Abstract
Background
To evaluate how a national policy of testing for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) regardless of symptoms was implemented during outbreaks in Dutch nursing homes in the second wave of the pandemic and to explore barriers and facilitators to serial testing.
Methods
We conducted a mixed‐method study of nursing homes in the Netherlands with a SARS‐CoV‐2 outbreak after 15 September 2020. Direct care staff and management from 355 healthcare organizations were invited to participate in a digital survey. A total of 74 out of 355 (20.9%) healthcare organizations participated and provided information about 117 nursing homes. We conducted 26 in‐depth interviews on the outbreak and the testing strategy used. We also conducted four focus group meetings involving managers, physicians, nurses, and certified health assistants. Recordings were transcribed and data were thematically analyzed.
Results
One hundred and four nursing homes (89%) tested residents regardless of their symptoms during the outbreak, and 85 nursing homes (73%) tested the staff regardless of their symptoms. However, interviews showed testing was sometimes implemented during later stages of the outbreak and was not always followed up with serial testing. Barriers to serial testing regardless of symptoms were lack of knowledge of local leaders with decisional making authority, lack of a cohort ward or skilled staff, and insufficient collaboration with laboratories or local public health services. Important facilitators to serial testing were staff willingness to undergo testing and the availability of polymerase chain reaction (PCR) tests.
Conclusions
Serial testing regardless of symptoms was only partially implemented. The response rate of 21% of nursing home organizations gives a risk of selection bias. Barriers to testing need to be addressed. A national implementation policy that promotes collaboration between public health services and nursing homes and educates management and care staff is necessary.
Keywords: COVID‐19, infection prevention and control, skilled nursing facility
Key points
Serial testing regardless of symptoms of residents and staff is partially implemented in Dutch nursing homes.
Implementation of serial severe acute respiratory syndrome coronavirus 2 testing in Dutch nursing homes during an outbreak was complicated by lack of collaboration with public health services, missing of a cohort ward and lack of knowledge of managers and physicians.
Only 21% of Dutch nursing home organizations participated in the survey, which gives a risk of selection bias.
Why does this paper matter?
Implementation of infection prevention and control guidelines is important for the prevention of outbreaks in nursing homes.
INTRODUCTION
In nursing homes (NHs), presymptomatic transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus that causes coronavirus disease (COVID‐19), has been well established. 1 , 2 , 3 , 4 Restricting SARS‐CoV‐2 testing to symptomatic individuals increases the risk of large‐scale outbreaks in NHs. Before vaccines were available, NH residents with COVID‐19 had a higher risk of morbidity and mortality 5 even if they were asymptomatic. 6 In May 2020, the Centers for Disease Control and Prevention (CDC) recommended testing all NH residents for SARS‐CoV‐2 if there was an outbreak in the facility. 7 They also recommended testing residents 5–7 days after exposure to a SARS‐CoV‐2‐positive individual even if the initial test was negative. A further recommendation was for asymptomatic healthcare personnel to be tested after exposure to a SARS‐CoV‐2‐positive individual and to self‐isolate for 14 days following the exposure. 8 Multiple NHs were able to keep outbreaks under control with serial testing of residents and staff. 9 , 10 , 11 , 12 , 13 , 14 , 15
On 27 August 2020, the national advisory board of the Dutch Government recommended unit‐wide weekly testing of all individuals rather than just symptomatic individuals in NHs during a SARS‐CoV‐2 outbreak. 16 However, these guidelines were not implemented until November by the Elderly Care Physician Society and until December by the National Coordination Communicable Disease Control. It is unknown whether serial testing was implemented in Dutch NHs and what the barriers and facilitators to testing were.
In other countries, reported barriers to facility‐wide serial testing during the first wave of the pandemic include insufficient availability of tests, limited personnel, insufficient financial resources, limited public health resources such as laboratory capacity, 17 , 18 , 19 , 20 and the discomfort of nasopharyngeal swabs. 21 In summer 2020, the availability of PCR tests and personal protective equipment (PEE) increased in the Netherlands, which eliminated some of these potential barriers.
Reported facilitators to facility‐wide serial testing were collaborations with local hospitals, hospital laboratories, and local public health officials. 20 , 22 , 23 In a survey of NH staff in the United States, 71.1% said regular testing was important, 24 indicating that healthcare workers are willing to get tested. In addition, a systematic review reported that preparing for an outbreak can prevent or mitigate the outbreak when it happens. 25
In this mixed‐method study of SARS‐CoV‐2 outbreaks during the second wave of the COVID‐19 pandemic, we addressed two research questions:
1. How many NHs with a SARS‐CoV‐2 outbreak implemented unit‐wide serial testing in all individuals, regardless of symptoms, during the outbreak?
