Abstract
Aims
Nocturnal acid breakthrough has been considered an unmet need of proton‐pump inhibitors. Tegoprazan, a novel potassium‐competitive acid blocker, is expected to show improved properties for this unmet need. This study was aimed to compare night‐time acid suppression by tegoprazan with that by vonoprazan or esomeprazole, and to explore the effect of CYP2C19 phenotypes on acid‐suppressive effects.
Methods
A randomized, open‐label, 3‐period, 6‐sequence crossover study was conducted. A single oral dose of tegoprazan 50 mg, vonoprazan 20 mg or esomeprazole 40 mg was administered at night in each period. Continuous intragastric pH was monitored at baseline and after each dosing.
Results
Sixteen healthy subjects (6 CYP2C19 extensive metabolizers, 5 intermediate metabolizers, 5 poor metabolizers) completed the study. After a single dose of tegoprazan, intragastric pH increased more rapidly to over 4 at approximately 1 hour compared to the other treatments, and elevated intragastric pH was maintained stably at night. Tegoprazan exhibited night‐time acid suppression for slightly but not significantly longer than vonoprazan, and greater than esomeprazole; % time at pH ≥ 4 at night was 66.0%, 60.5% and 36.1% for tegoprazan, vonoprazan and esomeprazole, respectively. Night‐time acid suppression by tegoprazan and vonoprazan was not dependent on CYP2C19 phenotypes, while that by esomeprazole tended to be influenced by CYP2C19 phenotypes.
Conclusion
Tegoprazan produced more rapid, potent and well sustained night‐time acid suppression vs. vonoprazan or esomeprazole when administered at night. Furthermore, tegoprazan showed no CYP2C19 phenotype dependency in acid suppression. It suggests the potential of tegoprazan, especially in preventing nocturnal acid breakthrough.
Keywords: CYP2C19 genetic polymorphisms, gastroesophageal reflux disease, nocturnal acid breakthrough, potassium‐competitive acid blocker, proton‐pump inhibitor
What is already known about this subject
Proton‐pump inhibitors (PPIs), the mainstay therapies for gastroesophageal reflux disease, have some unmet needs such as nocturnal acid breakthrough and CYP2C19 phenotype‐related variability in efficacy.
Considering more profound acid suppression than PPIs and elimination pathway irrelevant to CYP2C19, tegoprazan, a novel potassium‐competitive acid blocker, is expected to show improved properties for these unmet needs of PPIs.
What this study adds
Tegoprazan produced more rapid, potent and well sustained night‐time gastric acid suppression vs. vonoprazan or esomeprazole, suggesting the advantageous properties of tegoprazan in managing nocturnal acid breakthrough.
CYP2C19 phenotypes did not meaningfully influence gastric acid suppression by tegoprazan, indicating that the same dosing regimen of tegoprazan is applicable to all patients without consideration for CYP2C19 polymorphisms.
1. INTRODUCTION
Gastroesophageal reflux disease (GERD) is 1 of the most frequent gastric acid‐related disorders and is caused by abnormal reflux of gastric contents into the oesophagus, resulting in various symptoms, including heartburn and regurgitation. 1 Gastric acid suppression, especially maintaining gastric acidity at a pH ≥ 4.0, is important for controlling GERD, and proton‐pump inhibitors (PPIs) have been widely used as the most successful agents. 2 , 3 Nonetheless, PPIs, the mainstay therapies for GERD, have some unmet needs such as nocturnal acid breakthrough and CYP2C19 phenotype‐related variability in efficacy.
Treatment failures, particularly reflux episodes at night, which is known as nocturnal acid breakthrough, have been continuously noted in patients with GERD, even those on twice‐daily PPI therapies. 4 , 5 This failure might be provoked by class‐specific factors of PPIs, including short half‐lives (t1/2) and irreversible binding to H+/K+ ATPase, 6 , 7 which result in reduced exposure to proton pumps synthesized at night. In addition, gastric acid secretion has a circadian profile that peaks between 10 PM and 2 AM, which may represent a fundamental cause of this phenomenon. 8 Since nocturnal acid breakthrough may cause night‐time heartburn and/or wakening by cough or stuffiness, ultimately affecting sleep quality and daytime functioning, 9 , 10 it is important to control the gastric acid secretion at night in patients with GERD.
