Abstract
Head and neck cancer (HNC) affects over 890,000 people annually worldwide and has a mortality rate of 50%. Aside from poor survival, HNC pain impairs eating, drinking, and talking in patients, severely reducing quality of life. Different pain phenotype in patients (allodynia, hyperalgesia, and spontaneous pain) results from a combination of anatomical, histopathological, and molecular differences between cancers. Poor pathologic features (e.g., perineural invasion, lymph node metastasis) are associated with increased pain. The use of syngeneic/immunocompetent animal models, as well as a new mouse model of perineural invasion, provides novel insights into the pathobiology of HNC pain. Glial and immune modulation of the tumor microenvironment affect not only cancer progression but also pain signaling. For example, Schwann cells promote cancer cell proliferation, migration, and secretion of nociceptive mediators, whereas neutrophils are implicated in sex differences in pain in animal models of HNC. Emerging evidence supports the existence of a functional loop of cross-activation between the tumor microenvironment and peripheral nerves, mediated by a molecular exchange of bioactive contents (pronociceptive and protumorigenic) via paracrine and autocrine signaling. Brain-derived neurotrophic factor, tumor necrosis factor α, legumain, cathepsin S, and A disintegrin and metalloprotease 17 expressed in the HNC microenvironment have recently been shown to promote HNC pain, further highlighting the importance of proinflammatory cytokines, neurotrophic factors, and proteases in mediating HNC-associated pain. Pronociceptive mediators, together with nerve injury, cause nociceptor hypersensitivity. Oncogenic, pronociceptive mediators packaged in cancer cell–derived exosomes also induce nociception in mice. In addition to increased production of pronociceptive mediators, HNC is accompanied by a dampened endogenous antinociception system (e.g., downregulation of resolvins and µ-opioid receptor expression). Resolvin treatment or gene delivery of µ-opioid receptors provides pain relief in preclinical HNC models. Collectively, recent studies suggest that pain and HNC progression share converging mechanisms that can be targeted for cancer treatment and pain management.
Keywords: mouth neoplasm, facial pain, nociceptors, carcinoma, squamous cell, peripheral nerves
Head and neck cancer (HNC), the majority being squamous cell carcinoma (SCC), is a heterogeneous group of malignancies that can be broadly classified into human papillomavirus (HPV)–positive and HPV-negative subtypes (see Appendix for more details on HNC). Pain is the most common initial symptom that leads to the diagnosis of HNC (Cuffari et al. 2006) and affects 57% to 70% of patients before cancer treatment (Macfarlane et al. 2012). Pain is rated as the worst symptom and impairs oral function and interpersonal relations (Viet and Schmidt 2012). Pretreatment pain is an independent prognostic factor for survival; ineffective pain control correlates to decreased quality of life (QOL) and poor outcomes (Reyes-Gibby et al. 2014). Mild to moderate pain is commonly treated with nonsteroidal anti-inflammatory drugs (NSAIDs), while opioids remain the gold-standard treatment for severe pain relief. Patients with HNC develop opioid tolerance quickly, leading to dose escalation and significant side effects. A refined understanding of pathobiological mechanisms of HNC pain will aid in the development of effective nonopioid alternatives, which will improve both QOL and survival. In this review, we intend to give an overview of factors contributing to pain heterogeneity in patients and animal models available to study mechanisms of HNC pain. We will provide an update on recent progress regarding peripheral mechanisms of HNC pain, focusing on the interaction between the tumor microenvironment (TME) and peripheral nerves.
Heterogeneity of Pain in Patients
Pain experienced by patients with HNC is variable in phenotype and intensity (see Appendix for pain terminology and common questionnaires used to measure pain in patients with HNC). Patients with oral cancer particularly experience spontaneous pain, function-evoked pain, and function restriction, with function-evoked pain intensity significantly surpassing spontaneous pain intensity based on a validated, 8-item UCSF Oral Cancer Pain Questionnaire (UCSFOCPQ) (Kolokythas et al. 2007). Some patients also experience referred pain (Cuffari et al. 2006; Hechler et al. 2020) and widespread pain (van den Beuken-van Everdingen et al. 2007). A subset of patients with HNC exhibits symptoms suggestive of peripheral neuropathy, such as numbness, tingling, electrical shocks, formication, and otalgia (Okholm et al. 2018; Schmitd et al. 2018; Hechler et al. 2020).
Multiple factors contribute to such pain heterogeneity in HNC. Pain often worsens with HNC progression. Pathological conditions such as perineural invasion (PNI, a neoplastic invasion to the nerve), depth of invasion, tumor size, tumor stage, and nodal metastasis are correlated with increased pain prevalence and intensity (Macfarlane et al. 2012; Reyes-Gibby et al. 2014; Yeh et al. 2016; Bhattacharya et al. 2020; Hechler et al. 2020; Salvo et al. 2020; Naik et al. 2021). Tumor location also affects pain; oral cavity and oropharyngeal tumors have higher pain intensity and prevalence than tumors from other head and neck (HN) regions (Cuffari et al. 2006; Reyes-Gibby et al. 2014). HNC pain is often accompanied by other psychosocial comorbidities such as anxiety, depression, fatigue, and sleep disorders (Reyes-Gibby et al. 2014). Pain is further complicated by potential sexual dimorphism. Although HNC significantly affects more men than women (Auperin 2020), sex difference in HNC pain is controversial as some studies reported more severe pain in women (Hammerlid et al. 2001; Reyes-Gibby et al. 2014; Scheff et al. 2018), or in men, or no sex difference (Connelly and Schmidt 2004; Cuffari et al. 2006; Macfarlane et al. 2012). Such discrepancies may result from differences in sample size, patient population, and pain questionnaire used. Pain intensity in patients with tongue SCC with PNI seems to be similar between men and women based on the UCSFOCPQ (Yeh et al. 2016; Salvo et al. 2020).
Pain heterogeneity can also be attributed to the molecular complexity within the TME. However, clinical studies in this area are limited, and most studies are correlational with the causal effect waiting to be established in preclinical models. The generalizability of pain phenotype in patients requires the use of standardized questionnaires and a well-controlled patient population to minimize misleading conclusions. More or less, the notion that pain intensity and frequency covary with cancer progression suggests shared molecular mechanisms between pain and cancer progression. Indeed, many proteins/genes implicated in PNI in patients with HNC such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) also play a role in pain signaling (Chodroff et al. 2016; Schmitd et al. 2018; Zhang et al. 2019; Saidak et al. 2020; Grayson et al. 2021). Metastatic tumors from patients who report high pain overexpress oncogenic genes enriched for functions in extracellular matrix organization, angiogenesis, and axonogenesis (Bhattacharya et al. 2020).