2. What are the barriers and facilitators to unit‐wide serial SARS‐CoV‐2 testing in all individuals, regardless of symptoms, during an outbreak in NHs?
METHODS
This was a mixed‐method study. We distributed a digital survey among Dutch NHs. To get an in‐depth insight into how SARS‐CoV‐2 outbreaks were managed, we conducted semi‐structured interviews and focus group meetings. This study was part of a larger project evaluating SARS‐CoV‐2 outbreaks and related infection prevention policies in Dutch NHs during the second wave of the pandemic. The aims of the current study were planned in the main study. The complete protocol is available in Dutch upon request. An ethical waiver was obtained by the Medical Ethics committee of the Academic Medical Center.
Recruitment and participants
Most NHs in the Netherlands are part of a larger organization. Email addresses were collected from www.zorgkaartnederland.nl, designed by the Dutch Patient Federation. In January 2021, we sent an email to 355 healthcare organizations representing over 2500 NHs, inviting them to participate in the study. Nonresponders were reminded twice. We invited those NHs that reported a SARS‐CoV‐2 outbreak after 15 September, shortly after the new policy of weekly testing was advocated. At the peak of the second wave, 873 NHs reported an outbreak. 26
Eligible participants for the questionnaire and interview were staff involved in managing a SARS‐CoV‐2 outbreak and eligible participants for the focus group meetings were managers, elderly care physicians, 27 nurses, and certified nursing assistants. To ensure participants could exchange experiences on an equal footing, focus groups comprised participants of the same profession.
Survey design
Two pilot surveys were iteratively developed in June to August and November and December of 2020 together with managers and physicians of six NHs with a SARS‐CoV‐2 outbreak in the first wave of the pandemic. The survey was designed to evaluate the testing strategy and gain insight into the NH, the reported outbreak (number of tested and infected residents and staff), and the SARS‐CoV‐2 infection prevention policy and testing strategy (Text S1).
Interview and focus group design
We followed the Consolidated Criteria for Reporting Qualitative Research (Table S1).
To design the interview and focus groups, we used two frameworks. First, we organized probing questions based on the framework and results of Houghton. 28 Barriers and facilitators to following the infection prevention and control (IPC) guidelines for respiratory infectious diseases were organized in three levels: organizational, environmental, and individual. Second, we added probing questions based on the framework of Grol and Wensing. 29 On the organizational level, we focused on the social, political, and economic context; on the environmental level, we asked about innovation; and on the individual level, we asked about patient characteristics (Table S2).
Only participants who completed the online survey were interviewed, so staff could reflect on the answers given in the survey. The Dutch public health service is organized into 25 districts and we were able to interview NH staff from 21 of these districts. We started the interview with general questions about the organization and continued with questions about the outbreak and how it was mitigated (Text S2).
Staff focus group meetings included three open questions. The first was about outbreaks in the second wave: how did they happen and did increased testing and PPE help to mitigate the outbreaks? The second was what the staff needed to control SARS‐CoV‐2 outbreaks in NHs. The third focused on serial testing and staff members' experiences with this policy (Text S3).
Data collection
Data were collected between 12 January and 9 April 2021. The online survey was distributed using the online tool Castor 30 and we adhered to the guidelines of Good Clinical Practice. After obtaining informed consent, telephone interviews were conducted by JB, LT, and MSp. To ensure uniformity in interviewing, all interviews were by two researchers. Focus groups were conducted digitally with JB as moderator and LT and MSp as observers. Interviews and focus groups were recorded and transcribed.
Data analysis
Survey data were analyzed using SPSS (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp). Data from interviews and focus group meetings were thematically analyzed according to Braun. Data were analyzed based on theoretical assumptions and with an open approach. 31 First, JB, LT, and MSp read the transcripts to familiarize themselves with the data. After this, they coded three independent interviews inductively. After a team meeting about the initial coding, a coding scheme was developed. This coding scheme reflected the different levels of the framework: organizational, environmental, and individual. Experiences and policy choices were coded separately for the different IPC measures (preparation phase, testing, cohorting, and use of PPE). Next, JB, LT, and MSp coded the rest of the interviews and focus group meetings. Recurring barriers and facilitators to testing were presented at weekly team meetings to reach consensus on results. Data saturation was reached after the 17th interview. We used MAXQDA 2020 (VERBI Software) to analyze data.