CYP2C19 genetic polymorphisms lead to interpatient variabilities in efficacy or the incidences of adverse events related to PPI therapies. 11 Most PPIs are predominantly metabolized by CYP2C19, and thus the CYP2C19 genotype influences the inactivation of PPIs, with the resulting changes in their systemic exposure and acid‐suppressive effects. 11 Despite the lower involvement of CYP2C19 in metabolism than other PPIs, esomeprazole showed significant differences in the extent of acid suppression depending on CYP2C19 phenotypes in previous clinical studies. 12 , 13 Moreover, the efficacies of PPIs may be sensitive to ethnicities due to the substantial ethnic difference in the frequencies of CYP2C19 alleles. 14 , 15
Potassium‐competitive acid blockers (P‐CABs) are a novel antisecretory class of drugs for acid‐related diseases, and, currently, tegoprazan, vonoprazan and so on are commercially available. By producing more profound acid inhibition than PPIs due to prolonged t1/2 and reversible inhibition of H+/K+ ATPase, P‐CABs are anticipated to sufficiently manage nocturnal acid breakthrough. In fact, vonoprazan is superior to conventional PPIs in suppressing gastric acid secretion at night. 16 , 17 In addition, P‐CABs have elimination pathways irrelevant to CYP2C19, as they are mainly metabolized by CYP3A4. 17 , 18 In a previous study of vonoprazan, no difference in its efficacy was observed according to CYP2C19 phenotypes. 12 , 19
Tegoprazan is a newly developed P‐CAB approved for the treatment of GERD, gastric ulcers and Helicobacter pylori eradication in the Republic of Korea. Tegoprazan is rapidly absorbed with a time to reach a maximum concentration (Tmax) ranging from 0.5 to 1.5 hours and eliminated with a mean t1/2 of 3.7 to 5.4 hours. 20 , 21 Notably, it exhibits a relatively faster onset of action than other P‐CABs and PPIs. 20 , 21 Similar to vonoprazan, tegoprazan is also expected to show improved properties compared to PPIs, including nocturnal acid breakthrough and CYP2C19 phenotype‐dependent efficacy.
To date, however, no studies have directly compared acid‐suppressive effects, especially at night, among P‐CABs, including tegoprazan, and PPIs. Furthermore, the extent of night‐time gastric acid suppression by tegoprazan has never been explored stratified by CYP2C19 phenotypes. This study aimed to compare gastric acid suppression at night by tegoprazan 50 mg with that by vonoprazan 20 mg or esomeprazole 40 mg, and to explore whether the night‐time acid‐suppressive effect of each treatment was influenced by CYP2C19 phenotypes.
2. METHODS
2.1. Subjects and study design
The study protocol and informed consent form were reviewed and approved by the Korean Ministry of Food and Drug Safety and the Institutional Review Board of Seoul National University Hospital. This study was conducted in accordance with the Korean Good Clinical Practice guidelines and the tenets of the Declaration of Helsinki (ClinicalTrials.gov No. NCT04231136). Written informed consent was obtained from all subjects before any procedures related to this study were conducted.
Eligible subjects were healthy Koreans without H. pylori infection who were 19–65 years old with a body weight ≥45 kg and body mass index of 17.5–30.5 kg/m2. Additionally, subjects who were classified as CYP2C19 ultrarapid metabolizers carrying CYP2C19*17 allele, who had evidence or a history of gastrointestinal diseases likely to affect drug absorption, and/or whose aspartate aminotransferase or alanine aminotransferase level was >3 times the upper normal limit were excluded.
This study had a randomized, open‐label, single‐dose, 3‐treatment, 3‐period, 6‐sequence crossover design. Considering the exploratory nature of the study objectives, the calculation of study power was not considered for determining the sample size. Using the CYP2C19 phenotype as a stratification factor, 6 subjects per phenotype were randomly assigned to 1 of 6 sequences. According to the assigned sequence, subjects received 1 of the following 3 treatments with 150 mL of water at around 10 PM after at least 3 hours of fasting in each period: a single oral dose of tegoprazan 50 mg (K‐CAB Tab., HK inno. N Corp., Seoul, Republic of Korea), vonoprazan 20 mg (Vocinti Table 20 mg, Takeda Pharmaceutical Co., Ltd., Tokyo, Japan) or esomeprazole 40 mg (Nexium Table 40 mg, AstraZeneca Pharmaceutical Co., Ltd., Seoul, Republic of Korea; Figure 1). The wash‐out period between each treatment was 7 days, which was more than 5 times the t1/2 of all treatments. 19 , 20 , 22
FIGURE 1.

Study design. Night was defined as the period of 12 hours from 22:00 to 10:00 hours
2.2. CYP2C19 genotyping
Genomic DNA was extracted from whole blood using the Gentra Puregene Blood Kit (Qiagen, Hilden, Germany), as previously described. 23 Polymerase chain reaction (PCR) for amplification of the 5′‐untraslated region and exons 4 and 5 of CYP2C19 was performed with in‐house primers. PCR was performed with a Veriti 96‐Well Thermal Cycler (Thermo Fisher Scientific, Waltham, MA, USA) at 94°C for 3 minutes followed by 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds and 72°C for 1 minute. Direct sequencing of the PCR products was performed using an ABI Prism 3730xl DNA Analyzer (Thermo Fisher Scientific). According to the results, CYP2C19*1, *2, *3 and *17 alleles were identified. The subjects were categorized into 3 subgroups: extensive metabolizers (EM, including *1/*1 diplotypes); intermediate metabolizers (IM, including *1/*2 and *1/*3 diplotypes); and poor metabolizers (PM, including *2/*2, *2/*3 and *3/*3 diplotypes).