Preclinical Models of HNC Pain
Several animal models have been developed to study the molecular and cellular mechanisms underlying HNC pain (Table). Recent progress in preclinical models of HNC pain includes syngeneic/immunocompetent mice to model clinical pain and a sciatic nerve (SN) tumor model to specifically study pain associated with PNI. Sex differences in animal models are rigorously assessed. In addition to mechanical and thermal pain assessment at the tumor site, function-evoked pain, referred pain, spontaneous pain, and widespread pain are quantified in animal models to align with the complexity of pain experienced by patients.
Table.
Preclinical Models of HNC-Associated Pain.
| Model | Cell Line | Injection Site | Species | Pain Phenotypes | Behavioral Assessment Tool Used | References |
|---|---|---|---|---|---|---|
| Xenograft | Oral SCC (SCC-158) | Gingiva | Rats | Mechanical allodynia and thermal hyperalgesia in the whisker pad and submandibular skin | von Frey; hot plate | Hironaka et al. (2014); Nagamine et al. (2006) |
| Oral SCC (SCC-158) | Hind paw | Rats | Paw mechanical allodynia; paw thermal hyperalgesia; spontaneous pain | Dynamic plantar; aesthesiometer; Hargreaves test; paw flinching, licking, lifting | Shinoda et al. (2008) | |
| Oral SCC (HSC-2) | Tongue | Mice | Oral function impairment; mechanical hypersensitivity in the whisker pad; spontaneous pain | Food intake; von Frey; conditioned place preference | Chodroff et al. (2016) | |
| Oral SCC (HSC-2) | Paw | Mice | No pain | Feeding; von Frey; conditioned place preference | Chodroff et al. (2016) | |
| Oral SCC (HSC-3) | Tongue | Mice | Facial allodynia; oral function impairment; thermal hyperalgesia; widespread pain; spontaneous pain | von Frey; dolognawmeter; Hargreaves test; tail pinch; conditioned place preference | Tu, Jensen, et al. (2021); Ye et al. (2017); Scheff et al. (2019); Scheff et al. (2018) | |
| Oral SCC (HSC-3) | Hind paw | Mice | Mechanical allodynia; thermal hyperalgesia | von Frey; dynamic plantar aesthesiometer; Hargreaves test | Tu, Jensen, et al. (2021); Ye et al. (2011); Ye, Ono, et al. (2014) | |
| Oral SCC (HSC-3) | Sciatic nerve | Mice | Mechanical allodynia; spontaneous pain; motor defects | von Frey; conditioned place preference; paw licking, shaking; toe-spreading | Salvo et al. (2020) | |
| Allograft | HPV 16+ mouse oropharyngeal SCC | Hind paw | Mice | Paw mechanical allodynia; spontaneous pain | von Frey; Mouse Grimace Scale | Heussner et al. (2021) |
| Supernatant | Oral SCC (HSC-3, SCC-9, SCC-4) | Tongue | Mice | Oral function impairment | Dolognawmeter | Lam et al. (2015); Scheff et al. (2019); Scheff et al. (2018); Scheff et al. (2017); Scheff et al. (2020) |
| Oral SCC (HSC-3, HSC-2, SCC-9) | Hind paw | Mice | Paw mechanical allodynia; paw thermal hyperalgesia; spontaneous pain | von Frey; Hargreaves test; conditioned place preference | Ruparel et al. (2015); Scheff et al. (2020); Lam et al. (2015) | |
| Cancer-conditioned rat Schwann cells (RSC-96) | Hind paw | Mice | Paw mechanical allodynia | von Frey | Salvo et al. (2019) | |
| Cancer-conditioned human Schwann cells | Hind paw | Mice | Paw mechanical allodynia | von Frey | Salvo et al. (2021) | |
| Chemical induced | — | — | Mice | Oral function impairment; spontaneous pain | Dolognaweter; conditioned place preference | Tu, Jensen, et al. (2021); Scheff et al. (2018); Scheff et al. (2017) |
| Naturally occurring | — | — | Cats | Mechanical allodynia in the face; referred mechanical allodynia in the cornea; widespread pain | A pet owner administered quality-of-life questionnaire and visual assessment scoring tool; a clinician assessment questionnaire; electronic von Frey testing; Cochet-Bonnet aesthesiometry | Lai et al. (2021) |
HNC, head and neck cancer; HPV, human papillomavirus; SCC, squamous cell carcinoma.
The xenograft model was developed prior to syngeneic models and remains the most commonly used model in HNC pain research. In orthotopic xenograft models, human HNC cells are transplanted to the tongue, lips, or gingiva in immunocompromised mice; in nonorthotopic xenograft models, human HNC cells are often implanted into the mouse hind paw (Pineda-Farias et al. 2020). The benefits of using these transplantation models are high reproducibility, success rate, and efficiency. The intrinsic weakness of these models is that HNC is heterogeneous, and genetic differences between cell lines result in differences in tumor growth and pain phenotype. The orthotopic models exhibit resemblance to human anatomy and sensory innervations (Pineda-Farias et al. 2020). However, behavioral assessment and tumor monitoring in the orofacial region are more challenging than the nonorthotopic paw model. HNC disrupts food intake in patients, which is recapitulated in animal models (Chodroff et al. 2016; Grayson et al. 2021). A major disadvantage to the immunocompromised xenograft model is that T cells, important to both pain modulation and cancer progression, could not be studied. Recently, a syngeneic model of HPV+ oropharyngeal SCC cells implanted to the hind paw of immunocompetent mice has been reported; these mice exhibit local mechanical allodynia and spontaneous pain (Heussner et al. 2021). Mouse-derived HPV– oral SCC cells (Nagaya et al. 2017) are also available, which can be inoculated into the tongue of immunocompetent mice to produce syngeneic, orthotopic HNC models.
A new xenograft model has been developed by inoculating human oral SCC cells into the mouse SN to study mechanisms of pain associated with PNI (Salvo et al. 2020). PNI is difficult to detect, as rigorous histopathological examination of the resected tumor is required. The SN-PNI model produces reproducible tumor infiltration into the nerve and is easy to access, manipulate, and quantify (Deborde et al. 2018). Similar to patients with PNI, mice with SN-PNI exhibit spontaneous pain, function-evoked pain, and motor defects (Salvo et al. 2020).
