Abstract
Objective
The authors conducted a systematic review and meta‐analysis of pharmacological interventions to diminish cognitive side effects of ECT.
Methods
Electronic databases of Pubmed, PsycInfo, Embase and Scopus were searched from inception through 1 April, 2021, using terms for ECT (e.g. electroconvulsive therapy), cognitive outcome (e.g. cogni*) and pharmacological intervention (e.g. calcium channel blocker and general terms, like protein). Original studies with humans receiving ECT were included, which applied pharmacological interventions in comparison with placebo or no additive intervention to diminish cognitive side effects. Data quality was assessed using Risk of Bias and GRADE. Random‐effects models were used. PROSPERO registration number was CRD42021212773.
Results
Qualitative synthesis (systematic review) showed 52 studies reporting sixteen pharmacological intervention‐types. Quantitative synthesis (meta‐analysis) included 26 studies (1387 patients) describing twelve pharmacological intervention‐types. Low‐quality evidence of efficacy was established for memantine (large effect size) and liothyronine (medium effect size). Very low‐quality evidence shows effect of acetylcholine inhibitors, piracetam and melatonin in some cognitive domains. Evidence of no efficacy was revealed for ketamine (very low‐quality), herbal preparations with anti‐inflammatory properties (very low to low‐quality) and opioid receptor agonists (low‐quality).
Conclusion
Memantine and liothyronine are promising for further research and future application. Quality of evidence was low because of differences in ECT techniques, study populations and cognitive measurements. These findings provide a guide for rational choices of potential pharmacological intervention research targets to decrease the burden of cognitive side effects of ECT. Future research should be more uniform in design and attempt to clarify pathophysiological mechanisms of cognitive side effects of ECT.
Keywords: cognitive outcome, electroconvulsive therapy, meta‐analysis, pharmacological interventions, systematic review
Summations
This review provides a full overview of the range of pharmacological interventions tested for diminishing cognitive side effects of electroconvulsive therapy (ECT).
Memantine and liothyronine show some efficacy in decreasing cognitive side effects of ECT and are suggested as high priorities for future research.
In vulnerable patients suffering a high burden of cognitive side effects in ECT, memantine or liothyronine may be considered as potential additional treatment in clinical practice, because of the evidence that they may decrease these cognitive effects.
Limitations
Overall quality of established evidence in our systematic review was low, mostly because of small sample sizes and several risks of bias.
Conclusions are based on heterogeneous studies in terms of study population, type of cognitive outcome and ECT parameters hampering generalizability.
1. INTRODUCTION
Electroconvulsive therapy (ECT) is highly effective in treating major depressive disorder (MDD), with response‐rates around 70% and remission‐rates around 50% even in treatment‐resistant patients. 1 , 2 Still, ECT is often regarded as a treatment of last resort, partly because of concerns about cognitive side effects. Memory loss after ECT has been reported in 22%–79% of patients. 3 Autobiographical retrograde amnesia may be detectable even six months after ECT. 4 Although, at a group level, global cognitive functioning at least will return to baseline after ECT, studies show considerable inter‐individual variability. 5 Also, a discrepancy between subjective and objective cognitive side effects has been reported. 6 Cognitive side effects may contribute to the stigma of ECT and rejection of this effective treatment option. 7 Therefore, prevention or treatment of cognitive side effects will improve tolerability and may increase treatment motivation.
Cognitive side effects manifest in distinct cognitive functions, mainly in memory functions such as retrograde and anterograde amnesia. Global cognitive functioning, attention, executive and visuo‐constructive functioning are less affected. 4 , 8 Multiple theories try to explain the pathophysiology of cognitive effects in ECT, including roles of changes in immunological, hormonal and neurotrophic factors, as well as alterations in electrical brain activity, permeability of the blood brain barrier, brain perfusion and neuroplasticity. 9 Based on these presumed pathophysiological mechanisms, several prevention and treatment options for ECT‐induced cognitive side effects have been proposed. However, international clinical guidelines do not recommend any of such pharmacological interventions in ECT. 10 , 11 , 12 Earlier systematic reviews and meta‐analyses only reviewed specific drug (groups) or global cognitive functioning, without comprehensively examining all pharmacological interventions or all cognitive outcomes. 13 , 14 Thus, an overview of the full range of studied interventions targeting cognitive side effects of ECT is lacking.
1.1. Aim of the study
We present a systematic review and meta‐analysis of published studies on pharmacological interventions aimed at diminishing cognitive side effects of ECT.
2. MATERIAL AND METHODS
2.1. Search strategy and selection criteria
Cochrane Guidelines for Systematic Review and Meta‐analysis were used. 15 The review is reported following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (E‐Table 1 in the Supplement) 16 and registered in PROSPERO (CRD42021212773).
2.2. Data sources and searches
Electronic databases of Pubmed, PsycInfo, Embase and Scopus were searched by the first two authors (JV, MvK) from inception through 1 April, 2021, and included terms for electroconvulsive therapy (e.g. electroconvulsi*, electroshock, ECT), cognitive outcome (e.g. cogni*, amnes*, neuropsychological) and pharmacological intervention (i.e. a broad selection of previously examined types of drugs, like calcium channel blocker, and general terms, like protein). The full search strategy is available in EMethods in the Supplement.
2.3. Study selection
Studies were included if they (i) were a primary original study; (i) used a human population receiving ECT; (iii) used pharmacological interventions administered during the ECT‐course; (iv) applied placebo or no additive intervention as control condition and (v) measured the cognitive outcome on continuous cognitive scales. Publication year was not restricted. To gain a full overview of available evidence, all study designs and population diagnoses were allowed in the qualitative synthesis. Subsequently, only randomized, controlled, non‐crossover trials (RCTs) using a square‐wave pulse stimulus were included in the quantitative synthesis. To improve reproducibility, 55% of all identified titles, abstracts and full articles were independently examined by two reviewers (JV, MvK). Disagreements were settled by consensus.
2.4. Data extraction
Data extraction was performed by three independent reviewers (JV, MvK and JvW), each performing 37% of total extraction, which created overlap to ensure homogenous methods and consistency. The Cochrane Risk of Bias Tool 2 was used, 17 and overall quality of outcomes was rated using Grading of Recommendations, Assessment, Development and Evaluations (GRADE). 18 Imprecision was rated ‘large’ if confidence intervals (CI) crossed the clinical decision threshold of effect size (SMD, Hedges’ g < 0.5), or in case of less than 300 patients per outcome variable. ‘Very large’ imprecision was scored if less than 50 patients were included. In cases of missing data, we attempted to contact the first authors of studies for additional information.
2.4.1. Extracted patient and ECT characteristics
From all eligible studies, we extracted first author, year of publication, country, setting, psychiatric diagnoses, method to determine diagnoses, symptoms severity scores (e.g. Hamilton Depression Rating Scale [HDRS] score), mean age, distribution of sex, dose and frequency of intervention, type of control condition and known ECT‐parameters influencing cognitive side effects (e.g. ECT‐device, electrode placement, pulse amplitude [current], pulse width, anaesthetic, muscle relaxant and seizure durations 19 ).
2.4.2. Extracted cognitive outcome variables
To meet the primary goal of this systematic review and meta‐analysis, the cognitive tests used, the cognitive functions, timing of measurements and scores of cognitive outcomes were extracted (if available). Raw continuous values of cognitive scales at baseline and follow‐up were noted (i.e. means, standard deviations, standard errors or F‐scores). To synthesize the available evidence, outcome measurements were grouped according to timing. In line with previous ECT‐research, 20 these time‐intervals were ‘immediate’ (i.e. ≦ 24 h after ECT‐session), ‘short‐term’ (i.e. ≥24 h and ≦14 days after ECT‐course) and ‘medium‐term’ (i.e. between 24 days and two months after the ECT‐course; we chose 24 days, because no studies reported outcomes between 14 and 24 days).
