Abstract
Human bone marrow stromal cell (hBMSC)–derived exosomes are promising therapeutics for inflammatory diseases due to their unique microRNA (miRNA) and protein cargos. Periodontal diseases often present with chronicity and corresponding exuberant inflammation, which leads to loss of tooth support. In this study, we explored whether hBMSC exosomes can affect periodontitis progression. hBMSC exosomes were isolated from cell culture medium through sequential ultracentrifugation. miRNAs and proteins that were enriched in hBMSC exosomes were characterized by RNA sequencing and protein array, respectively. hBMSC exosomes significantly suppressed periodontal keystone pathogen Porphyromonas gingivalis–triggered inflammatory response in macrophages in vitro. Transcriptomic analysis suggested that exosomes exerted their effects through regulating cell metabolism, differentiation, and inflammation resolution. In vivo, weekly exosome injection into the gingival tissues reduced the tissue destruction and immune cell infiltration in rat ligature-induced periodontitis model. Collectively, these findings suggest that hBMSC-derived exosomes can potentially be used as a host modulation agent in the management of periodontitis.
Keywords: exosomes, cell therapy, human bone marrow stromal cells, periodontal inflammation, miRNA, experimental periodontitis
Introduction
Periodontal disease is one of the most common inflammatory diseases, affecting ~50% of American adults over the age of 30 y (Eke et al. 2012). The disease is initiated by a dysbiotic microbial community; however, it is the host immune response to microbial insult that ultimately causes tissue damage (Darveau 2010; Hajishengallis 2015). Specifically, a chronic and exuberant inflammatory response induces the overproduction of cytokines and matrix processing enzymes (i.e., matrix metalloproteinases [MMPs] and collagenases) and pathological activation of osteoclasts (OCs), leading to irreversible tissue degradation, bone destruction, and loss of attachment (Kirkwood et al. 2007).
Cell therapies based on mesenchymal stem cells (MSCs) have shown promise in treating a broad range of diseases and injuries, including periodontitis (Hynes et al. 2012; Lin et al. 2015). While encouraging results were reported in early clinical trials, the application of MSC therapy is limited by issues such as high cost, difficulties in transportation to and storage in dental offices, immune rejection to allogeneic cells, and potential tumorigenesis (Ankrum et al. 2014; Lin et al. 2015). Although it was originally proposed to occur, the differentiation of implanted MSCs to target cell types is a relatively rare event (Discher et al. 2009; Karp and Leng Teo 2009; Ankrum et al. 2014). Increasing evidence suggests that the therapeutic value of MSCs is derived from the biologics that they secret, which stimulate cell proliferation and differentiation, enhance angiogenesis, and modulate host immune responses during wound healing (Karp and Leng Teo 2009; Ankrum et al. 2014).
Exosomes are nanoscale lipid bilayer vesicles (~100 nm in diameter) secreted by most cell types (Raposo and Stoorvogel 2013; Colombo et al. 2014). They are naturally occurring delivery vehicles that encapsulate a diverse array of bioactive molecules such as microRNAs (miRNAs), cytokines, and growth factors. When exosomes travel to other cells in the local environment or distant organs, they regulate cellular activities by delivering their cargo (Valadi et al. 2007). Functioning as “messengers” in cell–cell communications, exosomes produced by stem cells are involved in many physiological processes, including embryonic development, the immune response, and wound healing (Tkach and Théry 2016). Being an important component of the MSC secretome, MSC-derived exosomes have been shown to ameliorate the progression of a variety of diseases such as hypoxia-induced pulmonary hypertension, silicosis, and allogenic skin graft rejection through immune modulatory and anti-inflammatory effects (Harrell et al. 2019).
MSC conditional medium has long been investigated for treating periodontitis, but MSC exosomes have just started to draw researchers’ attention. Very recently, it was reported that MSC exosomes can inhibit periodontal bone loss and promote tissue regeneration (Chew et al. 2019; Nakao et al. 2021; Zhang et al. 2021). Most of these exosomes were harvested from preconditioned or modified craniofacial MSCs, and controversial results were also reported (Xu and Wang 2017). In this study, we sought to characterize the miRNAs and proteins contained in human bone marrow stromal cell (hBMSC)–derived exosomes, examine the modulatory effects of these exosomes in vitro on Porphyromonas gingivalis (Pg)–induced inflammatory response, and determine if they can suppress periodontal inflammation and prevent alveolar bone loss.