Nitroquinoline 1-oxide (4NQO)–induced carcinogenesis exhibits multistage carcinogenicity, which allows for the study of pain from the development through progression. Animals can develop multiple lesions in the oral cavity and along the gastrointestinal tract (Pineda-Farias et al. 2020). 4NQO induces SCC with exophytic or invasive components associated with exophytic, papillary SCCs or invasive SCCs without exophytic histology (Naik et al. 2021). Only the papillary SCC tongue cancer subtype is associated with nociceptive behaviors. Increased tumor size is associated with greater nociceptive behavior in the 4NQO mouse model. PNI is rarely observed in this model.
To study the effect of cancer-derived mediators on acute pain, supernatant collected from oral cancer cells or cancer stromal cells is injected into the mouse hind paw or the orofacial region. Cells can be transfected, and antagonists or neutralizing antibodies can be added to the supernatant. This model can be used to study the contribution of a specific cell type or mediator to the development of pain. The major disadvantage is that it does not recapitulate the complexity of the TME or the chronic nature of cancer pain.
Oral SCC is a common and naturally occurring condition in domestic cats that exhibits similar features to HPV– oral SCC in humans (Lai et al. 2021). Using a combination of pet owner–administered QOL questionnaire and visual assessment scoring tool, a vet assessment questionnaire, and 2 mechanical quantitative sensory testing (QST) methods (electronic von Frey and Cochet–Bonnet), the authors show that cats with tongue cancer exhibit increased mechanical sensitivity around the face and cornea, as well as reduced mechanical thresholds at distant body sites, which can be reversed by the analgesic buprenorphine.
Peripheral Mechanisms
Pain reduction after surgical resection of the tumor, which strongly suggests the importance of peripheral mechanisms underlying HNC-associated pain (Viet and Schmidt 2012). Here we summarize recent findings on 3 major changes (neuroanatomical, electrophysiological, and TME-derived mediators) in the TME that may contribute to the development and maintenance of HNC pain. Some of these changes as discussed below are likely to be model, cell line, sex, or pain phenotype specific; future studies are need to evaluate the generalizability of these findings and to validate the mediators involved.
Neuroanatomical Changes
Tumor Hyperinnervation
The HNC TME is innervated by both primary sensory afferents and sympathetic nerves (Fig. 1; also see Appendix for a brief introduction). The TME secretes neurogenic factors, axon guidance molecules, and extracellular vesicles that induce axonogenesis, which could produce increased nerve innervation to the tumor (Madeo et al. 2018; Bhattacharya et al. 2020; Silverman et al. 2021). Densely innervated tumors are associated with increased metastasis and pain (Mantyh et al. 2002; Madeo et al. 2018; Silverman et al. 2021). It has been shown that tumor cells promote axonogenesis by driving neuronal reprogramming (a phenotypic switch converting sensory to sympathetic innervation) and neurogenesis by recruiting neural progenitor cells or cancer stem cells (Silverman et al. 2021). The effect of neuronal reprogramming and neurogenesis on cancer pain is currently unknown.
Figure 1.
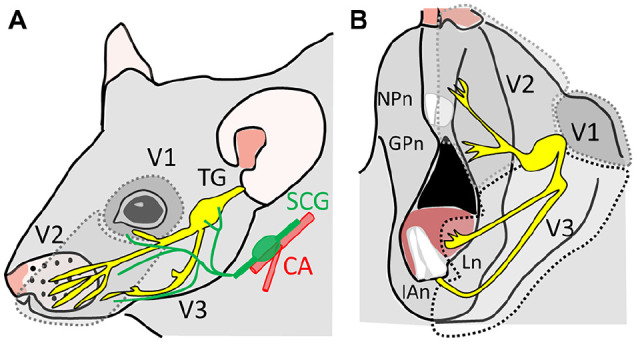
Sensory and sympathetic innervation of the head and neck region. The head and neck region is mainly innervated by 3 branches of the trigeminal ganglion (TG). (A) The lateral-upper view of the innervation territories of the V1 and V2. CA, carotid arteries; SCG, superior cervical sympathetic ganglia. (B) Lateral-lower view of the innervation territory of the V2 and V3 division of the TG. The tongue is innervated by the lingual nerve. GPn, greater palatin nerve; IAn, inferior alveolar nerve; Ln, lingual nerve; NPn, nasopalatine nerve.
Nerve Injury and Schwann Cell Abnormality in PNI
Although neuropathic symptoms are highly suggestive of cancer-induced nerve injury in patients, direct evidence of nerve injury in patients is lacking. In the tongue SCC mouse model produced by HSC-2 inoculation, the nerve injury marker ATF-3 is not upregulated (Grayson et al. 2019), suggesting the lack of nerve damage. Direct evidence of nerve injury and Schwann cell (SC) abnormalities was recently shown in the SN-PNI model. PNI results in swelling of the nerve and a significant reduction in axon count of A- and C-fibers. A-fibers exhibit patterns of myelin degeneration, splitting, and abnormal infoldings into the axons and SC cytoplasm (Salvo et al. 2020). How these anatomic changes affect sensory function needs to be examined further.
Electrophysiological Changes
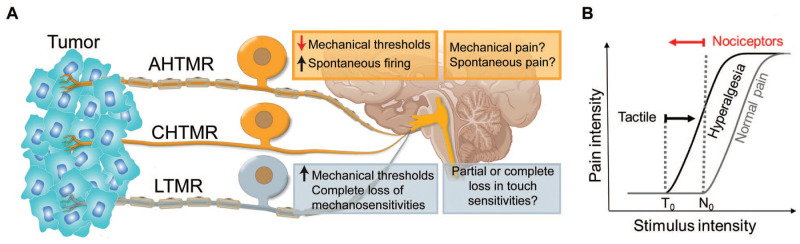
The HN region is innervated by both tactile (low-threshold mechanoreceptor [LTMR]) and nociceptive (high-threshold mechanoreceptor [HTMR]) nerve fibers (Fig. 2A), which are susceptible to change in the TME (Grayson et al. 2019; Gutierrez et al. 2021). Oral SCC cells (HSC-2) inoculated into the tongue produce increased spontaneous firing as well as mechanical sensitization of both slow (C) and fast (A) HTMRs without affecting the tactile afferents (LTMRs) (Grayson et al. 2019). Tongue SCC may also convert mechanically insensitive fibers into a mechanically sensitive phenotype (Grayson et al. 2019). These changes in mechanical sensitivities of nociceptors provide possible mechanistic explanations of tumor-induced pain (Fig. 2A) as HTMR spontaneous firing has been implicated for spontaneous pain, and HTMR sensitization could explain mechanical allodynia or hyperalgesia (von Hehn et al. 2012).
Figure 2.
