Abstract
Advances in small-scale robotics and nanotechnology are providing previously unimagined opportunities for new diagnostic and therapeutic approaches with high precision, control, and efficiency. We designed microrobots for tetherless biofilm treatment and retrieval using iron oxide nanoparticles (NPs) with dual catalytic-magnetic functionality as building blocks. We show 2 distinct microrobotic platforms. The first system is formed from NPs that assemble into aggregated microswarms under magnetic fields that can be controlled to disrupt and retrieve biofilm samples for microbial analysis. The second platform is composed of 3-dimensional (3D) micromolded opacifier-infused soft helicoids with embedded catalytic-magnetic NPs that can be visualized via existing radiographic imaging techniques and controlled magnetically inside the root canal, uninterrupted by the soft and hard tissues surrounding the teeth in an ex vivo model. These microrobots placed inside the root canal can remove biofilms and be efficiently guided with microscale precision. The proof-of-concept paradigm described here can be adapted to target difficult-to-reach anatomical spaces in other natural and implanted surfaces in an automated and tether-free manner.
Keywords: nanotechnology, biofilm(s), endodontics, antimicrobials/antimicrobial resistance, diagnostic systems, drug delivery
Microrobots, dynamic automated systems with controlled locomotion and functionality, have been explored in diverse applications in biomedicine. Existing proof-of-concept studies have demonstrated the ability of microrobots to access narrow spaces (capillaries), perform cell manipulation and stimulation, transfer genes selectively, and perform in vivo biopsy (Leong et al. 2009; Kawahara et al. 2013; Sakuma et al. 2013; Qiu et al. 2015; Zhang et al. 2019). Other proposed applications include targeted drug delivery using biodegradable and retrievable robots, precision surgery, biosensing, and detoxification (Li et al. 2017). Unlike traditional robots requiring large motors for function, controlled actuation and navigation of microrobots is achieved through self-propulsion or external control through magnetic, ultrasonic, optical, thermal, and electrical actuation (Li et al. 2017; Ma and Sánchez 2017). Magnetically controlled microrobots have shown diverse applications in biomedicine, including targeted drug/gene delivery, minimally invasive surgery, and imaging-guided therapy (Li et al. 2017; Zhou et al., 2021). Recently, targeted and tetherless stem cell delivery to the brain has been achieved using magnetic control of microrobots conjugated with stem cells (Jeon et al. 2021). Furthermore, microrobots have been maneuvered intravascularly for targeted drug delivery against tumor cells (malignant glioma) in vivo (Zhang et al. 2021).
While microrobots have been developed for specific tasks in the medical field, applications in oral and craniofacial health care remain sparse. The few systems that have been introduced are large-scale haptic robots (e.g., YOMI) to help increase predictability and precision during dental implant surgery (Wu et al. 2019). However, there are ample opportunities for implementing microrobots and automation to develop new therapeutic approaches. Potential applications include automated removal of dental biofilms (plaque) on tooth surfaces, orthodontic appliances, and implants. Microrobotic platforms can be also developed to allow precision-guided therapies to promote soft tissue and bone regeneration. By leveraging the cargo loading ability of microrobots, therapeutic applications can be tailored for drug, stem cell, or growth factor delivery in different oral-craniofacial sites, from deep periodontal pockets and the apical region of the canal to difficult-to-reach temporomandibular spaces to locally stimulate osteogenic or stem cell differentiation. Recent advances in nanotechnology and robotics have enabled the integration of magnetocatalytic nanoparticles with microrobotics principles to target and eradicate biofilms in clinically challenging settings (Li et al. 2019; Zhou et al. 2021). Here, we exploit the catalytic and magnetic properties of iron oxide nanoparticles (NPs) to introduce a microrobotics platform designed for biofilm treatment and diagnostics (Fig. 1A) using endodontic biofilm models as an exemplar proof-of-concept application.
