Abstract
Purpose
64Cu and 67Cu radioisotopes have nuclear characteristics suitable for nuclear medicine applications. The production of 64Cu is already well established. However, the production of 67Cu in quantities suitable to conduct clinical trials is more challenging as it leads to the coproduction of other Cu isotopes, in particular 64Cu. The aim of this study is to investigate the possibility of using a CuCl2 solution with a mixture of 67/64Cu radioisotopes for therapeutic purposes, providing an alternative solution for the cyclotron production problem.
Methods
Copper radioisotopes activities were calculated by considering proton beam irradiation of the following targets: (i) 70Zn in the energy range 70–45 MeV; (ii) 68Zn in the energy range 70–35 MeV; (iii) a combination of 70Zn (70–55 MeV) and 68Zn (55–35 MeV). The contribution of each copper radioisotope to the human‐absorbed dose was estimated with OLINDA/EXM software using the biokinetic model for CuCl2 published by ICRP 53. The total absorbed dose generated by the 67/64CuCl2 mixture, obtained through different production routes, was calculated at different times after the end of the bombardment (EOB). A simple spherical model was used to simulate tumors of different sizes containing uniformly distributed 67/64Cu mixture and to calculate the absorbed dose of self‐irradiation. The biological damage produced by 67Cu and 64Cu was also evaluated through cellular dosimetry and cell surviving fraction assessment using the MIRDcell code, considering two prostate cancer cell lines with different radiosensitivity.
Results
The absorbed dose to healthy organs and the effective dose (ED) per unit of administered activity of 67CuCl2 are higher than those of 64CuCl2. Absorbed dose values per unit of administered activity of 67/64CuCl2 mixture increase with time after the EOB because the amount of 67Cu in the mixture increases. Survival data showed that the biological damage caused per each decay of 67Cu is greater than that of 64Cu, assuming that radionuclides remain accumulated in the cell cytoplasm. Sphere model calculations demonstrated that 64Cu administered activity must be about five times higher than that of 67Cu to obtain the same absorbed dose for tumor mass between 0.01 and 10 g and about 10 times higher for very small spheres. Consequently, the 64CuCl2‐absorbed dose to healthy organs will reach higher values than those of 67CuCl2. The supplemental activity of the 67/64CuCl2 mixture, required to get the same tumor‐absorbed dose produced by 67CuCl2, triggers a dose increment (DI) in healthy organs. The waiting time post‐EOB necessary to keep this DI below 10% (t 10%) depends on the irradiation methods employed for the production of the 67/64CuCl2 mixture.
Conclusions
A mixture of cyclotron produced 67/64Cu radioisotopes proved to be an alternative solution for the therapeutic use of CuCl2 with minimal DI to healthy organs compared with pure 67Cu. Irradiation of a 70Zn+68Zn target in the 70–35 MeV proton energy range for 185 h appears to be the best option from among all the production routes investigated, as it gives the maximum amount of activity, the shortest t 10% (10 h), and less than 1% of 61Cu and 60Cu impurities.
Keywords: 67CuCl2 , copper radioisotope mixture, copper radioisotope production, cyclotron physics/radionuclide production, internal dosimetry, radiation dosimetry and risk, theranostic copper radioisotopes
1. INTRODUCTION
Copper is an essential element for a multitude of biological processes, being a catalytic cofactor of many enzymes and a key structural component of functional proteins with fundamental roles in cellular biology. 1 Copper also plays a key role in cell replication and growth, and it has been found to be deeply involved in cancer development and progression. The potential role of Cu2+ ions and their ability to selectively target cancerous cells was recently assessed. 2 Preliminary results showed a high uptake of 64Cu2+ in prostate cancer cells, demonstrating the great diagnostic potential of 64CuCl2 for cancer. 3 The therapeutic potential of 64CuCl2 was also assessed in malignant melanoma 4 and glioblastoma tumor‐bearing mice 5 and a high tumor uptake of 67CuCl2 was observed in colorectal tumor‐bearing mice. 6 Despite only two preliminary reports have demonstrated a therapeutic effect of 64CuCl2 in patients affected by relapsing malignancies (i.e. glioblastoma, prostate and uterine cancer), 7 , 8 these findings suggest that both 64CuCl2 and 67CuCl2 could be used to further treat these types of tumors in future.
The five copper radioisotopes with the nuclear characteristics most suitable for nuclear medicine applications are 60Cu, 61Cu, 62Cu, 64Cu, and 67Cu. 9 Among them, 60Cu (t 1/2 = 23.7 m), 61Cu (t 1/2 = 3.333 h), and 62Cu (t 1/2 = 9.673 m) are pure positron emitters; 67Cu (t 1/2 = 61.83 h) decays emitting a combination of β– particles with E max = 0.56 MeV (100%) and γ‐rays at 92 keV (23%) and 185 keV (48%), suitable for SPECT imaging, and could thus be used as a theranostic agent; 64Cu (t 1/2 = 12.7 h) decays mostly through the emission of β– (38%), β+ (18%) particles and Auger electrons, so it can find both diagnostic and therapeutic applications. 64Cu‐based therapy can be advantageous if the radionuclide is incorporated into the cell nucleus as its Auger electron emission could deliver a very high dose to the DNA, killing the cells.
While 64Cu radiopharmaceuticals are employed in the clinical diagnosis of some types of tumors, 10 the limited availability of 67Cu 11 has to date severely restricted its use, despite its promising results in radioimmunotherapy, 12 , 13 , 14 peptide receptor radionuclide therapy, 15 , 16 and PSMA targeting therapy. 17 , 18
The production of 64Cu is well‐established, and it is mainly based on the use of 64Ni or 68Zn targets, irradiated by proton or deuteron beams. 19
The production of 67Cu is instead more challenging and still under investigation, 20 as emerged from the dedicated Coordinated Research Project (CRP) promoted by the International Atomic Energy Agency (IAEA). 21 It emerges from recent publications on 67Cu production 22 , 23 , 24 , 25 that the use of highly enriched target materials results in a pure final product at the end of irradiation with the 68Zn(γ,p)67Cu, 70Zn(p,α)67Cu, and 70Zn(d,x)67Cu reactions at low energy (E p < 35 MeV, E d < 27 MeV). All nuclear reactions concerned have low cross‐section values (below 30 mb), leading to a rather low 67Cu yield. In order to increase the proton‐based production of 67Cu, it is necessary to use 68Zn‐ or 70Zn‐enriched targets and irradiations at intermediate beam energies (i.e., larger than 30 MeV). However, this approach leads to the coproduction of Cu isotopic impurities, in particular 64Cu. As Cu isotopes cannot be separated by standard (i.e., radiochemical) methods, this is a concern from a pharmaceutical point of view. According to the European Pharmacopeia, the radionuclidic purity of a radiopharmaceutical must indeed be greater than 99%. In general, this limit guarantees that the dose increase due to the impurities remains below 10%. 26 , 27 If 64Cu is considered an impurity, it will be then necessary a long waiting time after the irradiation of targets to achieve the required radionuclidic purity, losing most of the 67Cu produced activity. However, as both 67Cu and 64Cu have promising therapeutic characteristics, 64Cu could not be considered as an impurity, but, on the contrary, as a therapeutic coadjuvant of 67Cu, with also the possibility of exploiting its β+ emission for the monitoring of the radiopharmaceutical uptake and the biodistribution in the body by PET imaging, with higher accuracy compared to the SPECT imaging allowed by the γ‐emissions of 67Cu. Therefore, a combination of the two radionuclides is worthwhile to be investigated.