Electrophysiological alterations of primary sensory neurons in oral cancer. (A) The tumor microenvironment (TME) is innervated by both high-threshold mechanoreceptors (slow CHTMRs or fast AHTMRs; nociceptors) and low-threshold mechanoreceptors (LTMRs; tactile afferents). Hypothetical mechanistic links between primary afferent neuron electrophysiological properties and pain phenotype: LTMR desensitization (an increase in mechanical thresholds) and complete loss of mechanosensitivity in LTMR subpopulations could explain partial or complete loss in touch sensitivities in some patients. HTMR sensitization (a decrease in mechanical thresholds) could explain for mechanical pain. Spontaneous firing in nociceptors could explain spontaneous pain experienced by patients. (B) Graphical representation of the shift in pain thresholds during pain states and the observed peripheral effects. No, nociceptive threshold; To, tactile threshold. LTMR thresholds are increased and HTMR thresholds are decreased in mouse sciatic nerves with PNI. A hyperalgesic state to mechanical stimulation is hypothetically an outcome of an increased excitatory input resulting from HTMR sensitization and a decreased inhibitory input resulting from LTMR desensitization to the pain circuitry.
In the mouse SN-PNI model, HTMRs are mechanically sensitized, while LTMRs are desensitized (Salvo et al. 2020), which is consistent with other cancer and noncancerous models with neuropathic pain (Boada et al. 2019; Boada et al. 2020; Gutierrez et al. 2021). Possible nerve damage caused by PNI is also reflected by decreased conduction velocity of A-fibers and a loss of mechanically sensitive fibers (Salvo et al. 2020). The behavioral sequelae of LTMR desensitization and loss of mechanically sensitive fibers have yet to be determined. Some patients with HNC report orofacial pain together with partial or complete sensory loss (e.g., numbness), which are in line with HTMR sensitization and LTMR desensitization. LTMR desensitization, however, could paradoxically enhance pain. As proposed by Boada et al. (2020), chronic pain is a result of an enhanced HTMR input and a decreased LTMR input (loss of inhibition) to the pain circuitry (Fig. 2B).
TME-Derived Mediators
The TME is composed of tumor and tumor stromal cells, with the latter including immune cells, SCs, fibroblasts, and endothelial cells (Fig. 3). Tumor and tumor stromal cells secrete a variety of pronociceptive and antinociceptive mediators (Fig. 3), most of which are also implicated in cancer progression. Pronociceptive molecules found in the TME, including endothelins, prostaglandins, adenosine triphosphate (ATP), NGF, transmembrane protease, serine 2 (TMPRSS2) released from oral SCC cells, and TRPV1 expressed in sensory neurons, have been reviewed previously (Viet and Schmidt 2012). New studies have found that oral SCC cells release legumain (Tu, Jensen, et al. 2021), cathepsin S (Cat-S) (Tu, Inoue, et al. 2021), TRP-activating lipids (Ruparel et al. 2015), BDNF (Chodroff et al. 2016), tumor necrosis factor (TNF) (Scheff et al. 2017; Salvo et al. 2021), and A disintegrin metalloprotease domain 17 (ADAM17) (Scheff et al. 2020) and show a decrease in resolvin signaling (Ye et al. 2018), which jointly contribute to activation and sensitization of nociceptors. It should be noted that most of these mediators were identified in animal models using female mice. Molecules like BDNF exhibit sex dimorphism in oral cancer pain (Grayson et al. 2021).
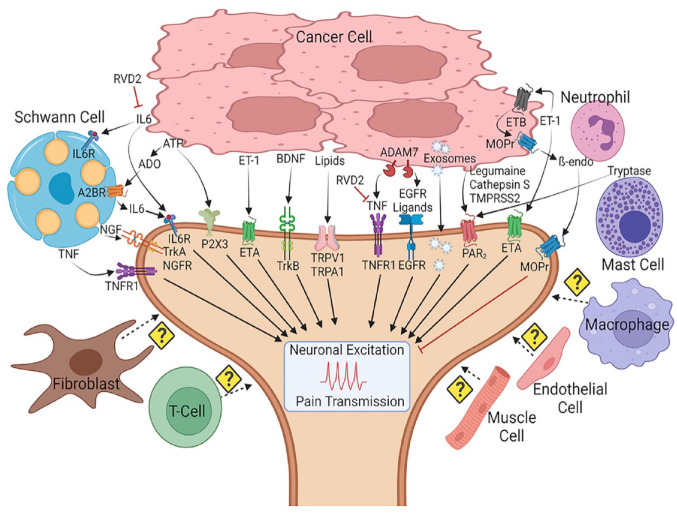
Figure 3.
Tumor microenvironment (TME) and TME-derived mediators in cancer progression and associated pain. The TME is a highly interactive environment that includes cancer cells, immune cells, fibroblasts, endothelial cells, muscle cells, Schwann cells, and the primary afferent nerves. The TME secretes an array of nociceptive mediators and exosomes that lead to neuronal excitation and pain transmission. A few endogenous antinociceptive, anti-inflammatory mechanisms such as µ-opioid signaling and prosolving lipids are suppressed in the TME. The role of fibroblast, macrophages, endothelial cells, T cells, and muscle cells in oral cancer pain is unknown. β-endo, β-endorphin; A2BR, adenosine receptor 2B; ADO, adenosine; MOPr, µ-opioid receptor; TNF, tumor necrosis factor α; TNFR1, tumor necrosis factor receptor 1.
Peripheral glia SCs have emerged as a new player in the TME (Fig. 4). SCs and HNC cells reciprocally interact and influence cancer cell proliferation, migration, invasion, and dispersion (Ein et al. 2019; Salvo et al. 2019; Salvo et al. 2021; Ye et al. 2021). Cancer invasion and growth within the nerve break down the extracellular matrix and compress the nerve, resulting in physical damage to the nerve and injury response in SCs (Salvo et al. 2020). Oral SCC-activated SCs release nociceptive mediators such as TNF, NGF, and interleukin 6 (IL-6); the supernatant collected from oral SCC activates SCs, inducing pain-like behaviors in mice (Salvo et al. 2019; Salvo et al. 2021). However, in nonpainful premalignant pancreatic neoplasia, SC activation and increased IL-6 have been implicated to suppress pain by blocking central glial activation (Demir et al. 2016).