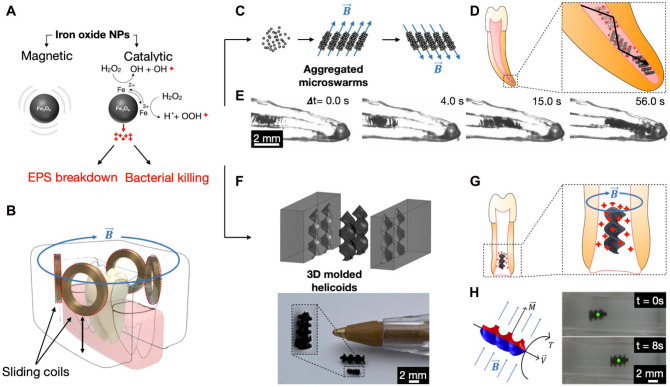
Figure 1.
Therapeutic and diagnostic microrobotic platforms. (A) Multifunctional magnetocatalytic nanoparticle (NP) building blocks for the 2 microrobotic platforms. (B) Schematic of controllable magnetic apparatus for human alveolar process. (C) Formation of aggregated microswarms driven by controlled magnetic fields. (D) Schematic diagram displaying the aggregated microswarms directed toward the apical region of the canal and releasing bioactives simultaneously. (E) Aggregated microswarms are directed toward the apical region of root canal 3D replica. (F) Top panel: molding of NPs embedded 3D helicoids. Bottom panel: miniaturized 3D molded helicoids. (G) Schematic of 3D helicoids guided to the apical region of incompletely formed root releasing bioactives/drugs. (H) Left panel: when a magnetic torque is applied (T), the magnetic dipole moment of the helicoid (M) will seek to align itself with the magnetic field (B), which in turn will lead to the generation of a linear velocity of the helicoid (V). Right panel: controlled locomotion of the 3D helicoid as a result of the generated linear velocity from the applied magnetic torque.
Targeting Endodontic Biofilms
Incomplete root canal disinfection remains the main cause of treatment failure, leading to persistent endodontic infections and apical periodontitis in conventional antimicrobial and regenerative procedures (Nair et al. 1990; Ricucci and Siqueira 2010; Verma et al. 2017). The anatomical complexities in the root canal system hinder effective removal of biofilms (Ricucci et al. 2009; Vera et al. 2012), while approaches to diagnose or assess disinfection efficacy are limited (Abusrewil et al. 2020). New disinfection modalities such as photon-induced, photoacoustic streaming; passive ultrasonic irrigation; antimicrobial nanoparticles; and photodynamic therapy have been proposed and/or used for endodontic biofilm treatment (Căpută et al. 2019; Plotino et al. 2019; Anagnostaki et al. 2020; Raura et al. 2020). However, these approaches lack controlled targeting of the anatomical complexities and are unable to retrieve biofilm samples for diagnostics. New technologies could enable multifunctionality to access difficult-to-reach surfaces and perform biofilm killing, removal, and microbial detection simultaneously for effective and precise endodontic therapy. Such multimodal approaches may be achieved using microrobotics.
Magnetically Driven Catalytic Antibiofilm Robots
In this study, we use iron oxide NPs as the fundamental building blocks to create distinctive microrobots to kill, degrade, and retrieve biofilms from confined spaces found in the root canal system, a challenging anatomical space in the oral cavity. The average diameter of the iron oxide nanoparticles is estimated to be 464.9 nm with a standard deviation of 36.08 nm (Appendix Fig. 1). Iron oxide NPs have catalytic properties that arise from intrinsic enzyme-like (peroxidase-like) activity (Gao et al. 2007) that activates H2O2 to generate bioactive molecules in situ to disrupt oral biofilms (Fig. 1A) (Liu et al. 2018; Naha et al. 2019). Furthermore, iron oxide NPs are widely used in nanomedicine due to their minimal cytotoxicity, excellent physicochemical properties, stability in aqueous solutions, and biocompatibility (Malhotra et al. 2020). One of the first nanoparticle formulations to be approved by the Food and Drug Administration (FDA) for clinical use was an iron oxide nanoparticle contrast agent for magnetic resonance imaging (Feridex, Bayer Healthcare Pharmaceuticals Inc), while another similar preparation (Feraheme, AMAG Pharmaceuticals, Inc) was subsequently approved for treatment of iron-deficiency anemia. Our previous histopathological analysis of gingival, mucosal, and other tissues, including major organs such as liver and kidney, showed no signs of harmful effects, such as proliferative changes, inflammatory responses, or necrosis, indicating high histocompatibility of both in-house and FDA-approved iron oxide NP formulations with H2O2 treatment (Liu et al. 2018; Naha et al. 2019; Huang et al. 2022). Furthermore, NPs can be directed to precise locations using magnetic fields that are generated by permanent magnets or electromagnets (Geilich et al. 2017; Hwang et al. 2019). Magnetic actuation allows tether-free controlled motion, enables a wide variety of robotic locomotion strategies, and can readily and harmlessly penetrate biological and synthetic materials and direct robots’ motion in confined spaces (Fig. 1B) (Chen et al. 2017; Wang et al. 2021).