The energy of released particles is an important parameter to be evaluated for cancer therapy with β emitters because therapeutic effectiveness can be low if electron penetration ranges are greater than the tumor dimensions. Generally, tumors come in a variety of sizes, ranging from a single or a few cells to large tumors with radii of several centimeters. A radionuclide that releases a high absorbed dose to large tumors may be nonoptimal for small ones because a substantial fraction of the β‐particle energy will be delivered to healthy tissues adjacent to the tumors. Therefore, an optimal tumor diameter range for each radionuclide has been identified in order to produce an effective treatment. 28 , 29 Wheldon et al. 28 were the first to propose the use of a panel of β–‐emitting radionuclides for clinical scenarios involving a vast number of tumors and metastases of different sizes. The authors reported that the overall level of variation in the probability of cure of tumors with extensive differences in radii could be reduced when using β– emitters with different β end‐point energies. 28 A clinical study, using a combined 90Y/177Lu‐DOTATATE therapy, demonstrated that the combination of the two radionuclides with differing β– energy and, therefore, a different maximum range in tissues (2.27 MeV and 10 mm for 90Y, and 0.497 MeV and 2–4 mm for 177Lu, respectively), produced longer overall patient survival than a single radioisotope treatment. 30 Nevertheless, it is important to underline that the chemical properties of the same molecule, labeled with different radionuclides, are not identical. The radiolabeled molecules seem to be similar, but can present different stability and biodistribution, because each element has a specific chemical demand arising from its fundamental characteristics such as the atomic number, charge, and radius, which result in a distinct coordination number and geometry. 31 The advantage of using a radionuclide cocktail with isotopes of the same element is that their labeled conjugates will have the same stability and biodistribution due to identical chemical properties. In case of 64Cu and 67Cu, despite their different decay schemes, the β– end‐point energies are quite similar (0.65310 and 0.56170 MeV for 64Cu and 67Cu, respectively). Therefore, a mixture of the two radionuclides is not expected to provide a therapeutic benefit for treating tumors of different sizes, as demonstrated by the similar therapeutic potential of 64Cu and 67Cu on a per‐decay basis by both in vitro and in vivo studies. 32 , 33 However, supposing that the presence of 64Cu will not adversely affect the absorbed dose to healthy organs compared with the administration of pure 67Cu, the possibility of using a mixture of 67Cu and 64Cu for therapeutic purposes will provide an alternative solution to the 67Cu supply.
This work investigated the production of 67Cu/64Cu using proton beams up to 70 MeV in three scenarios: (i) the use of 70Zn targets in the energy range 70–45 MeV; (ii) the use of 68Zn targets in the energy range 70–35 MeV; (iii) the use of a combination of 70Zn (70–55 MeV) and 68Zn (55–35 MeV) targets, as presented in the INFN patent. 34
To assess the possibility of using a mixture of 67/64Cu radioisotopes for therapeutic purposes, the contribution of each radioisotope to the human‐absorbed dose after the administration of the CuCl2 solution was estimated using the biokinetic model published by ICRP 53 35 with the OLINDA/EXM software's adult male/female reference phantoms. 36 The total absorbed dose from a CuCl2 solution containing a mixture of both radioisotopes was then calculated considering different production methods at different times after the end of bombardment (EOB). Furthermore, a simple model was used to simulate tumors as isolated unit density spheres immersed in an infinite unit density medium and to calculate the absorbed dose attributable to self‐irradiation for the activity uniformly distributed into the spheres. Cellular dosimetry and cell surviving fraction were also evaluated assuming the administration of 67CuCl2 or 64CuCl2 to two prostate cancer cell lines with different radiosensitivity to determine the biological damage produced by each radioisotope.
2. MATERIALS AND METHODS
2.1. Copper‐67 and Copper‐64 production yields
The production of 67Cu, 64Cu, 61Cu, and 60Cu radionuclides was calculated with the IAEA tool ISOTOPIA, 37 taking into account the following priority list for the selection of nuclear cross sections (xs):
the IAEA recommended values 38 ;
the experimental values available in the literature and the EXFOR database 39 ;
the TALYS estimated trend available in the TENDL library. 40
These criteria led to the following configuration for the different scenarios: (A) a 68Zn target with a proton beam energy in the range 70–35 MeV (the exit energy for a 6.2 mm thick 68Zn target): 67Cu and 64Cu activities were calculated by taking the IAEA xs recommended data into account, 38 61Cu activity by considering experimental xs values, 39 , 41 and 60Cu activity considering TENDL nuclear model predictions 40 ; (B) a 70Zn target with a proton beam in the energy range 70–45 MeV (the exit energy for a 5.08 mm thick 70Zn target): 67Cu and 64Cu activities were calculated by considering experimental xs data, 25 , 39 61Cu activity was estimated based on the use of the TENDL library, 40 and 60Cu production was not foreseen; (C) the combined 70Zn+68Zn target: in the energy range 70–55 MeV (70Zn target), 67Cu and 64Cu activities were calculated by considering experimental data, 25 , 39 61Cu activity was based on the use of TENDL‐predicted cross sections, 40 while 60Cu production was not foreseen; in the energy range 35–55 MeV (68Zn target), 67Cu and 64Cu activities were calculated by taking IAEA data into account, 38 61Cu activity was based on the use of experimental values, 41 and 60Cu activity by considering the TENDL library. 40
The yield for all the different nuclear reaction routes concerned was estimated by considering a proton beam current of 1 µA and irradiation times of 62 h (corresponding to a saturation factor [SF] of about 50% of 67Cu), 124 h (67Cu SF ≈ 75%) and 185 h (67Cu SF ≈ 88%) as irradiation parameters.
2.2. Biokinetic model of CuCl2
The biokinetic model published by ICRP 53 35 was used to estimate the total number of disintegrations in the main human source organs after administration of xx CuCl2.
According to a general first‐order kinetic model, and assuming an immediate uptake into the organs, the fractional activity in a source organ S at time t, A s(t), after administration of the activity A 0 is given by the relationship:
| (1) |
where F s is the fractional distribution to organ or tissue S, m is the number of elimination components, and ai is the fraction of F S eliminated with effective half‐life Ti ,eff, which can be calculated from the corresponding biological half‐life Ti and the physical half‐life T p of the radioisotope:
| (2) |
The model parameters to calculate copper uptake in the main human source organs such as the liver, brain, kidneys, pancreas, and in the entire body are reported in Table S1. 35 The normalized cumulated activity is then calculated according to the formula:
| (3) |
2.3. Dosimetric calculations applied to human phantoms
Dosimetric calculations for xx CuCl2 were performed with the Organ Level Internal Dose Assessment (OLINDA) software code version 2.2.0, 36 , 42 based upon the RADAR method for internal dose estimation, 43 aiming at obtaining both the absorbed doses per unit of administered activity in each organ and the effective dose (ED). The normalized cumulated activity in the source organs obtained with the ICRP 53 biokinetic model 35 and both female and male NURBS‐type phantoms, 44 based on the standardized masses defined by ICRP 89, 45 were used as input for the calculations with the OLINDA software. Effective dose equivalent (EDE) and ED values were calculated by using the three different tissue‐weighting factors sets, recommended by ICRP 26, 46 ICRP 60, 47 and ICRP 103. 48
Finally, the absorbed doses to different healthy organs (D organ,t) and the total ED (EDt) per unit administered activity caused by the mixture of copper radioisotopes obtained from different production methods were calculated at different times after EOB, using the following equations:
| (4) |
| (5) |
where fxx Cu (t) is the fraction of total activity corresponding to xx Cu radioisotope at the time t after EOB and D organ, xx Cu and ED xx Cu are the absorbed dose to an organ and the ED due to unit administered activity of XX CuCl2.
2.4. Dosimetric calculations applied to a macroscopic tumor (sphere model)
The OLINDA software's sphere model module was used to simulate tumors as isolated unit density spheres immersed in an infinite unit density medium. This module allows for the evaluation of the absorbed dose solely from self‐irradiation for activity uniformly distributed throughout the spheres. Data are available for discrete sphere masses ranging from 0.01 to 6000 g. Calculations for smaller spheres were performed using the MIRDcell programme, 49 evaluating self‐doses to spheres ranging from 10 µm of diameter (mass: 5 × 10–10 g) up to 2.5 mm (mass: 8 × 10–3 g). Both programmes were used to calculate the absorbed doses for 67Cu and 64Cu radionuclides, which were then compared with the data for 177Lu.
The tumor‐absorbed dose generated by the mixture of copper radioisotopes obtained from different irradiations was also calculated at different times after EOB. Calculations were performed assuming an immediate uptake of the 67/64CuCl2 mixture in the tumor and disregarding biological elimination. The percentage of the number of nuclear transformations (%nt) occurring within the tumor due to each xx Cu radioisotope in the mixture was evaluated on the basis of the total activity fraction corresponding to each xx Cu radioisotope at the time of injection and the physical half‐life of the radioisotope:
| (6) |
The tumor‐absorbed dose for the 67/64CuCl2 mixture was then obtained by weighting the absorbed dose of each xx Cu radioisotope according to the fraction of decays inside the sphere.