RESULTS
Implementation of serial testing
74/355 (20.9%) healthcare organizations participated in the study: 72 in the online questionnaire, providing data from 117 NHs reporting an outbreak of which 24 also participated in focus groups and interviews. The remaining two healthcare organizations participated in the focus groups without completing a questionnaire (Figure S1). Responding and nonresponding NHs were compared in size and location: no differences were found (Table S3). A total of 53% of the NHs reported one outbreak, 23.9% reported two outbreaks, and 23.1% reported three outbreaks or more during the second wave. During the most recent outbreak, a median of eight residents (IQR 3;19) and 30 staff members (IQR 14;75) tested positive for SARS‐CoV‐2 (Table 1).
TABLE 1.
Characteristics of participating staff and facilities
| Characteristic | |
|---|---|
| Respondents participating in survey f , N | 84 |
| Female, N | 59 |
| Profession of survey respondent, % | 15 |
| Elderly care physician | 30 |
| Manager | 14 |
| Board member or secretary | 16 |
| Nurse | 20 |
| Administrator | 5 |
| Certified health assistant | |
| Facilities participating in survey, N | 117 |
| Urbanity, % | |
| Urban a | 12.8 |
| Medium‐sized cities b | 53.0 |
| Rural c | 34.2 |
| Number of residents, % | |
| ≤60 | 33.3 |
| 61–120 | 40.2 |
| ≥121 | 26.5 |
| Number of staff, % | |
| ≤75 | 25.6 |
| 76–150 | 36.8 |
| 150–225 | 17.9 |
| ≥226 | 19.7 |
| Shared bed–bathroom, % | |
| Bedroom | 2.6 |
| Bathroom |
38.5 63.2 |
| None | |
| Number of outbreaks of SARS‐CoV‐2 after 15 September 2020, % | |
| 1 | 53.0 |
| 2 | 23.9 |
| ≥3 | 23.1 |
| Number of residents with a positive test g during the last outbreak, median (IQR d ) | 8 (3,19) |
| Number of staff with a positive test g for SARS‐CoV‐2 during last outbreak, median (IQR d ) e | 30 (14,75) |
| Facilities experiencing an outbreak of SARS‐CoV‐2 in spring–summer 2020, % | 38.5 |
>2500 addresses/km2.
1000–2500 addresses/km2.
<1000 addresses/km2.
Interquartile range (25%;75%).
Three missing.
Eight surveys were answered by two respondents.
PCR or antigen test.
Figure 1 and Table S4 illustrate the SARS‐CoV‐2 testing policies implemented by NHs. Respondents could check multiple boxes about their testing strategies. NHs performed unit‐ or facility‐wide testing of all residents regardless of symptoms (104/117) more often than they did all staff (85/117) (Figure 1). Of NHs performing unit‐wide testing of residents (84/117), 72.6% repeated this at least once a week. Of NHs performing unit‐wide testing of staff (64/117), 62.5% repeated this at least once per week. Table S4 shows that several testing strategies were implemented simultaneously, which was also reflected in the interviews. In some cases, serial testing was only implemented after multiple cases had been identified.
FIGURE 1.
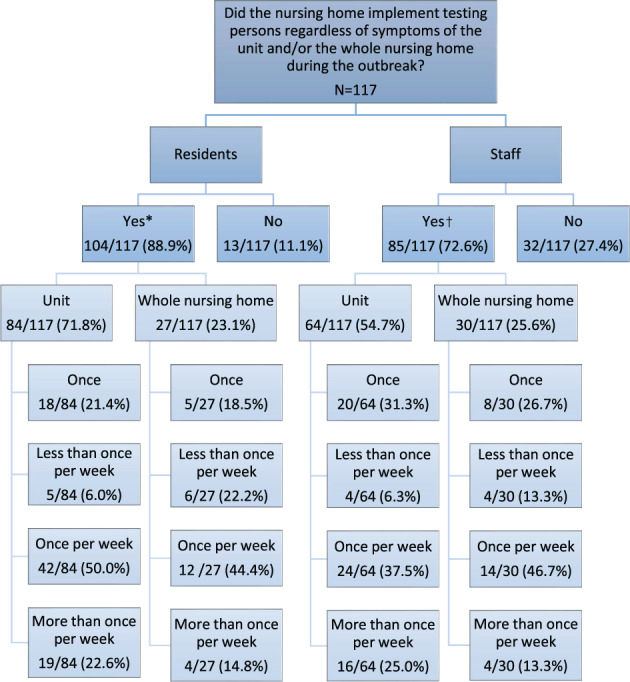
Implementation of SARS‐CoV‐2 testing policy by nursing homes participating in surveys. *Answered question “Which testing policy for residents was implemented at this outbreak?” with “we test residents of the whole unit regardless of symptoms” and/or “we test all residents of the nursing home regardless of symptoms”. 7/104 nursing homes implemented both strategies. †Answered question “Which testing policy for staff was implemented at this outbreak?” with “we test staff of the whole unit regardless of symptoms” and/or “we test all staff of the nursing home regardless of symptoms”. 9/85 nursing homes implemented both strategies
Barriers and facilitators to serial testing regardless of symptoms
We conducted 24 interviews about 26 outbreaks (one healthcare organizations interviewed about 3 different outbreaks). We conducted four focus group meetings with 49 participants from 21/25 health service districts in the Netherlands (Table 2). We identified organizational, environmental, and individual barriers and facilitators to serial testing regardless of symptoms (Figure 2 and Table 3). These factors interacted with each other as described below.