Materials and Methods
Methods for Western blot, exosome uptake, 1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindotricarbocyanine Iodide (DiR) labeling of exosomes, protein array, experimental periodontitis (EP) and exosome treatment in rats, micro–computed tomography (CT) analysis, histological analysis, next-generation RNA sequencing, and statistical analysis are presented in Appendix. Please refer to Appendix Table 1 for details of key resources.
hBMSC Culture
hBMSCs (RoosterBio) were CD166+CD105+CD90+CD73+/CD14–CD34–CD45– and able to differentiate into adipocytes and osteoblasts. Cells within 5 passages were used in our experiments following previously published protocol (Lin et al. 2022). They were expanded in High Performance Basal Medium (RoosterBio) supplemented with Media Booster GTX and 1% Pen/Strep/Fungizone (HyClone). When cells were 90% confluent at passage 4, expansion medium was aspirated and cells were washed 3 times with warm phosphate-buffered saline (PBS). Subsequently, exosome-depleted full medium (FM), which contained Dulbecco’s Modified Eagle’s Medium (DMEM), 10% exosome-depleted fetal bovine serum (FBS) and 1% Pen/Strep/Fungizone, was added for 2 d. To prepare exosome-depleted medium, commercial exosome-depleted FBS (Gibco) was used or regular FBS was diluted and centrifuged at 100,000 g, 4°C for 16 h.
Exosome Isolation
Exosomes were isolated by sequential ultracentrifugation (Théry et al. 2006). Briefly, hBMSC culture medium was centrifuged at 500 g for 10 min, 2,000 g for 10 min, and 10,000 g for 30 min to remove cell debris and large organelles. After that, exosomes were collected from supernatants by ultracentrifugation at 100,000 g for 70 min (50.2Ti rotor; Beckman Coulter). The pellets were washed in 0.9% NaCl and centrifuged at 100,000 g for 70 min to further remove medium protein contaminants. Exosome pellets were then resuspended in 0.9% NaCl and stored at −80°C until further use. Regarding the particle size and number, exosome samples were diluted in 0.2% paraformaldehyde (PFA) and measured by ZetaView BASIC NTA–Nanoparticle Tracking Video Microscope PMX-120.
Transmission Electron Microscopy
For the transmission electron microscopy (TEM) analysis to observe the pelleted exosomes, ~10 µL exosomes in 0.9% NaCl solution was loaded on a copper grid. After the solution was completely dried, negative staining was performed (Théry et al. 2006) and the copper grid was loaded to a Jeol JEM-1230 TEM.
Messenger RNA, miRNA, and Cytokine Production in Macrophages after Exosome Treatment
RAW 264.7 cells (ATCC) were seeded at a density of 25,000 cells/well in a 48-well plate in culture medium (DMEM + 10% heat-inactivated (HI)-FBS + 1% penicillin-streptomycin (PS)). The next day, the medium was changed and cells were challenged with Escherichia coli lipopolysaccharide (LPS) (0.1 µg/mL; Sigma-Aldrich) or heat-killed Pg bacteria (multiplicity of infection [MOI] = 66) for 12 h with exosomes (2 µg/mL) or 0.9% NaCl. Interleukin (IL)–6 cytokine production was measured in cell culture supernatants using mouse IL-6 enzyme-linked immunosorbent assays (ELISAs) (Biolegend). RNA was extracted by the miRNeasy kit (Qiagen), and 100 ng RNA was used for reverse transcription with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystem). Quantitative reverse transcription polymerase chain reactions (RT-PCRs) (PerfeCTa SYBR Green FastMix; Quanta Bio) were performed to measure the gene expression and Gapdh was used as an endogenous control. Primer sequences are shown in Appendix Table 2. To measure the expression of miRNAs, reverse transcription reactions were done with miScript II RT kits (Qiagen) and PCRs were performed with a universal primer (Qiagen) and a primer targeting specific miRNA (Appendix Table 3). SNORD68 was used as endogenous control for miRNA RT-PCRs.
Results
Characterization of Exosomes Derived from hBMSCs
Although hBMSC exosomes were heterogeneous under TEM (Fig. 1A), ZetaView NTA measurement showed that most of these vesicles were approximately 100 to 150 nm in diameter (Fig. 1B), with a concentration of 2.45 × 108 particles/µg protein (Fig. 1C). Exosome-specific markers such as tetraspanin protein CD81 and CD63 were highly enriched, as well as TSG101. Meanwhile, β-actin and HSP70 were at higher levels in the cell lysates (Fig. 1D).