2.5. Cellular dosimetry and survival
MIRDcell software 49 was used to compare the biological damage caused by 67Cu or 64Cu radionuclides. This programme makes it possible to determine the cellular radiation absorbed doses as well as the surviving fraction of cells in a 3D multicellular cluster after radionuclide treatment. Calculations were performed considering all β and conversion electron emissions with a contribution to the total energy emitted per nuclear transformation greater than 0.1%. Calculations considered the full energy spectrum for β particles. Cellular S values (mean absorbed dose per unit cumulated activity in the source region) were obtained using a model that considers the cell as two concentric spheres with a 10 and 4 µm radius, representing the whole cell (c) and its nucleus (n), respectively. The cell size was selected based upon the mean size of some of the most studied cancer cell lines, 50 whereas the cell nucleus size was calculated by using the assumption that the nucleus volume is approximately 8% of the whole cell volume. 51 The region between both spheres represents the cytoplasm (cy), whereas the surface of the outer larger sphere represents the cell surface.
The cellular S value is a dose factor that is determined by the radioisotope used and the spatial relationship between the target and the source region. In this work, cellular S values were obtained assuming that radioactivity was uniformly distributed inside one of the cell regions (source region) and taking into account different distances between the target and the source cells (from 20 to 124 µm). Two types of treatment were studied: the first one assuming that the entire cell was both the source and target region, whereas the second one assuming that the cell nucleus was the target region and the cytoplasm the source region. Finally, calculated S values were used to obtain the absorbed dose (D) to the target region using the following equation:
| (7) |
where N source is the number of disintegrations in the source region per unit of administered activity (Bq‐h/Bq).
The MIRDcell programme was used to estimate survival for each treatment, assuming a cluster of cells with a spherical shape and a radius of 124 µm, containing 1021 cells with a distance of 20 µm between centers of neighboring cells, and considering that only 50% of the cells were labeled with radioactivity. The programme randomly selects labeled cells in the cluster. Cell activity can vary from zero up to a maximum activity, which in this study was set at 0.02 Bq per cell. The time‐integrated activity coefficient, also known as residence time, representing the cumulative number of nuclear transformations (Bq‐h) occurring in the source region per unit administered activity A 0 (Bq), was set at 100 h for both 67Cu and 64Cu radionuclides.
The surviving fraction was obtained by using the linear quadratic model, which assumes that each cell is killed due to the inactivation of two or more targets and considers two possibilities: lethal damage when the cell injury is irreparable, and sublethal damage when the injury is reparable by the cell itself. Then cell survival curve can be determined through two components, αD, which accounts for the linear behavior (proportional to the radiation dose, related to the irreparable injury), and βD, 2 which is proportional to the square of the radiation dose (related to the repairable damage). Survival curves were obtained taking into account the absorbed dose generated by both the radiation emitted within the same cell (self‐dose [D self]) and the radiation emitted by neighboring cells (cross dose [D cross]), using the next equation:
| (8) |
where α self and β self and α cross and β cross are the linear quadratic parameters that characterize the cellular response to D self and to D cross, respectively. Calculations were carried out using α self = α cross = α and β self = β cross = β and choosing α and β values reported for two types of prostate cancer cell lines with different radiosensitivity, LNCaP (α = 1.081 and β = 0) and PC3 (α = 0.551 and β = 0.021). 52
The biological damage caused by Cu‐radionuclides was also compared with that obtained with 177Lu, currently the most used radionuclide in theranostics. Therefore, the MIRDcell programme was also run under the same conditions by considering the radionuclide 177Lu.
3. RESULTS
3.1. 67Cu and 64Cu production yields
The production yields of 67Cu, 64Cu and the radioisotopic impurities 61Cu and 60Cu are reported in Table 1. Production yields were estimated considering the proton irradiation of both 68Zn and 70Zn targets for the different scenarios and irradiation times described.
TABLE 1.
Calculated yields (MBq/µA) of 67Cu,64Cu,61Cu, and 60Cu radionuclides obtained at the EOB through the proton irradiation of 68Zn and 70Zn targets for the different scenarios and irradiation times, the waiting time necessary to achieve a 67Cu radionuclidic purity of 99% and the amount of 67Cu activity at this time
| Irr. time (h) | 67Cu at EOB (MBq/µA) | 64Cu at EOB (MBq/µA) | 61Cu at EOB (MBq/µA) | 60Cu at EOB (MBq/µA) | t 99% (h) | 67Cu at t99% (MBq/µA) | |
|---|---|---|---|---|---|---|---|
| 68Zn: 70–35 MeV | 62 | 1240.1 | 6512.0 | 1140.1 | 26.5 | 145 | 244.1 |
| 124 | 1859.4 | 6732.9 | 1140.1 | 26.5 | 136 | 404.8 | |
| 185 | 2165.2 | 6740.4 | 1140.1 | 26.5 | 133 | 487.5 | |
| 70 Zn: 70–45 MeV | 62 | 1751.7 | 7506.7 | 11.7 | – | 139 | 368.7 |
| 124 | 2626.5 | 7761.4 | 11.7 | – | 131 | 604.8 | |
| 185 | 3058.5 | 7770.0 | 11.7 | – | 128 | 728.3 | |
| 70 Zn: 70–55 MeV + 68 Zn: 55–35 MeV | 62 | 1881.3 | 5825.0 | 40.0 | 0.0012 | 132 | 428.3 |
| 124 | 2820.9 | 6022.6 | 40.0 | 0.0012 | 123 | 710.5 | |
| 185 | 3284.9 | 6029.3 | 40.0 | 0.0012 | 120 | 855.6 |
Table 1 demonstrates that both 67Cu and 64Cu are produced in all the scenarios investigated and their amount increases with the irradiation time. The activity of 64Cu is always greater than that of 67Cu at the EOB. However, due to the different half‐lives of the two radioisotopes, the percentage amount of 64Cu activity in the total decreases with time after irradiation, whereas the percentage amount of 67Cu activity increases (see Figure 1). However, as also reported in Table 1, considering 64Cu as an impurity (besides 61Cu and 60Cu) with respect to the 67Cu production process, the waiting time necessary to achieve a radionuclidic purity higher than 99% (t 99%) would be quite long (between 120 and 145 h, depending upon the irradiation conditions), causing a decay of about 75–80% of the 67Cu produced activity. Both 61Cu and 60Cu radioisotopic impurities are produced by the irradiation of the 68Zn target (for both the 70–35 and 55–35 MeV energy ranges), whereas only 61Cu is generated by the irradiation of the 70Zn target for both 70–45 and 70–55 MeV. The fraction of total activity due to both 61Cu and 60Cu radionuclides is, however, lower than 1% at EOB for the irradiation of the 70Zn target alone or in combination with the 68Zn target. The fraction of total activity due to 61Cu plus 60Cu radionuclides for the irradiation of the 68Zn target at 70–35 MeV is about 12–13%. However, this percentage decreases with time, achieving 1% of total activity from 16 to 17 h after the EOB due to the short half‐lives of both 61Cu and 60Cu radionuclides.
FIGURE 1.
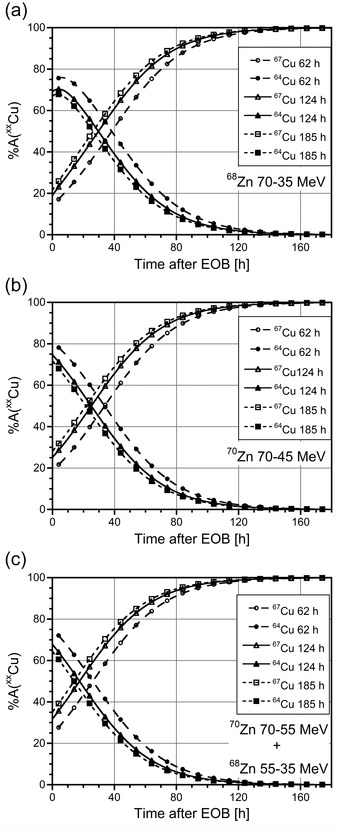
Percentage of activity due to 67Cu and 64Cu radionuclides as a function of time postirradiation, obtained with a 1 µA proton beam and different irradiation times (circles: 62 h; triangles: 124 h; squares: 185 h) of (a) a 68Zn target in the energy range 70–35 MeV; (b) a 70Zn target in the energy range 70–45 MeV; (c) a composite 70Zn–68Zn target in the energy range 70–55 and 55–35 MeV, respectively.
