Abstract
Background
A relation between the left ventricular assist device inflow cannula (IC) malposition and pump thrombus has been reported. This study aimed to investigate if the pump position, derived from chest X‐rays in HeartMate 3 (HM3) patients, correlates with neurological dysfunction (ND), ischemic stroke (IS), hemorrhagic stroke (HS) and survival.
Methods
This analysis was performed on routinely acquired X‐rays of 42 patients implanted with a HM3 between 2014 and 2017. Device position was quantified in patients with and without ND from frontal and lateral X‐rays characterizing the IC and pump in relation to spine, diaphragm or horizontal line. The primary end‐point was freedom from stroke and survival one‐year after HM3 implantation stratified by pump position.
Results
The analysis of X‐rays, 33.5 (41.0) days postoperative, revealed a significant smaller IC angle of HM3 patients with ND versus no ND (0.1° ± 14.0° vs. 12.9° ± 10.1°, p = 0.005). Additionally, the IC angle in the frontal view, IS: 4.1 (20.9)° versus no IS: 13.8 (7.5)°, p = 0.004 was significantly smaller for HM3 patients with IS. Using receiver operating characteristics derived cut‐off, IC angle <10° provided 75% sensitivity and 100% specificity (C‐statistic = 0.85) for predicting IS. Stratified by IC angle, freedom from IS at 12 months was 100% (>10°) and 60% (<10°) respectively (p = 0.002). No significant differences were found in any end‐point between patients with and without HS. One‐year survival was significantly higher in patients with IC angle >10° versus <10° (100% vs. 71.8%, p = 0.012).
Conclusions
IC malposition derived from standard chest X‐rays serves as a risk factor for ND, IS and worse survival in HM3 patients.
Keywords: inflow cannula position, mechanical circulatory support, neurological dysfunction, predictor, ventricular assist device
Inflow cannula malposition (inflow cannula angle <10°) derived from standard chest X‐rays serves as a risk factor for neurological dysfunction, ischemic stroke and worse survival in HeartMate 3 patients.
1. INTRODUCTION
With advancements in device technology and improvements in patient selection and care, the average survival time for patients with permanent mechanical circulatory support is approaching 5 years. 1 However, long‐term success of this therapy is compromised by a considerable number of adverse events, 2 with stroke being one of the most feared complications. 3 Although the risk of pump thrombosis is reduced in patients with HeartMate 3 (HM3; Abbott Inc., Chicago, IL, USA) compared with contemporary technologies 4 and the long‐term stroke incidence is 3.3 times lower compared to the HeartMate II (HM3: 0.04 vs. HeartMate II: 0.13 events per patient‐year), the occurrence of stroke of any type (ischemic stroke [IS] or hemorrhagic stroke [HS]) or any functional severity (disabling and non‐disabling) is predictive of poor clinical outcome. 5
Inflow cannula (IC) alignment has been identified as an important device‐specific risk factor for specific adverse events and also for patient prognosis. 6 , 7 , 8 For example, device positioning (pocket depth 9 , 10 , 11 and IC angle 7 , 11 , 12 , 13 , 14 ) was identified as an risk factor for GI bleeding 15 for HeartMate II (Abbott Inc., Chicago, IL, USA) patients. Whereas clinical data on the effect of pump position for centrifugal VADs are scarce, clinical evidence suggests an effect of pump position on pump thrombosis, compromised unloading and GI bleeding in HVAD (Medtronic Inc., Minneapolis, MN, USA) patients. 8 , 16 , 17
Recently, it has been shown that correlations exist between computer tomography (CT) angles and radiographs 17 ; therefore, assessment and identification of pump position related risk factors can be easily accomplished by routine chest radiographs. 18 Even though, only two reports among HM3 patients linked death and heart failure readmission 19 or composite adverse events 20 to pump position.
The present study was performed to determine if the IC position derived from standard chest X‐rays can predict strokes and worse survival in HM3 patients.
2. METHODS
2.1. Study population
Fifty‐seven consecutive patients undergoing HM3 LVAD implantation from June 2014 to December 2017 were included in this retrospective, singe‐center analysis. Two surgeons performed all LVAD implantations with “sew‐then‐core” technique of the sewing ring at the anatomical apex. Freedom from any stroke and survival were followed for one year after implantation. The study protocol (EK Nr: 1769/2018, waiver of informed consent) was approved by the Institutional Review Board.
2.2. X‐ray measurements
Posterior‐anterior (PA) and lateral (LAT) chest X‐rays were retrospectively collected and measured by an independent expert who was blinded to the clinical outcomes at the time of analysis. DICOM data were imported with ImageJ 21 and geometric measurements were performed in both PA and LAT projections (Figure 1). The first standing X‐ray was used for analysis, and X‐rays were checked for postural inconsistencies by verifying for alignment of the spine with the vertical axis, symmetric position of the clavicular heads and symmetric arm posture. Pump depth (distance between the dome of the right hemi diaphragm and the bottom of the pump body), projected IC length and IC angle (angle of the IC against a horizontal reference line) were analyzed similarly to previously reported data. 8 , 19 Additionally, elliptical approximations of the IC tip and the area of the projected pump body including the short and long elliptical axis, 17 were evaluated in both projections. In the PA X‐rays, the horizontal line was checked for possible rotation of the patient and corrected if necessary (>1° rotation) by defining it perpendicular to the spline. In cases where the IC was not visible and hidden behind the pump body, these values could not be evaluated, but the IC visibility was noted. In addition, significantly different pump position parameters of control versus IS or HS patients in the first standing X‐rays were analyzed 6 and 12 months after implantation to assess possible positional changes.