Figure 1.
Characterization of mesenchymal stem cell exosomes. (A) Transmission electron microscopy image of exosomes. (B) The sizes of exosomes were measured by ZetaView NTA. (C) Particle numbers from 3 different exosome batches were measured and normalized to protein concentration. (D) Protein markers were evaluated by Western blot. (E) Exosomes were labeled by PKH26 and the unbound dye was washed off by ultracentrifugation. Labeled exosomes were added to RAW cells (2 µg/mL) for 18 h, and the cells were imaged by florescent microscopy. 10× and 63× presented the lenses that were used. Blue: DAPI. Red: PKH26.
Uptake of Exosomes by Recipient Cells
To test if exosomes can be transferred to other cells, exosomes were incubated with a fluorescent dye PKH26. The labeled exosomes were concentrated by ultracentrifugation and added into mouse macrophage RAW264.7 cells. Eighteen hours later, the uptake of exosomes by recipient cells was clearly visualized in fluorescent microscopy (Fig. 1E). Higher magnification showed that labeled exosomes were in cytoplasm. The control (0.9% NaCl mixed with PKH26) only resulted in limited background staining, suggesting that most unbound dye was washed off during ultracentrifugation.
Small RNA and Cytokines Were Enriched in hBMSC Exosomes
Since exosomes may exert their functions by transferring RNA and protein cargos, we performed RNA sequencing and protein array to determine their RNA and cytokine profiles. A distinct RNA pattern was observed in hBMSC exosomes as compared to total cell RNA, in which small RNAs (<200 nt) were highly enriched (Fig. 2A). Small RNA sequencing further showed that the most abundant (with the highest normalized reads) exosomal miRNAs included miR-100-5p, miR-125b-5p, miR21-5p, miR-10a-5p, and so on (Fig. 2B). A group of miRNAs, including miR-451a, miR-486-5p, miR-7641, miR-4792, and miR-6087, was found to be highly enriched in exosomes compared to that in cells (Fig. 2C). In a focused human cytokine and growth factor array, 31 of 120 cytokines and growth factors in the array were detected in exosomes, whereas 48 were found in the cell culture conditioned media supernatant after the exosomes were pelleted (Fig. 2D). Some of these proteins, such as FGF6, IGF1, IL-1ra, IL-16, and IL-3, were more concentrated in exosomes.
Figure 2.
The RNA and protein components of human bone marrow stromal cell (hBMSC) exosomes. (A) RNA from hBMSCs and their exosomes were extracted, and distinct patterns between cellular and exosomal RNA were noticed. Small RNAs were highly enriched in exosomes. (B) Top 10 most abundant microRNAs (miRNAs) in hBMSC exosomes identified by RNA sequencing. Their quantities in cells are also listed. (C) Top 5 most enriched miRNAs in exosomes. (D) Cytokines and growth factors in hBMSC exosomes and supernatants. Thirty-one of 120 cytokines and growth factors in the array were detected in exosomes, whereas 48 were found in the medium supernatant.
hBMSC Exosomes Suppressed Inflammatory Response In Vitro
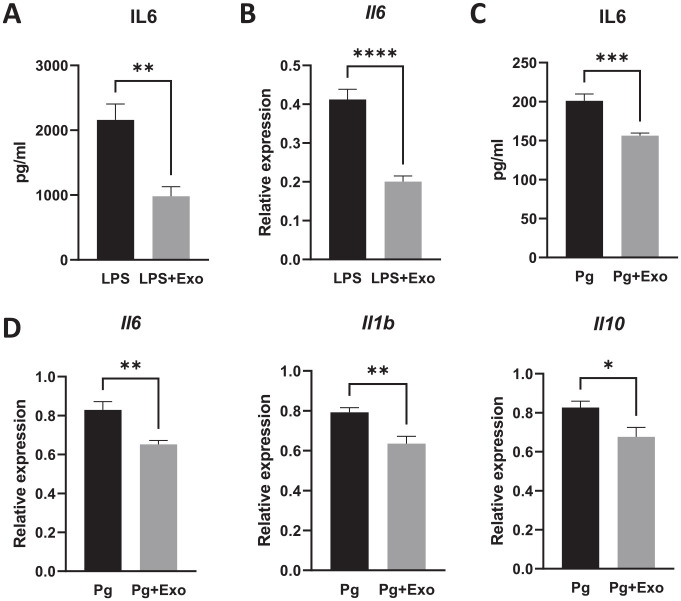
Harvested hBMSC exosomes have an anti-inflammatory effect. E. coli LPS stimulated the secretion of proinflammatory cytokines such as IL-6 in RAW264.7 macrophages. However, exosome treatment resulted in ~50% reduction in IL-6 protein production (Fig. 3A), and this effect was dose dependent (Appendix Fig. 2). RT-PCR confirmed that the Il6 messenger RNA (mRNA) was dramatically decreased (Fig. 3B). Heat-killed Pg also triggered a robust inflammatory response in macrophages. Similarly, Pg-induced IL-6 protein production in macrophages was significantly reduced after exosome treatment (Fig. 3C), as well as the expression of cytokine genes, including Il6, Il1b, and Il10 (Fig. 3D).