It should be recalled that the irradiation of 70Zn targets at low energy (30–10 MeV range) only produces 67Cu, yet the amount of activity obtained is rather low: 258.5 MBq/µA for 62 h of irradiation, 387.6 MBq/µA for 124 h, and 451.4 MBq/µA for 185 h, corresponding to about 15% or 65% of the 67Cu activity obtained at EOB or at t 99%, respectively, irradiating 70Zn at higher energy (70–45 MeV). For this reason, this scenario was not included in the current work.
3.2. Dosimetry of XX CuCl2
Table 2 illustrates the normalized cumulated activity in the main source organs, calculated for the copper radioisotopes 67Cu, 64Cu, 61Cu, and 60Cu according to the formula (3). The normalized cumulated activity in the rest of the body corresponds to the difference between the cumulated activity evaluated in the total body and the sum of the cumulated activity recorded in the main source organs.
TABLE 2.
Normalized cumulated activity calculated for 67Cu, 64Cu, 61Cu, and 60Cu according to the ICRP 53 biokinetic model
| (MBq‐h/MBq) | ||||
|---|---|---|---|---|
| Organ | 67Cu | 64Cu | 61Cu | 60Cu |
| Brain | 7.10 | 1.74 | 0.47 | 0.06 |
| Liver | 32.4 | 9.65 | 2.91 | 0.37 |
| Kidneys | 0.71 | 0.17 | 0.05 | <0.01 |
| Pancreas | 0.14 | 0.03 | 0.01 | <0.01 |
| Rest of the body | 30.60 | 5.80 | 1.30 | 0.14 |
These results show that the predominant uptake of CuCl2 is in the liver, since this organ is involved in the storage and subsequent redistribution of copper ions to other tissues. Consequently, the hepatobiliary system is the most relevant elimination pathway of excess copper ions from the organism.
Table 3 shows the results of dosimetric calculations performed using both the ICRP 89 male and female phantoms 45 for 67Cu–, 64Cu–, 61Cu–, and 60Cu–Cl2, respectively. ED values were obtained with the more recent tissue‐weighting factors given by ICRP 103. 48 In addition, EDE and ED values were obtained using the given ICRP 26 46 and ICRP 60 47 tissue‐weighting factors in order to compare them to EDE results published by ICRP 53 35 for a hermaphroditic phantom and to other published data.
TABLE 3.
Organ doses (mGy/MBq), effective dose equivalent (EDE), and effective doses (ED) due to 67Cu–, 64Cu–, 61Cu–, and 60Cu–Cl2 for the ICRP 89 male and female phantoms calculated with OLINDA 2.2.0 software
| Radioisotope | 67Cu | 64Cu | 61Cu | 60Cu | ||||
|---|---|---|---|---|---|---|---|---|
| Half‐life | 61.83 h | 12.72 h | 3.333 h | 23.7 min | ||||
| Target organ | Male | Female | Male | Female | Male | Female | Male | Female |
| Adrenals | 0.148 | 0.171 | 0.0522 | 0.0581 | 0.0665 | 0.0729 | 0.0355 | 0.0394 |
| Brain | 0.483 | 0.537 | 0.108 | 0.12 | 0.0840 | 0.0931 | 0.0327 | 0.0362 |
| Breasts | – | 0.065 | – | 0.0155 | – | 0.0143 | – | 0.00686 |
| Esophagus | 0.086 | 0.107 | 0.0232 | 0.0321 | 0.0258 | 0.0364 | 0.0126 | 0.0178 |
| Eyes | 0.059 | 0.072 | 0.0133 | 0.0169 | 0.0119 | 0.0154 | 0.00548 | 0.00704 |
| Gallbladder wall | 0.195 | 0.157 | 0.0731 | 0.0514 | 0.0949 | 0.0620 | 0.0499 | 0.0309 |
| LLI wall/left colon | 0.066 | 0.077 | 0.0164 | 0.0187 | 0.0161 | 0.0179 | 0.00769 | 0.00835 |
| Small intestine | 0.066 | 0.080 | 0.0164 | 0.02 | 0.0160 | 0.0198 | 0.00759 | 0.00936 |
| stomach wall | 0.081 | 0.091 | 0.0227 | 0.0244 | 0.0247 | 0.0258 | 0.0122 | 0.0125 |
| ULI wall/right colon | 0.088 | 0.095 | 0.0256 | 0.0262 | 0.0286 | 0.0280 | 0.0141 | 0.0139 |
| Rectum | 0.053 | 0.064 | 0.0111 | 0.0133 | 0.00891 | 0.0106 | 0.00387 | 0.00454 |
| Heart wall | 0.089 | 0.089 | 0.0266 | 0.0234 | 0.0300 | 0.0243 | 0.0148 | 0.0116 |
| Kidneys | 0.263 | 0.301 | 0.0659 | 0.077 | 0.0598 | 0.0714 | 0.0261 | 0.0316 |
| Liver | 1.780 | 2.270 | 0.482 | 0.612 | 0.415 | 0.523 | 0.168 | 0.211 |
| Lungs | 0.078 | 0.094 | 0.0217 | 0.0261 | 0.0241 | 0.0290 | 0.012 | 0.0144 |
| Ovaries | – | 0.067 | – | 0.0143 | – | 0.0119 | – | 0.00525 |
| Pancreas | 0.149 | 0.206 | 0.0413 | 0.0624 | 0.0420 | 0.0689 | 0.0194 | 0.0332 |
| Prostate | 0.054 | – | 0.0116 | – | 0.00965 | – | 0.00427 | ‐ |
| Salivary glands | 0.061 | 0.070 | 0.0141 | 0.0162 | 0.0128 | 0.01450 | 0.00585 | 0.00662 |
| Red marrow | 0.053 | 0.062 | 0.0143 | 0.0166 | 0.0145 | 0.0168 | 0.00701 | 0.00805 |
| Osteogenic cells | 0.080 | 0.084 | 0.0137 | 0.015 | 0.0125 | 0.0145 | 0.00549 | 0.00648 |
| Spleen | 0.062 | 0.077 | 0.0152 | 0.0195 | 0.0146 | 0.0191 | 0.00694 | 0.00877 |
| Testes | 0.047 | – | 0.00902 | – | 0.00653 | – | 0.00265 | – |
| Thymus | 0.063 | 0.076 | 0.015 | 0.0177 | 0.0146 | 0.0170 | 0.00694 | 0.00807 |
| Thyroid | 0.055 | 0.064 | 0.0121 | 0.0142 | 0.0106 | 0.0122 | 0.00478 | 0.00542 |
| Urinary bladder wall | 0.052 | 0.063 | 0.0107 | 0.0118 | 0.00850 | 0.00911 | 0.00364 | 0.00382 |
| Uterus | – | 0.066 | – | 0.0139 | – | 0.0114 | – | 0.00499 |
| Total Body | 0.101 | 0.134 | 0.0231 | 0.0327 | 0.0185 | 0.0286 | 0.00757 | 0.0124 |
| EDE (ICRP26) (mSv/MBq) | 0.204 | 0.258 | 0.0542 | 0.0677 | 0.0502 | 0.0612 | 0.0220 | 0.0265 |
| ED (ICRP60) (mSv/MBq) | 0.149 | 0.189 | 0.0391 | 0.0497 | 0.0356 | 0.0450 | 0.0155 | 0.0195 |
| ED (ICRP103) (mSv/MBq) | 0.131 | 0.168 | 0.0351 | 0.0444 | 0.0329 | 0.0410 | 0.0146 | 0.0180 |
The absorbed doses calculated for 67Cu and 64Cu radioisotopes with OLINDA 2.2.0 using the male phantom are generally in agreement with values reported by ICRP 53 for the hermaphroditic phantom. The most significant divergences were found for absorbed dose values in the adrenals and in the total body. Higher differences were found for absorbed doses calculated with the female phantom compared with the hermaphroditic one. Consequently, the EDE values calculated for the male phantom (0.204 mSv/MBq for 67Cu and 0.0542 mSv/MBq for 64Cu) are quite similar to the values published by ICRP 53 (0.22 mSv/MBq for 67Cu and 0.053 mSv/MBq for 64Cu), whereas EDE values calculated for the female phantom are higher (0.258 mSv/MBq for 67Cu and 0.0677 mSv/MBq for 64Cu). Comparing the results calculated with OLINDA for both phantoms, it can be observed that the absorbed doses are higher for female than for male phantoms, as already reported for other radiopharmaceuticals. 26 , 53 , 54 In this case, the difference is also due to the fact that the same organ cumulated activities were used for the male and female phantoms. The dosimetric estimation in humans proved that, with both radioisotopes, the liver received the highest dose, followed by the brain and the kidneys. Due to its longer half‐life, the absorbed doses due to 67Cu are higher than those due to 64Cu by a factor of between 3 and 6, depending upon the organ. This resulted in a 3.8‐fold increased value of ED or EDE, for both female and male phantoms.