The flexibility of “NP building blocks” allows formation of reconfigurable aggregated microswarms, inclusion in 3-dimensional (3D)–molded soft helicoids, or even direct 3D printing. Here, we develop 2 platforms. The first system uses magnetic forces to concentrate NPs and form structured aggregates (Fig. 1C). These aggregated microswarms are catalytically active robotic structures that are driven by controlled magnetic fields to the apical region of the tooth (Fig. 1D, E). They are magnetically driven to mechanically remove and retrieve the disrupted biofilms. The second platform is designed to be amenable for micromolding with 3D printing techniques to create specific shapes for intracanal locomotion. Miniaturized, helical robots are fabricated from a matrix of biocompatible hydrogel within which NPs are embedded. The robot shape features a double helix having 1.5 turns swept around a central axis. The molds are fabricated using a stereolithography 3D printer (Fig. 1F). Navigation of microrobots is controlled by rotating magnetic fields generated by electromagnets to propel through fluids in the desired direction (Fig. 1H) (Hwang et al. 2019). The 3D molded helicoids guided to the apical region can be used to transport bioactives or drugs and release them on-site (i.e., apical region) (Fig. 1G), which may lead to multipurpose applications.
Aggregated Microswarms
Iron oxide NPs can reversibly form aggregated microswarms by applying and removing the external magnetic fields on-demand. Once the swarms are formed, collective behavior can be achieved through precise control over the magnetic fields. Functioning as a dynamic collective unit under the magnetic field control, aggregated microswarms can perform complex tasks and can reconfigure and adapt to the confined space (Dong et al. 2021). Using 3D printed teeth replicas generated based on micro–computed tomography (CT) scans of natural human teeth (Moraes et al. 2019), our data show that the aggregated microswarms can be moved and directed by the magnetic field and adapt to the variable canal geometry to reach the apical region. The size of the aggregates between the canal walls had a wide range of sizes (from 154.5 to 844.4 µm). By measuring the microswarms’ width in a corono-apical direction, the range was between 37.8 and 1235.9 µm (Fig. 1E). To demonstrate the ability of aggregated microswarms to disrupt biofilm and retrieve biofilm samples, we initially prepared fluorescently labeled (SYTO 9) 72-h mixed-species biofilms containing Streptococcus gordonii, Enterococcus faecalis, Fusobacterium nucleatum, and Actinomyces israelii (initial inoculum size ~2 × 105, ~2 × 105, ~2 × 108, and ~1 × 108 CFU/mL, respectively, grown anaerobically in BHI media supplemented with 5 mg/mL yeast extract, 0.5 mg/mL L-cysteine HCl, 5 µg/mL hemin, and 0.5 µg/mL vitamin K1), on a glass surface. The NPs (1–2 mg/mL) were introduced near the biofilm surface and catalytically activated by adding 3% H2O2 for bacterial killing/extracellular polymeric substances (EPS) degradation followed by magnetic actuation. Suspended NPs readily formed aggregated assemblies when actuated using a neodymium iron boron magnet (diameter = 6 mm). In addition, we found that as the microswarms mechanically disrupted biofilms, bacterial cells became bound into the aggregated NPs (Fig. 2A, Appendix Fig. 2). This indicates that the aggregated microswarms can be used to retrieve biofilms for the purpose of sampling.
