Abstract
Background
Individuals who experience emotional, physical, or sexual abuse as children suffer from higher rates of major depressive disorder, drug abuse, and suicide. Early life interventions such as peer support groups can be beneficial to adolescents who experience trauma, suggesting that social support is important in facilitating rehabilitation and promoting resiliency to stress. Although there are some animal paradigms that can model how peer-peer interactions influence stress-reactivity, less is known about how individual stress experiences influence the effectiveness of social buffering.
Methods
The vicarious social defeat stress (VSDS) paradigm allows for the assessment of two different stress modalities, physical (PS) and emotional (ES) stress, which confer different levels of stress with similar biological and behavioral outcomes. Using a modified VSDS paradigm in which pairs of mice experience ES and PS together we can begin to evaluate how stress exposure influences the buffering efficacy of social relationships. Adolescent mice (postnatal day 35) were randomly combined into dyads and were allocated into either mutual experience or cohabitation pairs. Within each dyad, one mouse was assigned to the physically stressed (PS) condition and was repeatedly exposed to an aggressive CD1 mouse while the other mouse was designated as the partner. In the mutual experience dyads the partner mice witnessed the defeat bout (ES) while in the cohabitation dyads the partner was separated from the PS mouse and returned after the 10 min defeat bout was terminated (non-stressed). After 10 days of defeat, mice were tested in the social interaction test (SIT), the elevated plus maze (EPM), and the forced swim test (FST).
Results
PS-exposed mice in the cohabitation dyads, but not those in the mutual experience dyads, showed significantly more avoidance of a novel CD1 aggressor or c57BL/6 mouse, in the SIT. Surprisingly, both partner conditions showed avoidance to a CD1. Interestingly, non-stressed partner mice spent less time in the open arms of the EPM, suggesting increased anxiety; only PS-exposed mice in cohabitation dyads showed more time spent immobile in the FST, indicative of increased learned helplessness.
Conclusions
These data suggest that the efficacy of social buffering can be mediated by individual stress experience.
Keywords: adolescence, stress, chronic social defeat stress, vicarious social defeat stress, stress transmission, social buffering
Introduction
Adolescence is a complex developmental period during which individuals begin to establish social relationships that influence how stressful situations may be managed.1,2 Ultimately, these experiences shape and establish proper coping mechanisms. Early life stressors encompass a variety of domains including physical, emotional, and psychological insults, all of which can induce long-lasting detrimental effects. 3 Reports documenting domestic abuse cases from households with multiple children suggest that in about 50% of cases there is at least one sibling that is also being emotionally, physically, or sexually abused. 4 Notably, imbalances in the stress experience between siblings has been found to influence long-term stress perception. While there seems to be some concordance in the depression rates among abused siblings5,6 there is much more variability in mood-related outcomes among siblings if one perceives that they themselves were targeted or unfairly treated compared to others. 7 Therefore, it is likely that various factors, including environmental contexts and stressor magnitude, dictate how situations are processed and whether social support is an effective buffer. Although evidence suggests that social support can attenuate some of the maladaptive consequences of stress, 8 whether the relationship between adolescents exposed to various levels of stress is influenced by an individual’s stress experience, is largely unknown.
Animal models of stress susceptibility, particularly the vicarious social defeat stress (VSDS) model, have confirmed that indirectly experiencing stress (ie, emotional/psychological stress [ES]), can promote behavioral and biological outcomes similar to those produced by directly experiencing it (ie, physical stress [PS]). 9 In this model, a mouse (PS) is repeatedly exposed to aggressive bouts with a larger, more aggressive mouse while another mouse witnesses (ES) the bouts without ever coming into direct contact with the PS or aggressor mouse. However, while the VSDS model is very useful for evaluating social avoidance as a function of stress modality, the experimental mice are individually housed in an antagonistic environment, thus the model does not allow for assessing the consequences of mutually experienced stress. Using a modified resident-intruder stress paradigm to evaluate whether group or isolated housing would influence social avoidance, Li et al found that group housing could attenuate stress-induced social avoidance in ES-, but not PS-exposed mice, 10 demonstrating that social environment does indeed influence stress susceptibility. Nevertheless, alternative paradigms are necessary to evaluate stress-induced deficits that arise from complex enviormental contexts and social relationships, including the influence of non-stressed cohabitants. To this end, we modified the VSDS paradigm by incorporating a partner mouse to the defeat (mutual experience [PS + ES] vs. cohabitation [PS + non-stressed]) to gain a better understanding of how stress influences the buffering capacity of different social relationships. We hypothesized that mice in the mutual experience stress group would have an attenuated stress response, whereas the non-stressed mice in the cohabitation group would have an aggravated stress response due to the presence of a PS-exposed partner.