As regards the 61Cu impurity, it can be observed that despite the almost fourfold shorter half‐life, the absorbed doses and the ED or EDE values due to this radioisotope are quite similar to those due to 64Cu. This is a result of the higher total energy emitted by 61Cu for nuclear transformation (1.1327 MeV/nt for 61Cu and 0.3102 MeV/nt for 64Cu 55 ). Due to the high energy emitted for nt (4.8087 MeV/nt 55), the absorbed doses of 60Cu are not negligible, despite its very short half‐life.
3.3. Tumor dosimetry (sphere model)
Dose factors obtained with the OLINDA and MIRDcell codes for spheres of mass larger and smaller than 0.01 g, respectively, were used to calculate the absorbed dose to the spheres, considering 1 nt/µm3 (that is 1012 nt per g of tissue). The results obtained for 67Cu, 64Cu, and 177Lu radionuclides are plotted in Figure 2. It can be noted that the absorbed doses due to 67Cu and 177Lu are almost identical for small spheres up to 10 g of mass. This is due to the emitted energy per decay in the form of electrons, which is quite similar for the two radionuclides (0.1504 MeV/nt for 67Cu and 0.1479 MeV/nt for 177Lu). The same holds true for their mean β– energy (0.1359 MeV for 67Cu and 0.1333 MeV for 177Lu) as reported in Table S2, which describes the main decay characteristics of the 64Cu, 67Cu, and 177Lu radionuclides. 55 Since a 10 g sphere 56 absorbs almost all of the energy released by both radionuclides’ electron emission, the absorbed dose for 67Cu becomes larger than that for 177Lu beyond this size. This is due to the contribution of photons whose emission is higher for 67Cu than for 177Lu (see Table S2). The lower value of emitted energy per decay in the form of electrons in the case of 64Cu (0.1248 MeV/nt) explains the lower absorbed dose values of this radionuclide for the smaller spheres. The ratio of the absorbed dose due to the two copper radionuclides D 67Cu/D 64Cu is about 1.1 for the 10 g sphere, increasing to 1.2 for the 0.01 g sphere. This value rises strongly as the mass of the spheres decreases, reaching a maximum of about 2.3 for a sphere of 4 × 10–6 g (200 µm diameter) as a result of the higher mean energy of electron emission by 64Cu compared to that of 67Cu (see Table S2). Because of the rather similar total emitted energy per decay (0.2657 MeV/nt for 67Cu and 0.3102 MeV/nt for 64Cu), the absorbed dose for the two copper radioisotopes only converges for spheres larger than 103 g.
FIGURE 2.
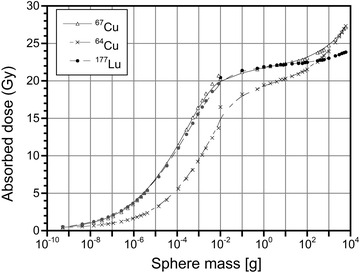
Absorbed dose (Gy) to spheres resulting from a uniform concentration of events (1 decay for µm3) due to 67Cu, 64Cu, and 177Lu radioisotopes.
3.4. Cellular dosimetry and survival
Cellular S values calculated for 67Cu, 64Cu, and 177Lu for each target region, nucleus (n), and the whole cell, assuming that the radionuclide was uniformly distributed in one of the source regions, the cytoplasm (cy) or the entire cell (c), demonstrated that in all cases, the self S values are the highest. These values decrease as the distance between the source and target cells increases (see Table S3). In general, the calculated 177Lu and 67Cu S values are similar because, as previously discussed, the emitted energy per decay in the form of both radionuclides’ electrons is comparable. Consequently, the mean absorbed doses to cells obtained after treatments with 177Lu and 67Cu at parity of number of disintegrations were also relatively similar (see Figure 3(a)). As expected, higher differences were found between the mean absorbed doses produced by 67Cu and 64Cu treatments (see Figure 3(a)).
FIGURE 3.
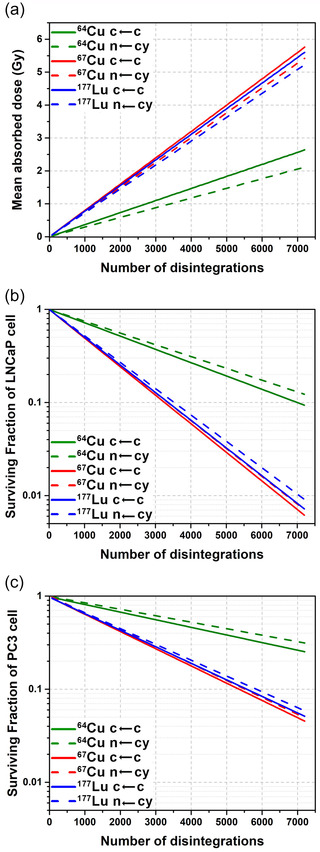
(a) Mean absorbed doses to cells obtained after treatment with 67Cu, 64Cu, and 177Lu and the surviving fractions of (b) LNCaP cells and (c) PC3 cells.
Mean cell absorbed doses obtained for both LNCaP and PC3 cell lines were the same since only one cell model was used for both of them, however, some differences were found between their surviving fractions (Figures 3(b) and 3(c)). The surviving fraction of LNCaP cells after treatment with 177Lu or 67Cu was less than 10% and 1% considering 3500 and 7000 disintegrations per cell, respectively. Nevertheless, more than 6000 disintegrations are required to reduce the surviving fraction of the more radioresistant PC3 cells to 10% (see Figure 3(c)). A much larger number of disintegrations is required to achieve the same level of cell survival in the case of 64Cu treatments.
3.5. Dosimetry of the 67/64CuCl2 mixture
The absorbed doses to healthy organs generated by the 67/64CuCl2 mixture per unit of administered activity were calculated for the male adult ICRP 89 phantom for different production conditions at different times after the EOB. As can be observed in Figure 4, in all cases the absorbed dose to the liver (the most irradiated organ) increases with time. This is due to the increasing contribution of 67Cu (see Figure 1) and to its higher value of absorbed dose compared to that of 64Cu (see Table 3), approaching the value of 1.78 mGy/MBq, corresponding to 100% 67Cu in the mixture.
FIGURE 4.
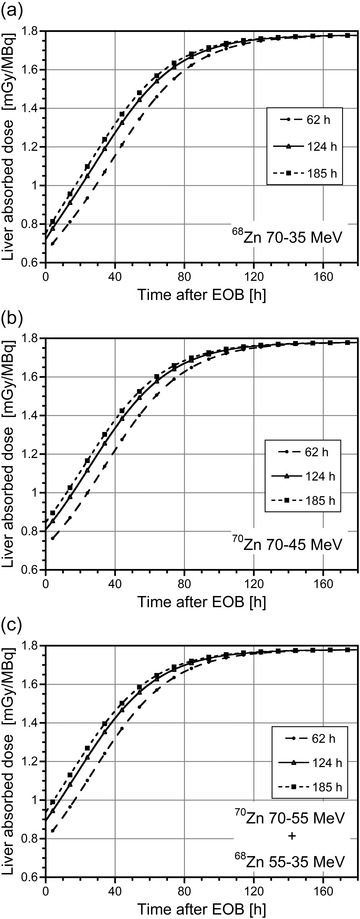
Absorbed dose to the liver per unit of administered activity for the male adult ICRP 89 phantom as a function of time postirradiation due to injection of the 67/64CuCl2 mixture obtained with 1 µA proton beam and different irradiation times (circles: 62 h; triangles: 124 h; squares: 185 h) of (a) a 68Zn target in the energy range 70–35 MeV; (b) a 70Zn target in the energy range 70–45 MeV; (c) a composite 70Zn–68Zn target in the energy range 70–55 and 55–35 MeV, respectively.