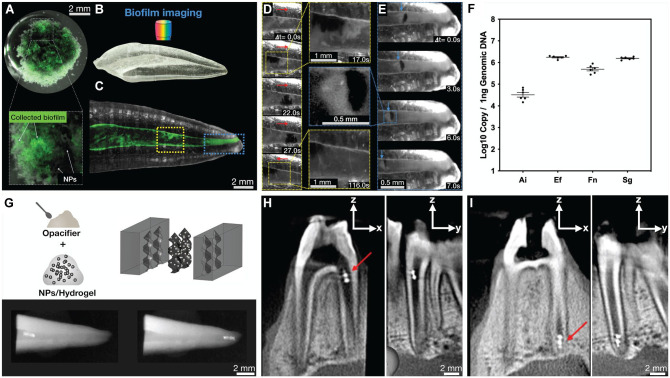
Figure 2.
Biofilm removal, microbial detection, and robot tracking. (A) Mixed-species endodontic biofilm after disruption and collection by aggregated microswarms with a magnet. Bottom panel shows the entrapped biofilm within the aggregated microswarms. (B) The 3-dimensional (3D) printed teeth replica used for biofilm disruption and collection experiments. (C) Fluorescently labeled mixed-species biofilm inside root canal space of 3D replicas with colored boxes indicating areas of biofilm removal in panels D and E, and time-lapse videos were generated using a fluorescence upright stereo microscope with a 1× objective (numerical aperture, 0.25). (D) Time-series images showing aggregated microswarms disrupting biofilm on the wall of the canal. Red arrows indicate the locomotion direction of aggregated microswarms. (E) Time-lapse images showing aggregated microswarms retrieving a biofilm sample from the apical segment of the canal. Blue arrows indicate the location of the biofilm during retrieval. (F) Retrieval of biofilm samples allows for species identification analysis. Ai, Actinomyces israelii; Ef, Enterococcus faecalis; Fn, Fusobacterium nucleatum; Sg, Streptococcus gordonii. (G) Incorporation of opacifiers within the hydrogel helicoids improves radiographic visualization of the helicoids; bottom panel shows dental radiographic images of opacifier-infused 3D helicoids in the coronal (left bottom) and apical parts of the canal (bottom right panel) of an extracted tooth. (H) Sagittal and coronal views of a cone beam computed tomography (CBCT) scan of the ex vivo jaw model showing an opacifier-infused 3D helicoid (red arrow) in the coronal aspect of the canal. (I) Sagittal and coronal views of CBCT images showing opacifier-infused 3D helicoids (red arrow) in the apical region of the canal after magnetic actuation in an apical direction. This figure is available in color online.
To test the ability of the microswarms to disrupt and retrieve samples from the apical regions, we prepared 14-d mixed-species biofilms (same bacterial species and inoculum size as described above) inside vertically positioned 3D printed teeth replicas (media were changed after 24 h, then after every 48 h) (Fig. 2B, C). The NP suspension was introduced in the coronal aspect of the canal (approximately 0.5 mg/mL). The microswarms were actuated and precisely controlled using an electromagnetic apparatus (Fig. 1B) that generates a transverse magnetic field (60 mT) using coaxially positioned electromagnets with iron cores, allowing them to move in a coronal and apical direction to disrupt biofilm inside the canal. The aggregated microswarm had an average size of 797.3 µm with a standard deviation of 153.12 µm between the canal walls and an average size of 1196.6 µm with a standard deviation of 92.1 µm along the corono-apical direction (Fig. 2D). Moreover, aggregated microswarms successfully retrieved a portion of the apical biofilm samples for analysis. The size of the aggregated microswarm retrieving a biofilm sample from the apical region in Figure 2E was 553.4 µm between the canal walls and 165.9 µm corono-apically (Fig. 2E). The retrieved biofilms were collected and subjected to standard quantitative polymerase chain reaction analysis (Fig. 2F). We found that all 4 species were readily detected using species-specific probes. In addition, all nanoparticles appeared to be removed based on visualization using a stereo-zoom microscope. However, elemental analyses such as inductively coupled plasma–optical emission spectrometry and scanning electron microscopy with energy-dispersive spectroscopy are needed to further assess nanoparticle removal.