2.5. Statistical analysis and data synthesis
This study comprised (i) qualitative synthesis of studies meeting the general inclusion criteria, (ii) quantitative synthesis of RCTs reporting sufficient data of comparable measures of cognitive outcome in one or two studies and (iii) meta‐analysis of RCTs reporting sufficient data of comparable measures of cognitive outcome in three or more studies (see Figure 1).
FIGURE 1.
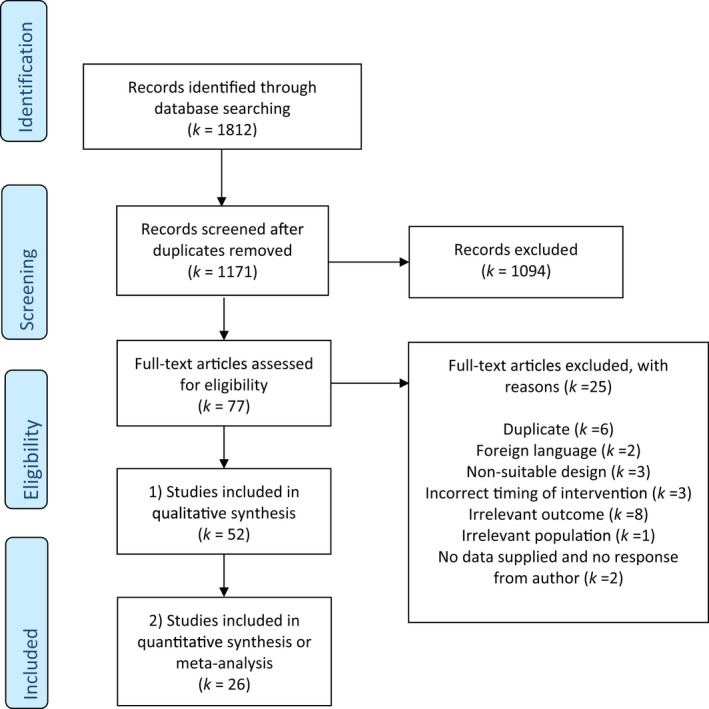
Flowchart of study selection
Descriptive statistics were used to report on the included studies. Using random‐effects models, effect estimates with 95%‐CI were calculated using mean differences (MD) in outcomes with a single type of cognitive measure. Also, for all outcomes, we calculated the standardized mean difference (SMD, Hedges’ g) to gain a measure of effect size. All SMDs were calculated by the difference between conditions at each of the different post‐ECT timepoints. SMDs were considered small ((0.2 ⩽ SMD < 0.5), medium (0.5 ⩽ SMD < 0.8) or large (SMD ⩾ 0.8). 21 Analyses were performed using Review Manager (version 5.4). 22
To synthesize available evidence, pharmacological interventions were pooled in groups according to the supposed mechanism of action. Because cognitive status would be influenced by specific psychiatric diagnoses, 23 , 24 we analysed studies with exclusively MDD, multiple diagnoses, mania and schizophrenia, separately. We combined unipolar depressive episode and studies reporting ‘depression’ without further specification. Level of evidence was characterized per intervention group as evidence for effect, evidence for no effect and insufficient evidence. Statistical heterogeneity was assessed by the I2 statistic with 95%‐CI, Chi‐squared tests with p‐values and by inspection of forest plots. If p < 0.10 and I2 > 50%, heterogeneity was considered to be substantial and, consequently, this outcome analysis was degraded in GRADE.
3. RESULTS
In total, 1812 articles were identified of which 1171 were unique (see Figure 1). After screening, 77 articles appeared suitable for full text inspection. Of these, 52 studies met criteria for inclusion in the qualitative synthesis. Of these, 26 articles met criteria for inclusion in the quantitative synthesis.
Characteristics of studies included in the qualitative synthesis (k = 52) are summarized in Table 1, showing 23 different pharmacological interventions. These interventions were merged into sixteen treatment groups according to mechanisms of action. Further details of the qualitative synthesis are presented in E‐Table 2 in the Supplement.
TABLE 1.
Characteristics of studies included in the qualitative synthesis (k = 52) and meta‐analysis (k = 26) on pharmacological interventions aimed at diminishing cognitive side effects of electroconvulsive therapy
| Qualitative synthesis | Meta‐analysis | |
|---|---|---|
| Number of studies (k) | 52 | 26 |
| Number of patients (n total) | 2320 | 1387 |
| Country (k, percent) | ||
| United States | 14; 27% | 4; 15% |
| Iran | 13; 25% | 11; 42% |
| China | 6; 12% | 4; 15% |
| Israel | 5; 10% | 1; 4% |
| Sweden | 3; 6% | 1; 4% |
| India | 2; 4% | 1; 4% |
| Great Britain | 2; 4% | 1; 4% |
| Australia | 1; 2% | 1; 4% |
| Kuwait | 1; 2% | 1; 4% |
| The Netherlands | 1; 2% | 0 |
| Japan | 1; 2% | 0 |
| Greece | 1; 2% | 0 |
| South Africa | 1; 2% | 0 |
| Norway | 1; 2% | 0 |
| Year of publication (median; range) | 2002; 1968‐2020 | 2013; 1978‐2020 |
| Mean age (in years; median; range) | Not available a | 40.9; 29.5‐65.7 |
| Sex (percentage female; range) | Not available a | 47%; 0‐67% |
| Included psychiatric disorder | ||
| Unipolar depressive episode | 21; 40% | 8; 30% |
| Various diagnoses | 17; 33% | 7; 27% |
| Depressive episode without further specification | 11; 21% | 9; 35% |
| Mania | 1; 2% | 1; 4% |
| Schizophrenia | 2; 4% | 1; 4% |
|
Electrode placement (k, percent) | ||
| Bifrontotemporal | 33; 63% | 15; 58% |
| Right unilateral according to d'Elia | 8; 15% | 6; 22% |
| Mix of unilateral and bifrontotemporal | 3; 6% | 2; 7% |
| Not specified | 8; 15% | 3; 11% |
Not reported because of missing demographic data in many studies.
The following paragraphs concern the studies included in the quantitative synthesis (k = 26), together describing results on twelve pharmacological interventions.
3.1. Study quality, risk of bias and GRADE
All included studies (k = 26) were scrutinized regarding bias because of randomization process, deviations from the intended interventions, missing outcome data, measurement of outcome and selection of reported results. Risk of bias for each included study is depicted in E‐Figure 1 in the Supplement. Evidence for all outcomes started high because of the RCT design. Most studies (k = 23, 88%) compared the pharmacological intervention with placebo. However, all evidence had to be downgraded at least one level because of imprecision (risk of bias, publication/reporting bias, imprecision and/or inconsistency according to GRADE; E‐Table 2). Checking trial registrations revealed a high risk of bias in selection of the reported results in three trials (11%). Funnel plots and statistical methods to assess the publication bias could not be applied, because there were insufficient studies for all comparisons. In sum, all evidence was appeared of moderate to very low‐quality.