FIGURE 1.
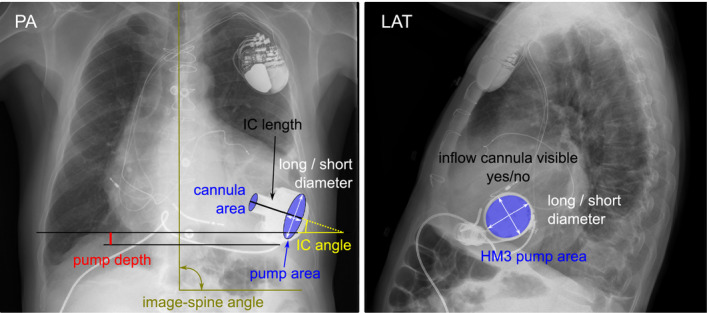
Definition of X‐ray measurements. HM3, HeartMate 3; LAT, lateral; PA, postero‐anterior [Color figure can be viewed at wileyonlinelibrary.com]
2.3. Follow‐up
In all patients, anticoagulation was initiated postoperatively with heparin with a goal activated partial thromboplastin time (aPTT) of 45–50 s for 24 h and gradually increased to maintain a target aPTT of 55–65 s. Then patients were transitioned to an oral anticoagulation regime with a vitamin K antagonist (phenprocoumon) after removal of the chest tubes (INR target range 2.0–2.5) and, if no contraindication existed, on the third postoperative day (POD) antiplatelet therapy with 100 mg aspirin was started. During the one‐year follow‐up, data including deaths or heart transplants and freedom from IS, HS or any neurological dysfunction (ND) (= IS, HS or transient ischemic attack), as defined by the Interagency Registry for Mechanical Circulatory Support were collected. 22 IS or HS were diagnosed as any new, temporary or permanent, focal or global ND ascertained by a standard neurological history and examination administered by a neurologist or other qualified physician and documented with appropriate diagnostic tests and consultation note; or an abnormality identified by surveillance neuroimaging (CT scan). 22
2.4. Study design
The primary endpoint in this study was the influence of pump position parameters on the freedom from any stroke and survival one‐year after HM3 implantation. Secondary endpoints were the descriptive analysis of pump positions in patients with and without IS or HS during the follow‐up period.
2.5. Statistical analysis
Statistical analyses were performed with SPSS for Windows Release 26.0.0 (IBM, NY, USA). Descriptive statistics are presented as mean ± standard deviation (SD) for continuous variables and number (percentage) for categorical variables. Where continuous variables were non‐normally distributed, data is presented as median and interquartile range (IQR). Normal distribution was assessed by the Shapiro–Wilk test. Fisher's exact test was used to assess for statistical significance of categorical variables, depending on the normal distribution student t‐test or Mann–Whitney U test for independent continuous variables. A repeated‐measures analysis of variance was performed for normally distributed longitudinal pump position (from first standing to 6 and 12 months after implantation); F‐values are reported. If Mauchly's test indicated that the assumption of sphericity was violated, Greenhouse–Geisser‐corrected values were used. Nonparametric longitudinal pump position was assessed with the Friedman test, and post hoc analyses with Wilcoxon signed‐rank tests were performed using a Bonferroni correction. Time‐to‐event analysis was performed by using Kaplan–Meier curves with p‐values reported using the log‐rank test. Patient follow‐up was censored when patients underwent heart transplantation, device explantation or expired. A receiver operating characteristics (ROC) curve analysis to assess the ability of pump position parameters from X‐rays to predict the occurrence of stroke was performed together with the area under the curve. Statistical significance was set at p < 0.05.
3. RESULTS
3.1. Patient characteristics
Of 57 patients who received HM3 LVAD implantation, 15 patients had to be excluded due to early death or heart transplantation before the first standing X‐rays (n = 10) or poor image quality (n = 5), and 42 patients were included in the study cohort. 23.8% received a VAD as bridge to transplant, 40.5% as destination therapy and 35.7% as bridge to candidacy. Mean age of the patients was 62.5 ± 8.5 years, body mass index was 28.4 ± 5.2 kg/m2 and 7.1% were female. Baseline demographics and comorbidities are shown in Table 1.
TABLE 1.
Baseline demographics and device position parameters
| n (%), median (IQR) or mean ± SD | HeartMate 3 study cohort (n = 42) |
|---|---|
| Patient characteristics | |
| Sex (female) | 3 (7.1) |
| Age at implant (years) | 62.5 ± 8.5 |
| BMI (kg/m2) | 28.4 ± 5.2 |
| INTERMACS level | |
| 1 | 8 (19.0) |
| 2 | 4 (9.5) |
| 3 | 7 (16.7) |
| 4–7 | 23 (54.8) |
| Cardiomyopathy | |
| Ischemic | 23 (54.8) |
| Dilated | 19 (45.2) |
| Strategy | |
| Destination therapy | 17 (40.5) |
| Bridge to transplantation | 10 (23.8) |
| Bridge to candidacy | 15 (35.7) |
| Implantation technique, minimal invasive | 10 (23.8) |
| Intraoperative bypass support | |
| HLM | 40 (95.2) |
| Off pump | 2 (4.8) |
| Prae ECLS | 8 (19.0) |
| Stroke history | 9 (22.0) |
| Diabetes | 14 (35.0) |
| Hypertension | 22 (56.4) |
| Atrial fibrillation | 25 (61.0) |
| Device position parameters | |
| POD first standing X‐ray (days) | 33.5 (41.0) |
| Posteroanterior measurements | |
| Pump area (mm2) | 1040.9 ± 552.2 |
| Short diameter (mm) | 24.1 ± 12.9 |
| Long diameter (mm) | 55.3 ± 1.4 |
| IC area (mm2) | 162.2 ± 92.9 |
| IC length (mm2) | 63.7 ± 3.2 |
| IC angle (°) | 10.9 (11.4) |
| Pump depth (mm) | 31.4 ± 20.3 |
| Lateral measurements | |
| Pump area (mm2) | 2583.4 (1074.8) |
| Short diameter (mm) | 55.8 (20.3) |
| Long diameter (mm) | 58.5 ± 2.5 |
| IC area (mm2) | 239.4 ± 52.9 |
| IC length (mm2) | 66.4 ± 2.1 |
| IC angle (°) | 2.1 ± 14.6 |
Abbreviations: BMI, body mass index; ECLS, extracorporeal life support; HLM, heart lung machine; IC, inflow cannula; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; POD, postoperative day.