3D-Molded Helicoids
Given the flexibility of the iron oxide NPs as building blocks, we also developed a molded robotic system using 1 mm negative molds designed in SolidWorks and 3D printed using a ProJet 6000 HD stereolithography printer (3D Systems). Helicoid 3D-molded robots (2 helices wrapped around a central axis) are formed by embedding NPs within a thermo-reversible granulated agar gel (Difco; BD Biosciences) in a final soft robot composition that contains 25% glycerol and 3% (w/v) agar gel within which 10% NPs are embedded. The NP-embedded gel is used to fill the negative molds to create 3D-molded soft helicoids with a size of ~700 µm by 1.5 mm. When directed by the magnetic fields, the 3D-molded helicoids can propel with high efficacy (Hunter et al. 2018) (Fig. 1H). When a magnetic torque is applied, a 3D-molded helicoid rotates around its central axis. This results in forwarding locomotion due to the chiral geometry and corkscrew-like action of the helicoid. Notably, 3D-molded helicoids can be cargo loaded (i.e., drugs) to achieve targeted drug delivery through on-demand drug release (Chiang et al. 2012). The loading and releasing of drugs from microrobots have been shown to be feasible using different approaches, as reviewed by Erkoc et al. (2019). For example, the cargo can be encapsulated in protective microcapsules produced via glass capillary microfluidics using double emulsions. Drugs loaded within the microrobots can be released on-demand by the application of rapid oscillation of a magnetic field as shown previously (Chiang et al. 2012). Here, we loaded the helicoid with a chemical (opacifier) to address an important application hurdle while showing feasibility of incorporating additional substances into the robotic structure.
A practical challenge facing microrobotics is the difficulty of tracking inside the body (Vilela et al. 2018; Nguyen et al. 2021). Real-time update of position would aid precise navigation systems, ensure targeting, and confirm that microrobots are performing their desired function. To address the challenge of real-time tracking and locating the microrobots, bismuth oxide opacifiers were incorporated 3% (w/v) within the matrix of 3D-molded helicoids to enhance their radiopacity (Fig. 2G, top panel). X-ray images using an intraoral radiographic sensor and a dental X-ray unit were obtained and showed the radiopaque 3D helicoids within the canal of the extracted tooth (Fig. 2G, bottom panel). Importantly, we demonstrated the feasibility of magnetic actuation of 3D-molded helicoids in an ex vivo model of an intact pig jaw model (Sierra for Medical Science, Inc.) uninterrupted by the soft and hard tissues surrounding the teeth (intact periodontium). In addition, we show an exciting prospect of controlled movement of the robots from the coronal to the apical region within the canal, as imaged with cone beam computed tomography (CBCT) (Fig. 2H, I), demonstrating clinical feasibility of intracanal imaging of microrobots. We have previously shown that the permeability of the hydrogel allows for the diffusion of H2O2 to react with embedded NPs to release reactive oxygen species (ROS) (Hwang et al. 2019). This property, together with the ability of 3D microrobots to controllably propel through fluids inside the canal, opens exciting opportunities for both biofilm disruption and drug delivery in situ.
The multiple functions demonstrated above may also lead to new methods for biofilm treatment and disinfection in regenerative endodontics where mechanical instrumentation is not desirable and treatment relies mainly on the chemical effect of antimicrobials (Fouad AF 2020). The 3D-molded soft helicoids can be precisely controlled and actuated within the root canal space of incompletely formed roots to deliver bioactives on-site to achieve chemical and mechanical disruption of biofilm, while being tracked and located with available radiographic imaging techniques. Notably, 3D-molded helicoids can be loaded with microcapsules that contain a clinically used antibiotic mixture (i.e., metronidazole and ciprofloxacin) (Ruparel et al. 2012; Chrepa et al. 2020). The cargo (antibiotics) could be released on-demand via application of rapid oscillation of the magnetic field; we will be assessing this possibility in future studies.