2.3. Intragastric pH monitoring
Continuous intragastric pH was monitored at baseline (Day −1) and after each dosing (Days 1, 8 and 15) using a Digitrapper PH‐Z recorder (Medtronic) and VersaFlex pH Catheters (Alpine Biomed Corporation/Natus Medical). The observed intragastric pH data up to 12 hours from the start of monitoring were used to calculate night‐time pharmacodynamic (PD) parameters, including the percentage of time that the intragastric pH was over 4 (% time at pH ≥ 4), median pH, mean pH and the percent decrease from baseline in the integrated gastric acidity (△ integrated gastric acidity [%]). △ Integrated gastric acidity (%) was calculated as [(integrated gastric acidity at baseline − integrated gastric acidity after dosing) / (integrated gastric acidity at baseline) × 100]. Night was defined as the period of 12 hours (22:00–10:00 h), 4 , 19 , 24 and 8‐hour night‐time interval (22:00–06:00 hours) was additionally explored.
2.4. Safety evaluation
Throughout the study, adverse events (AEs) were monitored, and clinical laboratory tests, vital signs, physical examinations and 12‐lead electrocardiograms were assessed. In addition, serum gastrin levels and pepsinogen I/II ratios were measured before and after each treatment. The investigators determined whether each finding from the safety evaluation was clinically significant and related to the treatment.
2.5. Statistical analysis
All statistical analyses were conducted using SAS software (version 9.4, SAS Institute Inc., NC, USA), and a P‐value <.05 was considered statistically significant. Baseline demographics were compared among CYP2C19 phenotypes using the Kruskal–Wallis test. The night‐time PD parameters were summarized by treatments and CYP2C19 phenotypes per treatment. Analysis of variance (ANOVA) was performed for the night‐time PD parameters of each treatment, and the extent of gastric acid suppression by tegoprazan 50 mg was compared with that by vonoprazan 20 mg or esomeprazole 40 mg. Additionally, ANOVA was performed for the night‐time PD parameters of each CYP2C19 phenotype per treatment, and the effect of CYP2C19 phenotypes on acid suppression by each treatment was explored by calculating the point estimates of mean differences between CYP2C19 phenotypes (CYP2C19 IM – CYP2C19 EM; CYP2C19 PM – CYP2C19 EM) with the corresponding 2‐sided 95% confidence intervals (CIs).
3. RESULTS
3.1. Study population
Nineteen healthy Korean subjects (7 EMs, 6 IMs and 6 PMs) were enrolled and randomized. Of those, 3 subjects withdrew from the study: 1 EM and 1 PM before the first dosing, and 1 IM before the second dosing. Consequently, 16 subjects (6 EMs, 5 IMs and 5 PMs) completed the study. There were no statistically significant differences in the demographics among CYP2C19 phenotypes (Table S1).
Intragastric pH was analysed in 16 subjects (6 EMs, 5 IMs and 5 PMs) who had completed the study and had intragastric pH data for >95% of the total monitoring time, and safety was evaluated in 17 subjects (6 EMs, 6 IMs and 5 PMs) who had been administered the treatment at least once.
3.2. PD
Tegoprazan 50 mg showed more rapid suppression of gastric acid secretion than vonoprazan 20 mg and esomeprazole 40 mg. Mean intragastric pH reached >4 at approximately 1 hour after a single dose of tegoprazan 50 mg, and at approximately 4 hour after a single dose of vonoprazan 20 mg or esomeprazole 40 mg. Afterwards, during the night, the elevated intragastric pH was consistently maintained above 4 for tegoprazan 50 mg and vonoprazan 20 mg; however, it dropped below 4 intermittently for esomeprazole 40 mg (Figure 2).
FIGURE 2.