Methods and Materials
Animals
Five-week-old (postnatal day [PD] 35) male c57BL/6J mice (Jackson Labs, Bar Harbor, Maine), and CD1 retired breeders (Charles River Strain code:022), were used in this study. Mice were allowed a one-week habituation period before any experimental manipulation and were housed at 23–25 °C on a 12 h light/dark cycle. Mice were housed in clear polypropylene boxes containing wood shavings. Upon arrival, c57BL/6J mice were housed four per cage, while CD1 mice were singly housed due to social incompatibility (ie, increased aggression). Experiments were conducted in compliance with the guidelines for the Care and Use of Laboratory Animals 11 and approved by the Texas A&M Institutional Animal Care and Use Committee.
Stress Paradigm
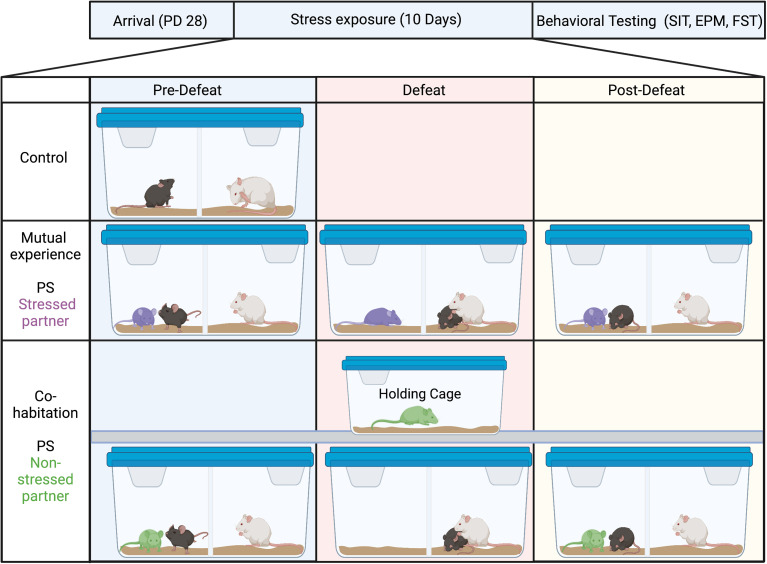
The VSDS paradigm was performed as previously described,9,12 with minor modifications. Two days prior to the start of the VSDS procedure, mice were paired with a weight-matched conspecific. Mice were randomly assigned to either mutual experience or cohabitation dyads. Within each mutual experience dyad, one mouse was assigned to experience ES and the other PS, for 10 consecutive days. Dyads were placed into the empty compartment of a divided hamster cage (48 cm × 26 cm × 15 cm) containing a CD1 aggressor. At the time of the social defeat bout, the mouse in the PS condition was placed into the CD1 compartment, while the mice in the ES condition only observed the defeat bout. After ten minutes, the PS-exposed mice were returned to the adjacent compartment and the pair was housed overnight next to the recently encountered aggressor. The cohabitation dyads differed only in that the partner mouse was removed and placed into a holding cage during the defeat bouts. Given that the partners in the cohabitation dyads do not directly or indirectly experience stress, they are deemed non-stressed partners while the partners in the mutual experience dyads are considered stressed partners. Mice in the control (CON) condition were single housed next to a CD1 but separated by a Plexiglas divider such that each mouse was housed in its own compartment (for a schematic of the experimental design please see Figure 1). A separate cohort of age-matched mice was subjected to a standard social defeat stress (SDS) paradigm 13 and used as a reference of what avoidance behavior is expected to look like in a defeated adolescent mouse.
Figure 1.
Experimental design. The vicarious social defeat stress paradigm was performed as previously described, with minor modifications. Mice were paired with a weight-matched conspecific and randomly assigned to either mutual experience or cohabitation dyads. Within each mutual experience dyad, one mouse was assigned to experience emotional (ES) and the other physical (PS) stress for 10 consecutive days. Pre-defeat, dyads were placed into the empty compartment of a divided hamster cage containing a CD1 aggressor. During the defeat bout the PS- mice were placed into the CD1 compartment, while the ES-exposed mice only observed the defeat bout. After ten minutes, the mice in the PS condition were returned to the adjacent compartment and the pair was housed overnight next to the recently encountered aggressor (post-defeat). The cohabitation dyads differed only in that the partner mouse was removed and placed into a holding cage during the defeat bouts. Given that the partners in the cohabitation dyads do not directly or indirectly experience stress, they are also considered non-stressed partners. Mice in the control (CON) condition were single housed next to a CD1 but separated by a Plexiglas divider such that each mouse was housed in its own compartment. Image Created with BioRender.com.