3.2. Patient and ECT characteristics
Detailed patient characteristics of the quantitative synthesis and meta‐analysis (k = 26) are presented in Table 1. Mean of sample sizes was 53 ± 33 patients (range: 18‐137 patients). Most studies originated from Iran (k = 11; 41%). In 42% (k = 11), patients were recruited from in‐patient settings, but mostly (k = 12, 46%) location of recruitment was not reported. Median of mean ages of included patients was 40.9 years (interquartile range [IQR]: 34‐45 years), and median frequency of female sex was 46% (IQR: 41‐62%). All studies showed heterogeneity in terms of diagnosis.
Several ECT‐devices were used, mostly brief‐pulse, square‐wave systems of MECTA (31%, k = 8; MECTA Corporation, Portland, USA) and Thymatron (31%, k = 8; Somatics Inc, Lake Bluff, USA). One study 25 , using sine‐wave stimuli was excluded, because this method was regarded obsolete and would influence cognitive functioning differently compared with square‐wave methods. Although fifteen studies did not specify the pulse width, ten studies (38%) used brief‐pulse stimulation (0.5–1.0 ms) and one study used ultra‐brief stimulation (0.3 ms). Regarding anaesthesia, most studies (50%; k = 13) described the use of propofol and succinylcholine. Most studies (58%; k = 15) used bifrontotemporal electrode placement and 23% (k = 6) used right unilateral ECT, which might have impacted cognitive outcomes substantially. In sum, included studies varied substantially regarding use of independent determinants of cognitive side effects after ECT.
3.3. Data synthesis
Studies examining the following pharmacological interventions were included in our quantitative synthesis and meta‐analysis: acetylcholine inhibitors, ketamine, thyroid pathway, piracetam, memantine, opioid receptor agonists, herbal preparations with anti‐inflammatory properties, melatonin, opioid receptor antagonists, calcium antagonists, L‐tryptophan and vasopressin analogues. Quantitative synthesis of other (miscellaneous) interventions (k = 23) was not possible.
3.4. Cognitive outcome measures
A variety of cognitive functions was reported as outcome measures, of which the global cognition outcome measures were used most frequently (54%, k = 14; i.e. Montreal Cognitive Assessment [MOCA] 26 or Mini‐Mental State Exam [MMSE] 27 ). More specific cognitive functions were immediate and delayed recall, general memory abilities, visuospatial memory, biographical memory, semantic memory, working memory, language, attention and executive functions. No study reported statistically significant differences at baseline. Studies appeared largely heterogeneous in timing of outcome measurements. Immediate cognitive outcome was measured in only one study. 28 Most other studies (65%, k = 17) examined short‐term outcome and 27% (k = 7) reported medium‐term outcome.
E‐Table 2 summarizes the cognitive outcome measures, grouped by pharmacological intervention and time‐intervals, of which the qualitative synthesis, quantitative synthesis and meta‐analysis will now be described.
3.4.1. Acetylcholine inhibitors (k = 9)
Qualitative synthesis
Four studies reported divergent effects on cognitive outcomes (see E‐Table 2 in the Supplement). 25 , 29 , 30 , 31
A total of five studies (n = 209) included in quantitative synthesis and meta‐analysis reported on 12 cognitive outcomes. 28 , 32 , 33 , 34 , 35
Quantitative synthesis
Eight outcomes were of low‐quality evidence, and one outcome of very low‐quality. One study (n = 30, galantamine) found a large effect on short‐term delayed recall (MD = 19.67 (4.32, 35.02)). 32 One study (n = 45) found evidence of a large effect of donepezil on immediate recall (MD = 15.70 [8.39, 23.01]) and medium effect on autobiographical memory (MD = 9.00 [1.90, 16.10]) immediately after the ECT‐course. 28 Regarding other cognitive outcomes reported by a single study, no significant effects were found.
Meta‐analysis
Two outcomes were of low‐quality evidence, and one of very low‐quality. Four studies (n = 184) showed evidence that acetylcholine inhibitors (i.e. galantamine, rivastigmine and donepezil) are associated with a medium effect on short‐term global cognitive outcome (SMD = 0.72 [0.11, 1.34]; forest plots of global cognitive outcomes in Figure 2). 32 , 33 , 34 , 35 However, no statistically significant effects were found when looking separately at studies enrolling patients with depressive episodes and various diagnoses.
FIGURE 2.
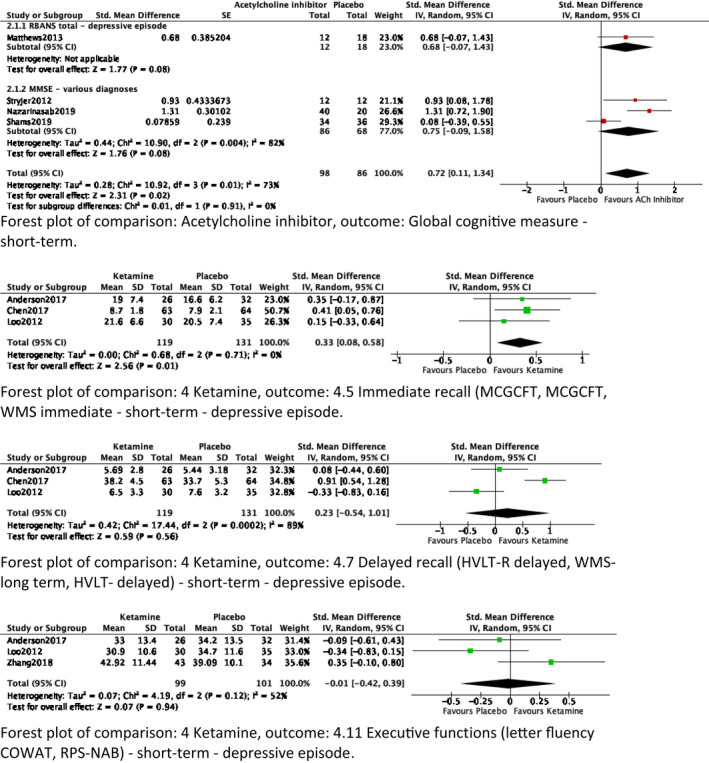
Forest plots of meta‐analyses
Forest plot of comparison: Acetylcholine inhibitor, outcome: Global cognitive measure ‐ short‐term. Forest plot of comparison: 4 Ketamine, outcome: 4.5 Immediate recall (MCGCFT, MCGCFT, WMS immediate) ‐ short‐term ‐ depressive episode. Forest plot of comparison: 4 Ketamine, outcome: 4.7 Delayed recall (HVLT‐R delayed, WMS‐long term, HVLT‐ delayed) ‐ short‐term ‐ depressive episode. Forest plot of comparison: 4 Ketamine, outcome: 4.11 Executive functions (letter fluency COWAT, RPS‐NAB) ‐ short‐term ‐ depressive episode.
3.4.2. Ketamine (k = 8)
Qualitative synthesis
Two studies (n = 112) established absence of correlations with cognitive outcome after ECT. 36 , 37
Quantitative synthesis and meta‐analysis
Six studies (n = 517) reported 19 outcomes, ranging from very low‐ to medium‐quality evidence (E‐Table 2). 38 , 39 , 40 , 41 , 42 , 43 None of the outcomes showed statistically significant effects on cognitive outcome, except for three studies reporting very low‐quality evidence for an association with small effect on short‐term immediate recall (SMD = 0.33 [0.08, 0.58]). 40 , 42 , 43
3.4.3. Memantine (k = 2)
Quantitative synthesis
Two studies (n = 78) were included in the meta‐analysis, reporting seven cognitive outcomes in patients with MDD 44 and in patients with various diagnoses. 45 Quality of the evidence ranged from very low to low. Memantine showed medium effect on short‐term global cognitive outcome (MD = 0.73 [0.25, 1.20]; Figure 2). Also, evidence was found of a statistically significant association with large effect on working memory (MD = 0.53 [0.12, 0.94]), as well as on immediate recall (MD = 1.10 [0.78, 1.42]). However, no effect on immediate recall was established when these MDs were combined. Data on medium‐term cognitive outcome were absent.