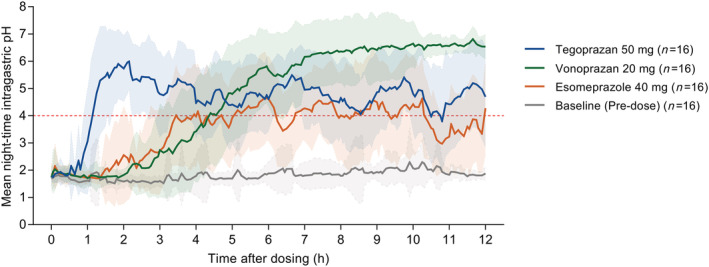
Mean night‐time intragastric pH–time profiles at baseline (predose) and after a single oral administration of tegoprazan 50 mg, vonoprazan 20 mg or esomeprazole 40 mg under fasted condition. The background shadow represents standard deviation. Night was defined as the period of 12 hours from 22:00 to 10:00 hours
Gastric acid suppression at night by tegoprazan 50 mg tended to be longer, not statistically significantly, than that by vonoprazan 20 mg (% time at pH ≥ 4 at night: 66.0% for tegoprazan vs. 60.5% for vonoprazan, P = .30), and was statistically greater than that by esomeprazole 40 mg (% time at pH ≥ 4 at night: 66.0% for tegoprazan vs. 36.1% for esomeprazole, P < .0001; Figure 3, Table 1). Night‐time median pH and mean pH values were >4 for tegoprazan 50 mg and vonoprazan 20 mg, but <4 for esomeprazole 40 mg (Table 1). During the 8 hours of night, tegoprazan 50 mg exerted greater acid suppression than vonoprazan 20 mg and esomeprazole 40 mg, which were similar trends to those during the entire night (Table S2).
FIGURE 3.

Mean night‐time percentage of time that the intragastric pH was over 4 (% time at pH ≥ 4) for (A) each treatment in all CYP2C19 phenotypes, and for (B) tegoprazan 50 mg, (C) vonoprazan 20 mg and (D) esomeprazole 40 mg according to CYP2C19 phenotypes. Night was defined as the period of 12 hours from 22:00 to 10:00 hours
TABLE 1.
Night‐time pharmacodynamic parameters after a single oral administration of tegoprazan 50 mg, vonoprazan 20 mg or esomeprazole 40 mg under fasted condition
| Treatment | n | Night (22:00–10:00 h) | |||||
|---|---|---|---|---|---|---|---|
| % time at pH ≥ 4 | Median pH | Mean pH | △ integrated gastric acidity (%) | ||||
| Baseline (predose) | Total | 16 | 1.3 ± 1.8 | 1.7 ± 0.1 | 1.8 ± 0.2 | ‐ | |
| CYP2C19 | EM | 6 | 1.3 ± 1.8 | 1.8 ± 0.1 | 1.9 ± 0.2 | ‐ | |
| IM | 5 | 1.7 ± 2.1 | 1.7 ± 0.1 | 1.8 ± 0.2 | ‐ | ||
| PM | 5 | 0.9 ± 1.8 | 1.7 ± 0.1 | 1.7 ± 0.2 | ‐ | ||
| Tegoprazan 50 mg | Total | 16 | 66.0 ± 15.7 | 4.9 ± 0.9 | 4.6 ± 0.7 | 88.0 ± 7.7 | |
| CYP2C19 | EM | 6 | 63.5 ± 15.2 | 5.0 ± 0.9 | 4.6 ± 0.6 | 86.7 ± 9.4 | |
| IM | 5 | 66.9 ± 22.6 | 4.8 ± 1.3 | 4.7 ± 0.8 | 91.2 ± 4.4 | ||
| PM | 5 | 68.1 ± 10.8 | 5.1 ± 0.8 | 4.7 ± 0.7 | 86.6 ± 8.7 | ||
| Vonoprazan 20 mg | Total | 16 | 60.5 ± 13.5 | 5.6 ± 1.2 | 4.7 ± 0.6 | 67.7 ± 15.8 | |
| CYP2C19 | EM | 6 | 62.9 ± 20.8 | 5.6 ± 1.7 | 4.8 ± 0.9 | 67.5 ± 16.5 | |
| IM | 5 | 57.9 ± 5.4 | 5.6 ± 1.0 | 4.6 ± 0.3 | 63.6 ± 18.9 | ||
| PM | 5 | 60.2 ± 9.9 | 5.6 ± 0.8 | 4.7 ± 0.5 | 72.1 ± 13.9 | ||
| Esomeprazole 40 mg | Total | 16 | 36.1 ± 14.7 | 3.1 ± 0.9 | 3.5 ± 0.5 | 66.4 ± 25.2 | |
| CYP2C19 | EM | 6 | 30.5 ± 12.1 | 2.7 ± 0.6 | 3.3 ± 0.5 | 53.2 ± 33.4 | |
| IM | 5 | 37.3 ± 15.7 | 2.9 ± 0.9 | 3.5 ± 0.6 | 77.2 ± 6.6 | ||
| PM | 5 | 41.6 ± 17.1 | 3.7 ± 0.9 | 3.7 ± 0.6 | 71.4 ± 22.4 | ||
Data are expressed as mean ± standard deviation.
EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer.
Baseline intragastric pH profiles at night were comparable among all CYP2C19 phenotypes (Table 1). Mean night‐time intragastric pH–time profiles after each treatment did not differ meaningfully according to CYP2C19 phenotypes (Figure S1). The extent of gastric acid suppression at night by tegoprazan 50 mg and vonoprazan 20 mg did not depend on CYP2C19 phenotypes; however, that by esomeprazole 40 mg showed trends of being influenced by CYP2C19 phenotypes, although not statistically significant (Figures 3 and 4, Table 1). Night‐time PD parameters for esomeprazole 40 mg tended to increase in order of EM, IM and PM.