The same time dependent behavior was found for the absorbed dose to other healthy organs and also for total ED (EDt), as can be observed in Figure 5 for the case of a mixture obtained from 70Zn target irradiation in the energy range 70–45 MeV. Similar results were obtained for the irradiation of the 68Zn target in the energy range 70–35 MeV and for the composite target 70Zn–68Zn in the energy range 70–35 MeV. The contribution of the 61Cu and 60Cu impurities to the liver‐absorbed dose and to the EDt was always less than 10% at the EOB, rapidly decreasing over time.
FIGURE 5.
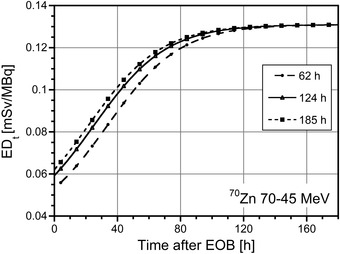
Total ED (ED t ) per unit of administered activity for the male adult ICRP 89 phantom as a function of time postirradiation due to injection of the 67/64CuCl2 mixture obtained with 1 µA proton beam and different irradiation times (circles: 62 h; triangles: 124 h; squares: 185 h) of a 70Zn target in the energy range 70–45 MeV.
The tumor‐absorbed dose attributable to the 67/64CuCl2 mixture, evaluated with the sphere model, was also calculated for different production conditions at different postirradiation times. The results obtained are plotted in Figure 6 for spheres of different mass and a uniform concentration of events (1 decay per µm3). The tumor‐absorbed dose increases with time when the 70Zn target is irradiated in the energy range 70–45 MeV for each tumor size, reaching a plateau value corresponding to 100% 67Cu in the mixture (Figure 6(b)). The absorbed doses are higher for the larger spheres: the absorbed dose for the 10 g sphere is about 15% higher at EOB and 10% higher at the plateau when compared to the 0.01 g sphere (see Figure S1(b)).
FIGURE 6.
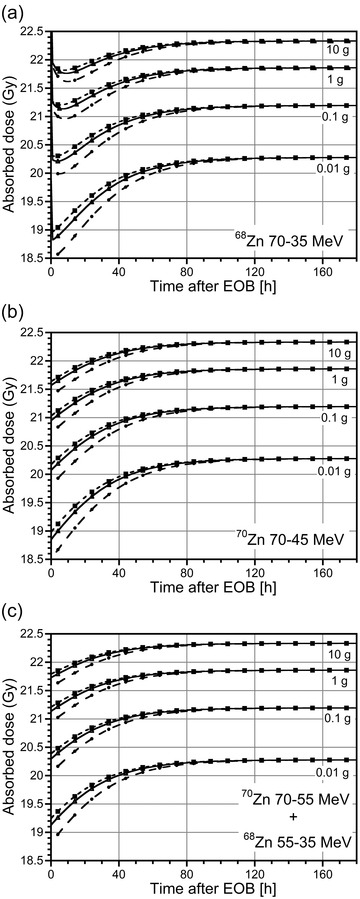
Absorbed doses (Gy) to spheres of different mass (0.01, 0.1, 1, and 10 g) resulting from a uniform concentration of events (1 decay per µm3) due to injection of the 67/64CuCl2 mixture obtained with 1 µA proton beam and different irradiation times (circles: 62 h; triangles: 124 h; squares: 185 h) of (a) a 68Zn target in the energy range 70–35 MeV; (b) a 70Zn target in the energy range 70–45 MeV; (c) a composite 70Zn–68Zn target in the energy range 70–55 and 55–35 MeV, respectively.
The relative increment in the absorbed dose at the plateau with respect to the EOB for 62 h of irradiation (situation corresponding to the largest variation) is less than 10% for the smaller spheres (0.01 and 0.1 g), and about 5% for the larger ones (1 and 10 g) (see Figure S2(b)). Similar results were obtained for the irradiation of the combined 70Zn–68Zn target (see Figure 6(c), S1(c), and S2(c)). Due to the decay of the 61Cu and 60Cu impurities, the tumor‐absorbed doses initially decrease with time when a 68Zn target is irradiated in the energy range 70–35 MeV; this behavior is more evident in the larger spheres (see Figure 6(a)). After a few hours, the absorbed doses increase again with time until they reach a plateau value, similar to the other irradiation conditions.
4. DISCUSSION
The 64CuCl2 dosimetric evaluation conducted in this study revealed that the liver was the organ that received the highest dose, as already reported in ICRP 53 and validated by other authors in human healthy volunteers, 57 prostate cancer patients, 3 , 58 and glioblastoma multiforme patients. 59 Table 4 depicts the comparison of our dosimetric data with those previously reported. The liver‐absorbed dose values calculated in this study are higher than those published in the literature, possibly due to the limited number of time points used to estimate radionuclide accumulation in preceding studies. Nevertheless, ED values are in line with those previously published.
TABLE 4.
Comparison of the liver‐absorbed dose and the effective dose calculated per unit of 64CuCl2 administered activity in human models
| Human model | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Capasso et al. 2015 | Righi et al. 2018 | Panichelli et al. 2016 | Avila‐Rodriguez et al. 2017 | ICRP 53 | This study (ICRP 89 phantom) | ||||
| Male | Male | Male | Male | Female | Mean | Hermaphroditic | Male | Female | |
|
Liver (μGy/MBq) |
294 | 271 | 321 | 310 | 421 | 366 | 480 | 482 | 612 |
| EDE ICRP26 (μSv/MBq) | – | – | 40 a | – | – | – | 53 | 54.2 | 67.7 |
| ED ICRP60 (μSv/MBq) | 33.8 | 31 | 40 a | 51.2 | 61.8 | 56.5 | – | 39.1 | 49.7 |
| ED ICRP103 (μSv/MBq) | – | 29.1 | – | – | – | – | – | 35.1 | 44.4 |
Calculated on the basis of the published organ dose.
Our 67CuCl2 dosimetric data are also comparable to those reported by ICRP 53, even if higher absorbed doses were calculated for the female phantom compared to the hermaphroditic one used by ICRP 53. It should also be noted that the adoption of the most recent ICRP 103 tissue weighting factors determines a substantial decrease of the ED values, for both male and female phantoms and for both 64CuCl2 and 67CuCl2, compared with the EDE values based on the ICRP 26 data set used in ICRP 53 evaluation (see Table 3). The overall consistency of our dosimetric evaluation with published data is encouraging for the application of the same model to the 67/64CuCl2 mixture.
Absorbed doses to healthy organs per unit of administered activity of 67CuCl2 are higher by a factor of between 3 and 6 (3.7 for the liver) compared with those attributable to 64CuCl2, resulting in an ED coefficient that is 3.8 times higher (see Table 3). Nevertheless, given that for most organs the maximum tolerated dose (MTD) to radiation is in the order of some tens of Gy, and the MTD for the gonads and red bone marrow are as low as 1–2 Gy, 60 , 61 our dosimetric estimations suggest that it is feasible to administer 67CuCl2 therapeutic activities in the order of several GBq without jeopardizing the function of these organs. In the case of 67/64CuCl2, the amount of 67Cu in the mixture increases with time after the EOB and, therefore, the absorbed dose to healthy organs and ED values per unit of administered activity increase as well.
Absorbed dose calculations using the sphere model demonstrated that approximately the same total number of 67Cu and 177Lu radioactive decays are required for the same absorbed dose to a tumor of up to 10 g of mass (see Figure 2). In general, the biodistribution of 67Cu‐ and 177Lu‐radiopharmaceuticals will be different. However, assuming that the same fraction of administered activities (A 0) accumulates in the tumor for both radionuclides, and considering an immediate uptake without biological elimination, it follows that the same absorbed doses can be attained with 67Cu and 177Lu by scaling A 0 according to the radioisotope half‐lives (A 0 = nt ln2/T 1/2). Therefore, the required activity of 67Cu will be about 2.6 times higher than the activity of 177Lu. Given that a 10–20% higher value of radioactive decays is necessary in the case of 64Cu compared with 177Lu to produce the same absorbed doses for tumor masses ranging between 0.01 and 10 g, the required activity of 64Cu will be about 14–15 times higher than that of 177Lu. Consequently, when comparing the two copper radioisotopes, the 64Cu administered activity must be about 5.5 higher than that of 67Cu to get the same tumor‐absorbed dose in this range of sizes, causing the absorbed dose to healthy organs and ED to be higher with respect to 67CuCl2. The number of 64Cu disintegrations necessary to release the same absorbed dose attributed to 67Cu becomes about two times higher for very small spheres, necessitating up to 10 times higher 64Cu activity in these cases.