TABLE 2.
Characteristics of focus group interviews
| Characteristic | |
|---|---|
| Participant's interview, N | 31 |
| Female, N | 22 |
| Profession a , N | |
| Elderly care physician | 6 |
| Manager | 6 |
| Board member | 4 |
| Nurse | 6 |
| Certified health assistant | 4 |
| Administrator | 5 |
| Years of work experience, mean | 17 |
| Participants focus groups, N | 21 |
| Female, N | 19 |
| Profession a , N | |
| Elderly care physician | 5 |
| Manager | 8 |
| Nurse | 4 |
| Certified health assistant | 6 |
| Years of work experience, mean | 12 |
One elderly care physician and two managers participated in an interview and a focus group.
FIGURE 2.
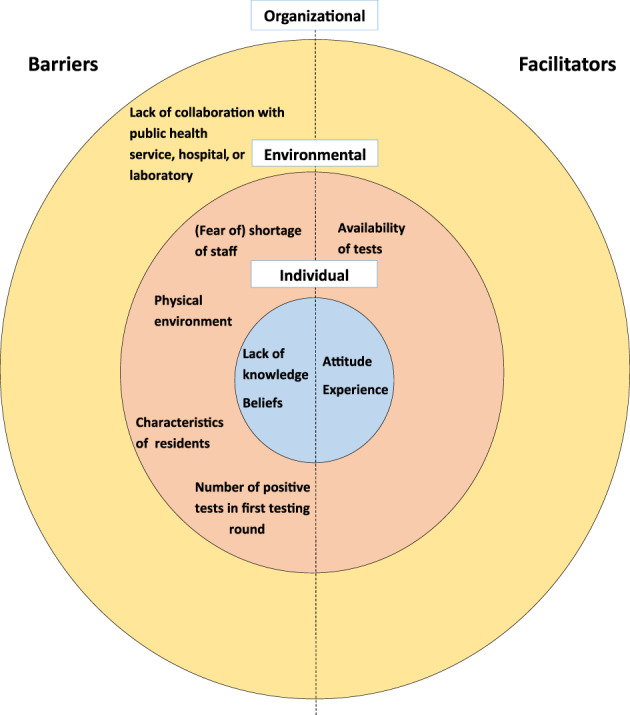
Barriers and facilitators for implementing serial facility‐wide testing of residents and staff of nursing homes on the organizational, environmental, and individual level
TABLE 3.
Barriers and facilitators to weekly testing regardless of symptoms according to interview and focus group participants
| Barriers–Facilitators | Sample brief quotation | |
|---|---|---|
| Organizational factors | ||
| Barrier | Lack of collaboration with external parties | “At one moment, the public health service had a call center with 2000 employees and they were not aware [of the latest guidelines]. […] We were very strict about implementing the guidelines, so we were struggling with the changing advice we were getting and the ambiguous explanations.” |
| Environmental factors | ||
| Barriers | Physical environment | “Our dedicated ward for positive residents was not large enough, then you have a problem” |
| (Fear of) shortage of staff | “We did not implement facility‐wide testing of resident and staff because it's a pragmatic argument. I think, what if ten staff members are positive, where do I get the staff who have to do the work?” | |
| Characteristics of residents | “we waited to test [residents with dementia] until a resident had symptoms or altered behavior, because a lot of residents had a problem with the swab of their throat, that was not OK” | |
| Number of positive tests in first testing round | “if the outbreak is small with only one or two residents, then we could keep it small, but if we had 20 positive tests at once […] then it is very different” | |
| Facilitator | Availability of tests | “The testing went smoothly and we had enough supplies” |
| Individual factors | ||
| Barrier | Lack of knowledge | “In our experience, we had a lot of false negative tests. People felt safe with a negative test, but after a few days they developed symptoms, so the testing did not do us any good.” |
| Beliefs | “We did not transfer the resident from one ward to another: if a resident has dementia and you take this resident out of their own environment, that is difficult.” | |
| Facilitator | Experience | “You learned a lot [from an earlier outbreak] so you know immediately what to do.” |
| Attitude | “there was a high willingness of staff to get tested […] they were afraid of corona […] and they did not want to infect colleagues or residents.” | |
Organizational level
A barrier to serial testing regardless of symptoms was the lack of collaboration with external parties. In the Netherlands, NHs are responsible for managing outbreaks of infectious diseases and the local public health service only assists on request or if the outbreak is large. Most NHs received guidance on hygiene and how to realize a cohort, but not on testing. Some NHs needed help managing their outbreak early on, but local public health services were often overloaded and unavailable. Furthermore, some NHs reported that local public health service staff were inexperienced and unable to advise on the latest testing guidelines.