FIGURE 4.

Point estimates and 95% confidence intervals of the mean differences between CYP2C19 phenotypes for night (A) percentage of time that the intragastric pH was >4 (% time at pH ≥ 4), (B) median pH and (C) mean pH after a single oral administration of tegoprazan 50 mg, vonoprazan 20 mg or esomeprazole 40 mg under fasted condition. Night was defined as the period of 12 hours from 22:00 to 10:00 hours
3.3. Safety
In all treatments, serum gastrin levels were elevated after dosing, but not clinically significant, and recovered closely to their baselines. The degree of increase in gastrin levels was not significantly different among treatments [△serum gastrin: 11.2 pg/mL for tegoprazan vs. 26.3 pg/mL for vonoprazan vs. 7.1 pg/mL for esomeprazole (P = .11)]. Additionally, the pepsinogen I/II ratio did not change meaningfully in any of the treatments.
Throughout the study, 3 treatment‐emergent AEs were reported in 3 subjects: 1 case (thermal burn; not related) in 1 subject after the administration of tegoprazan and 2 cases (dyspepsia and nausea; unlikely) in 1 subject after the administration of vonoprazan. All treatment‐emergent AEs were mild in intensity, and no serious AEs occurred. No clinically significant issues or changes in clinical laboratory tests, vital signs, physical examinations or 12‐lead electrocardiograms were observed.
4. DISCUSSION
Despite the use of various approaches, including twice daily PPI therapies, PPIs with longer t1/2 and the additional bedtime dosing of histamine 2 receptor antagonists, the management of nocturnal acid breakthrough with PPIs has experienced limitations. 4 , 5 , 25 , 26 , 27 As shown in this study, night‐time acid suppression by tegoprazan 50 mg and vonoprazan 20 mg, which are P‐CABs, was clearly greater than that by esomeprazole 40 mg, a PPI, and sufficient even with a single dose. It is consistent with the findings of a previous study of vonoprazan and esomeprazole, 28 and seems to be due to differences in the mechanisms of action between P‐CABs and PPIs, such as reversible vs. irreversible inhibition of H+/K+ ATPase. These results suggest the advantageous properties of P‐CABs, including tegoprazan, compared to PPIs, particularly in managing nocturnal acid breakthrough.
After a single dose of each treatment, times to reach intragastric pH ≥ 4 were observed at approximately 1 hour for tegoprazan 50 mg, and approximately 4 hours for vonoprazan 20 mg and esomeprazole 40 mg. The differences identified in this study are consistent with the results from previous studies. 20 , 28 , 29 Since all of the treatments are known to be rapidly absorbed with comparable Tmax values, 19 , 20 , 22 this phenomenon may have been ascribed to the mechanism of action of PPIs and the characteristics of each treatment. 20 , 30 , 31 Meanwhile, the necessity for on‐demand therapy to control the reflux symptoms of patients with GERD has steadily emerged. 32 In several studies, on‐demand therapy with vonoprazan exhibited similar efficacy to PPI maintenance therapy for patients with mild reflux oesophagitis or non‐erosive reflux disease, 33 , 34 which implies the potential of P‐CABs for the rapid control of the acute symptoms of GERD. In terms of on‐demand therapy for GERD, the faster onset of action of tegoprazan can act as a favourable feature, even expecting superiority to vonoprazan.
Although tegoprazan is known to be predominantly metabolized by CYP3A4 based on in vitro studies, the current study investigated the association between CYP2C19 phenotypes and the acid‐inhibitory effect of tegoprazan, considering the significance of CYP2C19 genetic polymorphisms in clinical settings. The plasma drug concentrations of each treatment were not measured because of clear exposure–response relationships for antisecretory agents, including P‐CABs and PPIs, as previously reported. 35 , 36 In consequence, CYP2C19 phenotypes did not meaningfully influence gastric acid suppression by tegoprazan, which indicates that tegoprazan undergoes negligible CYP2C19‐mediated metabolism in vivo. Based on these results, it is considered that CYP2C19 polymorphisms have no clinically significant effect on the efficacy of tegoprazan, thereby suggesting no necessity for dose adjustment according to CYP2C19 genotypes. The findings of this Korean study, evaluated in a balanced manner for CYP2C19 phenotype, may be generalized to other populations including Caucasian.