Figure 3.
Exosomes inhibited cytokine gene expression and protein production. (A) RAW264.7 macrophages were treated with Escherichia coli lipopolysaccharide (0.1 µg/mL) and exosomes (2 µg/mL) for 12 h. The interleukin-6 (IL-6) protein in culture medium was analyzed by enzyme-linked immunosorbent assay (ELISA). (B) Reverse transcription polymerase chain reaction (RT-PCR) showed that Il6 gene expression was suppressed after exosome treatment. (C, D) Heat-killed Porphyromonas gingivalis (Pg) was used to stimulated RAW264.7 cells for 12 h with and without exosomes (2 µg/mL). The IL-6 protein expression in culture medium was measured by ELISA (C) and the cytokine gene expressions of ll6, ll1b, and ll10 were measured by RT-PCR (D). *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001.
Impact of Exosomes on the Macrophage Transcriptome
To explore the underlying mechanisms of exosomes’ anti-inflammatory effect, we stimulated the macrophages with heat-killed Pg and treated them with exosomes. Through RNA sequencing and gene ontology analysis, we found that the biological activities most significantly affected by exosome treatment included cell metabolic processes, cell differentiation, biosynthetic processes, and the transforming growth factor β1 (TGFβ1) signaling pathway (Fig. 4A). RT-PCR further validated that a group of proinflammatory genes such as Mmp9, Ccl7, and Lst1 were significantly decreased by exosome treatment (Fig. 4B). Interestingly, cmklr1 (also known as ChemR23), a gene related to inflammation resolution, was dramatically increased after exosome treatment.
Figure 4.
The impact of exosomes on transcriptome. RAW264.7 cells were stimulated by heat-killed Pg with and without exosomes (2 µg/mL). (A) RNA sequencing was performed and the top 10 biological processes in gene ontology (GO) analysis were listed and ranked by false discovery rate (FDR). The raw P values were labeled on the side. (B) Reverse transcription polymerase chain reaction (RT-PCR) was applied to measure the expression of a panel of genes identified from RNA sequencing. NT means no Pg or exosome treatment. Pg represents heat-killed Porphyromonas gingivalis bacteria. Exo represents exosome treatment. *P < 0.05. **P < 0.01. ***P < 0.001.
hBMSC Exosomes Prevented Alveolar Bone Loss and Periodontal Inflammation in EP
To determine how long the exosomes remain localized in the periodontal tissues, we labeled exosomes with DiR, a near-infrared, lipophilic fluorescent dye, and injected them into gingival tissues around the first maxillary molar in rats. Through in vivo imaging, we found that DiR-labeled exosomes stayed in gingival tissues for at least a week (Appendix Fig. 1). Based on this result, hBMSC exosomes were injected into the gingival tissues once weekly when ligatures were placed to induce periodontitis (Fig. 5A). The accumulation of bacteria around the ligated left maxillary second molar (M2) led to inflammation and alveolar bone loss, which was shown by the decreased bone volume/tissue volume (BV/TV) compared to the nonligated right side (Fig. 5B). The normalized BV/TV of the saline group was 0.56, whereas the normalized BV/TV of the exosome treatment group was 0.68 (P < 0.05) (Fig. 5C), indicating more bone loss in the saline groups. Similarly, the normalized linear alveolar bone losses (ABLs) in saline and exosome treatment groups were 1.67 and 1.34, respectively (P = 0.06) (Fig. 5D), suggesting that less alveolar bone loss occurred after exosome treatment. The actual BV/TV and ABL at the ligated side are shown in Appendix Figure 3. In hematoxylin and eosin (H&E) staining, compared with saline, exosome injection resulted in less epithelium hyperplasia (Fig. 5E). Immune cell infiltration is a classic presentation of periodontitis. We quantified the infiltrated lymphocytes in the gingival connective tissue immediately underneath the epithelium and found that significantly less lymphocytes were present in the exosome treatment group (Fig. 5F). Interestingly, remnants of silk suture fibers or hair were noticed in the tissue, but they did not seem to have a correlation with the extent of the lymphocyte infiltration.