“At one moment, the public health service had a call center with 2000 employees and they weren't aware [of the latest guidelines]. […] We were very strict about implementing the guidelines, so we were struggling with the changing advice we were getting and the ambiguous explanations.” (Board member, interview 23).
Another barrier to serial testing in some NHs was the lack of collaboration with a laboratory or hospital to provide quick PCR test results. Some NHs reported waiting up to 72 h for a test result.
Environmental factors
A strong barrier to serial testing regardless of symptoms was the lack of a cohort ward in the NH. Multiple NHs could not create a separate ward because of limited staff or architectural constraints of the building. Some physicians and managers felt the test results had no consequences because residents would stay on the same ward anyway, so they did not test further. According to them, it was particularly difficult to isolate residents with dementia after a positive test result, so physicians did not see the advantages of repeated testing. In NHs with individual apartments, staff did not choose to test all residents regardless of symptoms—instead, they kept all residents in quarantine until 10 days after the last symptoms were detected. Characteristics of the outbreak and the residents were also considered when deciding to implement serial testing regardless of symptoms:
“Manager: if the outbreak is small with only one or two residents, then we could keep it small, but if we had 20 positive tests at once and residents walk around and are sick at the same time, then it is very different. I also think that if you [as an organization] choose to give residents with dementia the freedom to walk around the building you risk a lot of contagions.
Moderator: So, with the beliefs of the organization and the layout of the building, the serial testing had no benefit?
Manager: Yes.” (Manager 7, focus group managers).
Shortage of staff not only complicated the creation of a cohort ward but also prevented managers from testing staff regardless of symptoms.
“We did not implement facility‐wide testing of resident and staff because it's a pragmatic argument. I think, what if ten staff members are positive, where do I get the staff who have to do the work?” (Manager, interview 10).
A facilitator to serial testing was the widespread availability of PCR tests—only one NH reported having to wait two days for enough tests. In addition, no NH reported shortage of PPE.
Individual factors
Almost all participants felt they had to choose between the safety and quality of life of their residents. The outcome of this decision depended on the prior experience, knowledge, and beliefs of the participant. For example, if serial testing had been successful in managing a previous outbreak, then NHs were more likely to implement weekly testing regardless of symptoms in a future outbreak. However, if the managers and physicians had limited knowledge then misconceptions could be a barrier to serial testing. This influenced the decisions made on the organizational level. For example, some believed that if one resident tested positive, then all the other residents would test positive as well and it would be no use testing everyone more than once.
“Interviewer: But after the initial testing of the unit, did you continue with testing after a few days?
Physician: No, because in all three common areas residents were positive, so we assumed that all residents in these units were positive.” (Physician, interview 8).
Another misconception was that testing everyone would give more false‐negative test results. If new infections were detected after the initial testing, a number of managers and physicians felt that cases had been missed and that the testing was not reliable.
“Manager 2: In our experience, we had a lot of false negative tests. People felt safe with a negative test, but after a few days they developed symptoms, so the testing did not do us any good.
Moderator: Because you tested all residents and staff after a week?
Manager 2: We tested because somebody got symptoms, so we had a lot of false negatives…
Moderators: But the outbreak did not mitigate after the serial testing?
Manager: Well, we only tested because of symptoms and then we found new cases…” (Focus group managers).
Some managers and physicians believed that testing and transferring residents with dementia would reduce the quality of life too much and that it would be better to continue care as usual instead of testing all residents and isolating positive individuals to a cohort ward.
“We did not transfer the resident from one ward to another: if a resident has dementia and you take this resident out of their own environment, that is difficult.” (Secretary of board, interview 12).
Participants reported that some residents would refuse to be tested but not if a familiar member of staff performed the test.
An important facilitator was the positive attitude of direct care staff toward testing during an outbreak. Staff wanted to know if they were positive and wanted to protect their family, friends, and residents.