Social Interaction Test
The social interaction test (SIT) was performed as previously described, 13 with minor modifications. Briefly, the SIT was done as a three-session test consisting of a “no target”, a “target-CD1” session, and a “target-c57BL6/J” session. In the “no target” session, the mice were allowed to freely explore an open field arena (42 cm × 42 cm × 42 cm) for 2.5 min. For the “target-CD1” session, the mouse was removed, and a novel CD1 male mouse was placed into a wire mesh cage, situated in an 8 cm wide space along one side of the arena (ie, “interaction zone”). The experimental mouse was placed back into the arena for 2.5 min and the time spent in the interaction zone was measured. Similarly, during the “target-c57BL6/J” session, a novel c57BL6/J was placed into a clean enclosure and the experimental mouse was returned for another 2.5 min session. A novel mouse was used for either social target. Introduction of the novel CD1 or c57BL6/J was counterbalanced to avoid potential sequence effects.
Elevated Plus Maze
The elevated plus maze (EPM) is commonly used to measure anxiety-like behavior and was performed as previously described. 14 The EPM apparatus is elevated approximately 1 m off the ground and consists of two perpendicular intersecting runways (6 cm × 25 cm); one runway has no walls (open arms) while the other runway has walls on either side (closed arms; 25 cm tall). Mice are placed into the center of the intersecting runways and are allowed to freely explore the maze for 5 min. Mice tend to prefer the safety of the closed arms but will eventually begin to explore the open runway. Increased time spent in the closed arms is interpreted as increased anxiety-like behavior.
Forced Swim Test
Historically, the forced swim test (FST) has been used as determinant of learned helplessness 15 and has been suggested to have high predictive validity of drug antidepressant efficacy. Although the validity of this task has been recently debated, its utility as part of a behavioral battery can still be considered, and its results cautiously interpreted. As previously described, mice were individually placed into a 4 L glass beaker (27 cm × 18 cm) containing 3 L of water (23 ± 2 °C), for 6 min. Eventually, mice adopt an immobile posture characterized by motionless floating and the cessation of struggling behaviors. All sessions were video recorded and total immobility was used as the main behavioral output. Mice with more time spent immobile reflect a state of depression-like behavior.
Corticosterone Analysis
A separate cohort of mice was sacrificed 24 h after 10 days of stress and an SI test. Trunk blood from each mouse was collected in tubes containing Ethylenediaminetetraacetic acid (EDTA). Whole blood samples were centrifuged at 1200 × g for 10 min. Plasma was separated for analysis using a corticosterone (CORT) enzyme-linked immunoassay (ELISA), per the manufacturer’s instructions (Enzo Life sciences). Briefly, plasma was diluted to 1:40 using the provided diluent and added to the wells of an antibody-coated 96-well plate. The plate was washed with a provided wash buffers and the optical density was read using a florescence/colorimetric plate-reader. Corticosterone levels were calculated by comparing the obtained values to the optical density values of prepared corticosterone standards.
Statistical Analysis
Social interaction (SI) with either social target was analyzed with a repeated, one-way analysis of variance (ANOVA), with stress condition (cohabitation or mutual experience) as the independent variable, and SI time, as the dependent variable. Pre-planned analyses comparing the time spent in the interaction zone between the dyad mice and their respective controls (CON conditions) was performed. Significant effects were followed up with Bonferroni post hoc test for multiple comparisons. CORT analysis and behavioral outputs were analyzed by one-way ANOVA, with time spent in open or closed arms (EPM) and time spent immobile (FST) as the dependent variables, respectively. Significant interactions or main effects were followed up with Bonferroni post-hoc tests. Pearson’s r was used to evaluate the correlation of SI time within the dyads. Statistical significance was defined as p < .05. Data are expressed as the mean ± standard error of the mean. Prism 9 was used for all statistical analyzes (GraphPad Software; San Diego, California).