3.2. X‐ray pump position parameters
Depending on the postoperative course, the average POD of the first standing X‐ray was 33.5 (41.0) days (Table 1). In the PA projection the average pump area was 1040.9 ± 552.2 mm2, IC area 162.2 ± 92.9 mm2, IC angle 10.9 (11.4)°, and pump depth 31.4 ± 20.3 mm. In the LAT projection median pump area was 2583.4 (1074.8) mm2 and mean IC area 239.4 ± 52.9 mm2 and IC angle 2.1° ± 14.6°. In addition, the proportion with a non‐visible IC (Figure 1, right) in LAT X‐rays was 67.6%.
3.3. Pump position and effect on stroke and clinical outcomes
Of the pump position parameters measured, the IC angle in PA view was significantly different between patients with any ND (n = 12) versus no ND (n = 30) (0.1° ± 14.0° vs. 12.9° ± 10.1°, p = 0.005).
When stratified by IS (Table 2), the IC angle in the PA view was significantly smaller for patients with IS: 4.1 (20.9)° versus no IS: 13.8 (7.5)°, p = 0.004, and did not change over time in any period: F(2,4) = 0.28, p = 0.77 (see Table S1). An example of PA X‐rays of two patients without (IC angle 10.8°) and with IS (IC angle 0.2°) is presented in Figure 2. Pump area and depth, short and long diameter as well as IC area and projected IC length did not show significant differences in patients with and without IS in PA X‐rays. Only one of the IS patients had a visible IC in the LAT X‐ray, so the average sagittal IC area, projected length and angle were not assessed (Table 2).
TABLE 2.
Device position parameters stratified by ischemic stroke and intracranial hemorrhage
| Device position parameters median (IQR) or mean ± SD | Ischemic stroke (n = 8) | No ischemic stroke (n = 34) | p‐value |
|---|---|---|---|
| Posteroanterior measurements | |||
| Pump area (mm2) | 944.8 ± 530.3 | 1066.7 ± 565.3 | 0.61 |
| Short diameter (mm) | 21.9 ± 12.5 | 24.7 ± 13.2 | 0.61 |
| Long diameter (mm) | 55.4 ± 1.6 | 55.3 ± 1.3 | 0.87 |
| IC area (mm2) | 143.6 ± 85.2 | 130.9 ± 71.9 | 0.54 |
| IC length (mm2) | 62.4 ± 2.5 | 63.5 ± 4.0 | 0.57 |
| IC angle (°) | 4.1 (20.9) | 13.8 (7.5) | 0.004 |
| Pump depth (mm) | 25.1 ± 18.5 | 32.9 ± 20.7 | 0.34 |
| Lateral measurements | |||
| Pump area (mm2) | 2458.9 (1050.5) | 2624.5 (1076.1) | 0.55 |
| Short diameter (mm) | 55.6 (19.6) | 56.1 (20.3) | 0.84 |
| Long diameter (mm) | 56.7 ± 2.5 | 58.7 ± 2.5 | 0.13 |
| IC area (mm2) | – | 241.8 ± 54.88 | – |
| IC length (mm2) | – | 66.7 ± 1.9 | – |
| IC angle (°) | – | 2.5 ± 15.3 | – |
| Device position parameters median (IQR) or mean ± SD | Intracranial hemorrhage (n = 9) | No intracranial hemorrhage (n = 33) | p‐value |
|---|---|---|---|
| Posteroanterior measurements | |||
| Pump area (mm2) | 1040.3 ± 565.8 | 1043 ± 535.6 | 0.99 |
| Short diameter (mm) | 24.0 ± 13.2 | 24.4 ± 12.9 | 0.95 |
| Long diameter (mm) | 55.1 ± 1.8 | 55.3 ± 1.3 | 0.65 |
| IC area (mm2) | 162.1 ± 90.6 | 162.2 ± 95.0 | 0.99 |
| IC length (mm2) | 63.1 (4.0) | 64.7 (2.7) | 0.35 |
| IC angle (°) | 8.8 (32.3) | 12.8 (9.9) | 0.08 |
| Pump depth (mm) | 27.2 ± 19.9 | 32.5 ± 20.6 | 0.49 |
| Lateral measurements | |||
| Pump area (mm2) | 2050.5 (1183.9) | 2626.4 (1004.3) | 0.19 |
| Short diameter (mm) | 45.0 (21.8) | 56.3 (18.2) | 0.26 |
| Long diameter (mm) | 56.9 ± 2.6 | 58.8 ± 2.4 | 0.07 |
| IC area (mm2) | 260.8 ± 55.6 | 228.6 ± 51.8 | 0.35 |
| IC length (mm2) | 65.7 ± 2.4 | 66.7 ± 2.0 | 0.49 |
| IC angle (°) | −7.9 ± 9.2 | 7.1 ± 14.6 | 0.09 |
Abbreviation: IC, inflow cannula.