Figure 5.
Exosome treatment ameliorated periodontal bone loss in vivo. (A) Experiment timeline. Ligatures were placed around the left maxillary second molar (M2) for 3 wk. Exosomes or saline were injected to gingival tissues once a week. (B) Representative micro–computed tomography images of the ligated and nonligated maxillae. (C) Normalized bone volume/tissue volume (BV/TV) representing the ratio between treatment (left side) versus healthy control (right side). (D) Normalized linear alveolar bone loss (ABL) representing the ratio of treatment (left side)/healthy control (right side). (E) Hematoxylin and eosin staining. Upper panels, low magnification (the scale bars were 200 µm). Lower panels, high magnification of the areas in the yellow boxes (the scale bars were 50 µm). (F) Quantitative measurement of infiltrated lymphocytes in the interproximal gingival tissues.
Discussion
In the present study, we isolated hBMSC exosomes and characterized their RNA and protein components. Similar to other studies (Lai et al. 2010; Munshi et al. 2019; Liu et al. 2020), we found that these exosomes contained high levels of exosome markers such as CD81, CD63, and TSG101. However, the exosomes were heterogeneous in shape and size, suggesting the possibility that more than 1 species was present in the preparation. It is interesting that CD9, another common marker for exosomes, was not found in our samples (data not shown), which may be associated with the donors and culture conditions (Kordelas et al. 2014; Tracy et al. 2019). Exosomal small RNA, miRNA in particular, has emerged as important messengers of cell–cell/cell–matrix communication (Tkach and Théry 2016). In our study, exosomal miRNAs shared a certain degree of similarity with total cellular miRNAs as the most abundant cellular miRNAs were also present in exosomes with significant volumes. However, a list of miRNAs enriched hundreds of times in exosomes compared to cells, supporting the hypothesis that a selective mechanism occurs during the miRNA packaging into exosomes. A similar phenomenon was observed in other extracellular vesicles, such as vesicles isolated from the extracellular matrix (Lin et al. 2016; Lin et al. 2018). Cytokines and growth factors are other bioactive cargos in exosomes (Toh et al. 2018). We were able to detect a panel of growth factors and cytokines from exosome samples, and some of them, such as FGF6 and IGF1, had a higher concentration in exosomes than that in the suspension component of the conditional medium. Not all the cytokines and growth factors that were found in the culture conditioned medium existed in exosomes. Therefore, the cytokine and growth factors in exosomes are unlikely from “contamination” by medium proteins.
hBMSC exosomes had a robust immunomodulation effect in our experiments, which suppressed the inflammatory cytokine expression in vitro and prevented immune cell infiltration and bone resorption in vivo. Our results are in line with the increasing evidence that hBMSC exosomes, the large molecular weight component of the MSC secretome, are capable of recapitulating the anti-inflammatory function of MSCs (Harrell et al. 2019; Ha et al. 2020). Although allogeneic MSCs have been widely studied for inflammation attenuation and tissue regeneration because of their immune evasive nature, MSCs express major histocompatibility complex (MHC) class I molecules and may still trigger strong immune response/rejection in MHC mismatched recipients, resulting in low cell survival rate in recipients after MSC treatment (Ankrum et al. 2014). As a cell-free product, MSC exosomes can overcome such concerns. In addition, MSC exosomes may suppress the immune response by reducing the expression of costimulatory molecules and MHC class II proteins in dendritic cells (Harrell et al. 2019). In our study, xenogeneic exosomes were applied to rats without major side effects, supporting the advantage of exosome therapy. We observed that DiR-labeled exosomes stayed in gingival tissues for at least a week. Although we could not completely rule out the possibilities of dye transfer and nonspecific staining in this type of experiment, the fact that weekly injection regimen resulted in a significant protective effect in periodontium supported that exosomes are relatively stable, probably due to their complex structure and nanoscale size. Such properties may be advantageous when compared to other immune modulation agents based on small molecular compounds and antibodies. Interestingly, our results were different from a previous report that exosomes derived from rat maxillary BMSCs enhanced alveolar bone deterioration (Xu and Wang 2017). We believed that such a difference may due to the different stem cell origins, culture systems, exosome isolation methods, and exosome concentration.