Results
Social Interaction Test
SIT: CD1
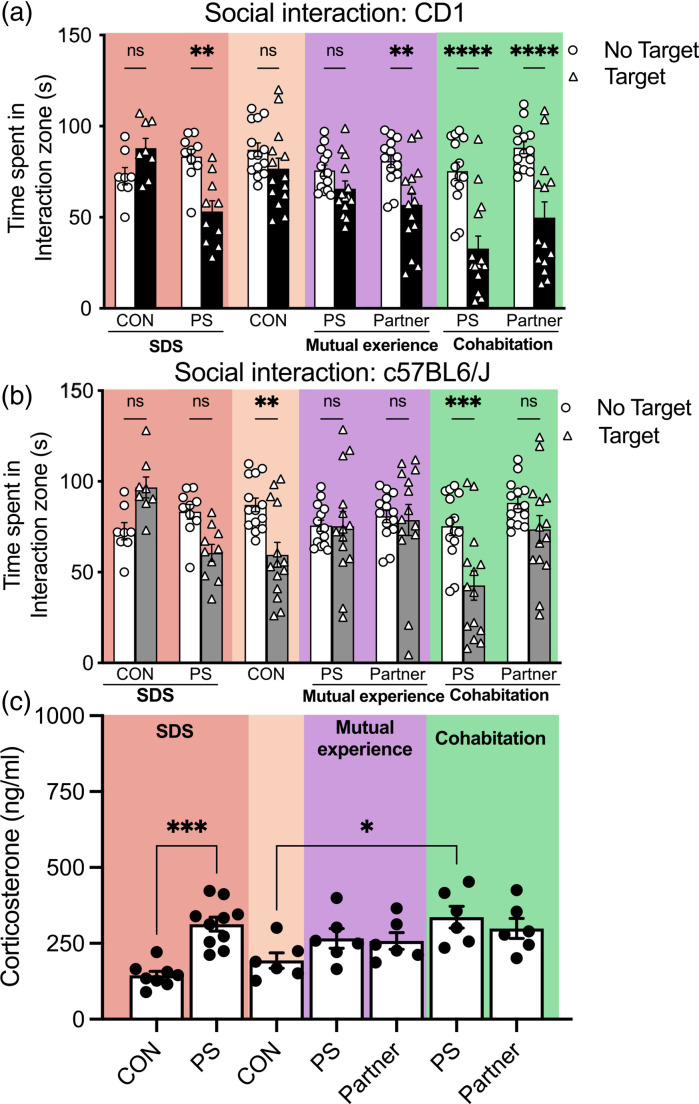
Following ten days of stress exposure, adolescent mice were exposed to a SIT with a CD1 as the social target (n = 6-12/group; Figure 2a). A repeated measures ANOVA revealed a significant difference in SI as a function of stress condition (F(6,81) = 4.83; p < .05) and the presence of a social target (F(1,81) = 58.6; p < .05), as well as an interaction between the variables (F(6,81) = 7.27; p < .05). As expected, adolescent mice exposed to 10 days of SDS show avoidance of a novel CD1 mouse (p < .05). Interestingly, in the dyad defeats, both partner conditions spent significantly less time with the novel CD1 regardless of whether they experienced stress (p < .05). Interestingly, PS-exposed mice with a non-stressed partner (cohabitation), spent significantly less time interacting with the novel aggressor (p < .05). Mice in the CON condition equally explored the interaction zone with or without a CD1 present (p > .05).
Figure 2.
Having a stress-exposed partner buffers stress-induced avoidance in mice exposed to physical stress. Following ten days of stress exposure, adolescent mice were tested in the social interaction test with a CD1 (a) or a c57BL6/J (b) as the social target. Both partner mice spent significantly less time with the novel CD1 regardless of whether they experienced stress (p < .05). Interestingly, the PS-exposed mice with a partner that did not witness the defeat spent less time interacting with the novel CD1, which was attenuated in PS-exposed mice that had a mutually experiencing partner. The mice in the CON condition showed equal levels of exploration in the interaction zone with or without a CD1 present (p > .05). Only the non-stressed partner mice (co-habituating partners) showed avoidance to a novel conspecific. Stress-induced CORT modulation was significantly increased in adolescent mice exposed to SDS (c) compared to their respective controls (p < .05). Similarly, PS mice in the co-habitation dyads showed significantly higher CORT expression compared to their control counterparts (p < .05) *p < .05, **p < .01, ***p < .001.
SIT: c57BL6/J
After ten days of stress exposure, adolescent mice were tested in the SIT with a c57BL6/J as the social target (n = 6-12/group; Figure 2b). A repeated measures ANOVA revealed a significant difference in SI as a function of social target (F(1,81) = 12.74; p < .05) and stress condition (F(6,81) = 3.26; p < .05) and an interaction between the variables (F(6,81) = 5.22; p < .05). Specifically, mice exposed to the standard social defeat stress (SDS) show avoidance of a novel conspecific (p < .05) while in the dyad groups, PS-exposed mice with a non-stressed partner (cohabitation partners) avoided a novel c57BL6/J mouse (p < .05). Taken together the SI results suggest that having a social partner selectively attenuated social avoidance, particularly in PS-exposed mice with stressed partners.
