging‐based body composition analysis and the systemic inflammatory response in patients with cancer: a systematic review. Cancers. 2019;11(9):1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13(3):260‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malietzis G, Johns N, Al‐Hassi HO, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016;263(2):320‐325. [DOI] [PubMed] [Google Scholar]
- 25. Zoico E, Corzato F, Bambace C, et al. Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriat. 2013;57(3):411‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beasley LE, Koster A, Newman AB, et al. Inflammation and race and gender differences in computerized tomography‐measured adipose depots. Obesity. 2009;17(5):1062‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murnane LC, Forsyth AK, Koukounaras J, et al. Myosteatosis predicts higher complications and reduced overall survival following radical oesophageal and gastric cancer surgery. Eur J Surg Oncol. 2021;47:2295‐2303. 10.1016/j.ejso.2021.02.008 [DOI] [PubMed] [Google Scholar]
- 28. Reisinger KW, Derikx JPM, van Vugt JLA, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. 2016;35(4):924‐927. [DOI] [PubMed] [Google Scholar]
- 29. Margadant CC, Bruns ERJ, Sloothaak DAM, et al. Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur J Surg Oncol. 2016;42(11):1654‐1659. [DOI] [PubMed] [Google Scholar]
- 30. Herrod PJJ, Boyd‐Carson H, Doleman B, et al. Quick and simple; psoas density measurement is an independent predictor of anastomotic leak and other complications after colorectal resection. Tech Coloproctol. 2019;23(2):129‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simonsen C, de Heer P, Bjerre ED, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta‐analysis. Ann Surg. 2018;268(1):58‐69. [DOI] [PubMed] [Google Scholar]
- 32. Levolger S, van Vugt JLA, de Bruin RWF, IJzermans JNM. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102(12):1448‐1458. [DOI] [PubMed] [Google Scholar]
- 33. Tankel J, Yellinek S, Vainberg E, et al. Sarcopenia defined by muscle quality rather than quantity predicts complications following laparoscopic right hemicolectomy. Int J Colorectal Dis. 2020;35(1):85‐94. [DOI] [PubMed] [Google Scholar]
- 34. West MA, Dijk DPJ, Gleadowe F, et al. Myosteatosis is associated with poor physical fitness in patients undergoing hepatopancreatobiliary surgery. J Cachexia Sarcopenia Muscle. 2019;10(4):860‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berkel AEM, Bongers BC, Kotte H, et al. Effects of community‐based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg. 2022;275(2):e299‐e306. 10.1097/sla.0000000000004702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barberan‐Garcia A, Ubre M, Roca J, et al. Personalised prehabilitation in high‐risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. 2018;267(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 37. Gillis C, Fenton TR, Sajobi TT, et al. Trimodal prehabilitation for colorectal surgery attenuates post‐surgical losses in lean body mass: a pooled analysis of randomized controlled trials. Clin Nutr. 2019;38(3):1053‐1060. [DOI] [PubMed] [Google Scholar]
- 38. Ramírez‐Vélez R, Ezzatvar Y, Izquierdo M, García‐Hermoso A. Effect of exercise on myosteatosis in adults: a systematic review and meta‐analysis. J Appl Physiol. 2021;130(1):245‐255. [DOI] [PubMed] [Google Scholar]
- 39. Stump CS, Henriksen EJ, Wei Y, Sowers JR. The metabolic syndrome: role of skeletal muscle metabolism. Ann Med. 2006;38(6):389‐402. [DOI] [PubMed] [Google Scholar]
- 40. Shaw CS, Clark J, Wagenmakers AJM. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr. 2010;30(1):13‐34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