The transcriptome data enable a better understanding of the effect of hBMSC exosomes on macrophages. It is not surprising to see that many inflammatory markers such as Ccl7, Lst1, and Mmp9 were downregulated by exosomes. Interestingly, metabolic processes and cell differentiation were the most affected biological activities after exosome treatment, which suggested that exosomes may regulate the M1 to M2 transformation of macrophages, leading them toward an inflammation resolution status. Indeed, Cmklr1(ChemR23), the receptor for Resolvin E1, was dramatically increased in macrophages by exosomes. It has been shown that overexpression of Cmklr1 in mice led to less inflammation and bone loss in periodontal tissues (Gao et al. 2013).
The molecular mechanism mediating the immune modulation by exosomes is unclear. Exosomal miRNAs can downregulate the expression of inflammatory genes in recipient cells by posttranscription mechanisms. Among the top 10 most abundant miRNAs, 7 of them are negative regulators of the inflammatory response, including miR-100-5p, miR-125b-5p miR-21-5p, miR-199a-5p, miR-10a-5p, let-7b-5p, and miR-27b-3p (Appendix Table 2). Furthermore, 4 of the top 5 most enriched exosomal miRNAs have anti-inflammatory effects (Appendix Table 4). Okamoto et al. (2018) showed that miR-451a, which is enriched more than 400 times in exosomes, attenuates inflammatory responses of macrophage and dendritic cells. Overexpression of miR-486-5p repressed the expression of inflammatory cytokines and matrix-degrading enzymes triggered by LPS (Chai et al. 2019). To investigate the role of miRNA in the immune-modulatory effect by exosomes, we measured the levels of the most abundant or enriched exosomal miRNAs in target cells. Some of these miRNAs (i.e., miR-100-5p and miR-451a) appear to increase in target cells after exosome treatment (Appendix Fig. 4); however, no statistical significance was seen. We selected those miRNAs in our RT-PCR experiments based on their abundance in the exosomes. It is still possible that other exosomal miRNAs were mediating the immune modulation effect. A miRNA sequencing in the target cells will be needed in the future.
Other bioactive molecules in exosomes may contribute to the immune modulation effect as well. The cytokines and growth factors in exosomes have the potential to elicit a timely response in recipient cells through binding to their receptors. We did not characterize the lipid component of hBMSC exosomes. However, others reported that the membrane lipid defines the subtypes of MSC exosomes (Lai and Lim 2019), and the lipid mediators in exosomes may be involved in their inflammation-resolving function (Pizzinat et al. 2020). It has been well documented that extracellular vesicles can manipulate the local environment and tissue healing through their enriched enzymes such as MMPs (Schmitz et al. 1996; Maeda et al. 2001), glutathione peroxidase 1 (GPX-1) (Yan et al. 2017), and NADPH oxidase 2 (Hervera et al. 2018). Collectively, hBMSC exosomes may provide a mixture of therapeutic components that mirrors the complexity of the physiological signals from MSCs during wound healing.
Another limitation of our study is that we only used BMSCs from 1 donor. Actually, this donor was selected from 7 available ones at the time based on the extensive characterization data provided by the manufacturer. BMSCs from all 7 donors had similar cell surface markers, expressed a similar amount of angiogenic cytokines (FGF, HGF, IL-8, PDGF, TIMP1, TIMP2, TNFa, and VEGF), and could be differentiated to adipocytes, osteoblasts, and chondrocytes. Despite some variances, they all produced detectable amounts of immunomodulatory molecule indoleamine 2,3-dioxygenase (IDO) after induction. Because of such similarities, we believe that the cells we used, although they were from 1 donor, still can provide us general information about the effects of BMSC exosomes on periodontal inflammation. In addition, the miRNA data set generated from our RNA sequencing was in line with findings from other groups (Chen et al. 2010). Wu et al. (2019) also showed that miR-100-5p, miR-223-3p, and miR-21-5p were the most abundant MSC exosomal miRNAs and exosomal miR-100-5p ameliorated osteoarthritis via inhibiting mTOR. We did not identify any specific cargo molecule that was responsible for the immunomodulatory effect in our study. However, if such key molecules are discovered in the future, we can use them to screen the donors to minimize the potential donor effect.