SIT: Partner Correlations
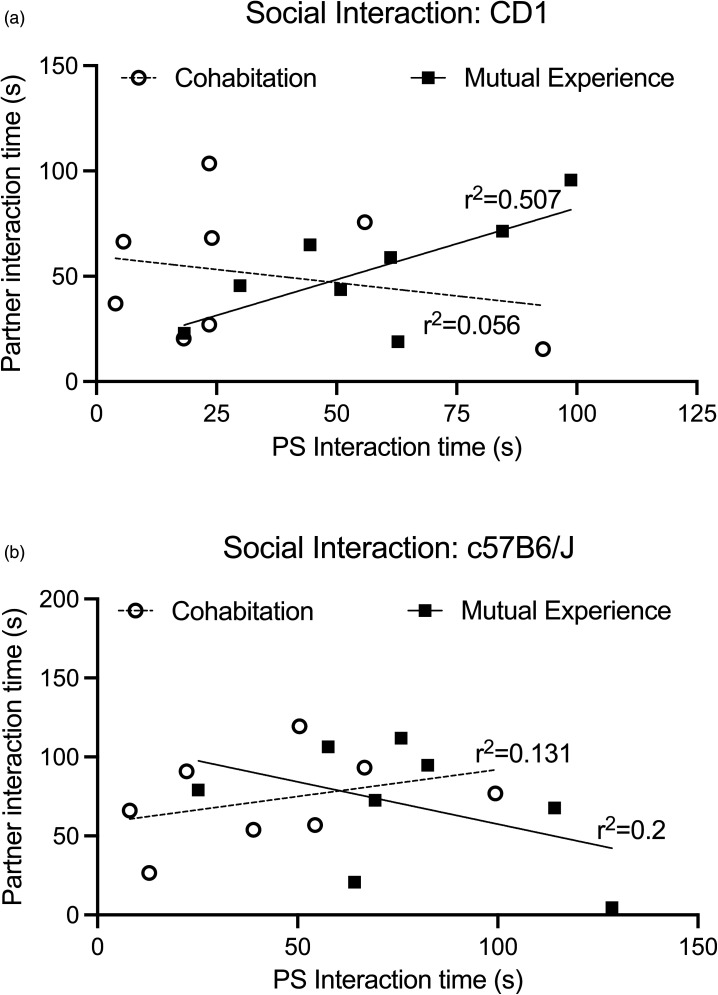
To gain a better understanding of how SI scores were related within the dyads, a correlation analysis was used to determine whether there were any similarities in interaction time between the partner mice. Pearson’s r correlation revealed that mutual experience dyads showed a stronger concurrence in SI score when interacting with a novel CD1 (r = 0.7125; r2 = 0.5076; 95% confidence interval: 0.01568-0.9435; p < .05), compared to co-habitation dyads (r = -0.2380; r2 = 0.05663; 95% confidence interval: −0.8073-0.5607; p < .05; Figure 3a). Interestingly, there was no significant relationship within the mutual experience dyads (r = -0.4482; r2 = 0.2009; 95% confidence interval: −0.8762-0.3749; p < .05) or the cohabitation dyads (r = 0.3622; r2 = 0.1312; 95% confidence interval: −0.4599-0.8499; p < .05) when interacting with a novel c57BL6/J (Figure 3b).
Figure 3.
Interaction correlations within the dyads. A correlation analysis revealed that mutual experience dyads showed a stronger concurrence in SI score when interacting with a novel CD1 (p < .05), compared to cohabitation dyads (a). There was no significant relationship within the mutual experience or cohabitation dyads when interacting with a novel c57BL6/J (b).
Corticosterone Assay
To obtain another measure of stress response, blood was taken 24hrs after the SI test and corticosterone levels were measured (n = 6-10/group; Figure 2c). As expected, adolescent mice showed increased CORT levels. A one-way ANOVA revealed that CORT expression varied as a function of stress condition (F(6,41) = 6.973, p > .05; Figure 2c). Post hoc comparisons between stress conditions and their respective controls revealed that adolescent mice exposed to SDS had higher CORT expression compared to their respective controls (p < .05). Similarly, all other experimental groups showed CORT modulation, however, only PS-exposed mice in the cohabitation dyads showed significantly higher CORT expression compared to their control counterparts (p < .05). Interestingly, CON-exposed mice from the standard SDS which are housed with a c57BL/6j show less CORT induction than the CON-exposed mice housed next to a CD1 (p > .05) Although not statistically significant, this highlights that control housing conditions can play a role in modulating behavioral responses.