3.4.4. Thyroid pathway (k = 5)
Qualitative synthesis
Three studies did not meet criteria for inclusion in our quantitative synthesis; however, two of these were small crossover trials, which reported a statistically significant effect on cognitive outcome. 46 , 47 One trial supplied insufficient data for quantitative synthesis, but described a significantly positive effect on global cognitive outcome (see E‐Table 2 in the Supplement). 48
Quantitative synthesis
Two RCTs (n = 50) with one cognitive outcome measure showed low‐quality evidence that liothyronine was associated with a medium effect on short‐term general memory abilities in patients with MDD after ECT (SMD = 0.73 [0.15, 1.30]). 49 , 50
3.4.5. Piracetam (k = 4)
Qualitative synthesis
Two trials did not meet criteria for inclusion in the quantitative synthesis; one study because of insufficient data 51 and the other because it used a crossover design. 52 Neither studies established an effect of piracetam on cognitive outcome.
Quantitative synthesis
All evidence was of very low‐quality. Two RCTs (n = 68), both including patients with multiple diagnoses, tested piracetam for its efficacy in improving short‐term cognitive outcome measured with five outcomes. 53 , 54 One RCT (n = 30) found a statistically significant association with a large effect on short‐term general memory abilities (MD = 20.20 [6.89, 33.51]). 53 However, the other RCT (n = 38) showed no effect on four other short‐term cognitive measures. 54
3.4.6. Melatonin (k = 2)
Quantitative synthesis
One study (n = 40), reporting very low‐quality evidence on two cognitive outcomes, tested melatonin in patients with MDD. The trial reported a statistically significant large effect on global cognitive outcome (MD = 2.95 [1.95, 3.95]; E‐Table 2) and short‐term immediate recall after ECT (MD = 0.55 [0.17, 0.93]). 55
3.4.7. Additional interventions
Qualitative synthesis
One study (n = 319) reported a significant association of using nortriptyline during ECT with a better short‐term cognitive outcome. 56 One non‐blinded trial (n = 20) found an association of pemoline (a stimulant drug) with better short‐term outcome on global memory. 57 Two studies, one crossover trial 58 and one non‐randomized trial, 59 found no evidence of effect of anticholinergic agents on immediate cognitive outcome. Three crossover trials 60 , 61 , 62 and one trial supplying insufficient data for quantitative synthesis 62 found no effect of pharmacological interventions targeting the cortisol pathway. One crossover trial (n = 15) did not detect an effect of myo‐inositol. 63 Adrenergic antagonists had no effect on cognitive outcome in one crossover trial (n = 10). 64 One crossover study (n = 8) studying the effect of calcium antagonists found no statistically significant association with immediate cognitive outcome. 65 One crossover trial (n = 9) found no effect of vasopressin analogues, but one case‐series (n = 2) found positive effect on immediate delayed recall. 66 , 67
Quantitative synthesis
All evidence was of very low‐ to low‐quality. One trial (n = 37) reported no effect of opioid receptor antagonists. 68 Calcium antagonists showed statistically significant effects, as tested in one single small trial (n = 26) with five cognitive outcomes. 69 One study (n = 44) found no effect of L‐tryptophan on two measures of cognitive outcome. 70 One trial (n = 32) showed no effect of vasopressin analogues. 71 Two RCTs (n = 149) found no effect of opioid receptor agonists on short‐term cognitive outcome after ECT. 72 , 73 Two RCTs (n = 137) tested herbal preparations with anti‐inflammatory properties and found no effect on four cognitive outcomes at short‐term. 74 , 75
4. DISCUSSION
This is the first systematic literature review and meta‐analysis of the full range of pharmacological interventions used to attempt to diminish cognitive side effects of ECT. Quantitative synthesis reveals low‐quality evidence for a large effect of memantine and a medium effect of liothyronine. Furthermore, very low‐quality evidence—regarding short‐term cognitive outcomes—suggests possible effects of acetylcholine inhibitors, piracetam and melatonin. Otherwise, quantitative synthesis and meta‐analysis reveals evidence of no cognitive improvement with ketamine (very low‐quality), herbal preparations with anti‐inflammatory properties (very low‐quality) and opioid receptor agonists (low‐quality) after ECT.
Given the high burden of cognitive side effects in some patients, 5 , 76 this review and meta‐analysis strongly encourages further research on memantine and liothyronine in their efficacy to diminish short‐term cognitive side effects for ECT‐patients. Overall, effect sizes of these interventions appear medium to large, and thus, potentially may have important implications for daily clinical practice. However, international clinical ECT guidelines do not include any recommendations to use these scientifically substantiated interventions. 10 , 11 , 12 , 77 , 78 Moreover, guidelines do not advise against the use of agents that may have proven, although in (very) low‐quality studies, ineffective. Therefore, these results also may provide a guide for clinicians in the use of potentially beneficial pharmacological interventions, in case ECT‐patients show substantial cognitive side effects during treatment or in attempting to prevent such effects in particularly vulnerable patients (see Table 2).
TABLE 2.
Recommendation for priority of further research of potential agents to diminish or prevent cognitive side effects in electroconvulsive therapy, based on qualitative synthesis and meta‐analysis of the systematic literature review
| Recommendation for further study | Intervention | Effect size | Quality of evidence (GRADE) |
|---|---|---|---|
| High priority | Memantine | Large | Low |
| Liothyronine | Medium | Low | |
| Medium priority | Acetylcholine inhibitors | None to large | Very low |
| Melatonin | Medium to large | Very low | |
| Piracetam | None to large | Very low | |
|
Interventions targeting cortisol Pathway a |
|||
| Myo‐inositol a | |||
| Adrenergic antagonists a | |||
| Calcium antagonists a | |||
| Vasopressin analogues a | |||
| Opioid receptor antagonists a | |||
| Low priority | Ketamine | None | Very low |
| Anti‐inflammatory herbal preparations | None | Very low | |
| Opioid receptor agonists | None | Low | |
No effect size or GRADE was calculated since studies did not meet criteria for inclusion in meta‐analysis.
Our systematic literature search finds several patterns throughout history regarding the study of pharmacological interventions in ECT. First, the majority of studies are published in the last ten years, especially in non‐Western countries. Second, only a handful of research groups seems to have investigated this subject, strengthening our observation that generalization of the findings across the globe is very limited to date. An influential systematic review of ECT‐efficacy included 73 RCTs, 79 while our study yields noticeably fewer studies (k = 26). Also, evidence from 40% of the included outcomes is rated as low and 54% as very low. These arguments may reflect low priority in studying cognitive side effects of ECT. Moreover, limited knowledge is available regarding underlying ECT‐specific mechanisms of cognitive side effects. Hypotheses on the effects of melatonin, memantine, piracetam and acetylcholine inhibitors derive primarily from research of Alzheimer’s disease. 80 , 81 , 82 Furthermore, potential interventions influencing the cortisol pathway have only been studied in the 1970’s, which is understandable because evidence regarding cortisol dysregulation and MDD emerged in these years. 83 However, no further studies have been published since.