However, the biological effect of 64Cu would be much higher than that of 177Lu or 67Cu if this radionuclide were incorporated into the cell nucleus, close to the DNA, because the 64Cu Auger electrons would produce high‐density ionizations and high‐energy deposition in a few nanometers. Consequently, the biological effectiveness of Auger electrons emitted inside the cell nucleus could be similar to that of α particles, but it would be minimal if the particles were emitted outside the nucleus. Therefore, to calculate the survival fraction of cells after treatment with an Auger‐electron‐emitting radionuclide localized inside the nucleus cell, it is generally necessary to make a distinction between self‐dose and cross‐dose parameters (see Equation 8). 62 It was discovered that a protein called Atox1 could transport copper into the cell's nucleus, 63 but it was recently reported that CuCl2 could be accumulated inside the nucleus only if it is present in cytotoxic concentrations. 64 Given that the concentrations of administered radiopharmaceuticals are several orders of magnitude below cytotoxic concentrations, the amount of Cu in the cell's nucleus would be minimal. Consequently, we used the same α and β values for self‐doses and cross doses to calculate the surviving fraction for all the radionuclides studied.
The evaluation of mean cell absorbed doses and cell survival after both treatments with all radionuclides studied revealed that, when it was assumed that radioactivity was distributed evenly throughout the cell, higher values of absorbed doses were obtained compared to the more realistic approach which considered the cytoplasm as the source region (see Figure 3(a)). The small differences between the mean absorbed doses obtained with both kinds of treatments for 177Lu or 67Cu do not change the biological effects, since the cell surviving fractions of both treatments are almost identical (see Figures 3(b) and 3(c)). Treatment with 64Cu, however, cause lower values of absorbed doses to the cells, producing less biological damage because it was considered that, in these treatment conditions, CuCl2 is not concentrated inside the cell's nucleus (see Figures 3(b) and 3(c)). Therefore, in these hypotheses, not only a greater amount of 64Cu activity must be injected to obtain the same number of 67Cu decays, due to the different radioisotopes half‐lives, but also an additional activity must be administered to obtain the same absorbed dose levels, and consequently the same cell survival. When considering the 67/64CuCl2 mixture, the supplemental activity necessary to get the same tumor‐absorbed dose produced by 67CuCl2 depends on the time of administration, since the 67Cu concentration in the mixture increases with time after EOB (see Figure 1), as does the relative absorbed dose resulting from a uniform concentration of events, D mix(t)/D 67Cu (see Figure S2). For example, with D mix(t = 0)/D 67Cu ≈ 0.9 for the 0.01 g sphere and an irradiation time of 62 h, approximately 10% more decays of the mixture are required at EOB when compared with those of 67CuCl2 in order to produce the same absorbed dose. The number of decays occurring in the sphere per unit of administered activity of the mixture, ntmix/A 0, are given by the equation:
| (9) |
where k is a proportionality constant, representing the fraction of Cu radioisotopes accumulating inside the tumor.
The percentage of 64Cu activity in the mixture obtained by the irradiation of the 70Zn target in the energy range 70–45 MeV is about 80% at EOB, giving the coefficient nt67Cu/ntmix(t = 0) = 2.78, which decreases with time after EOB. By considering this coefficient's ratio and the relative absorbed dose attributed to the mixture D mix(t)/D 67Cu, it is possible to calculate the increase in the activity of the 67/64CuCl2 mixture necessary to obtain the same absorbed dose in the sphere as when using 67CuCl2:
| (10) |
This suggests that the administered activity of the 67/64CuCl2 mixture must be almost three times higher than that of 67CuCl2 at EOB in order to obtain an equivalent absorbed dose to the 0.01 g sphere.
The dose increment (DI) caused by the use of the 67/64CuCl2 mixture rather than 67CuCl2 can be estimated by multiplying the A mix/A 67Cu coefficient for the liver‐absorbed dose per unit of administered activity or the EDt value per unit of administered activity (see Figures 4 and 5). For the considered scenario, the increase in the liver‐absorbed dose and in the ED is about 25% at EOB, decreasing to almost 10% approximately 30 h after EOB. Setting the maximum DI limit to 10% after administering the 67/64CuCl2 mixture, the waiting time required to reach this limit (t 10%) after the EOB can be used to compare the quality of the different 67/64CuCl2 mixtures. Table 5 shows the values of t 10% and the total activity available at that time, evaluated for the different scenarios and taking the sphere of 0.01 g of mass as a reference. For all the different scenarios, the percentage of 67Cu activity at t 10% is about 45% and the A mix/A 67Cu coefficient at this time is about 1.8. As irradiation time rises, the amount of available total activity increases and the t 10% decreases in all cases (see Table 5). A comparison of the amount of activity of 67/64CuCl2 available at t 10% with that of 67CuCl2 at t 99%, reported in Table 1, clearly indicates the advantage of administering the radionuclidic mix instead of the pure 67Cu radioisotope, even taking into account that a greater amount of mixing activity is required. It should be noted that the estimated production yields of all the radionuclides of interest are based on the hypothesis of 100% isotopically enriched target material. However, the material available on the international market for use as a target may have a lower enrichment level (materials with enrichment levels higher than 98.7% for 70ZnO and 99% for 68ZnO are currently available) and different amounts of Cu isotopes will be produced based on the specific target composition. Given that the natural abundance of 70Zn is only 0.61% and that of 68Zn is 18.45%, 65 the price of these enriched materials varies, with the price of 70Zn approximately four times more expensive than that of 68Zn. From a technical point of view, it is customary to recover and reuse costly enriched materials in the routine production of radionuclides. 19
TABLE 5.
Minimum waiting time necessary after EOB to keep the dose increment lower than 10% (t 10%) and the activity (MBq/µA) of the 67Cu and 67Cu + 64Cu mixture at that time obtained through the proton irradiation of 68Zn and 70Zn targets for different scenarios and irradiation times
| Irr. time (h) | t 10%(h) | 67Cu + 64Cu(MBq/µA) | 67Cu(MBq/µA) | |
|---|---|---|---|---|
| 68Zn 70–35 MeV | 62 | 35 | 1801.8 | 837.6 |
| 124 | 26 | 3018.5 | 1389.3 | |
| 185 | 23 | 3594.2 | 1673.1 | |
| 70Zn 70–45 MeV | 62 | 30 | 2711.6 | 1251.4 |
| 124 | 21 | 4542.8 | 2075.6 | |
| 185 | 18 | 5409.0 | 2499.6 | |
| 70Zn 70–55 MeV + 68Zn 55–35 MeV | 62 | 22 | 3223.4 | 1470.1 |
| 124 | 13 | 5400.9 | 2438.3 | |
| 185 | 10 | 6430.0 | 2936.5 |
5. CONCLUSIONS
This study assessed the feasibility of using a 67/64Cu radioisotope mixture for therapeutic purposes by calculating the total absorbed dose into unit density spheres through the simulation of small‐sized tumors after administration of a 67/64CuCl2 solution. Owing to the increased contribution of 67Cu in the mixture, it was found that the DI resulting from the administration of the 67/64CuCl2 mixture rather than 67CuCl2 decreases with time after EOB. The post‐EOB waiting time required to reduce this increment to below 10% (t 10%) depends upon the choice of target and irradiation conditions. The irradiation of a multilayer target composed of 70Zn+68Zn for 185 h appears to be the best option for CuCl2 administration from among all the production parameters studied, since maximum activity was obtained under this condition with the shortest t 10% (10 h) and less than 1% calculated percentages of 61Cu and 60Cu impurities. Based on these results, we can conclude that the use of a 67/64Cu mixture for therapy could be an advantage because the larger amount of available activity will allow to treat more patients and to reduce the cost of the treatment.
FUNDING
This work was supported by the COME experiment (funded by INFN) as part of the activities of the LARAMED project of the INFN‐LNL and it was also included in the framework of the IAEA Coordinated Research Project (CRP) on “Therapeutic Radiopharmaceuticals Labelled with New Emerging Radionuclides (67Cu, 186Re, 47Sc)” (IAEA CRP No. F22053).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Supporting information
ACKNOWLEDGMENTS
Authors would like to thank the staff at the LNL for the support and at the ARRONAX facility for arranging the irradiation runs performed for cross sections measurements.
Open Access Funding provided by Universita degli Studi di Padova within the CRUI‐CARE Agreement.