DISCUSSION
Serial testing all individuals in an NH regardless of symptoms had been implemented largely in Dutch NHs. Most participating NHs had performed unit‐ or facility‐wide testing of residents (89%) and staff (73%) regardless of their symptoms during a SARS‐CoV‐2 outbreak. However, interviews showed that testing regardless of symptoms is often implemented later on in an outbreak instead of after the first positive PCR test result. Another finding was that testing was often not repeated. Important barriers to serial testing regardless of symptoms were the insufficient collaboration with laboratories or public health services, the lack of a cohort ward, not enough skilled staff, and insufficient knowledge leading to incorrect tradeoffs between safety and quality of life of residents among managers and physicians. Important facilitators were availability of PCR tests and willingness of direct care staff to undergo SARS‐CoV‐2 tests.
Implementing a testing strategy late in an outbreak is consistent with the findings of Lee 32 on how diseases are transmitted and controlled in NHs: outbreak mitigation was hampered by delayed notification of an outbreak, late implementation of IPC guidelines, and delayed recognition of outbreaks. Many NHs that successfully mitigated an outbreak with facility‐wide testing were supported by the local hospital, laboratory, or public health services. 10 , 13 , 33 , 34 Other studies have shown that a cohort ward or being able to isolate positive residents and having enough staff was key facilitators to mitigating an outbreak. 10 , 13 , 34 , 35 This is in agreement with our findings. In the last years, Dutch NHs have been built and renovated to resemble a home environment. However, infection prevention did not play a role and resulted in the experienced problems of not being able to cohort or isolate positive residents. Similar concerns have been addressed in the United States. 36
A hypothesized barrier to serial testing is a fear of staff shortages if too many people test positive. 17 To our knowledge, we are the first study, which showed managers avoided to test staff to prevent shortages. This may promote an outbreak among staff members resulting in more staff shortages and more positive cases in the long run. To overcome this barrier, managers and physicians need to be educated on the rationale of serial testing regardless of symptoms and need to be supported by the local public health service. In addition, misconceptions and lack of knowledge among managers and physicians are important barriers to serial testing regardless of symptoms of residents. They believed that testing and cohorting would be too much of a burden for residents. However, respondents who did implement serial testing of residents did not report these negative effects. This lack of appropriate knowledge means managers and physicians are unconsciously incompetent, and therefore, cannot adequately weigh the risk of a large outbreak against the quality of life of residents. Managers and physicians need to make sure they have appropriate knowledge of IPC guidelines for implementation in their NH. Ethical objections to visiting restrictions 37 , 38 and moving positive residents 39 have been described before. However, implementing facility‐wide testing and isolation or movement of positive residents during early stages of an outbreak could limit virus transmission, minimizing the need for future restrictions. This allows residents to maintain contact with their relatives and the NH to continue normal care, which is especially important for residents with dementia.
We observed almost no shortages in PCR tests and PPE in our participating NHs during the second wave of the pandemic. This contrasts with the shortages reported during SARS‐CoV‐2 outbreaks in NHs during the first wave of the pandemic. 18 , 19 , 20 The willingness of direct care staff to undergo SARS‐CoV‐2 testing is consistent with previous literature 24 , 40 , 41 and was explained by a desire not to infect family or residents.
A strength of our study is the sampling of healthcare organizations in the Netherlands with NH care distributed over rural and urban areas and from small and large organizations. The sample size of 117 assumes 95% confidence with an error of 10% of the survey results 42 21% of Dutch NHs organizations participated, which gives a major risk of selection bias: NHs with more staff and who were not dealing with a current outbreak were more likely to participate. Another limitation is that we did not validate our findings with the public health services, residents, and their informal caregivers.
The findings of our study are important because NH residents are vulnerable to infectious diseases. 43 The barriers to serial testing we describe suggest four ways to prevent infection in NHs. First, NHs need to collaborate with the public health service and the local hospital or laboratory. Second, NHs need to be designed so that infected residents can be isolated in case of an outbreak. Third, management and direct care staff need to be trained and educated on IPC guidelines to avoid misconceptions. Last, NHs need sufficient staff to implement infection prevention measures.
CONFLICT OF INTEREST
Authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Study concept and design: Judith H. van den Besselaar, Martin Smalbrugge, Fleur M.H.P.A Koene, Cees M.P.M. Hertogh, Bianca M. Buurman. Acquisition of data: Judith H. van den Besselaar, Marije Spaargaren, Loes Termeulen. Analysis and did the interpretation of data: Judith H. van den Besselaar, Marije Spaargaren, Loes Termeulen, Martin Smalbrugge, Cees M.P.M. Hertogh. Preparation of manuscript: Judith H. van den Besselaar, Marije Spaargaren, Martin Smalbrugge, Fleur M.H.P.A Koene, Loes Termeulen, Cees M.P.M. Hertogh, Bianca M. Buurman.