In conclusion, this study characterized the biological cargos of hBMSC exosomes and demonstrated that these exosomes suppressed the inflammatory response of macrophages triggered by Pg in vitro. Furthermore, hBMSC exosomes inhibited periodontal inflammation and subsequent alveolar bone loss in experimental periodontitis in vivo. Our results suggest that hBMSC exosomes may be a viable host immunomodulatory therapeutics for periodontitis.
Author Contributions
C. Yue, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J. Cao, A. Wong, J.H. Kim, contributed to design, data acquisition, analysis, and interpretation, drafted the manuscript; S. Alam, G. Luong, S. Talegaonkar, contributed to data acquisition, analysis, and interpretation, drafted the manuscript; Z. Schwartz, B.D. Boyan, W.V. Giannobile, S.E. Sahingur, contributed to conception, design, and data interpretation, critically revised the manuscript; Z. Lin, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345221084975 for Human Bone Marrow Stromal Cell Exosomes Ameliorate Periodontitis by C. Yue, J. Cao, A. Wong, J.H. Kim, S. Alam, G. Luong, S. Talegaonkar, Z. Schwartz, B.D. Boyan, W.V. Giannobile, S.E. Sahingur and Z. Lin in Journal of Dental Research
Acknowledgments
We thank Montserrat Samso for the excellent assistance in the TEM imaging. We thank Mingxu Sun, Noorpreet Kaur, Sheriz Chisley-Strickler, and Frances White for their excellent technical assistance. ZetaView and confocal microscopy were performed at the VCU Massey Cancer Center Microscopy Core Facility and supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. Study data will be available from the corresponding author on reasonable request.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health (NIH) grant R03DE027146 and a startup fund from Virginia Commonwealth University School of Dentistry to Z. Lin.
ORCID iD: W.V. Giannobile  https://orcid.org/0000-0002-7102-9746
https://orcid.org/0000-0002-7102-9746
References
- Ankrum JA, Ong JF, Karp JM. 2014. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 32(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Si H, Song J, Chong Y, Wang J, Zhao G. 2019. miR-486-5p inhibits inflammatory response, matrix degradation and apoptosis of nucleus pulposus cells through directly targeting FOXO1 in intervertebral disc degeneration. Cell Physiol Biochem. 52(1):109–118. [DOI] [PubMed] [Google Scholar]
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. 2010. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 38(1):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew JRJ, Chuah SJ, Teo KYW, Zhang S, Lai RC, Fu JH, Lim LP, Lim SK, Toh WS. 2019. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 89:252–264. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Théry C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 30:255–289. [DOI] [PubMed] [Google Scholar]
- Darveau RP. 2010. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 8(7):481–490. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. 2009. Growth factors, matrices, and forces combine and control stem cells. Science. 324(5935):1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance Workgroup. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 91(10):914–920. [DOI] [PubMed] [Google Scholar]
- Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, Van Dyke TE, Gyurko R. 2013. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol. 190(2):689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, Jung JY, Choi H, Lee JH, Sung S, et al. 2020. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 9(5):1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. 2015. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. 2019. Mesenchymal stem cell–derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 8(12):1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervera A, De Virgiliis F, Palmisano I, Zhou L, Tantardini E, Kong G, Hutson T, Danzi MC, Perry RB, Santos CXC, et al. 2018. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat Cell Biol. 20(3):307–319. [DOI] [PubMed] [Google Scholar]
- Hynes K, Menicanin D, Gronthos S, Bartold PM. 2012. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. 59(1):203–227. [DOI] [PubMed] [Google Scholar]
- Karp JM, Leng Teo GS. 2009. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 4(3):206–216. [DOI] [PubMed] [Google Scholar]
- Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. 2007. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 43:294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. 2014. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 28(4):970–973. [DOI] [PubMed] [Google Scholar]
- Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al. 2010. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4(3):214–222. [DOI] [PubMed] [Google Scholar]