Significant p < 0.05 are indicated in bold.
FIGURE 2.
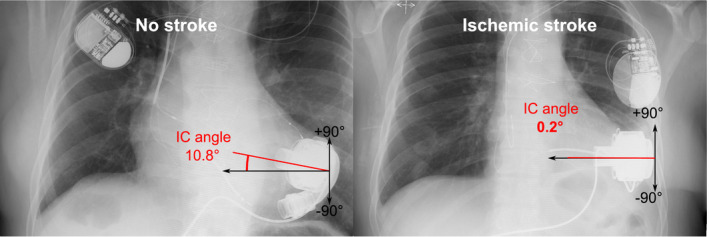
Example of postero‐anterior chest X‐rays of patients without (left) and with ischemic stroke (right). IC angle, inflow cannula angle [Color figure can be viewed at wileyonlinelibrary.com]
With ROC curve analysis (Figure 3), an IC angle <10° assessed in PA X‐rays provided 75% sensitivity and 100% specificity (C‐statistic = 0.85) as the optimal cut‐off to differentiate the risk of meeting the primary endpoint of this study (IS one‐year after HM3 implantation). Stratified by IC angle, freedom from any IS up to 12 months after HM3 implantation was 100% (>10°) and 60.0% (<10°) respectively (p = 0.002, Figure 4A). The one‐year survival rate was 100% (IC angle >10°) and 71.8% (IC angle <10°), respectively (p = 0.012, Figure 4B). Baseline characteristics and laboratory parameters were not statistically different between patients with an IC angle of less than or more than 10° and are summarized in the supplementary online data, Table S2. Invasive hemodynamic measurements were available in twenty‐eight patients (Table S2). Patients with IC angle <10° had significantly higher (p = 0.02) central venous pressure (CVP) and numerically higher pulmonary capillary wedge pressure (PCWP), but not significantly (p = 0.23).
FIGURE 3.
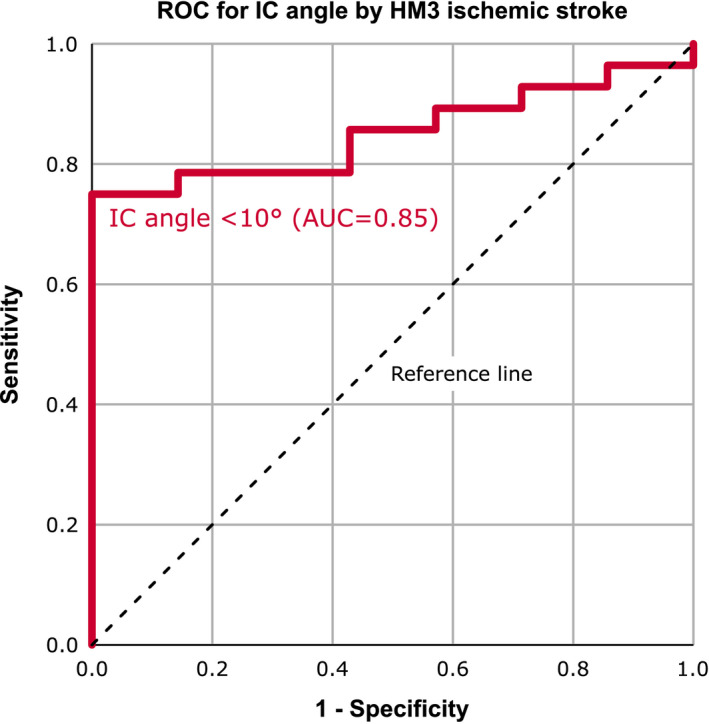
Receiver operator characteristics of inflow cannula angle for predicting ischemic stroke one‐year after HeartMate 3 implantation. AUC, area under the curve; HM3, HeartMate 3; IC angle, inflow cannula angle; ROC, receiver operator characteristics [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
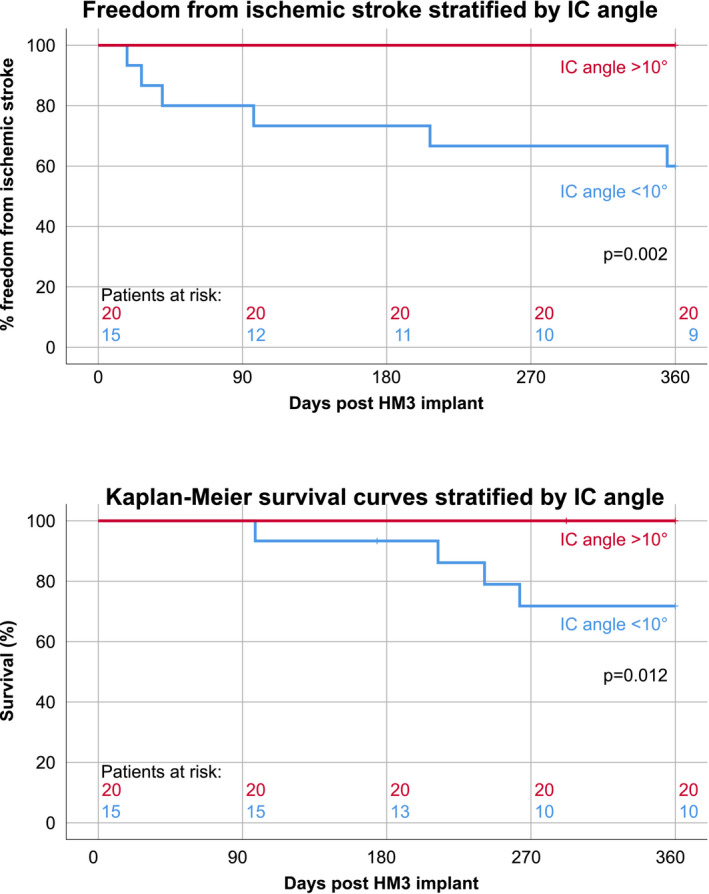
Freedom from ischemic stroke (top) and clinical outcomes (bottom) stratified by inflow cannula angle one‐year after HeartMate 3 implantation. HM3, HeartMate 3; IC angle, inflow cannula angle [Color figure can be viewed at wileyonlinelibrary.com]
Stratification of the cohort by HS, did not show significant differences in patients with and without HS in any X‐ray parameter, although in PA view the IC angle indicated a trend toward smaller values in patients with HS: 8.8 (32.3)° versus no HS: 12.8 (9.9)°, p = 0.08. On LAT X‐rays, the proportion of patients with visible IC (57.1%) was also comparable in the HS and no HS cohort (26.7%, p = 0.18).