Summary and Perspectives
We introduce a new concept for precision biofilm treatment using microrobotic platforms with potential for both diagnostics and therapeutics in the root canal system, a clinically challenging anatomical space in the oral cavity. We demonstrate the feasibility of tetherless magnetic control uninterrupted by the presence of the intact periodontium surrounding the tooth. We use iron oxide nanoparticles as fundamental building blocks, which enables significant design flexibility. NPs can form aggregated microswarms by controlled magnetic fields, which are capable of navigating inside the tooth canal and simultaneously disrupting biofilms and retrieving biological information for pathogen detection using microbiological DNA analysis. Alternatively, NPs can be incorporated into 3D-molded soft helicoids that are precisely driven to the apical third of the canal. In addition, incorporation of opacifiers into the helicoids provides opportunities for real-time tracking using existing radiographic imaging. Inclusion of additional chemicals opens possibilities for drug loading, transport, and on-site release in a controllable manner. However, additional research is needed to assess reliability and reproducibility of this system (particularly using automated routines) as well as compare its efficacy against current disinfection modalities. Initial studies show that aggregated microswarms display higher killing activity against mixed-species biofilms when compared to 3% sodium hypochlorite (Appendix Fig. 3).
We envision further development of microrobots that can be precisely guided to reach the apical area and used to deliver bioactives or drugs in situ to achieve both chemical disinfection and tissue regeneration. Future studies may expand the possibilities for robotics applications to detect, treat, and remove biofilms associated with other infectious diseases and biofouling of dental/medical devices or implants. We hope that this topic, which remains relatively unexplored in the dental field, can stimulate the utilization of the latest advances in small-scale robotics and nanotechnology that are providing previously unimagined opportunities for new diagnostic and therapeutic approaches with high precision, control, and efficiency.
Author Contributions
A. Babeer, contributed to conception, design, data acquisition, and interpretation, drafted and critically revised the manuscript; M.J. Oh, contributed to conception, design, data acquisition, and analysis, drafted and critically revised the manuscript; Z. Ren, contributed to conception, design, data acquisition, and analysis, drafted the manuscript; Y. Liu, B. Karabucak, contributed to conception and design, critically revised the manuscript; F. Marques, A. Poly, contributed to design, drafted the manuscript; E. Steager, H. Koo, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_00220345221087149 for Microrobotics for Precision Biofilm Diagnostics and Treatment by A. Babeer, M.J. Oh, Z. Ren, Y. Liu, F. Marques, A. Poly, B. Karabucak, E. Steager and H. Koo in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Institute for Dental and Craniofacial Research (NIDCR) grant R01 DE025848 (H. Koo) and R56 DE029985 (E. Steager). M.J. Oh is a recipient of the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (No. NRF-2021R1A6A3A03044553). Y. Liu and Z. Ren are recipients of the Colgate–Palmolive Fellowship. Z. Ren is supported by the NIDCR Postdoctoral Training Program under award number R90DE031532. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
ORCID iDs: Z. Ren  https://orcid.org/0000-0002-3553-1958
https://orcid.org/0000-0002-3553-1958
References
- Abusrewil S, Alshanta OA, Albashaireh K, Alqahtani S, Nile CJ, Scott JA, McLean W. 2020. Detection, treatment and prevention of endodontic biofilm infections: what’s new in 2020? Crit Rev Microbiol. 46(2):194–212. [DOI] [PubMed] [Google Scholar]