- Lai RC, Lim SK. 2019. Membrane lipids define small extracellular vesicle subtypes secreted by mesenchymal stromal cells. J Lipid Res. 60(2):318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, McClure MJ, Zhao J, Ramey AN, Asmussen N, Hyzy SL, Schwartz Z, Boyan BD. 2018. MicroRNA contents in matrix vesicles produced by growth plate chondrocytes are cell maturation dependent. Sci Rep. 8(1):3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Rios HF, Cochran DL. 2015. Emerging regenerative approaches for periodontal reconstruction: a systematic review from the AAP regeneration workshop. J Periodontol. 86(2 Suppl):S134–S152. [DOI] [PubMed] [Google Scholar]
- Lin Z, Rodriguez NE, Zhao J, Ramey AN, Hyzy SL, Boyan BD, Schwartz Z. 2016. Selective enrichment of microRNAs in extracellular matrix vesicles produced by growth plate chondrocytes. Bone. 88:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Wong A, Alam S. Extraction of exosomes and exosomal miRNA from mesenchymal stem cells. 2022. Methods Mol Biol. 2455:333–341. [DOI] [PubMed] [Google Scholar]
- Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, Fan B, Zhao H, He H, Zhang F. 2020. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol. 235(11):8010–8022. [DOI] [PubMed] [Google Scholar]
- Maeda S, Dean DD, Gay I, Schwartz Z, Boyan BD. 2001. Activation of latent transforming growth factor beta1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J Bone Miner Res. 16(7):1281–1290. [DOI] [PubMed] [Google Scholar]
- Munshi A, Mehic J, Creskey M, Gobin J, Gao J, Rigg E, Muradia G, Luebbert CC, Westwood C, Stalker A, et al. 2019. A comprehensive proteomics profiling identifies NRP1 as a novel identity marker of human bone marrow mesenchymal stromal cell–derived small extracellular vesicles. Stem Cell Res Ther. 10(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, Chen C, Liu D, Watanabe Y, Hayashi C, et al. 2021. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 122:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Fukushima Y, Kouwaki T, Daito T, Kohara M, Kida H, Oshiumi H. 2018. MicroRNA-451a in extracellular, blood-resident vesicles attenuates macrophage and dendritic cell responses to influenza whole-virus vaccine. J Biol Chem. 293(48):18585–18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzinat N, Ong-Meang V, Bourgailh-Tortosa F, Blanzat M, Perquis L, Cussac D, Parini A, Poinsot V. 2020. Extracellular vesicles of MSCs and cardiomyoblasts are vehicles for lipid mediators. Biochimie. 178:69–80. [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 200(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JP, Dean DD, Schwartz Z, Cochran DL, Grant GM, Klebe RJ, Nakaya H, Boyan BD. 1996. Chondrocyte cultures express matrix metalloproteinase mRNA and immunoreactive protein; stromelysin-1 and 72 kDa gelatinase are localized in extracellular matrix vesicles. J Cell Biochem. 61(3):375–391. [DOI] [PubMed] [Google Scholar]
- Théry C, Amigorena S, Raposo G, Clayton A. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. Chapter 3:Unit 3.22. [DOI] [PubMed] [Google Scholar]
- Tkach M, Théry C. 2016. Communication by extracellular vesicles: where we are and where we need to go. Cell. 164(6):1226–1232. [DOI] [PubMed] [Google Scholar]
- Toh WS, Lai RC, Zhang B, Lim SK. 2018. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 46(4):843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy SA, Ahmed A, Tigges JC, Ericsson M, Pal AK, Zurakowski D, Fauza DO. 2019. A comparison of clinically relevant sources of mesenchymal stem cell–derived exosomes: bone marrow and amniotic fluid. J Pediatr Surg. 54(1):86–90. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 9(6):654–659. [DOI] [PubMed] [Google Scholar]
- Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li T, Chen H, Huang S, Fu Z, Li J, et al. 2019. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 206:87–100. [DOI] [PubMed] [Google Scholar]
- Xu SY, Wang ZL. 2017. Bone marrow mesenchymal stem cell–derived exosomes enhance osteoclastogenesis during alveolar bone deterioration in rats. RSC Adv. 7(34):21153–21163. [Google Scholar]
- Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. 2017. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 25(2):465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, Shen Z, Qin W, Lin Z, Huang S. 2021. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 13(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345221084975 for Human Bone Marrow Stromal Cell Exosomes Ameliorate Periodontitis by C. Yue, J. Cao, A. Wong, J.H. Kim, S. Alam, G. Luong, S. Talegaonkar, Z. Schwartz, B.D. Boyan, W.V. Giannobile, S.E. Sahingur and Z. Lin in Journal of Dental Research