4. DISCUSSION
Although there is general consensus that the IC of LVADs should be positioned parallel to the septal wall in the direction of the mitral valve 13 , 23 in order to maintain a neutral intraventricular septum position, 24 it remains unclear whether the device position specifically for the HM3 is a modifiable risk factor for stroke. Suboptimal pump position may be associated with left ventricular flow fields that foster intraventricular thrombus formation. 8 , 23 , 25 Although several experimental 25 and computational 26 , 27 studies have identified relationships between flow and thromboembolic complications the mechanistic relationship remains unclear and needs further investigation. Pump and IC malposition potentially leads to a vicious circle of flow changes, creation of stagnation zones with subsequent thrombus deposition, pannus formation at the IC and suction events followed by low pump flow with elevated residence time of blood particles. 27 , 28 All of this can ultimately lead to the formation of a thrombus intraventricular, 29 pre‐, intra‐, or post‐LVAD, which can detach and contribute to the development of stroke. Clinical radiographs are standard for regular outpatient follow‐up and, compared with CT scans, are more cost‐effective with a lower radiation dose and no contrast exposure. As recently reported, 15 X‐rays are a simple method to identify patients with pump malposition, as several radiographic parameters correlate well with the deviation of the IC to the mitral valve in the three‐chamber view of CT scans.
The key findings of this study, which examines the relationship between HM3 pump position, stroke, and survival, are (a) smaller IC angle in patients with IS and any ND, (b) a small IC angle (<10°) is a significant predictor of IS after HM3 implantation, and (c) the major clinical implication of risk stratification as early as one month after HM3 implantation based solely on the IC angle determined by X‐rays.
The potential clinical impact of IC angle determined by X‐rays, has been suspected by Imamura et al. 19 who reported—on the other end—that larger coronal angles (CCA >28°) as a significant predictor of death or heart failure readmission. A well‐positioned pump is expected to result in improved hemodynamics with better unloading and reduced right ventricular function. 19 However, these large reported values (median CCA of 40°) were not found in our entire cohort with a median IC angle of 10.9 (11.4)°. Therefore, combining these findings and the results of our study, an IC angle range between 10° to 28° seems optimal for excellent clinical outcomes with the HM3. Further studies are warranted to identify and test possible risk‐adapted medical therapies to improve outcomes in patients with pump malposition, which was beyond the scope of this work.
Previously reported preoperative risk factors for stroke 30 such as female gender (IC angle <10°: 13.3% vs. IC angle >10°: 5%, p = 0.56) and type of cardiomyopathy (IC angle <10°: 53.3% ischemic vs. IC angle >10°: 60% ischemic, p = 0.48), did not differ between patients with IC angle less or more than 10° (Table S2). Interestingly, numerically more patients (p = 0.10) with an IC angle >10° had a history of stroke (31.6% vs. IC angle <10°: 6.7%). Although stroke history has been previously reported as a risk factor for stroke during LVAD support 31 one would expect a higher rate of stroke in these patients, but since the opposite trend was observed, proper pump position seems to be more relevant than stroke prior to implantation.
As demonstrated by Imamura et al. 8 , 19 IC position plays also an important role in enabling cardiac unloading and achieving optimal hemodynamics, consequently also our results indicate improved hemodynamics in patients with an IC angle >10°, as determined by invasively measured CVP (10.9 ± 2.4 mm Hg vs. IC angle <10°: 13.1 ± 2.8 mm Hg, p = 0.02) and numerically lower PCWP.
Similarly to the results of Shih et al., 20 in the longitudinal sub‐group analysis (n = 23), IC angle did not change significantly in any period (χ 2(2) = 0.09, p = 0.96) (Table S1), highlighting the clinical implication of risk stratification already at the first standing X‐ray after HM3 implantation based on the IC angle only.