Worthwhile potential agents for further study may derive from our review and meta‐analysis. Insufficient evidence is available regarding the use of opioid receptor antagonists, calcium antagonists, L‐tryptophan, vasopressin analogues, anticholinergic agents, interventions targeting the cortisol pathway, myo‐inositol, pemoline, nortriptyline and adrenergic antagonists, partly because most studies included very small samples (e.g. <10 participants, see E‐Table 3 in the Supplement). Therefore, many studies were underpowered. Moreover, no replications or follow‐up RCTs have been reported yet, and conclusions are not possible. Potential treatment or preventive modalities of cognitive side effects in ECT, still worth further study, are prioritized based upon our systematic review and summarized in Table 2.
Current hypotheses of the pathophysiology of cognitive side effects in ECT span multiple candidate mechanisms. Recent studies have found potential roles of oxidative stress, inflammation, neurotrophic factors, immunological factors, hormones, alterations in electrical brain activity, permeability of the blood brain barrier, brain perfusion, changes in functional networks with a lag of integration of new neurons and volume changes of the hippocampus. 9 , 84 , 85 Further study of the worthwhile pharmacological interventions in this review may help elucidate the mechanisms of cognitive side effects in ECT. In addition, the study of novel candidates may contribute to further understanding of these mechanisms. Erythropoietin, currently under investigation, 86 is hypothesized to reduce cognitive side effects of ECT by reducing inflammation and oxidative stress, and inducing greater hippocampal activation and reinforcement of dorsolateral prefrontal activity networks. Also, preventing postictal vasoconstriction accompanied with cerebral hypoperfusion by using blood vessel dilating agents (e.g. calcium antagonists, cyclooxygenase‐2 [COX‐2] inhibitors) is suggested to reduce postictal phenomena, such as postictal cognitive dysfunction. 87
In this systematic review and meta‐analysis, modern and robust techniques are applied. 15 , 17 Another strength is the wide inclusion strategy, avoiding exclusion in advance of potential pharmacological interventions. However, our results must be considered in light of some limitations. First, we searched for data regarding ECT variables, which would have determined cognitive side effects inevitably (i.e. electrode placement, pulse width, anaesthesia). 19 Unfortunately, such data were lacking to correct for in our analyses. We expect considerable differences in ECT techniques between studies because of broad inclusion of study year and country. Moreover, 71% (k = 19) of the included studies in the quantitative synthesis appeared from only three countries, which probably may reduce worldwide generalizability of our findings. Second, substantial clinical heterogeneity existed in our included studies (e.g. regarding studied populations, sample sizes, investigated cognitive functions, types of cognitive tests and time‐intervals). Third, included studies applied ketamine as induction for anaesthesia, in contrast to the other interventions which were dosed in between ECT sessions which may hamper the comparability. Fourth, the majority of studies (54%) used the MMSE or MOCA to measure cognitive outcome. These cognitive screens may be unable to capture subtle changes because of ceiling effects, especially in younger patients. 88 Lastly, diagnostic types of depressive episodes were not always available, which decreased comparability between studies and increased statistical heterogeneity.
More uniformity in future research is advised to limit clinical heterogeneity, as is shown in our review. First of all, ECT variables such as electrode placement, pulse width and anaesthetic regime should be reported, and—ideally—only homogeneous patient groups should be included. Regarding the outcome measures, we advise adherence to standardized time‐intervals (i.e. immediate [within 24 h after the ECT‐session], short‐term [within two weeks after the ECT‐course], medium‐term [two weeks to three months after the ECT‐course], long‐term [3–6 months after the ECT‐course] and very long‐term (> 6 months after the ECT‐course]). Moreover, we suggest defining standard instruments for each specific cognitive function. We advise to minimally include the following cognitive functions in test batteries: global cognitive functioning (e.g. MOCA 89 ), immediate and delayed recall (e.g. Rey Auditory Verbal Learning Test) and executive functioning (e.g. Category and Letter fluency). Additionally, we suggest functions of attention (e.g. Trail Making Test A), cognitive flexibility (e.g. Trail Making Test B), working memory (e.g. WAIS‐IV backward numbers), autobiographical memory (e.g. Columbia Autobiographical Memory Interview, given an improved new scoring system 90 , 91 , 92 ), processing speed (e.g. STROOP) and subjective memory (e.g. Subjective Assessment of Memory, SAMI). Though, because of high attrition rates in these often severely ill populations, future research may focus on short tools with documented sensitivity to cognitive side effects. 6
In conclusion, this systematic review and meta‐analysis shows urgency to further study the efficacy of memantine and liothyronine in improving short‐term cognitive outcome after ECT. Acetylcholine inhibitors, piracetam and melatonin may also show potency to diminish cognitive side effects of ECT in some cognitive domains, although they are less promising. Studies appear clinically heterogeneous. Because of the sometimes very high patient burden of cognitive side effects, and some evidence for the efficacy and safety of these interventions during ECT, memantine and liothyronine may be considered for use in clinical practice in vulnerable patients.
CONFLICT OF INTERESTS
Charles H. Kellner, MD, receives fees from UpToDate for writing about ECT, royalties from Cambridge University Press for a textbook on ECT, and honoraria from Northwell Health for teaching an ECT‐course. The other authors declare no competing interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/acps.13397.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Study concept and design were drafted by EV, JV, MvK and JvW. JV, MvK and JvW collected data. JV and MO performed data analysis. All authors had full access to all the data in the study. JV, MvK and JvW verified the data set. Data interpretation and drafting of the manuscript were performed by all authors. All authors had full responsibility for the decision to submit for publication. EV en JvW contributed equally regarding the supervision of this study.
Verdijk JPAJ, van Kessel MA, Oud M, et al. Pharmacological interventions to diminish cognitive side effects of electroconvulsive therapy: A systematic review and meta‐analysis. Acta Psychiatr Scand. 2022;145:343–356. doi: 10.1111/acps.13397
Esmée Verwijk and Jeroen A. van Waarde contributed equally to this work
Funding information
There was no funding source for this study.
DATA AVAILABILITY STATEMENT
The full data sheet is available upon request from the corresponding author.
REFERENCES
- 1. van den Broek WW, de Lely A, Mulder PG, Birkenhäger TK, Bruijn JA. Effect of antidepressant medication resistance on short‐term response to electroconvulsive therapy. J Clin Psychopharmacol. 2004;24(4):400‐403. doi: 10.1097/01.jcp.0000130551.70878.56 [DOI] [PubMed] [Google Scholar]
- 2. van Diermen L, van den Ameele S, Kamperman AM, et al. Prediction of electroconvulsive therapy response and remission in major depression: meta‐analysis. Br J Psychiatry. 2018;212(2):71‐80. doi: 10.1192/bjp.2017.28 [DOI] [PubMed] [Google Scholar]
- 3. Rose D, Fleischmann P, Wykes T, Leese M, Bindman J. Patients' perspectives on electroconvulsive therapy: systematic review. BMJ. 2003;326(7403):1363. doi: 10.1136/bmj.326.7403.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verwijk E, Comijs HC, Kok RM, Spaans HP, Stek ML, Scherder EJ. Neurocognitive effects after brief pulse and ultrabrief pulse unilateral electroconvulsive therapy for major depression: a review. J Affect Disord. 2012;140(3):233‐243. doi: 10.1016/j.jad.2012.02.024 [DOI] [PubMed] [Google Scholar]
- 5. Obbels J, Verwijk E, Vansteelandt K, et al. Long‐term neurocognitive functioning after electroconvulsive therapy in patients with late‐life depression. Acta Psychiatr Scand. 2018;138(3):223‐231. doi: 10.1111/acps.12942 [DOI] [PubMed] [Google Scholar]
- 6. Hammershøj LG, Petersen JZ, Jensen HM, Jørgensen MB, Miskowiak KW. Cognitive adverse effects of electroconvulsive therapy: a discrepancy between subjective and objective measures? J ECT. 2021. doi: 10.1097/yct.0000000000000797 [DOI] [PubMed] [Google Scholar]
- 7. Wells K, Scanlan JN, Gomez L, et al. Decision making and support available to individuals considering and undertaking electroconvulsive therapy (ECT): a qualitative, consumer‐led study. BMC Psychiatry. 2018;18(1):236. doi: 10.1186/s12888-018-1813-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar S, Mulsant BH, Liu AY, Blumberger DM, Daskalakis ZJ, Rajji TK. Systematic review of cognitive effects of electroconvulsive therapy in late‐life depression. Am J Geriatr Psychiatry. 2016;24(7):547‐565. doi: 10.1016/j.jagp.2016.02.053 [DOI] [PubMed] [Google Scholar]
- 9. Singh A, Kar SK. How electroconvulsive therapy works?: Understanding the neurobiological mechanisms. Clin Psychopharmacol Neurosci. 2017;15(3):210‐221. doi: 10.9758/cpn.2017.15.3.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence (NICE) . Guidance on the use of electroconvulsive therapy. Updated 2009. https://www.nice.org.uk/guidance/ta59. Accessed April 16th, 2021.