This study showed that the acid‐suppressive effects of tegoprazan and vonoprazan are insensitive to CYP2C19 polymorphisms, whereas that of esomeprazole is not, although not statistically significant. The absence of statistical significance in the acid‐inhibitory effect of esomeprazole according to CYP2C19 phenotypes might be attributed to the relatively small sample size. Likewise, a previous similar‐size study with 19 subjects produced the findings that % time at pH ≥ 4 for esomeprazole tended to be influenced by CYP2C19 phenotypes, but not statistically significantly. 37 When estimated with a 80% statistical power at a 5% level of significance based on the results of the current study, over 26 subjects per phenotype would be needed to achieve statistically significant differences between CYP2C19 phenotypes in % time at pH ≥ 4 for esomeprazole.
The current study is the first to compare night‐time gastric acid suppression by tegoprazan, vonoprazan and esomeprazole. However, these results should be translated with caution after considering some limitations of the study. First, this exploratory study included only H. pylori‐negative healthy subjects, not patients with gastrointestinal disorders, including GERD. Because several studies have proposed that the H. pylori status is related to the extent of gastric acid secretion and the presence of nocturnal acid breakthrough, 5 , 38 the results should be extrapolated carefully to patients, especially with H. pylori infection. Second, the extent of acid suppression was assessed after a single administration of each treatment. But the results appear meaningful to identify the trends observed for different treatments in this study, and further confirmation studies with multiple doses may be required. Based on dose‐dependent acid inhibition by the treatments as previously reported, the trends following multiple doses are expected to be comparable with those in this single‐dose study. 19 , 20 , 36 Finally, all subjects received their assigned treatment at night, around 10 PM; however, evening dosing is slightly different from real clinical settings in which antisecretory agents are usually taken.
In conclusion, tegoprazan 50 mg produced more rapid, potent and well sustained night‐time gastric acid suppression vs. vonoprazan 20 mg or esomeprazole 40 mg when it was administered at night and showed no CYP2C19 phenotype dependency in acid suppression. These results suggest the potential of tegoprazan, especially in preventing nocturnal acid breakthrough.
COMPETING INTERESTS
Seokuee Kim, Bongtae Kim and Geun Seog Song are employees of HK inno. N Corp. The other authors report no conflicts of interest in this work.
CONTRIBUTORS
Eunsol Yang, Boram Kim, Yechan Kim, Sung Sup Park, Kyung‐Sang Yu, In‐Jin Jang and SeungHwan Lee performed the research. Eunsol Yang and SeungHwan Lee analysed the data. Eunsol Yang, Seokuee Kim, Bongtae Kim, Kyung‐Sang Yu, In‐Jin Jang and SeungHwan Lee designed the research. Eunsol Yang and SeungHwan Lee wrote the manuscript. All the authors reviewed and revised the paper. All the authors reviewed and revised the paper. All the authors approved the final version of the manuscript.
Supporting information
TABLE S1 Demographics of subjects according to CYP2C19 phenotypes.TABLE S2 Night‐time pharmacodynamic parameters during the 8‐hour interval after a single oral administration of tegoprazan 50 mg, vonoprazan 20 mg or esomeprazole 40 mg under fasted condition.FIGURE S1 Mean night‐time intragastric pH–time profiles after a single oral administration of (A) tegoprazan 50 mg, (B) vonoprazan 20 mg and (C) esomeprazole 40 mg according to CYP2C19 phenotypes.
ACKNOWLEDGEMENT
This study was funded in full by HK inno. N Corp., Seoul, Republic of Korea.
Yang E, Kim S, Kim B, et al. Night‐time gastric acid suppression by tegoprazan compared to vonoprazan or esomeprazole. Br J Clin Pharmacol. 2022;88(7):3288-3296. doi: 10.1111/bcp.15268
The authors confirm that the Principal Investigator for this paper is In‐Jin Jang and that he had direct clinical responsibility for patients.
Funding information HK inno. N Corp.
DATA AVAILABILITY STATEMENT
The individual de‐identified participant data supporting published results are available with approval from the corresponding author on reasonable request, at any time after publication.