SPONSOR'S ROLE
None.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGEMENTS
We thank all participants for their time and contribution to this study.
van den Besselaar JH, Spaargaren M, Smalbrugge M, et al. Implementation of a national testing policy in Dutch nursing homes during SARS‐CoV‐2 outbreaks. J Am Geriatr Soc. 2022;70(4):940‐949. doi: 10.1111/jgs.17687
Funding informationThis work was supported by the Dutch Ministry of Health, Welfare, and Sport.
REFERENCES
- 1. Goldberg SA, Lennerz J, Klompas M, et al. Presymptomatic transmission of severe acute respiratory syndrome coronavirus 2 among residents and staff at a skilled nursing facility: results of real‐time polymerase chain reaction and serologic testing. Clin Infect Dis. 2021;72:686‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS‐CoV‐2 infections in residents of a long‐term care skilled nursing facility ‐ King County, Washington, march 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van den Besselaar JH, Sikkema RS, Koene F, et al. Are presymptomatic SARS‐CoV‐2 infections in nursing home residents unrecognized symptomatic infections? Sequence and metadata from weekly testing in an extensive nursing home outbreak. Age Ageing. 2021;50:1454‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutten JJS, van Loon AM, van Kooten J, et al. Clinical suspicion of COVID‐19 in nursing home residents: symptoms and mortality risk factors. J Am Med Dir Assoc. 2020;21:1791‐1797.e1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bigelow BF, Tang O, Toci GR, et al. Transmission of SARS‐CoV‐2 involving residents receiving dialysis in a nursing home ‐ Maryland, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1089‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Testing Guidelines for Nursing Homes: interim SARS‐CoV‐2 Testing Guidelines for Nursing Home Residents and Healthcare Personnel. Centers for Disease Control and Prevention.
- 8. Interim Guidance on Testing Healthcare Personnel for SARS‐CoV‐2. Centers for Disease Control and Prevention.
- 9. Blain H, Rolland Y, Tuaillon E, et al. Efficacy of a test‐retest strategy in residents and health care personnel of a nursing home facing a COVID‐19 outbreak. J Am Med Dir Assoc. 2020;21:933‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Escobar DJ, Lanzi M, Saberi P, et al. Mitigation of a coronavirus disease 2019 outbreak in a nursing home through serial testing of residents and staff. Clin Infect Dis. 2021;72:e394‐e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garibaldi PMM, Ferreira NN, Moraes GR, et al. Efficacy of COVID‐19 outbreak management in a skilled nursing facility based on serial testing for early detection and control. Braz J Infect Dis. 2021;25:101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karmarkar EN, Blanco I, Amornkul PN, et al. Timely intervention and control of a novel coronavirus (COVID‐19) outbreak at a large skilled nursing facility‐San Francisco, California. Infect Control Hosp Epidemiol. 2020;2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ehrlich HY, Harizaj A, Campbell L, et al. SARS‐CoV‐2 in nursing homes after 3 months of serial, Facilitywide point prevalence testing, Connecticut, USA. Emerg Infect Dis. 2021;27:1288‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krone M, Noffz A, Richter E, Vogel U, Schwab M. Control of a COVID‐19 outbreak in a nursing home by general screening and cohort isolation in Germany, march to may 2020. Euro Surveill. 2021;26:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Psevdos G, Papamanoli A, Barrett N, et al. Halting a SARS‐CoV‐2 outbreak in a US veterans affairs nursing home. Am J Infect Control. 2021;49:115‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Jonge H. Advice of national advisory board of the Dutch Government of 27th of August. In: Ministy of Health WaS, ed. https://www.rijksoverheid.nl/documenten/kamerstukken/2020/09/01/brief-inzake-omt-advies-27-augustus-2020, 2020.