Because pump position alone may not be the only risk factor in LVAD patients, other factors must also be considered, but these were not primarily investigated in this study. In any case, the development of stroke is multifactorial, and in pump types other than HM3, infection, 32 inadequate anticoagulation, 33 or pump thrombosis 17 have been described as additional risk factors. On the day of stroke (POD 178.6 ± 164.5), mean arterial blood pressure (78.9 ± 9.7 mm Hg) and LDH (285.5 ± 47.7 U/L) were within normal range, and IS patients (n = 8) had elevated systemic inflammatory parameters, but only 12.5% had previous sub‐therapeutic anticoagulation one week before the event (Table S3), and all IS patients received 100 mg of aspirin daily without prior change in antiplatelet medication. These findings support recent evidence of synergistic effects of platelet activation mediated by shear forces and inflammatory activation of endothelial cells, which may explain endothelial cell‐platelet adhesion and the prothrombotic function of platelets as a potential contributor to intraventricular thrombosis, 29 and point to a small contribution of aspirin to protect against thromboembolic events with HM3 support. 34 , 35
Further, pump malposition as a potential contributing factor in the development of hemorrhagic events was not confirmed, as no significant differences between patients with and without HS were found in any X‐ray parameter. Although the mean IC angle in PA projection was lower in patients with versus without HS 8.8 (32.3)° versus 12.8 (9.9)°, it did not reach statistical significance (p = 0.08). IC visibility was used as a simple parameter to identify anterior or posterior pump rotation in the LAT X‐rays, and showed a trend of higher proportion of patients with a visible IC (57.1%) in those who suffered from HS versus no HS (26.7%, p = 0.18). This trend may be explained at least partially by the nature of their clinical interconnection, IS being oftentimes a predecessor of a hemorrhagic event. 36 Also in our analysis of the any ND cohort, 5 patients with HS suffered from IS beforehand. Thus a clear‐cut separation and differentiation can be difficult, but overall, the increased risk of IS in patients with pump malposition (IC angle <10°) might be explained by deposition of thrombus material due to close wall washout and ingestion. 37
Finally, the current one‐size‐fits‐all surgical approach could be replaced by a personalized LVAD implant strategy 28 based on careful assessment of the individual patient's left ventricular geometry (heart sizes), intrathoracic anatomy, and the interplay between both for optimal IC positioning. 38 , 39 Although optimal cannulation seems clear and logical, it is often influenced by these patient‐specific differences, therefore, various surgical techniques including pre‐ and peri‐operative planning of the coring site by CT 40 and (3D)‐TEE 41 also including virtual planning and 3D‐printing, 39 , 42 insertion of a needle at the appropriate cannulation site, 43 and creation of a pouch similar to an abdominal pocket 44 as well as the use of exoskeletons appear promising. 38 Whether surgical access via a median sternotomy or minimal invasive approaches for HM3 implantation can have an impact on the IC angle and thus ultimately on the risk of stroke needs further analysis. Based on the results of our study, no preference for a strategy could be found, as the proportion of patients implanted via a median sternotomy (75.0% vs. 76.5%, p = 0.93) was comparable in both patients with and without IS.
Our study has limitations, including its retrospective design, the limitation of excluding patients because they did not reach the appropriate postoperative time points because of death or heart transplantation. Furthermore, data analysis was limited to analysis to patients from a single center and, in particular, the rather small sample size may have been another confounding factor, and therefore a larger, prospective randomized control study should provide a more detailed description of pump malposition as risk factor for stroke than was possible in this study.
In conclusion, X‐rays show significantly smaller HM3 IC angle in patients with IS and any ND. A small IC angle (<10°) identified from X‐rays serves as a risk factor of IS and worse survival after HM3 implantation.
CONFLICT OF INTEREST
TS has served as a consultant and advisor for Medtronic Inc. and Abbott Inc. MG has served as a consultant for Berlin Heart. DW has served as a proctor and advisor for Medtronic Inc. and Abbott Inc. HS has served as an advisor for Medtronic Inc. and has received research grants from Medtronic Inc. DZ has served as a proctor, advisor, and speaker for Medtronic Inc., Abbott Inc., Berlin Heart, Edwards, and Abiomed and has received research and travel grants from Medtronic Inc. and Abbott Inc. All other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Concept and design: all authors; Data collection and analysis: Thomas Schlöglhofer, Philipp Aigner, Marcel Migas, Dietrich Beitzke, Kamen Dimitrov, Franziska Wittmann, Dominik Wiedemann, Julia Riebandt; Funding: Heinrich Schima, Günther Laufer, Daniel Zimpfer; Drafting article: Thomas Schlöglhofer and Philipp Aigner; All authors performed critical revision of the article and approved the final version.