REFERENCES
- 1. Kellerman R, Kintanar T. Gastroesophageal Reflux Disease. Prim Care. 2017;44(4):561‐573. doi: 10.1016/j.pop.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 2. Chen J, Brady P. Gastroesophageal Reflux Disease: Pathophysiology, Diagnosis, and Treatment. Gastroenterol Nurs. 2019;42(1):20‐28. doi: 10.1097/sga.0000000000000359 [DOI] [PubMed] [Google Scholar]
- 3. Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro‐oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59‐67. doi: 10.1159/000200917 [DOI] [PubMed] [Google Scholar]
- 4. Peghini PL, Katz PO, Bracy NA, Castell DO. Nocturnal recovery of gastric acid secretion with twice‐daily dosing of proton pump inhibitors. Am J Gastroenterol. 1998;93(5):763‐767. doi: 10.1111/j.1572-0241.1998.221_a.x [DOI] [PubMed] [Google Scholar]
- 5. Tutuian R, Castell DO. Nocturnal acid breakthrough ‐ approach to management. MedGenMed. 2004;6(4):11‐11. [PMC free article] [PubMed] [Google Scholar]
- 6. Yacyshyn B, Thomson A. The clinical importance of proton pump inhibitor pharmacokinetics. Digestion. 2002;66(2):67‐78. doi: 10.1159/000065588 [DOI] [PubMed] [Google Scholar]
- 7. Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin Proc. 2018;93(2):240‐246. doi: 10.1016/j.mayocp.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 8. Vaughn BV, Rotolo S, Roth HL. Circadian rhythm and sleep influences on digestive physiology and disorders. ChronoPhysiol Ther. 2014;4:67‐77. doi: 10.2147/CPT.S44806 [DOI] [Google Scholar]
- 9. Farup C, Kleinman L, Sloan S, et al. The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health‐related quality of life. Arch Intern Med. 2001;161(1):45‐52. doi: 10.1001/archinte.161.1.45 [DOI] [PubMed] [Google Scholar]
- 10. Shaker R, Castell DO, Schoenfeld PS, Spechler SJ. Nighttime heartburn is an under‐appreciated clinical problem that impacts sleep and daytime function: the results of a Gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol. 2003;98(7):1487‐1493. doi: 10.1111/j.1572-0241.2003.07531.x [DOI] [PubMed] [Google Scholar]
- 11. El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. 2018;14(4):447‐460. doi: 10.1080/17425255.2018.1461835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther. 2016;43(10):1048‐1059. doi: 10.1111/apt.13588 [DOI] [PubMed] [Google Scholar]
- 13. Sahara S, Sugimoto M, Uotani T, et al. Twice‐daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice‐daily omeprazole, rabeprazole or lansoprazole. Aliment Pharmacol Ther. 2013;38(9):1129‐1137. doi: 10.1111/apt.12492 [DOI] [PubMed] [Google Scholar]
- 14. Petrović J, Pešić V, Lauschke VM. Frequencies of clinically important CYP2C19 and CYP2D6 alleles are graded across Europe. Eur J Hum Genet. 2020;28(1):88‐94. doi: 10.1038/s41431-019-0480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorji PW, Tshering G, Na‐Bangchang K. CYP2C9, CYP2C19, CYP2D6 and CYP3A5 polymorphisms in South‐East and East Asian populations: A systematic review. J Clin Pharm Ther. 2019;44(4):508‐524. doi: 10.1111/jcpt.12835 [DOI] [PubMed] [Google Scholar]
- 16. Oshima T, Miwa H. Potent Potassium‐competitive Acid Blockers: A New Era for the Treatment of Acid‐related Diseases. J Neurogastroenterol Motil. 2018;24(3):334‐344. doi: 10.5056/jnm18029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shibli F, Kitayama Y, Fass R. Novel Therapies for Gastroesophageal Reflux Disease: Beyond Proton Pump Inhibitors. Curr Gastroenterol Rep. 2020;22(4):1‐13. doi: 10.1007/s11894-020-0753-y [DOI] [PubMed] [Google Scholar]
- 18. Kagami T, Yamade M, Suzuki T, et al. Comparative Study of Effects of Vonoprazan and Esomeprazole on Antiplatelet Function of Clopidogrel or Prasugrel in Relation to CYP2C19 Genotype. Clin Pharmacol Ther. 2018;103(5):906‐913. doi: 10.1002/cpt.863 [DOI] [PubMed] [Google Scholar]
- 19. Sakurai Y, Nishimura A, Kennedy G, et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Rising TAK‐438 (Vonoprazan) Doses in Healthy Male Japanese/non‐Japanese Subjects. Clin Transl Gastroenterol. 2015;6(6):e94 doi: 10.1038/ctg.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han S, Choi HY, Kim YH, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ‐12420), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2019;50(7):751‐759. doi: 10.1111/apt.15438 [DOI] [PubMed] [Google Scholar]
- 21. Yoon DY, Sunwoo J, Shin N, et al. Effect of meal timing on pharmacokinetics and pharmacodynamics of tegoprazan in healthy male volunteers. Clin Transl Sci. 2021;14(3):934‐941. doi: 10.1111/cts.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hassan‐Alin M, Andersson T, Bredberg E, Röhss K. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol. 2000;56(9–10):665‐670. doi: 10.1007/s002280000206 [DOI] [PubMed] [Google Scholar]