- 11. National Institute for Health and Care Excellence (NICE). Depression in adults: recognition and management. Updated 2009. https://www.nice.org.uk/guidance/cg90. Accessed April 16th, 2021. [PubMed]
- 12. Gelenberg AJ, Chair MD & Marlene P et al. Practice Guideline For The Treatment of Patients With Major Depressive Disorder. American Psychiatric Association. Updated 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed April 16th 2021.
- 13. Niu Y, Ye D, You Y, Wu J. Prophylactic cognitive enhancers for improvement of cognitive function in patients undergoing electroconvulsive therapy: a systematic review and meta‐analysis. Medicine (Baltimore). 2020;99(11):e19527. doi: 10.1097/md.0000000000019527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGirr A, Berlim MT, Bond DJ, Chan PY, Yatham LN, Lam RW. Adjunctive ketamine in electroconvulsive therapy: updated systematic review and meta‐analysis. Br J Psychiatry. 2017;210(6):403‐407. doi: 10.1192/bjp.bp.116.195826 [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. 2021;15‐3. Updated February 2021. Available from: http://www.training.cochrane.org/handbook
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint–preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Phys Ther. 2009;89(9):873‐880. [PubMed] [Google Scholar]
- 17. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 18. Schünemann H. Brożek J, Guyatt G, Oxman A. (editors). GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013. Updated October 2013. Available from: http://guidelinedevelopment.org/handbook
- 19. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71‐83. doi: 10.1016/j.brs.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verwijk E, Comijs HC, Kok RM, et al. Short‐ and long‐term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315‐324. doi: 10.1017/s1041610213001932 [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013. [Google Scholar]
- 22. Review Manager (RevMan) . The Cochrane Collaboration. Version 5.4. 2020.
- 23. Xu G, Lin K, Rao D, et al. Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: a longitudinal, naturalistic study. J Affect Disord. 2012;136(3):328‐339. doi: 10.1016/j.jad.2011.11.029 [DOI] [PubMed] [Google Scholar]
- 24. Solís‐Vivanco R, Rangel‐Hassey F, León‐Ortiz P, Mondragón‐Maya A, Reyes‐Madrigal F, de la Fuente‐Sandoval C. Cognitive impairment in never‐medicated individuals on the schizophrenia spectrum. JAMA Psychiatry. 2020;77(5):543‐545. doi: 10.1001/jamapsychiatry.2020.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dutta LCB, Sarkar CP, Andrade C. Efficacy of donepezil for the attenuation of memory deficits associated with electroconvulsive therapy. Psychiatry Res. 2020;293:113397. doi: 10.1016/j.psychres.2020.113397 [DOI] [PubMed] [Google Scholar]
- 26. Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 27. Folstein MF, Folstein SE, McHugh PR, "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 28. Prakash J, Kotwal A, Prabhu HRA. Therapeutic and prophylactic utility of the memory‐enhancing drug donepezil hydrochloride on cognition of patients undergoing electroconvulsive therapy: a randomized controlled trial. Journal of ECT. 2006;22(3):163‐168. [DOI] [PubMed] [Google Scholar]
- 29. Levin Y, Elizur A, Korczyn AD. Physostigmine improves ECT‐induced memory disturbances.Neurology. 1987;37(5):871‐875. [DOI] [PubMed] [Google Scholar]
- 30. Matthews JD, Blais M, Park L, et al. The impact of galantamine on cognition and mood during electroconvulsive therapy: a pilot study. J Psychiatr Res. 2008;42(7):526–531. [DOI] [PubMed] [Google Scholar]
- 31. Van Schaik AM, Rhebergen D, Henstra MJ, Kadouch DJ, Van Exel E, Stek ML. Cognitive impairment and electroconvulsive therapy in geriatric depression, what could be the role of rivastigmine? A case series. Clin Pract. 2015;5(3):68‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthews JD, Blais MA, Park LT, et al. A double‐blind, placebo‐controlled study of the impact of galantamine on anterograde memory impairment during electroconvulsive therapy. J ECT. 2013;29(3):170‐178. [DOI] [PubMed] [Google Scholar]
- 33. Nazarinasab M, Behrouzian F, Hajatzadeh M. Evaluation of donepezil and rivastigmine administration on the cognitive deficits induced by electroconvulsive therapy: a randomized, double‐blind clinical trial. Fam Med Prim Care Rev. 2019;21(3):243‐248. doi: 10.5114/fmpcr.2019.88383 [DOI] [Google Scholar]
- 34. Shams‐Alizadeh N, Maroufi A, Sofla AQC, Ghaderi E, Hassanzadeh K. Effect of donepezil on cognitive deficits associated with electroconvulsive therapy: a randomized triple‐blind clinical trial. Clin Neuropharmacol. 2019;42(2):27‐31. doi: 10.1097/WNF.0000000000000323 [DOI] [PubMed] [Google Scholar]
- 35. Stryjer R, Ophir D, Bar F, Spivak B, Weizman A, Strous RD. Rivastigmine treatment for the prevention of electroconvulsive therapy‐induced memory deficits in patients with schizophrenia. Clin Neuropharmacol. 2012;35(4):161–164. [DOI] [PubMed] [Google Scholar]
- 36. Altinay M, Karne H, Anand A. Administration of sub‐anesthetic dose of ketamine and electroconvulsive treatment on alternate week days in patients with treatment resistant depression: a double blind placebo controlled trial. Psychopharmacol Bull. 2019;49(1):8‐16. [PMC free article] [PubMed] [Google Scholar]
- 37. Zhong X, He H, Zhang C, et al. Mood and neuropsychological effects of different doses of ketamine in electroconvulsive therapy for treatment‐resistant depression. J Affect Disord. 2016;01(201):124‐130. [DOI] [PubMed] [Google Scholar]
- 38. Dong J, Min S, Qiu H, Chen Q, Ren L. Intermittent administration of low dose ketamine can shorten the course of electroconvulsive therapy for depression and reduce complications: a randomized controlled trial. Psych Res. 2019;281;112573. [DOI] [PubMed] [Google Scholar]
- 39. Zhang M, Rosenheck R, Lin X, et al. A randomized clinical trial of adjunctive ketamine anesthesia in electro‐convulsive therapy for depression. J Affect Disord. 2018;227:372‐378. [DOI] [PubMed] [Google Scholar]
- 40. Chen Q, Min SU, Hao X, et al. Effect of low dose of ketamine on learning memory function in patients undergoing electroconvulsive therapy‐A randomized, double‐blind, controlled clinical study. J ECT. 2017;33(2):89‐95. [DOI] [PubMed] [Google Scholar]