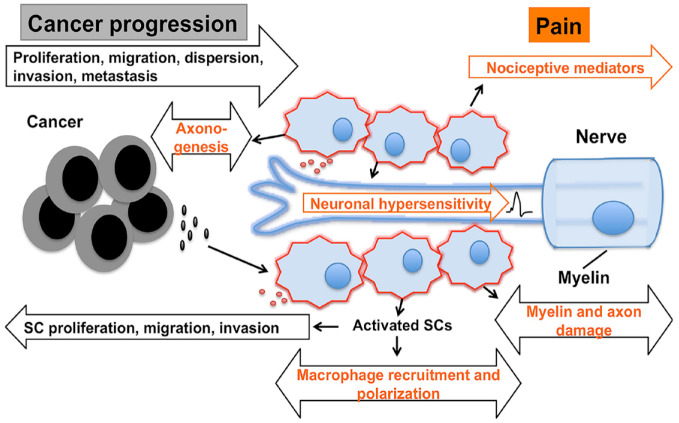
Figure 4.
Schwann cells as a new player in tumor microenvironment (TME) and head and neck cancer (HNC) pain. Cancer TME sends an injury signal to activate Schwann cells (SCs). Activated SCs lose myelin; switch to a migratory, proliferative repair phenotype; and secrete mediators that promote cancer proliferation, migration, dispersion, invasion, and metastasis. Activated SCs release nociceptive mediators, and cancer invading into the nerve further leads to myelin/axon damage, causing neuronal hypersensitivity, nerve injury, and neuropathic pain, in addition to direct neuronal activation and sensitization by cancer mediators. SCs are known to promote axonogenesis and recruitment of macrophages following nerve injury, which could be another mechanism by which SCs can further contribute to HNC progression and associated pain.
Pronociceptive Mediators
Legumain and Cat-S
The presence of protease-activated receptor 2 (PAR2) on sensory neurons and the ability of PAR2 to recruit TRPV1 and other known channels in pain signaling, coupled with the release of many proteases such as trypsin and TMRSS2 by the tumors in HNC, make PAR2 a key target in the study of oral cancer pain (Viet and Schmidt 2012). Recent studies have added to the list of PAR2-activating proteases released by oral cancer cells (Tu, Inoue, et al. 2021; Tu, Jensen, et al. 2021). Legumain, a cysteine protease found in late endosomes and lysosomes in cells, is identified as a biased agonist of PAR2 that induces calcium mobilization, cAMP formation, and activation of protein kinase D (PKD) and protein kinase A (PKA) but not β-arrestin recruitment or PAR2 endocytosis (Tu, Jensen, et al. 2021). Active legumain is elevated in oral SCC patient tumors and evokes nociceptive behaviors in mice. Selective Par2 gene deletion in nociceptive neurons, legumain inhibitors, or legumain gene deletion in SCC cells reduced nociceptive behaviors in several mouse models of oral cancer. Legumain-induced neuronal hyperexcitability in trigeminal neurons is successfully prevented by Par2 deletion, legumain inhibitors, and inhibitors of adenylyl cyclase or PKA. Cat-S is another cysteine protease that is highly active in oral SCC and the TME that activates PAR2 (Tu, Inoue, et al. 2021). Cat-S–induced pain is dependent on the neuronal expression of PAR2 as Cat-S evokes pain in wild-type mice but not in mice in which PAR2 is knocked out in Nav1.8-positive sensory neurons (Tu, Inoue, et al. 2021). These studies show that proteases released by the growing tumor not only work to rearrange the extracellular environment but also can directly activate sensory neurons innervating the surrounding tissue through PAR2 activation. Therefore, blockade of legumain, Cat-S, and other PAR2-cleaving proteases, instead of PAR2 alone, could be a promising therapeutic approach for oral cancer pain.
TNF-α
TNF-α is a “master regulator” cytokine that initiates inflammation and the production of a cascade of other proinflammatory cytokines. Cytokines downstream of TNF-α such as NGF and IL-6 have also been implicated as mediators of oral cancer pain (Ye et al. 2011; Viet and Schmidt 2012; Ye et al. 2018; Salvo et al. 2021). Oral SCC cells and tissues from 4NQO-induced tongue SCC are enriched with TNF-α (Scheff et al. 2017); inhibition of TNF-α signaling reduces orofacial nociception and decreases the number of CD3+ T cells infiltrating into the tongue (Scheff et al. 2017). TNF-α also activates SCs and stimulates SCs to release TNF-α and NGF, which further amplifies oral cancer pain (Salvo et al. 2021).
BDNF
Oral SCC cells release BDNF, and its receptor tropomyosin receptor kinase B (TrkB) is expressed in tumor-innervating lingual neurons (Chodroff et al. 2016; Grayson et al. 2021). Pharmacologically blocking TrkB or deleting TrkB in the trigeminal ganglion (TG) via small interfering RNA (siRNA) administration reversed tumor-induced orofacial pain and mechanical hypersensitivity of AHTMRs in male mice (Chodroff et al. 2016; Grayson et al. 2021). In contrast, female mice exhibited enhanced cancer pain to BDNF-TrkB blockade, opposite to the analgesic effect seen in males (Grayson et al. 2021).
ADAM17
ADAM17 is a membrane-bound enzyme that cleaves more than 80 cell surface proteins, including ligands of the epidermal growth factor receptor (EGFR) family, cytokines (e.g., TNFα), and cytokine receptors (IL-6R and TNFR). In an attempt to screen for pain mediators that are responsible for oral cancer pain, gene expression was compared between nociceptive oral SCC cells and nonnociceptive cell lines (Scheff et al. 2020). ADAM17 and EGFR were identified as 2 potential targets for oral cancer pain. Blocking EGFR reduced oral SCC supernatant–induced nociceptive behaviors in an acute oral SCC pain model.
TRP-activating lipids
Some types of lipids are known to activate or sensitize TRPV1, which is implicated in oral cancer pain (Nagamine et al. 2006; Shinoda et al. 2008; Ye, Bae, et al. 2014). Lipids extracted from oral SCC cell lines HSC-2 and HSC-3 evoke spontaneous pain in rats (Ruparel et al. 2015). However, only lipid extractions from HSC-2 and HSC-4, but not HSC-3, produce thermal hyperalgesia and mechanical allodynia (Ruparel et al. 2015). TRPV1 antagonists block spontaneous and thermal pain evoked by lipid extracts but have no effect on mechanical sensitivity. In contrast, TRPA1 antagonists reverse lipid extract-induced thermal hypersensitivity without affecting lipid-induced spontaneous pain or mechanical allodynia (Ruparel et al. 2015).