Elevated Plus Maze
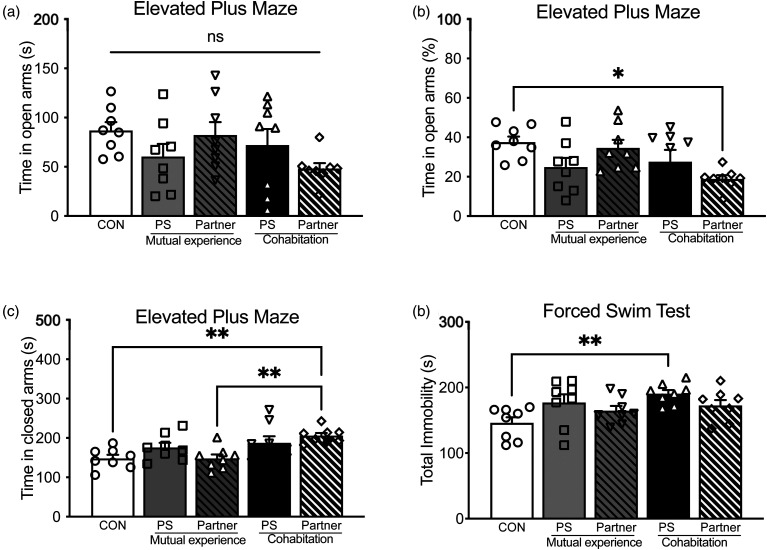
To identify changes in anxiety-like behavior, mice were tested in the EPM 48 h after the last defeat bout (n = 8/group; Figure 4). A one-way ANOVA revealed that raw time spent in the open arms of the EPM did not vary as a function of stress condition (F(4,35) = 1.7, p > .05; Figure 4a), however to account for any time spent in the intersecting center zone of the maze, time in the open arms was recalculated as a percent of time between the open and closed arms ([open arm time/(open arm + closed arm time)]*100) and revealed a difference in percent time spent in the open arms (F(4,35) = 3.259; p > .05; Figure 4b); post hoc analysis showed that the mice in the non-stressed partner condition (cohabitation) spent significantly more time in the closed arms compared to mice in the CON condition and the stressed partner mice (mutual experience) (p < .05, respectively). Further, there was a significant difference in the raw time spent in the closed arms of the EPM (F(4,35) = 4.9, p < .05; Figure 4c). Post hoc tests revealed a significant difference between the non-stressed partner (cohabitation) and CON-exposed mice (p < .05). These data indicated that while we consider the cohabitation partner a “non-stressed” condition, there is a clear maladaptive response to their environment.
Figure 4.
Non-stressed partners show increased anxiety-like behavior. Mice were tested in the elevated plus maze (EPM) 48 h after the last defeat. Raw time spent in the open arms of the EPM was not significantly different between the groups (a); however, time spent as a percent of time in each arm ([open arm time/(open arm + closed arm time)]*100), revealed that non-stressed partners spent less time in the open arms of the EPM (b). The non-stressed partner also had significantly more time spent in the closed arms compared to stressed partner mice and CON mice (p < .05, respectively; c). **p < .01, ***p < .001. To assess depression-like behavior, mice were tested in the forced swim test 48 h after the last defeat bout (d). Physically stressed mice with a non-stressed partner spent significantly more time immobile compared to the mice in the CON condition (p < .05). **p < .01.
Forced Swim Test
Mice were tested in the FST 48 h after the last defeat bout (n = 8/group; Figure 4d). A one-way ANOVA showed that total time spent immobile varied as a function of stress condition (F(4,35) = 3.466, p < 0.05). Post hoc analysis revealed that the mice in the PS condition with a non-stressed partner (cohabitation) spent significantly more time immobile compared to mice in the CON condition (p < .05). Increased time spent immobile in these mice is indicative of increased learned helplessness.
Discussion
Previous studies have found that social support can influence the severity of long-term depression-related behaviors in adolescents. 16 However, the relationship between stress-induced social deficits and the capacity of social support to buffer against these deficits is complex and difficult to model. 17 Also, there is often a greater focus on the effects of social support in subjects that have directly experienced stress and much less is known about how shared stress may influence perceived stress severity or how an individual’s stress experience may influence receptiveness to social buffering. To that end, the current set of experiments were designed to assess whether having a social partner could buffer the maladaptive consequences of different stress modalities. Further, we assessed whether having a stress-exposed partner would influence anxiety- and depression-like behavior in stressed and non-stressed counterparts. Previous work has confirmed that among various mood-related deficits, mice exposed to social defeat (PS) avoid novel social targets (c57BL6/J or CD1) and develop short- and long-term behavioral and biological maladaptations to stressful stimuli.13,18,19 Later work also demonstrated that adult and adolescent mice that do not experience stress directly (emotional stress; ES) also develop long term mood-related deficits.12,20,21 Taking advantage of the VSDS model, we assessed the tendency for social interaction in dyads where one mouse was physically defeated (PS) while their partner mouse either witnessed the social defeat (mutual experience) or was removed from the defeat cage and returned once the stressor was terminated (cohabitation). We found that PS-exposed mice in the mutual experience dyads benefited from having a social counterpart, as they did not display social avoidance, nor did they exhibit increased anxiety/learned helplessness-related behaviors. However, this benefit did not fully extend to both members of the dyad; the partner in the mutual experience condition displayed social avoidance to a CD1. While the mechanisms underlying these findings need to be investigated further, it is possible that the more severely stressed PS-exposed mice developed a tendency to affiliate with their non-threatening partner as a strategy to ameliorate the consequences of being defeated. Notably, studies in humans have shown that the level of mutual affective empathy displayed during a pain-sharing task predicted future prosocial inclination. 22 This would suggest that having an unaggressive partner present in the cage may confer some level of protection and facilitate affiliative behavior. Additionally, increased affiliative behavior by the PS-exposed mouse could also help explain the persistent avoidant behavior toward the CD1, but not the c57B6/J, seen in the stressed partners. That is, it is likely that dyads that are more affiliative have a better exchange of communication allowing them to understand alarm or distress calls between one another. This hypothesis is supported by previous work showing that stress transmission could be mediated, at least in part, by the exchange of chemosensory signals, via anogenital sniffing for example, 23 which requires intact partner investigation.