- 41. Zou L, Min S, Chen Q, Li X, Ren L. Subanesthetic dose of ketamine for the antidepressant effects and the associated cognitive impairments of electroconvulsive therapy in elderly patients‐A randomized, double‐blind, controlled clinical study. Brain Behav. 2020:e01775. doi: 10.1002/brb3.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anderson IM, Blamire A, Branton T, et al. Ketamine augmentation of electroconvulsive therapy to improve neuropsychological and clinical outcomes in depression (Ketamine‐ECT): a multicentre, double‐blind, randomised, parallel‐group, superiority trial. Lancet Psychiatry. 2017;4(5):365‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loo CK, Katalinic N, Garfield JBB, Sainsbury K, Hadzi‐Pavlovic D, Mac‐Pherson R. Neuropsychological and mood effects of ketamine in electroconvulsive therapy: a randomised controlled trial. J Affect Disord. 2012;142(1‐3):233‐240. [DOI] [PubMed] [Google Scholar]
- 44. Abbasinazari M, Adib‐Eshgh L, Rostami A, Beyraghi N, Dabir S, Jafari R. Memantine in the prevention or alleviation of electroconvulsive therapy induces cognitive disorders: a placebo controlled trial. Asian J Psychiatry. 2015;01(15):5‐9. [DOI] [PubMed] [Google Scholar]
- 45. Alizadeh NS, Maroufi A, Jamshidi M, Hassanzadeh K, Gharibi F, Ghaderi E. Effect of memantine on cognitive performance in patients under electroconvulsive therapy a double‐blind randomized clinical trial. Clin Neuropharmacol. 2015;38(6):236‐240. [DOI] [PubMed] [Google Scholar]
- 46. Khan A, Mirolo MH, Claypoole K, et al. Effects of low‐dose TRH on cognitive deficits in the ECT postictal state. Am J Psychiatry. 1994;151(11):1694‐1696. doi: 10.1176/ajp.151.11.1694 [DOI] [PubMed] [Google Scholar]
- 47. Zervas IM, Pehlivanidis AA, Papakostas YG, Markianos M, Papadimitriou GN, Stefanis CN. Effects of TRH administration on orientation time and recall after ECT. J ECT. 1998;14(4):236‐240. [PubMed] [Google Scholar]
- 48. Mohagheghi A, Arfaie A, Amiri S, Nouri M, Abdi S, Safikhanlou S. Preventive effect of liothyronine on electroconvulsive therapy‐induced memory deficit in patients with major depressive disorder: a double‐blind controlled clinical trial. Biomed Res Int. 2015;2015:1‐5. doi: 10.1155/2015/503918.503918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Masoudzadeh A, Yahyavi ST, Rashidi H, Mohammadpour RA, Kiani R. Use of liothyronine in preventing electroconvulsive therapy‐induced memory impairment: evaluation. Psychiatrist. 2013;37(2):49‐53. doi: 10.1192/pb.bp.111.038398 [DOI] [Google Scholar]
- 50. Stern RA, Nevels CT, Shelhorse ME, Prohaska ML, Mason GA, Prange AJ Jr. Antidepressant and memory effects of combined thyroid hormone treatment and electroconvulsive therapy: preliminary findings. Biol Psychiat. 1991;30(6):623‐627. [DOI] [PubMed] [Google Scholar]
- 51. Ghafur MS, Saadat M, Maraci MR, Bagherian RS, Mazaheri M. Comparison between the effect of liothyronine and piracetam on personal information, orientation and mental control in patients under treatment with ECT. Indian J Psychiat. 2012;54(2):154‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mindus P, Cronholm B, Levander SE. Does piracetam counteract the ECT induced memory dysfunctions in depressed patients? Acta Psychiatr Scand. 1975;51(5):319‐326. [DOI] [PubMed] [Google Scholar]
- 53. Ezzat DH, Ibraheem MM, Makhawy B. The effect of piracetam on ECT‐induced memory disturbances. Br J Psychiatry. 1985;147(6):720‐721. [DOI] [PubMed] [Google Scholar]
- 54. Tang WK, Ungvari GS, Leung HCM. Effect of piracetam on ECT‐induced cognitive disturbances: a randomized, placebo‐controlled, double‐blind study. Journal of ECT. 2002;18(3):130‐137. [DOI] [PubMed] [Google Scholar]
- 55. Hamdieh M, Abbasinazari M, Badri T, Saberi‐Isfeedvajani M, Arzani G. The impact of melatonin on the alleviation of cognitive impairment during electroconvulsive therapy: a double‐blind controlled trial. Neurol Psychiat Brain Res. 2017;01(24):30‐34. [Google Scholar]
- 56. Sackeim HA, Dillingham EM, Prudic J, et al. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: Short‐term efficacy and adverse effects. Archives General Psychiatry. 2009;66(7):729–737. doi: 10.1001/archgenpsychiatry.2009.75 [DOI] [PubMed] [Google Scholar]
- 57. Small IF, Sharpley P, Small JG. Influences of cylert upon memory changes with ECT. Am J Psychiatry. 1968;125(6):837‐840. doi: 10.1176/ajp.125.6.837 [DOI] [PubMed] [Google Scholar]
- 58. Kelway B, Simpson KH, Smith RJ, Halsall PJ. Effects of atropine and glycopyrrolate on cognitive function following anaesthesia and electroconvulsive therapy (ECT). Int Clin Psychopharmacol. 1986;1(4):296‐302. [DOI] [PubMed] [Google Scholar]
- 59. Calev A, Drexler H, Tubi N, et al. Atropine and cognitive performance after electroconvulsive therapy. Convuls Ther. 1991;7(2):92‐98. [PubMed] [Google Scholar]
- 60. d'Elia G, Frederiksen SO. ACTH4‐10 and memory in ECT‐treated patients and untreated controls. II. Effect on retrieval. Acta Psychiatr Scand. 1980;62(5):429‐435. [DOI] [PubMed] [Google Scholar]
- 61. Frederiksen SO, d'Elia G, Holsten F. Influence of ACTH 4–10 and unilateral ECT on primary and secondary memory in depressive patients. Eur Arch Psychiatry Neurolog Sci. 1985;234(5):291‐294. [DOI] [PubMed] [Google Scholar]
- 62. Small JG, Small IF, Milstein V, Dian DA. Effects of ACTH 4–10 on ECT‐induced memory dysfunctions. Acta Psychiatr Scand. 1977;55(4):241‐250. [DOI] [PubMed] [Google Scholar]
- 63. Levine J, Pomerantz T, Stier S, Belmaker RH. Lack of effect of 6 g inositol treatment of post‐ECT cognitive function in humans. J Psychiatr Res. 1995;29(6):487‐489. [DOI] [PubMed] [Google Scholar]
- 64. Sakamoto A, Ogawa R, Suzuki H, Kimura M, Okubo Y, Fujiya T. Landiolol attenuates acute hemodynamic responses but does not reduce seizure duration during maintenance electroconvulsive therapy. Psychiatry Clin Neurosci. 2004;58(6):630‐635. doi: 10.1111/j.1440-1819.2004.01322.x [DOI] [PubMed] [Google Scholar]
- 65. Cohen MR, Swartz CM. Absence of nimodipine premedication effect on memory after electroconvulsive therapy. Neuropsychobiology. 1991;24(4):165‐168. [DOI] [PubMed] [Google Scholar]