Supporting information
Supplementary Material
Schlöglhofer T, Aigner P, Migas M, Beitzke D, Dimitrov K, Wittmann F, et al. Inflow cannula position as risk factor for stroke in patients with HeartMate 3 left ventricular assist devices. Artif. Organs. 2022;46:1149–1157. 10.1111/aor.14165
Funding information
Partially funded by the Austrian National Bank (Anniversary Fund, Project Number 17314)
REFERENCES
- 1. Molina EJ, Shah P, Kiernan MS, Cornwell WK, Copeland H, Takeda K, et al. The society of thoracic surgeons intermacs 2020 annual report. Ann Thorac Surg. 2021;111(3):778–92. 10.1016/j.athoracsur.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 2. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–6. [DOI] [PubMed] [Google Scholar]
- 3. Blitz A. Pump thrombosis—a riddle wrapped in a mystery inside an enigma. Ann Cardiothorac Surg. 2014;3:450–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehra MR, Uriel N, Naka Y, Cleveland JC, Yuzefpolskaya M, Salerno CT, et al. A fully magnetically levitated left ventricular assist device — final report. N Engl J Med. 2019;380:1618–27. [DOI] [PubMed] [Google Scholar]
- 5. Colombo PC, Mehra MR, Goldstein DJ, Estep JD, Salerno C, Jorde UP, et al. Comprehensive analysis of stroke in the long‐term cohort of the MOMENTUM 3 study: a randomized controlled trial of the HeartMate 3 versus the HeartMate II cardiac pump. Circulation. 2019;139:155–68. [DOI] [PubMed] [Google Scholar]
- 6. Maltais S, Kilic A, Nathan S, Keebler M, Emani S, Ransom J, et al. PREVENtion of HeartMate II pump thrombosis through clinical management: the PREVENT multi‐center study. J Heart Lung Transplant. 2017;36:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Kilic A, Ransom J, Maltais S, Sun B, Entwistle JW, Bailey S, et al. Pump position impacts HeartMate II left ventricular assist device thrombosis. ASAIO J. 2019;65:227–32. [DOI] [PubMed] [Google Scholar]
- 8. Imamura T, Adatya S, Chung B, Nguyen A, Rodgers D, Sayer G, et al. Cannula and pump positions are associated with left ventricular unloading and clinical outcome in patients with heartware left ventricular assist device. J Card Fail. 2018;24:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adamson RM, Bower BL, Sundareswaran KS, Farrar DJ, Dembitsky WP. Radiologic assessment of HeartMate II position: minimal pump migration after long‐term support. J Heart Lung Transplant. 2015;34:1617–23. [DOI] [PubMed] [Google Scholar]
- 10. Adamson RM, Mangi AA, Kormos RL, Farrar DJ, Dembitsky WP. Principles of HeartMate II implantation to avoid pump malposition and migration. J Card Surg. 2015;30:296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taghavi S, Ward C, Jayarajan SN, Gaughan J, Wilson LM, Mangi AA. Surgical technique influences HeartMate II left ventricular assist device thrombosis. Ann Thorac Surg. 2013;96:1259–65. [DOI] [PubMed] [Google Scholar]
- 12. Bhama JK, Bansal A. Left ventricular assist device inflow cannula position may contribute to the development of HeartMate II left ventricular assist device pump thrombosis. Ochsner J. 2018;18:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han JJ, Gaffey AC, Sooppan R, Hung G, Venkataraman C, Phillips E, et al. HeartMate II left ventricular assist device geometry on chest radiograph does not correlate with risk of pump thrombosis. ASAIO J. 2016;62:128–32. [DOI] [PubMed] [Google Scholar]
- 14. Kazui T, Zhang A, Greenberg J, et al. Left ventricular assist device inflow angle and pump positional change over time adverse impact on left ventricular assist device function. Ann Thorac Surg. 2016;102:1933–40. [DOI] [PubMed] [Google Scholar]
- 15. Imamura T, Nguyen A, Chung B, Rodgers D, Sarswat N, Kim G, et al. Association of inflow cannula position with left ventricular unloading and clinical outcomes in patients with HeartMate II left ventricular assist device. ASAIO J. 2019;65:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imamura T, Narang N, Nitta D, et al. HVAD cannula position and hemocompatibility‐related adverse events. Ann Thorac Surg. 2020. 10.1016/j.athoracsur.2019.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aigner P, Schlöglhofer T, Plunger L,, Beitzke D, Wielandner A, Schima H, et al. Pump position and thrombosis in ventricular assist devices: correlation of radiographs and CT data. Int J Artif Organs. 2021;44(12):956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathew RP, Alexander T, Patel V, Low G. Chest radiographs of cardiac devices (part 2): ventricular assist devices. South Afr J Radiol. 2019;23:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imamura T, Narang N, Nitta D, Fujino T, Nguyen A, Kim G, et al. Optimal cannula positioning of HeartMate 3 left ventricular assist device. Artif Organs. 2020;44(12). 10.1111/aor.13755 [DOI] [PubMed] [Google Scholar]
- 20. Shih H, Butler C, Melehy A, Ning Y, Kurlansky P, Kaku Y, et al. Serial assessment of HeartMate 3 pump position and inflow angle and effects on adverse events. Eur J Cardiothorac Surg. 2021;59(6):1166–73. 10.1093/ejcts/ezaa475 [DOI] [PubMed] [Google Scholar]
- 21. Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Interagency Registry for Mechanically Assisted Circulatory Support—Appendix A—Adverse event definitions 4.0. Available from: https://www.uab.edu/medicine/intermacs/intermacs‐documents
- 23. Sorensen EN, Kon ZN, Feller ED, Pham SM, Griffith BP. Quantitative assessment of inflow malposition in two continuous‐flow left ventricular‐assist devices. Ann Thorac Surg. 2018;105:1377–83. [DOI] [PubMed] [Google Scholar]