- 17. Blackman C, Farber S, Feifer RA, Mor V, White EM. An illustration of SARS‐CoV‐2 dissemination within a skilled nursing facility using heat maps. J Am Geriatr Soc. 2020;68:2174‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abbasi J. “Abandoned” nursing homes continue to face critical supply and staff shortages as COVID‐19 toll has mounted. JAMA. 2020;324:123‐125. [DOI] [PubMed] [Google Scholar]
- 19. Grabowski DC, Mor V. Nursing home Care in Crisis in the wake of COVID‐19. JAMA. 2020;324:23‐24. [DOI] [PubMed] [Google Scholar]
- 20. Jones K, Mantey J, Washer L, et al. When planning meets reality: COVID‐19 interpandemic survey of Michigan nursing homes. Am J Infect Control. 2021;49:1343‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dumyati G, Gaur S, Nace DA, Jump RLP. Does universal testing for COVID‐19 work for everyone? J Am Med Dir Assoc. 2020;21:1525‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris SC, Resnick AT, England SA, Stern SA, Mitchell SH. Lessons learned from COVID‐19 outbreak in a skilled nursing facility, Washington state. J Am Coll Emerg Phys Open. 2020;1:563‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stall NM, Farquharson C, Fan‐Lun C, et al. A hospital partnership with a nursing home experiencing a COVID‐19 outbreak: description of a multiphase emergency response in Toronto, Canada. J Am Geriatr Soc. 2020;68:1376‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hofschulte‐Beck SL, Hickman SE, Blackburn JL, Mack LM, Unroe KT. Attitudes and experiences of frontline nursing home staff toward coronavirus testing. J Am Med Dir Assoc. 2021;22:215‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Usher K, Durkin J, Gyamfi N, Warsini S, Jackson D. Preparedness for viral respiratory infection pandemic in residential aged care facilities: a review of the literature to inform post‐COVID‐19 response. J Clin Nurs. 2021;1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coronavirus Dashboard of the Dutch Government ‐ Total number of infected locations over time. 2020.
- 27. Koopmans RTCM, Lavrijsen JCM, Hoek JF, Went PBM, Schols JMGA. Dutch elderly care physician: a new generation of nursing home physician specialists. J Am Geriatr Soc. 2010;58:1807‐1809. [DOI] [PubMed] [Google Scholar]
- 28. Houghton C, Meskell P, Delaney H, et al. Barriers and facilitators to healthcare workers' adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev. 2020;2020:CD013582. 10.1002/14651858.CD013582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence‐based practice. Med J Aust. 2004;180:S57‐S60. [DOI] [PubMed] [Google Scholar]
- 30. Castor. pp. Castor is a cloud‐based clinical data management platform, enabling researchers to easily capture and integrate data from clinicians, patients, devices, wearables, and EHR systems.
- 31. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77‐101. [Google Scholar]
- 32. Lee MH, Lee GA, Lee SH, Park Y‐H. A systematic review on the causes of the transmission and control measures of outbreaks in long‐term care facilities: Back to basics of infection control. PLoS ONE. 2020;15:e0229911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oldfield BJ, DeCosta S, Petterson L, Lagarde S, Olson DP. A blueprint for community health center and nursing home partnership: testing for COVID‐19 among residents and staff at long‐term care facilities. J Health Care Poor Underserved. 2021;32:xi‐xviii. [DOI] [PubMed] [Google Scholar]
- 34. Montoya A, Jenq G, Mills JP, et al. Partnering with local hospitals and public health to manage COVID‐19 outbreaks in nursing homes. J Am Geriatr Soc. 2021;69:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seshadri S, Concannon C, Woods JA, McCullough KM, Dumyati GK. "It's like fighting a war with rocks": nursing home healthcare workers' experiences during the COVID‐19 pandemic. Infect Control Hosp Epidemiol. 2021;42:1020‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mody L, Bradley SF, Huang SS. Keeping the "home" in nursing home: implications for infection prevention. JAMA Intern Med. 2013;173:853‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bolcato M, Trabucco Aurilio M, Di Mizio G, et al. The difficult balance between ensuring the right of nursing home residents to communication and their safety. Int J Environ Res Public Health. 2021;18:2484. 10.3390/ijerph18052484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kusmaul N. COVID‐19 and nursing home Residents' rights. J Am Med Dir Assoc. 2020;21:1389‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gugliotta C, Gentili D, Marras S, et al. SARS‐CoV‐2 epidemics in retirement and nursing homes in Italy: a new preparedness assessment model after the first epidemic wave. Int J Environ Res Public Health. 2021;18:5712. 10.3390/ijerph18115712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White EM, Wetle TF, Reddy A, Baier RR. Front‐line nursing home staff experiences during the COVID‐19 pandemic. J Am Med Dir Assoc. 2021;22:199‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarabia‐Cobo C, Pérez V, de Lorena P, et al. Experiences of geriatric nurses in nursing home settings across four countries in the face of the COVID‐19 pandemic. J Adv Nurs. 2021;77:869‐878. [DOI] [PubMed] [Google Scholar]
- 42. Dillman DA, Smyth JD, Christian LM. Internet, Phone, Mail, and Mixed‐Mode Surveys: the Tailored Design Method. John Wiley & Sons, Incorporated; 2014. [Google Scholar]
- 43. Juthani‐Mehta M, Quagliarello VJ. Infectious diseases in the nursing home setting: challenges and opportunities for clinical investigation. Clin Infect Dis. 2010;51:931‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