- 66. Lerer B, Zabow T, Egnal N, Belmaker RH. Effect of vasopressin on memory following electroconvulsive therapy. Biol Psychiat. 1983;18(7):821‐824. [PubMed] [Google Scholar]
- 67. Weingartner H, Gold P, Ballenger JC, et al. Effects of vasopressin on human memory functions. Science. 1981;211(4482):601‐603. [DOI] [PubMed] [Google Scholar]
- 68. Motazedian S, Noorbakhsh S, Shams J, Jafari R, Faghihimohamadi M, Zahiroddin A. The effect of naltrexone on memory deficit followed by electroconvulsive therapy: A randomized, double‐blind, placebo‐controlled trial. Iran Red Crescent Med J. 2017;19(9):e59814. [Google Scholar]
- 69. Dubovsky SL, Buzan R, Thomas M, Kassner C, Cullum CM. Nicardipine improves the antidepressant action of ECT but does not improve cognition. J ECT. 2001;17(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 70. D'Elia G, Lehmann J, Raotma H. Influence of tryptophan on memory functions in depressive patients treated with unilateral ECT. Acta Psychiatr Scand. 1978;57(3):259‐268. [DOI] [PubMed] [Google Scholar]
- 71. Mattes JA, Pettinati HM, Stephens S, Robin SE, Willis KW. A placebo‐controlled evaluation of vasopressin for ECT‐induced memory impairment. Biol Psychiat. 1990;27(3):289‐303. [DOI] [PubMed] [Google Scholar]
- 72. Rezaei F, Nasseri K, Esfandiari GR, Sadeghi SM, Fathie M, Gharibi F. Remifentanil added to propofol for induction of anesthesia can reduce reorientation time after electroconvulsive therapy in patients with severe mania. J ECT. 2012;28(2):124‐127. doi: 10.1097/YCT.0b013e31824d1cea [DOI] [PubMed] [Google Scholar]
- 73. Sedighinejad A, Nabi BN, Haghighi M, et al. Electroconvulsive therapy‐related cognitive impairment and choice of anesthesia: the tipping point. J ECT. 2015;31(2):101‐104. doi: 10.1097/yct.0000000000000187 [DOI] [PubMed] [Google Scholar]
- 74. Akuchekian S, Layegh E, Najafi M, Barekatein M, Maracy MR, Zomorodi MH. Effects of herbal medicine on memory impairment in electroconvulsive therapy. J Res Med Sci. 2012;17(1 SPL.1):S59‐S64. [Google Scholar]
- 75. Mousavi SG, Mohsen G, Reza MM, Amrollah E, Majid B, Fariba N. Efficacy of memoral herbal on prevention of electroconvulsive therapy‐induced memory impairment in mood disorder patients (Isfahan ‐ Iran 2011). Int J Prevent Med. 2012;3(7):499‐503. [PMC free article] [PubMed] [Google Scholar]
- 76. Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32(1):244‐254. doi: 10.1038/sj.npp.1301180 [DOI] [PubMed] [Google Scholar]
- 77. van den Broek WW, TKB D, de Boer JP, et al. Richtlijn elektroconvulsietherapie. Nederlandse Vereniging voor Psychiatrie. Updated. 2010.
- 78. Weiss A, Hussain S, Ng B, et al. Royal Australian and New Zealand College of Psychiatrists professional practice guidelines for the administration of electroconvulsive therapy. Aust N Z J Psychiatry. 2019;53(7):609‐623. doi: 10.1177/0004867419839139 [DOI] [PubMed] [Google Scholar]
- 79. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta‐analysis. Lancet. 2003;361(9360):799–808. doi: 10.1016/s0140-6736(03)12705-5 [DOI] [PubMed] [Google Scholar]
- 80. Kishi T, Matsunaga S, Oya K, Nomura I, Ikuta T, Iwata N. Memantine for Alzheimer's disease: an updated systematic review and meta‐analysis. J Alzheimers Dis. 2017;60(2):401‐425. doi: 10.3233/jad-170424 [DOI] [PubMed] [Google Scholar]
- 81. Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta‐analysis. JAMA. 2003;289(2):210‐216. doi: 10.1001/jama.289.2.210 [DOI] [PubMed] [Google Scholar]
- 82. Wu YH, Swaab DF. The human pineal gland and melatonin in aging and Alzheimer's disease. J Pineal Res. 2005;38(3):145‐152. doi: 10.1111/j.1600-079X.2004.00196.x [DOI] [PubMed] [Google Scholar]
- 83. Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. Urinary free cortisol excretion in depression. Psychol Med. 1976;6(1):43‐50. doi: 10.1017/s0033291700007480 [DOI] [PubMed] [Google Scholar]
- 84. Argyelan M, Lencz T, Kang S, et al. ECT‐induced cognitive side effects are associated with hippocampal enlargement. Transl Psychiatry. 2021;11(1):516. doi: 10.1038/s41398-021-01641-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bassa A, Sagués T, Porta‐Casteràs D, et al. The neurobiological basis of cognitive side effects of electroconvulsive therapy: a systematic review. Brain Sci. 2021;11(10) :1273. doi: 10.3390/brainsci11101273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schmidt LS, Petersen JZ, Vinberg M, et al. Erythropoietin as an add‐on treatment for cognitive side effects of electroconvulsive therapy: a study protocol for a randomized controlled trial. Trials. 2018;19(1):234. doi: 10.1186/s13063-018-2627-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pottkämper JCM, Verdijk JPAJ, van Waarde JA. Measuring Blood Flow in the Brain After Epileptic Activity (SYNAPSE). Available from: https://clinicaltrials.gov/ct2/show/NCT04028596
- 88. Hebbrecht K, Giltay EJ, Birkenhäger TK, et al. Cognitive change after electroconvulsive therapy in mood disorders measured with the Montreal Cognitive Assessment. Acta Psychiatr Scand. 2020;142(5):413‐422. doi: 10.1111/acps.13231 [DOI] [PubMed] [Google Scholar]
- 89. Moirand R, Galvao F, Lecompte M, Poulet E, Haesebaert F, Brunelin J. Usefulness of the Montreal Cognitive Assessment (MoCA) to monitor cognitive impairments in depressed patients receiving electroconvulsive therapy. Psychiatry Res. 2018;259:476‐481. doi: 10.1016/j.psychres.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 90. Semkovska M, McLoughlin DM. Measuring retrograde autobiographical amnesia following electroconvulsive therapy: historical perspective and current issues. J ECT. 2013;29(2):127‐133. doi: 10.1097/YCT.0b013e318279c2c9 [DOI] [PubMed] [Google Scholar]
- 91. Semkovska M, O’Grady T. Unravelling autobiographical retrograde amnesia following bitemporal electroconvulsive therapy: effect of treatment versus effect of time. Psychology. 2017;8:611‐626. [Google Scholar]
- 92. Semkovska M, McLoughlin DM. Retrograde autobiographical amnesia after electroconvulsive therapy: on the difficulty of finding the baby and clearing murky bathwater. J ECT. 2014;30(3):187‐188. doi: 10.1097/yct.0000000000000122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The full data sheet is available upon request from the corresponding author.