- Anagnostaki E, Mylona V, Parker S, Lynch E, Grootveld M. 2020. Systematic review on the role of lasers in endodontic therapy: valuable adjunct treatment? Dent J. 8(3):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Căpută PE, Retsas A, Kuijk L, Chávez de, Paz LE, Boutsioukis C. 2019. Ultrasonic irrigant activation during root canal treatment: a systematic review. J Endod. 45(1):31–44.e13. [DOI] [PubMed] [Google Scholar]
- Chen X-Z, Hoop M, Mushtaq F, Siringil E, Hu C, Nelson BJ, Pané S. 2017. Recent developments in magnetically driven micro- and nanorobots. Appl Mater Today. 9:37–48. [Google Scholar]
- Chiang W-L, Ke C-J, Liao Z-X, Chen S-Y, Chen F-R, Tsai C-Y, Xia Y, Sung H-W. 2012. Pulsatile drug release from PLGA hollow microspheres by controlling the permeability of their walls with a magnetic field. Small. 8(23):3584–3588. [DOI] [PubMed] [Google Scholar]
- Chrepa V, Joon R, Austah O, Diogenes A, Hargreaves KM, Ezeldeen M, Ruparel NB. 2020. Clinical outcomes of immature teeth treated with regenerative endodontic procedures—a San Antonio study. J Endod. 46(8):1074–1084. [DOI] [PubMed] [Google Scholar]
- Dong Y, Wang L, Yuan K, Ji F, Gao J, Zhang Z, Du X, Tian Y, Wang Q, Zhang L. 2021. Magnetic microswarm composed of porous nanocatalysts for targeted elimination of biofilm occlusion. ACS Nano. 15(3):5056–5067. [DOI] [PubMed] [Google Scholar]
- Erkoc P, Yasa IC, Ceylan H, Yasa O, Alapan Y, Sitti M. 2019. Mobile microrobots for active therapeutic delivery. Adv Ther. 2(1):1800064. [Google Scholar]
- Fouad AF. 2020. Contemporary microbial and antimicrobial considerations in regenerative endodontic therapy. J Endod. 46(9):S105–S114. [DOI] [PubMed] [Google Scholar]
- Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, et al. 2007. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2(9):577–583. [DOI] [PubMed] [Google Scholar]
- Geilich BM, Gelfat I, Sridhar S, van de Ven AL, Webster TJ. 2017. Superparamagnetic iron oxide-encapsulating polymersome nanocarriers for biofilm eradication. Biomaterials. 119:78–85. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hsu JC, Koo H, Cormode DP. 2022. Repurposing ferumoxytol: diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics. 12(2):796–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter EE, Brink EW, Steager EB, Kumar V. 2018. 3D micromolding of small-scale biological robots. In: 2018 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS). Nagoya: IEEE. p. 1–6. https://ieeexplore.ieee.org/document/8481196/. [Google Scholar]
- Hwang G, Paula AJ, Hunter EE, Liu Y, Babeer A, Karabucak B, Stebe K, Kumar V, Steager E, Koo H. 2019. Catalytic antimicrobial robots for biofilm eradication. Sci Robot. 4(29):eaaw2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Park SH, Kim E, Kim J, Kim SW, Choi H. 2021. A magnetically powered stem cell-based microrobot for minimally invasive stem cell delivery via the intranasal pathway in a mouse brain. Adv Healthc Mater. 10(19):2100801. [DOI] [PubMed] [Google Scholar]
- Kawahara T, Sugita M, Hagiwara M, Arai F, Kawano H, Shihira-Ishikawa I, Miyawaki A. 2013. On-chip microrobot for investigating the response of aquatic microorganisms to mechanical stimulation. Lab Chip. 13(6):1070. [DOI] [PubMed] [Google Scholar]
- Leong TG, Randall CL, Benson BR, Bassik N, Stern GM, Gracias DH. 2009. Tetherless thermobiochemically actuated microgrippers. Proc Natl Acad Sci. 106(3):703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Esteban-Fernández de Ávila B, Gao W, Zhang L, Wang J. 2017. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci Robot. 2(4):eaam6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nickel R, Wu J, Lin F, van Lierop J, Liu S. 2019. A new tool to attack biofilms: driving magnetic iron-oxide nanoparticles to disrupt the matrix. Nanoscale. 11(14):6905–6915. [DOI] [PubMed] [Google Scholar]
- Liu Y, Naha PC, Hwang G, Kim D, Huang Y, Simon-Soro A, Jung H-I, Ren Z, Li Y, Gubara S, et al. 2018. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat Commun. 9(1):2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Sánchez S. 2017. Self-propelling micro-nanorobots: challenges and future perspectives in nanomedicine. Nanomed. 12(12):1363–1367. [DOI] [PubMed] [Google Scholar]