Antinociceptive Mediators
OPRM1
Endogenous opioids play an important role in nociceptive regulation; inhibition of this endogenous opioid system amplifies the nociceptive signal. OPRM1, the gene that encodes for the µ-opioid receptor (MOPr), is hypermethylated and transcriptionally silenced in human oral SCC (Viet et al. 2017). OPRM1 is also downregulated in TGs and brainstems of mice with oral SCC (Ye et al. 2017). Adenovirus-mediated reexpression of OPRM1 in oral SCC produces antinociception in a mouse cancer model (Viet et al. 2017). Consistent with previous findings, reexpression of OPRM1 in oral SCC line HSC-3 cells using a novel nonviral hybrid vector (HIV-1 Tat peptide sequence modified with histidine and cysteine residues combined with a cationic lipid) provides analgesia in both tongue SCC and paw SCC xenograft models; the analgesic effect can be reversed by local naloxone administration (Yamano et al. 2017). OPRM1-transfected HSC-3 cells secrete more β-endorphin than control HSC-3 cells (Yamano et al. 2017). Nonviral vectors are less cytotoxic than viral vectors and therefore could be a better therapeutic strategy for cancer pain.
Resolvins
Resolvin D-series (RvDs) are endogenous lipid mediators derived from ω-3 fatty acids that exhibit proresolution and anti-inflammatory actions. These lipid mediators have emerged as a novel class of therapeutics for diseases that involve inflammation, including cancer. In HSC-3 cells, genes encoding for RvD1 and RvD2 are downregulated compared to normal oral keratinocytes; RvD1 and RvD2 treatment in the cell culture inhibits oral cancer proliferation (Ye et al. 2018). RvD2, but not RvD1, results in a suppression of IL-6, C-X-C motif chemokine 10 (CXCL10), and reduction of tumor necrosis. RvD2 also provides short-lasting analgesia in xenograft oral cancer models (Ye et al. 2018).
Exosomes
Cancer-derived exosomes carry different cargo than noncancer-derived exosomes and play an important role in regulating TME by facilitating tumor initiation, progression, and immunosuppression. HNC-derived exosomes can promote axonogenesis, angiogenesis, and neuronal reprogramming (Madeo et al. 2018; Amit et al. 2020; Silverman et al. 2021). Exosomes released by oral SCC cells also promote oral cancer pain (Bhattacharya et al. 2020). Genes identified in oral SCC-derived exosomes have dual functions in both carcinogenesis and pain. Pain induced by the oral SCC supernatant can be reversed by depletion of exosomes (Bhattacharya et al. 2020).
Summary, Emerging Topics, and Future Directions
Converging evidence highlights several shared mechanistic pathways linking carcinogenesis and pain in HNC. Thus, targeting these shared pathways could be a worthwhile therapeutic approach to simultaneously treat cancer and cancer pain. The interplay between TME and peripheral nerves (neurons and Schwann cells) contributes to the pathological evolution of HNC that results in anatomical changes of the nerve (e.g., tumor hyperinnervation, PNI, nerve injury). Mediators that are released in exosomes and nonexosomes into the TME not only result in tumor progression but also cause molecular and electrophysiological changes in peripheral nerves, contributing to chronic HNC pain. However, our understanding of pathobiology underlying HNC pain remains limited. Below we listed a few emerging areas that are still await further studies.
Immune, Glia, and Sympathetic Modulation
Immune cells, glial cells, and sympathetic nerves are known to interact with each other and also exert direct actions on sensory neurons, playing significant roles for the onset, maintenance, and resolution of pain. However, studies on the involvement of these 3 components in HNC pain are very limited. There is a huge growing body of knowledge on immune regulation of HNC, which can be exploited to guide research on immune–glia interaction, immune–sympathetic interaction, and immune–sensory nerve interaction related to pain associated with HNC.
Central Mechanisms
Referred pain and widespread pain have been reported in patients with HNC (Cuffari et al. 2006; van den Beuken-van Everdingen et al. 2007; Hechler et al. 2020) and in animals with oral cancer (Chodroff et al. 2016; Ye et al. 2017; Lai et al. 2021), which suggest the involvement of central sensitization. Although referred pain could also be attributed to peripheral mechanisms such as satellite glia activation (Shinoda et al. 2019) and cross-sensitization of nerves adjacent to the nerve innervating the injury site (Boada et al. 2015; Gutierrez et al. 2021), microglia-mediated central sensitization has been reported in rats with tongue SCC (Tamagawa et al. 2016). The involvement of brainstem and other regions of the central nervous system in nociceptive processing and regulation in HNC pain is poorly understood.
Mechanisms of Sex Differences
Female exhibits more pain in a recent clinical cohort and in animal models of tongue SCC (Scheff et al. 2018; Scheff et al. 2019). The sex differences in tongue SCC-associated pain in mice are not strain specific and do not occur at the primary sensory neuron level (Scheff et al. 2019). Instead, neutrophils, recruited by granulocyte colony-stimulating factor (G-CSF), are more abundant in male mice and release endogenous opioids (Scheff et al. 2018; Scheff et al. 2019). Immune cells and glial cells, both at the peripheral and central level, are critically involved in the sex dimorphism of pain and remain as a largely unexplored area in HNC pain.
Pain in HPV+ HNC
Currently, only 1 study has probed the mechanisms underlying pain associated with HPV+ HNC. Unlike oral SCC models that are marked by inflammation and upregulation of proinflammatory cytokines, only 1 inflammatory cytokine, IL-1β, is significantly upregulated in mouse HPV16+ oropharyngeal SCC, but IL-1β did not contribute to the pain induced by the tumor (Heussner et al. 2021). This study suggests different mechanisms associated with pain between HPV+ and HPV– HNC.
Author Contributions
Y. Ye, D.D. Jensen, M.D. Boada, contributed to conception, design, and data analysis, drafted and critically revised the manuscript; C.T. Viet, H.L. Pan, W.M. Campana, M. Amit, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345221088527 for Advances in Head and Neck Cancer Pain by Y. Ye, D.D. Jensen, C.T. Viet, H.L. Pan, W.M. Campana, M. Amit and M.D. Boada in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by National Institutes of Health grants R01 DE029493 (Y. Ye) and P01 NS113852 (M.D. Boada).