- 23. Yang D, Kim B, Song DY, et al. Pitfalls of ABO Genotyping Based on Targeted Single Nucleotide Variant Analysis Due to a Nondeletional O Allele Lacking c.261delG: First Report of ABO*O.09.01 in Korea. Ann Lab Med. 2019;39(6):599‐601. doi: 10.3343/alm.2019.39.6.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK‐438 (vonoprazan), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41(7):636‐648. doi: 10.1111/apt.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peghini PL, Katz PO, Castell DO. Ranitidine controls nocturnal gastric acid breakthrough on omeprazole: a controlled study in normal subjects. Gastroenterology. 1998;115(6):1335‐1339. doi: 10.1016/s0016-5085(98)70010-1 [DOI] [PubMed] [Google Scholar]
- 26. Hunt RH, Armstrong D, Yaghoobi M, James C. The pharmacodynamics and pharmacokinetics of S‐tenatoprazole‐Na 30 mg, 60 mg and 90 mg vs. esomeprazole 40 mg in healthy male subjects. Aliment Pharmacol Ther. 2010;31(6):648‐657. doi: 10.1111/j.1365-2036.2009.04219.x [DOI] [PubMed] [Google Scholar]
- 27. Jeon HK, Kim GH. Can Nocturnal Acid‐breakthrough Be Reduced by Long‐acting Proton Pump Inhibitors? J Neurogastroenterol Motil. 2017;23(2):145‐148. doi: 10.5056/jnm17037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakurai Y, Mori Y, Okamoto H, et al. Acid‐inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects‐a randomised open‐label cross‐over study. Aliment Pharmacol Ther. 2015;42(6):719‐730. doi: 10.1111/apt.13325 [DOI] [PubMed] [Google Scholar]
- 29. Sunwoo J, Ji SC, Oh J, et al. Pharmacodynamics of tegoprazan and revaprazan after single and multiple oral doses in healthy subjects. Aliment Pharmacol Ther. 2020;52(11–12):1640‐1647. doi: 10.1111/apt.16121 [DOI] [PubMed] [Google Scholar]
- 30. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6):528‐534. doi: 10.1007/s11894-008-0098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scarpignato C, Hunt RH. Editorial: potassium‐competitive acid blockers for acid‐related diseases‐tegoprazan, a new kid on the block. Aliment Pharmacol Ther. 2019;50(8):960‐962. doi: 10.1111/apt.15480 [DOI] [PubMed] [Google Scholar]
- 32. Metz DC, Inadomi JM, Howden CW, van Zanten SJ, Bytzer P. On‐demand therapy for gastroesophageal reflux disease. Am J Gastroenterol. 2007;102(3):642‐653. doi: 10.1111/j.1572-0241.2006.00998.x [DOI] [PubMed] [Google Scholar]
- 33. Umezawa M, Kawami N, Hoshino S, et al. Efficacy of On‐Demand Therapy Using 20‐mg Vonoprazan for Mild Reflux Esophagitis. Digestion. 2018;97(4):309‐315. doi: 10.1159/000485795 [DOI] [PubMed] [Google Scholar]
- 34. Hoshikawa Y, Kawami N, Hoshino S, et al. Efficacy of on‐demand therapy using 20‐mg vonoprazan for non‐erosive reflux disease. Esophagus. 2019;16(2):201‐206. doi: 10.1007/s10388-018-00654-9 [DOI] [PubMed] [Google Scholar]
- 35. Sunwoo J, Oh J, Moon S, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP 14012, a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2018;48(2):206‐218. doi: 10.1111/apt.14818 [DOI] [PubMed] [Google Scholar]
- 36. Andersson T, Röhss K, Bredberg E, Hassan‐Alin M. Pharmacokinetics and pharmacodynamics of esomeprazole, the S‐isomer of omeprazole. Aliment Pharmacol Ther. 2001;15(10):1563‐1569. doi: 10.1046/j.1365-2036.2001.01087.x [DOI] [PubMed] [Google Scholar]
- 37. Hunfeld NG, Touw DJ, Mathot RA, et al. A comparison of the acid‐inhibitory effects of esomeprazole and pantoprazole in relation to pharmacokinetics and CYP2C19 polymorphism. Aliment Pharmacol Ther. 2010;31(1):150‐159. doi: 10.1111/j.1365-2036.2009.04150.x [DOI] [PubMed] [Google Scholar]
- 38. Smolka AJ, Schubert ML. Helicobacter pylori‐Induced Changes in Gastric Acid Secretion and Upper Gastrointestinal Disease. Curr Top Microbiol Immunol. 2017;400:227‐252. doi: 10.1007/978-3-319-50520-6_10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Demographics of subjects according to CYP2C19 phenotypes.TABLE S2 Night‐time pharmacodynamic parameters during the 8‐hour interval after a single oral administration of tegoprazan 50 mg, vonoprazan 20 mg or esomeprazole 40 mg under fasted condition.FIGURE S1 Mean night‐time intragastric pH–time profiles after a single oral administration of (A) tegoprazan 50 mg, (B) vonoprazan 20 mg and (C) esomeprazole 40 mg according to CYP2C19 phenotypes.
Data Availability Statement
The individual de‐identified participant data supporting published results are available with approval from the corresponding author on reasonable request, at any time after publication.