In contrast to the dyads in the mutual experience condition, we observed social avoidance of a novel c57BL/6J mouse by the PS-exposed mice in the cohabitation condition, suggesting that not having a partner present for the defeat leads to a more generalized maladaptive response to a social target. Non-stressed partner mice showed increased anxiety, which has been shown to lead to less affiliative behavior between conspecifics, and in this case, could have influenced the stress-buffering capacity of having a social partner. Additionally, we found that the non-stressed partner mice displayed avoidance to a CD1 but not a c57BL/6J mouse, despite never witnessing or directly experiencing a defeat. This result suggests that the non-stressed partner is not truly stress-naïve, which calls for further investigation into how stressful environments indirectly influence behavioral reactivity. Still, this finding is in accordance with previous studies demonstrating stress transmissibility to non-stressed counterparts.24,25 Further supporting this finding is that CORT was similarly modulated within the dyads. Although CORT modulation should not be used to qualify susceptibility or resilience, its expression can be used to indicate a stress response. Indeed, all mice in the dyads, as well as those exposed to SDS, showed varying degrees of CORT modulation even if not directly exposed to the defeat. Combined, these findings support our hypothesis that having a social partner could attenuate some stress-induced deficits, however, further research is needed to address how having a stressed social partner impacts responses to non-physical stressors (ie, emotional/psychological stress).
Importantly, the differences we observed in partner-buffering capacity suggest that the quality of social support is an important factor when establishing stress-buffering relationships. This agrees with findings from a previous study assessing the quality of childhood relationships as a predictor of major depressive disorder (MDD) in adulthood, which found that individuals who experienced early life trauma and had poor sibling relationships were more likely to develop mood-related disorders compared to individuals who reported having harmonious sibling relationships. 7 Similarly, individuals who rate themselves as having stable peer groups are less likely to have MDD as adults,7,26 further highlighting the importance of the quality of social support in the context of stress-buffering. Nevertheless, while there is clear overlap in the pathologies that arise from experiencing emotional/psychological or physical stressors, 9 these data underscore the need for better modeling of the various stress modalities which can lead to the different MDD phenotypes.
While our findings can be used to guide future assessments of peer-related social buffering, there are some limitations to our approach. First, we did not perform any advanced behavioral assessments of the interactions between the partners within each dyad at any stage of the experiment, thus, future work would benefit from the implementation of more sensitive behavioral assessment tools. In particular, it would be important to use behavioral tracking tools to extract more nuanced differences in how the dyads interact after each defeat bout. Second, we did not fully examine all possible social relationship combinations within a single behavioral model. We chose to use the VSDS model because it provides more controllability over the experimental conditions. For example, if the dyads had been comprised of two PS-exposed mice, it would have been difficult to control whether each mouse was receiving an equal level of physical stress from the same aggressor within the experimental time window. Nevertheless, future studies using stress models that incorporate other social relationships are needed to better understand how social buffering works in those contexts. Another important limitation of our study was that the dyads used in this experiment were not previously cage mates, and thus had yet to establish a hierarchy. This is important consideration, as a recent study found that hierarchy and a history of winning can influence stress-related behavior in male mice. 27 Finally, our study was conducted exclusively in male mice, thus it would be necessary to see how these peer relationships influence stress responding in female mice. However, while there are female models of social defeat stress28–31 they still require the use of male mice to elicit the stressor, which in this context, could change the nature of the dyad interactions.
Overall, our findings can help guide future studies aimed at assessing the behavioral and neurobiological consequences of exposure to different stress modalities while also highlighting the importance and complexity of establishing appropriate early life-relationships.
Footnotes
Data Availability Statement: Data can be made available by the corresponding author upon reasonable request.
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Texas A&M University, College of Liberal Arts, and the National Institute on Drug Abuse (grant number R01DA046794).