References
- Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA, Cai Y, Wang R, et al. 2020. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 578(7795):449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auperin A. 2020. Epidemiology of head and neck cancers: an update. Curr Opin Oncol. 32(3):178–186. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Janal MN, Veeramachaneni R, Dolgalev I, Dubeykovskaya Z, Tu NH, Kim H, Zhang S, Wu AK, Hagiwara M, et al. 2020. Oncogenes overexpressed in metastatic oral cancers from patients with pain: potential pain mediators released in exosomes. Sci Rep. 10(1):14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada MD, Gutierrez S, Aschenbrenner CA, Houle TT, Hayashida K, Ririe DG, Eisenach JC. 2015. Nerve injury induces a new profile of tactile and mechanical nociceptor input from undamaged peripheral afferents. J Neurophysiol. 113(1):100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada MD, Gutierrez S, Eisenach JC. 2019. Peripheral oxytocin restores light touch and nociceptor sensory afferents towards normal after nerve injury. Pain. 160(5):1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada MD, Martin TJ, Parker R, Houle TT, Eisenach JC, Ririe DG. 2020. Recovery from nerve injury induced behavioral hypersensitivity in rats parallels resolution of abnormal primary sensory afferent signaling. Pain. 161(5):949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodroff L, Bendele M, Valenzuela V, Henry M, Ruparel S. 2016. Express: BDNF signaling contributes to oral cancer pain in a preclinical orthotopic rodent model. Mol Pain. 12:1744806916666841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly ST, Schmidt BL. 2004. Evaluation of pain in patients with oral squamous cell carcinoma. J Pain. 5(9):505–510. [DOI] [PubMed] [Google Scholar]
- Cuffari L, Tesseroli de, Siqueira JT, Nemr K, Rapaport A. 2006. Pain complaint as the first symptom of oral cancer: a descriptive study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 102(1):56–61. [DOI] [PubMed] [Google Scholar]
- Deborde S, Yu Y, Marcadis A, Chen CH, Fan N, Bakst RL, Wong RJ. 2018. An in vivo murine sciatic nerve model of perineural invasion. J Vis Exp. 134:56857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir IE, Tieftrunk E, Schorn S, Saricaoglu OC, Pfitzinger PL, Teller S, Wang K, Waldbaur C, Kurkowski MU, Wormann SM, et al. 2016. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut. 65(6):1001–1014. [DOI] [PubMed] [Google Scholar]
- Ein L, Mei C, Bracho O, Bas E, Monje P, Weed D, Sargi Z, Thomas G, Dinh C. 2019. Modulation of BDNF-TRKB interactions on Schwann cell-induced oral squamous cell carcinoma dispersion in vitro. Anticancer Res. 39(11):5933–5942. [DOI] [PubMed] [Google Scholar]
- Grayson M, Arris D, Wu P, Merlo J, Ibrahim T, Fang-Mei C, Valenzuela V, Ganatra S, Ruparel S. 2021. Oral squamous cell carcinoma-released brain-derived neurotrophic factor contributes to oral cancer pain by peripheral tropomyosin receptor kinase B activation. Pain. 163(3):496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson M, Furr A, Ruparel S. 2019. Depiction of oral tumor-induced trigeminal afferent responses using single-fiber electrophysiology. Sci Rep. 9(1):4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez S, Eisenach JC, Boada MD. 2021. Seeding of breast cancer cell line (MDA-MB-231LUC+) to the mandible induces overexpression of substance P and CGRP throughout the trigeminal ganglion and widespread peripheral sensory neuropathy throughout all three of its divisions. Mol Pain. 17:17448069211024082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerlid E, Bjordal K, Ahlner-Elmqvist M, Boysen M, Evensen JF, Biorklund A, Jannert M, Kaasa S, Sullivan M, Westin T. 2001. A prospective study of quality of life in head and neck cancer patients. Part I: at diagnosis. Laryngoscope. 111(4 Pt 1):669–680. [DOI] [PubMed] [Google Scholar]
- Hechler B, Carlson ER, Heidel RE, Fahmy MD, McCoy JM. 2020. Are oral pain and otalgia predictive of perineural invasion in squamous cell carcinoma of the oral tongue? J Oral Maxillofac Surg Med Pathol. 78(8):1418–1426. [DOI] [PubMed] [Google Scholar]
- Heussner MJ, Folger JK, Dias C, Massri N, Dahdah A, Vermeer PD, Laumet G. 2021. A novel syngeneic immunocompetent mouse model of head and neck cancer pain independent of interleukin-1 signaling. Anesth Analg. 132(4):1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironaka K, Ozaki N, Hattori H, Nagamine K, Nakashima H, Ueda M, Sugiura Y. 2014. Involvement of glial activation in trigeminal ganglion in a rat model of lower gingival cancer pain. Nagoya J Med Sci. 76(3–4):323–332. [PMC free article] [PubMed] [Google Scholar]
- Kolokythas A, Connelly ST, Schmidt BL. 2007. Validation of the university of California San Francisco oral cancer pain questionnaire. J Pain. 8(12):950–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YE, Lascelles BDX, Nolan MW. 2021. Behavioral phenotyping of cancer pain in domesticated cats with naturally occurring squamous cell carcinoma of the tongue: initial validation studies provide evidence for regional and widespread algoplasticity. PeerJ. 9:e11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DK, Dang D, Flynn AN, Hardt M, Schmidt BL. 2015. TMPRSS2, a novel membrane-anchored mediator in cancer pain. Pain. 156(5):923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane TV, Wirth T, Ranasinghe S, Ah-See KW, Renny N, Hurman D. 2012. Head and neck cancer pain: systematic review of prevalence and associated factors. J Oral Maxillofac Res. 3(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo M, Colbert PL, Vermeer DW, Lucido CT, Cain JT, Vichaya EG, Grossberg AJ, Muirhead D, Rickel AP, Hong Z, et al. 2018. Cancer exosomes induce tumor innervation. Nat Commun. 9(1):4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. 2002. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2(3):201–209. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Ozaki N, Shinoda M, Asai H, Nishiguchi H, Mitsudo K, Tohnai I, Ueda M, Sugiura Y. 2006. Mechanical allodynia and thermal hyperalgesia induced by experimental squamous cell carcinoma of the lower gingiva in rats. J Pain. 7(9):659–670. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Nakamura Y, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Allen C, Kobayashi H. 2017. Syngeneic mouse models of oral cancer are effectively targeted by anti-CD44-based NIR-PIT. Mol Cancer Res. 15(12):1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik K, Janal MN, Chen J, Bandary D, Brar B, Zhang S, Dolan JC, Schmidt BL, Albertson DG, Bhattacharya A. 2021. The histopathology of oral cancer pain in a mouse model and a human cohort. J Dent Res. 100(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okholm C, Frendo M, Kiss K, von Buchwald C. 2018. Cheek numbness caused by perineural tumor invasion of the infraorbital nerve: a review of 3 diagnostically challenging cases. Am J Case Rep. 19:296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Farias JB, Saloman JL, Scheff NN. 2020. Animal models of cancer-related pain: current perspectives in translation. Front Pharmacol. 11:610894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS, Hanna EY. 2014. Survival patterns in squamous cell carcinoma of the head and neck: pain as an independent prognostic factor for survival. J Pain. 15(10):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel S, Bendele M, Wallace A, Green D. 2015. Released lipids regulate transient receptor potential channel (TRP)-dependent oral cancer pain. Mol Pain. 11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidak Z, Lailler C, Clatot F, Galmiche A. 2020. Perineural invasion in head and neck squamous cell carcinoma: background, mechanisms, and prognostic implications. Curr Opin Otolaryngol Head Neck Surg. 28(2):90–95. [DOI] [PubMed] [Google Scholar]