- 24. Dual SA, Anthamatten L, Shah P, Meboldt M, Schmid DM. Ultrasound‐based prediction of interventricular septum positioning during left ventricular support—an experimental study. J of Cardiovasc Trans Res. 2020;13(6):1055–64. 10.1007/s12265-020-10034-3 [DOI] [PubMed] [Google Scholar]
- 25. Aigner P, Schweiger M, Fraser K, Choi Y, Lemme F, Cesarovic N, et al. Ventricular flow field visualization during mechanical circulatory support in the assisted isolated beating heart. Ann Biomed Eng. 2020;48(2):794–804. 10.1007/s10439-019-02406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chivukula VK, Beckman JA, Prisco AR, Dardas T, Lin S, Smith JW, et al. Left ventricular assist device inflow cannula angle and thrombosis risk. Circ Heart Fail. 2018;11(4):e004325. 10.1161/CIRCHEARTFAILURE.117.004325 [DOI] [PubMed] [Google Scholar]
- 27. Ghodrati M, Maurer A, Schlöglhofer T, Khienwad T, Zimpfer D, Beitzke D, et al. The influence of left ventricular assist device inflow cannula position on thrombosis risk. Artif Organs. 2020;44(9):939–46. 10.1111/aor.13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li S, Beckman JA, Mahr C. Stroke in ventricular assist device patients: reducing complications and improving outcomes. ASAIO J. 2019;65:757–9. [DOI] [PubMed] [Google Scholar]
- 29. Apostoli A, Bianchi V, Bono N, Dimasi A, Ammann KR, Moiia YR, et al. Prothrombotic activity of cytokine‐activated endothelial cells and shear‐activated platelets in the setting of ventricular assist device support. J Heart Lung Transplant. 2019;38:658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Acharya D, Loyaga‐Rendon R, Morgan CJ, et al. INTERMACS analysis of stroke during support with continuous‐flow left ventricular assist devices: risk factors and outcomes. JACC Heart Fail. 2017;5(10):703–11. 10.1016/j.jchf.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bishawi M, Joseph J, Patel C, Schroder J, Daneshmand M, Bowles D, et al. Risk factors for stroke on left ventricular assist devices. J Card Surg. 2018;33(6):348–52. 10.1111/jocs.13718 [DOI] [PubMed] [Google Scholar]
- 32. Angleitner P, Matic A, Kaider A, Dimitrov K, Sandner S, Wiedemann D, et al. Blood stream infection and outcomes in recipients of a left ventricular assist device. Eur J Cardiothorac Surg. 2020;58(5):907–14. [DOI] [PubMed] [Google Scholar]
- 33. Cho S‐M, Hassett C, Rice CJ, Starling R, Katzan I, Uchino K. What Causes LVAD‐associated ischemic stroke? Surgery, pump thrombosis, antithrombotics, and infection. ASAIO J. 2019;65(8):775–80. [DOI] [PubMed] [Google Scholar]
- 34. Consolo F, Raimondi Lucchetti M, Tramontin C, Lapenna E, Pappalardo F. Do we need aspirin in HeartMate 3 patients? Eur J Heart Fail. 2019;21:815–7. [DOI] [PubMed] [Google Scholar]
- 35. Saeed O, Colombo PC, Mehra MR, Uriel N, Goldstein DJ, Cleveland J, et al. Effect of aspirin dose on hemocompatibility‐related outcomes with a magnetically levitated left ventricular assist device: an analysis from the MOMENTUM 3 study. J Heart Lung Transplant. 2020;39:518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J, Yang Y, Sun H, Xing Y. Hemorrhagic transformation after cerebral infarction: current concepts and challenges. Ann Transl Med. 2014;2(8):81. 10.3978/j.issn.2305-5839.2014.08.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rowlands GW, Antaki JF. High‐speed visualization of ingested, ejected, adherent, and disintegrated thrombus in contemporary ventricular assist devices. Artif Organs. 2020;44(11). 10.1111/aor.13753 [DOI] [PubMed] [Google Scholar]
- 38. Barabás IJ, Hartyánszky I, Kocher A, Merkely B. A 3D printed exoskeleton facilitates HeartMate III inflow cannula position. Interact Cardiovasc Thorac Surg. 2019;29(4):644–6. [DOI] [PubMed] [Google Scholar]
- 39. Miller JR, Singh GK, Woodard PK, Eghtesady P, Anwar S. 3D printing for preoperative planning and surgical simulation of ventricular assist device implantation in a failing systemic right ventricle. J Cardiovasc Comput Tomogr. 2020;14:e172–4. [DOI] [PubMed] [Google Scholar]
- 40. Acharya D, Aryal S, Loyaga‐Rendon R, Pamboukian SV, Tallaj J, Kirklin JK, et al. Use of computed tomography in preoperative planning for heartware left ventricular assist device placement. ASAIO J. 2019;65:70–6. [DOI] [PubMed] [Google Scholar]
- 41. Sheinberg R, Brady MB, Mitter N. Intraoperative transesophageal echocardiography and ventricular assist device insertion. Semin Cardiothorac Vasc Anesth. 2011;15(1–2):14–24. [DOI] [PubMed] [Google Scholar]
- 42. Szugye NA, Zafar F, Villa C, Lorts A, Morales DLS, Moore RA. 3D holographic virtual surgical planning for a single right ventricle fontan patient needing heartmate III placement. ASAIO J. 2021;67(12):e211–5. 10.1097/MAT.0000000000001487 [DOI] [PubMed] [Google Scholar]
- 43. Kirklin JK, Pagani FD, Goldstein DJ, John R, Rogers JG, Atluri P, et al. American Association for Thoracic Surgery/International Society for Heart and Lung Transplantation guidelines on selected topics in mechanical circulatory support. J Heart Lung Transplant. 2020;39(3):187–219. [DOI] [PubMed] [Google Scholar]
- 44. Adachi I, Guzmán‐Pruneda FA, Jeewa A, Fraser CD, Dean ME. A modified implantation technique of the HeartWare ventricular assist device for pediatric patients. J Heart Lung Transplant. 2015;34(1):134–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