ORCID iDs: Lyonna F. Parise https://orcid.org/0000-0002-7527-8977
Carlos A. Bolaños-Guzmán https://orcid.org/0000-0003-0655-703X
References
- 1.Waldinger RJ, Vaillant GE, Orav EJ. Childhood sibling relationships as a predictor of major depression in adulthood: a 30-year prospective study. Am J Psychiat. 2007;164(6):949–954. [DOI] [PubMed] [Google Scholar]
- 2.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. [DOI] [PubMed] [Google Scholar]
- 3.Thomason ME, Marusak HA. Toward understanding the impact of trauma on the early developing human brain. Neuroscience. 2017;342:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haney SB. Siblings are also at risk for abuse. Pediatrics. 2021;147(5):e2021049930. [DOI] [PubMed] [Google Scholar]
- 5.Kullberg M-L, Schie Cv, Sprang Ev, et al. It is a family affair: individual experiences and sibling exposure to emotional, physical and sexual abuse and the impact on adult depressive symptoms. Psychol Med. 2021;51(12):2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker CJ, Finkelhor D, Turner H. Exposure to parent assault on a sibling as a childhood adversity. Child Abuse Neglect. 2021;122:105310. [DOI] [PubMed] [Google Scholar]
- 7.Witte S, Fegert JM, Walper S. Sibling relationship pattern in the context of abuse and neglect: results from a sample of adult siblings. Child Abuse Neglect. 2020;106:104528. [DOI] [PubMed] [Google Scholar]
- 8.Mackin DM, Perlman G, Davila J, et al. Social support buffers the effect of interpersonal life stress on suicidal ideation and self-injury during adolescence. Psychol Med. 2017;47(6):1149–1161. [DOI] [PubMed] [Google Scholar]
- 9.Warren BL, Vialou VF, Iniguez SD, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Xu H, Wang W. An improved model of physical and emotional social defeat: different effects on social behavior and body weight of adolescent mice by interaction with social support. Frontiers Psychiatry. 2018;9:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Council NR. Guide for the Care and Use of Laboratory Animals - NCBI Bookshelf. 8th ed. Epub ahead of print 2011. DOI: 10.17226/12910. [DOI]
- 12.Sial OK, Warren BL, Alcantara LF, et al. Vicarious social defeat stress: bridging the gap between physical and emotional stress. J Neurosci Methods. 2016;258:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden SA, Covington HE, Berton O, et al. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery KC. The relation between fear induced by novel stimulation and exploratory drive. J Comp Physiol Psychol. 1955;48(4):254–260. [DOI] [PubMed] [Google Scholar]
- 15.Reed AL, Happe HK, Petty F, et al. Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacology. 2008;197(3):433–441. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong MI, Birnie-Lefcovitch S, Ungar MT. Pathways between social support, family well being, quality of parenting, and child resilience: what we know. J Child Fam Stud. 2005;14:269–281. [Google Scholar]
- 17.Kiyokawa Y, Hennessy MB. Comparative studies of social buffering: a consideration of approaches, terminology, and pitfalls. Neurosci Biobehav Rev. 2018;86:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berton O, McClung CA, DiLeone RJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan V, Han M-H, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. [DOI] [PubMed] [Google Scholar]
- 20.Warren BL, Sial OK, Alcantara LF, et al. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci. 2014;36(3–4):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.iniguez SD, Riggs LM, Nieto SJ, et al. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress (Amsterdam, Netherlands). 2014;17(3):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng W, Lou W, Huang X, et al. Suffer together, bond together: brain-to-brain synchronization and mutual affective empathy when sharing painful experiences. Neuroimage. 2021;238:118249. [DOI] [PubMed] [Google Scholar]
- 23.Sterley T-L, Baimoukhametova D, Füzesi T, et al. Social transmission and buffering of synaptic changes after stress. Nat Neurosci. 2018;21(3):393–403. [DOI] [PubMed] [Google Scholar]
- 24.Peen NF, Duque-Wilckens N, Trainor BC. Convergent neuroendocrine mechanisms of social buffering and stress contagion. Horm Behav. 2021;129:104933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carnevali L, Montano N, Tobaldini E, et al. The contagion of social defeat stress: insights from rodent studies. Neurosci Biobehav Rev. 2020;111:12–18. [DOI] [PubMed] [Google Scholar]
- 26.Cicchetti D. Resilience under conditions of extreme stress: a multilevel perspective. World Psychiatry. 2010;9(3):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeClair KB, Chan KL, Kaster MP, et al. Individual history of winning and hierarchy landscape influence stress susceptibility in mice. Elife. 2021;10:e71401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi A, Chung J-R, Zhang S, et al. Establishment of a repeated social defeat stress model in female mice. Sci Rep-uk. 2017;7(1):12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iniguez SD, Flores-Ramirez FJ, Riggs LM, et al. Vicarious social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry. 2018;83(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris AZ, Atsak P, Bretton ZH, et al. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacol. 2018;43(6):1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yohn CN, Dieterich A, Bazer AS, et al. Chronic non-discriminatory social defeat is an effective chronic stress paradigm for both male and female mice. Neuropsychopharmacology. 2019;44(13):2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]