- Malhotra N, Lee J-S, Liman RAD, Ruallo JMS, Villaflores OB, Ger T-R, Hsiao C-D. 2020. Potential toxicity of iron oxide magnetic nanoparticles: a review. Molecules. 25(14):3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes RDR, Santos TMPD, Marceliano-Alves MF, Pintor AVB, Lopes RT, Primo LG, Neves AA. 2019. Reciprocating instrumentation in a maxillary primary central incisor: a protocol tested in a 3D printed prototype. Int J Paediatr Dent. 29(1):50–57. [DOI] [PubMed] [Google Scholar]
- Naha PC, Liu Y, Hwang G, Huang Y, Gubara S, Jonnakuti V, Simon-Soro A, Kim D, Gao L, Koo H, et al. 2019. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano. 13(5):4960–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair PNR, Sjögren U, Krey G, Kahnberg K-E, Sundqvist G. 1990. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 16(12):580–588. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Go G, Jin Z, Darmawan BA, Yoo A, Kim S, Nan M, Lee SB, Kang B, Kim C, et al. 2021. A magnetically guided self-rolled microrobot for targeted drug delivery, real-time X-ray imaging, and microrobot retrieval. Adv Healthc Mater. 10(6):2001681. [DOI] [PubMed] [Google Scholar]
- Plotino G, Grande NM, Mercade M. 2019. Photodynamic therapy in endodontics. Int Endod J. 52(6):760–774. [DOI] [PubMed] [Google Scholar]
- Qiu F, Fujita S, Mhanna R, Zhang L, Simona BR, Nelson BJ. 2015. Magnetic helical microswimmers functionalized with lipoplexes for targeted gene delivery. Adv Funct Mater. 25(11):1666–1671. [Google Scholar]
- Raura N, Garg A, Arora A, Roma M. 2020. Nanoparticle technology and its implications in endodontics: a review. Biomater Res. 24(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricucci D, Siqueira JF. 2010. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod. 36(8):1277–1288. [DOI] [PubMed] [Google Scholar]
- Ricucci D, Siqueira JF, Bate AL, Pitt Ford TR. 2009. Histologic investigation of root canal–treated teeth with apical periodontitis: a retrospective study from twenty-four patients. J Endod. 35(4):493–502. [DOI] [PubMed] [Google Scholar]
- Ruparel NB, Teixeira FB, Ferraz CCR, Diogenes A. 2012. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 38(10):1372–1375. [DOI] [PubMed] [Google Scholar]
- Sakuma S, Sugita M, Arai F. 2013. Fabrication of nanopillar micropatterns by hybrid mask lithography for surface-directed liquid flow. Micromachines. 4(2):232–242. [Google Scholar]
- Vera J, Siqueira JF, Ricucci D, Loghin S, Fernández N, Flores B, Cruz AG. 2012. One- versus two-visit endodontic treatment of teeth with apical periodontitis: a histobacteriologic study. J Endod. 38(8):1040–1052. [DOI] [PubMed] [Google Scholar]
- Verma P, Nosrat A, Kim JR, Price JB, Wang P, Bair E, Xu HH, Fouad AF. 2017. Effect of residual bacteria on the outcome of pulp regeneration in vivo. J Dent Res. 96(1):100–106. [DOI] [PubMed] [Google Scholar]
- Vilela D, Cossío U, Parmar J, Martínez-Villacorta AM, Gómez-Vallejo V, Llop J, Sánchez S. 2018. Medical imaging for the tracking of micromotors. ACS Nano. 12(2):1220–1227. [DOI] [PubMed] [Google Scholar]
- Wang L, Meng Z, Chen Y, Zheng Y. 2021. Engineering magnetic micro/nanorobots for versatile biomedical applications. Adv Intell Syst. 3(7):2000267. [Google Scholar]
- Wu Y, Wang F, Fan S, Chow JK-F. 2019. Robotics in dental implantology. Oral Maxillofac Surg Clin N Am. 31(3):513–518. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Z, Gao C, Fan X, Pang Y, Li T, Wu Z, Xie H, He Q. 2021. Dual-responsive biohybrid neutrobots for active target delivery. Sci Robot. 6(52):eaaz9519. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yuan K, Zhang L. 2019. Micro/nanomachines: from functionalization to sensing and removal. Adv Mater Technol. 4(4):1800636. [Google Scholar]
- Zhou H, Mayorga-Martinez CC, Pané S, Zhang L, Pumera M. 2021. Magnetically driven micro and nanorobots. Chem Rev. 121(8):4999–5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_00220345221087149 for Microrobotics for Precision Biofilm Diagnostics and Treatment by A. Babeer, M.J. Oh, Z. Ren, Y. Liu, F. Marques, A. Poly, B. Karabucak, E. Steager and H. Koo in Journal of Dental Research