- Salvo E, Campana WM, Scheff NN, Nguyen TH, Jeong SH, Wall I, Wu AK, Zhang S, Kim H, Bhattacharya A, et al. 2020. Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain. 161(11):2592–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo E, Saraithong P, Curtin JG, Janal MN, Ye Y. 2019. Reciprocal interactions between cancer and Schwann cells contribute to oral cancer progression and pain. Heliyon. 5(2):e01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo E, Tu NH, Scheff NN, Dubeykovskaya ZA, Chavan SA, Aouizerat BE, Ye Y. 2021. TNFα promotes oral cancer growth, pain, and Schwann cell activation. Sci Rep. 11(1):1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff NN, Alemu RG, Klares R, 3rd, Wall IM, Yang SC, Dolan JC, Schmidt BL. 2019. Granulocyte-colony stimulating factor-induced neutrophil recruitment provides opioid-mediated endogenous anti-nociception in female mice with oral squamous cell carcinoma. Front Mol Neurosci. 12:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff NN, Bhattacharya A, Dowse E, Dang RX, Dolan JC, Wang S, Kim H, Albertson DG, Schmidt BL. 2018. Neutrophil-mediated endogenous analgesia contributes to sex differences in oral cancer pain. Front Integr Neurosci. 12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff NN, Ye Y, Bhattacharya A, MacRae J, Hickman DN, Sharma AK, Dolan JC, Schmidt BL. 2017. Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain. 158(12):2396–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff NN, Ye Y, Conley Z, Quan JW, Lam YVR, Klares R, III, Singh K, Schmidt BL, Aouizerat BE. 2020. A disintegrin and metalloproteinase domain 17-epidermal growth factor receptor signaling contributes to oral cancer pain. Pain. 161(10):2330–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitd LB, Scanlon CS, D’Silva NJ. 2018. Perineural invasion in head and neck cancer. J Dent Res. 97(7):742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Kubo A, Hayashi Y, Iwata K. 2019. Peripheral and central mechanisms of persistent orofacial pain. Front Neurosci. 13:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Ogino A, Ozaki N, Urano H, Hironaka K, Yasui M, Sugiura Y. 2008. Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J Pain. 9(8):687–699. [DOI] [PubMed] [Google Scholar]
- Silverman DA, Martinez VK, Dougherty PM, Myers JN, Calin GA, Amit M. 2021. Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res. 81(6):1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagawa T, Shinoda M, Honda K, Furukawa A, Kaji K, Nagashima H, Akasaka R, Chen J, Sessle BJ, Yonehara Y, et al. 2016. Involvement of microglial P2Y12 signaling in tongue cancer pain. J Dent Res. 95(10):1176–1182. [DOI] [PubMed] [Google Scholar]
- Tu NH, Inoue K, Chen E, Anderson BM, Sawicki CM, Scheff NN, Tran HD, Kim DH, Alemu RG, Yang L, et al. 2021. Cathepsin s evokes PAR2-dependent pain in oral squamous cell carcinoma patients and preclinical mouse models. Cancers (Basel). 13(18):4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu NH, Jensen DD, Anderson BM, Chen E, Jimenez-Vargas NN, Scheff NN, Inoue K, Tran HD, Dolan JC, Meek TA, et al. 2021. Legumain induces oral cancer pain by biased agonism of protease-activated receptor-2. J Neurosci. 41(1):193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. 2007. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 18(9):1437–1449. [DOI] [PubMed] [Google Scholar]
- Viet CT, Dang D, Aouizerat BE, Miaskowski C, Ye Y, Viet DT, Ono K, Schmidt BL. 2017. Oprm1 methylation contributes to opioid tolerance in cancer patients. J Pain. 18(9):1046–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viet CT, Schmidt BL. 2012. Biologic mechanisms of oral cancer pain and implications for clinical therapy. J Dent Res. 91(5):447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hehn CA, Baron R, Woolf CJ. 2012. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 73(4):638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano S, Viet CT, Dang D, Dai J, Hanatani S, Takayama T, Kasai H, Imamura K, Campbell R, Ye Y, et al. 2017. Ex vivo nonviral gene delivery of µ-opioid receptor to attenuate cancer-induced pain. Pain. 158(2):240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Bae SS, Viet CT, Troob S, Bernabe D, Schmidt BL. 2014. Ib4(+) and trpv1(+) sensory neurons mediate pain but not proliferation in a mouse model of squamous cell carcinoma. Behav Brain Funct. 10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Bernabe DG, Salvo E, Viet CT, Ono K, Dolan JC, Janal M, Aouizerat BE, Miaskowski C, Schmidt BL. 2017. Alterations in opioid inhibition cause widespread nociception but do not affect anxiety-like behavior in oral cancer mice. Neuroscience. 363:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, Gibbs JL, Schmidt BL. 2011. Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther. 10(9):1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Ono K, Bernabe DG, Viet CT, Pickering V, Dolan JC, Hardt M, Ford AP, Schmidt BL. 2014. Adenosine triphosphate drives head and neck cancer pain through P2X2/3 heterotrimers. Acta Neuropathol Commun. 2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Salvo E, Romero-Reyes M, Akerman S, Shimizu E, Kobayashi Y, Michot B, Gibbs J. 2021. Glia and orofacial pain: progress and future directions. Int J Mol Sci. 22(10):5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Scheff NN, Bernabe D, Salvo E, Ono K, Liu C, Veeramachaneni R, Viet CT, Viet DT, Dolan JC, et al. 2018. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology. 139:182–193. [DOI] [PubMed] [Google Scholar]
- Yeh CF, Li WY, Chu PY, Kao SY, Chen YW, Lee TL, Hsu YB, Yang CC, Tai SK. 2016. Pretreatment pain predicts perineural invasion in oral squamous cell carcinoma: a prospective study. Oral Oncol. 61:115–119. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu R, Jin R, Fan Y, Li T, Shuai Y, Li X, Wang X, Luo J. 2019. Integrating clinical and genetic analysis of perineural invasion in head and neck squamous cell carcinoma. Front Oncol. 9:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345221088527 for Advances in Head and Neck Cancer Pain by Y. Ye, D.D. Jensen, C.T. Viet, H.L. Pan, W.M. Campana, M. Amit and M.D. Boada in Journal of Dental Research