De Nardo L, Pupillo G, Mou L, Esposito J, Rosato A, Meléndez‐Alafort L. A feasibility study of the therapeutic application of a mixture of 67/64Cu radioisotopes produced by cyclotrons with proton irradiation. Med Phys. 2022;49:2709–2724. 10.1002/mp.15524
Laura Meléndez‐Alafort is the senior author.
Contributor Information
Laura De Nardo, Email: laura.denardo@unipd.it, Email: denardo@pd.infn.it.
Gaia Pupillo, Email: gaia.pupillo@lnl.infn.it.
Liliana Mou, Email: liliana.mou@lnl.infn.it.
Juan Esposito, Email: juan.esposito@lnl.infn.it.
Antonio Rosato, Email: antonio.rosato@unipd.it.
Laura Meléndez‐Alafort, Email: laura.melendezalafort@iov.veneto.it.
REFERENCES
- 1. Kaplan JH, Maryon EB. How mammalian cells acquire copper: an essential but potentially toxic metal. Biophys J. 2016;110(1):7‐13. 10.1016/j.bpj.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakravarty R, Chakraborty S. Dash A. 64Cu2+ Ions as PET Probe: an emerging paradigm in molecular imaging of cancer. Mol Pharm. 2016;13(11):3601‐3612. 10.1021/acs.molpharmaceut.6b00582. [DOI] [PubMed] [Google Scholar]
- 3. Capasso E, Durzu S, Piras S, et al. Role of 64CuCl2 PET/CT in staging of prostate cancer. Ann Nucl Med. 2015;29(6):482‐488. 10.1007/s12149-015-0968-4. [DOI] [PubMed] [Google Scholar]
- 4. Qin C, Liu H, Chen K, et al. Theranostics of malignant melanoma with 64CuCl2. J Nucl Med. 2014;55(5):812‐817. 10.2967/jnumed.113.133850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrari C, Niccoli Asabella A, Villano C, et al. Copper‐64 dichloride as theranostic agent for glioblastoma multiforme: a preclinical study. Biomed Res Int. 2015;2015. 10.1155/2015/129764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sugo Y, Hashimoto K, Kawabata M, et al. Application of 67 Cu produced by 68 Zn(n, n ′ p + d) 67 Cu to biodistribution study in tumor‐bearing mice. J Phys Soc Japan. 2017;86(2):023201. 10.7566/JPSJ.86.023201. [DOI] [Google Scholar]
- 7. Capasso E, Valentini MC, Mirzaei SP, Knoll CM. Radionuclide treatment with 64Cu‐Cl2 in patients with progressive malignant gliomas. Eur J Nucl Med Mol Imaging. 2015;42(S1). 10.1007/s00259-015-3198-z. (Abstract). [DOI] [Google Scholar]
- 8. Valentini G, Panichelli P, Villano C, Pigotti G, Martini D. 64CuCl2: new theranostic agent. Nucl Med Biol. 2014;41(7):638. 10.1016/J.NUCMEDBIO.2014.05.039. [DOI] [Google Scholar]
- 9. Rowshanfarzad P, Sabet M, Reza Jalilian A, Kamalidehghan M. An overview of copper radionuclides and production of 61 Cu by proton irradiation of nat Zn at a medical cyclotron. Appl Radiat Isot. 2006;64:1563‐1573. 10.1016/j.apradiso.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 10. Ahmedova A, Todorov B, Burdzhiev N, Goze C. Copper radiopharmaceuticals for theranostic applications. Eur J Med Chem. 2018;157:1406‐1425. 10.1016/j.ejmech.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 11. Smith NA, Bowers DL, Ehst DA. The production, separation, and use of 67Cu for radioimmunotherapy: a review. Appl Radiat Isot. 2012;70(10):2377‐2383. 10.1016/j.apradiso.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 12. Novak‐Hofer I, Schubiger PA. Copper‐67 as a therapeutic nuclide for radioimmunotherapy. Eur J Nucl Med. 2002;29(6):821‐830. 10.1007/s00259-001-0724-y. [DOI] [PubMed] [Google Scholar]
- 13. Hao G, Mastren T, Silvers W, Hassan G, Öz OK, Sun X. Copper‐67 radioimmunotheranostics for simultaneous immunotherapy and immuno‐SPECT. Sci Rep. 2021;11(1):1‐11. 10.1038/s41598-021-82812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keinänen O, Fung K, Brennan JM, et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc Natl Acad Sci U S A. 2020;117(45):28316‐28327. 10.1073/pnas.2009960117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cullinane C, Jeffery CM, Roselt PD, et al. Peptide receptor radionuclide therapy with 67 Cu‐CuSarTATE is highly efficacious against a somatostatin‐positive neuroendocrine tumor model. Published online 2020. 10.2967/jnumed.120.243543 [DOI] [PubMed]
- 16. Dearling JLJ, Van Dam EM, Harris MJ, Packard AB. Detection and therapy of neuroblastoma minimal residual disease using [ 64/67 Cu] Cu‐SARTATE in a preclinical model of hepatic metastases. EJNMMI Res. 2021;11:20. 10.1186/s13550-021-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelly JM, Amor‐Coarasa A, Nikolopoulou A, et al. Assessment of PSMA targeting ligands bearing novel chelates with application to theranostics: stability and complexation kinetics of 68Ga3 +, 111In3 +, 177Lu3 + and 225Ac3 +. Nucl Med Biol. 2017;55:38‐46. 10.1016/j.nucmedbio.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 18. Mcinnes LE, Cullinane C, Roselt PD, et al. Therapeutic efficacy of a bivalent inhibitor of prostate‐specific membrane antigen labeled with 67 Cu. Published online 2021. 10.2967/jnumed.120.251579 [DOI] [PMC free article] [PubMed]
- 19. International Atomic Energy Agency . Cyclotron produced radionuclides: emerging positron emitters for medical applications: 64Cu and 124I.; 2016. http://www.iaea.org/Publications/index.html
- 20. International Atomic Energy Agency . Therapeutic radiopharmaceuticals labelled with copper‐67, rhenium‐186 and scandium‐47; 2021.
- 21. Jalilian AR, Gizawy MA, Alliot C, et al. IAEA activities on 67Cu, 186Re, 47Sc theranostic radionuclides and radiopharmaceuticals. Curr Radiopharm. 2020;13(September). 10.2174/1874471013999200928162322. [DOI] [PubMed] [Google Scholar]
- 22. Qaim SM, Hussain M, Spahn I, Neumaier B. Continuing nuclear data research for production of accelerator‐based novel radionuclides for medical use: a mini‐review. Front Phys. 2021;9. 10.3389/fphy.2021.639290. [DOI] [Google Scholar]
- 23. Merrick MJ, Rotsch DA, Tiwari A, et al. Imaging and dosimetric characteristics of 67 Cu. Phys Med Biol. 2021;66(3). 10.1088/1361-6560/abca52. [DOI] [PubMed] [Google Scholar]
- 24. Nigron E, Guertin A, Haddad F, Sounalet T. Is 70Zn(d,x)67Cu the best way to produce 67Cu for medical applications? Front Med. 2021;8(July):1‐7. 10.3389/fmed.2021.674617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pupillo G, Mou L, Martini P, et al. Production of 67Cu by enriched 70Zn targets: first measurements of formation cross sections of 67Cu, 64Cu, 67Ga, 66Ga, 69mZn and 65Zn in interactions of 70Zn with protons above 45 MeV. Radiochim Acta. 2020;108(8):593‐602. 10.1515/ract-2019-3199. [DOI] [Google Scholar]
- 26. De Nardo L, Pupillo G, Mou L, et al. Preliminary dosimetric analysis of DOTA‐folate radiopharmaceutical radiolabelled with 47Sc produced through natV(p,x)47Sc cyclotron irradiation. Phys Med Biol. 2021;66(2):025003. 10.1088/1361-6560/abc811. [DOI] [PubMed] [Google Scholar]
- 27. Meléndez‐Alafort L, Ferro‐Flores G, De Nardo L, et al. Internal radiation dose assessment of radiopharmaceuticals prepared with cyclotron‐produced 99mTc. Med Phys. 2019;46(3). 10.1002/mp.13393. [DOI] [PubMed] [Google Scholar]
- 28. Wheldon TE, O'Donoghue JA, Barrette A, Michalowski AS. The curability of tumours of differing size by targeted radiotherapy using 131I or 90Y. Radiother Oncol. 1991;21(2):91‐99. 10.1016/0167-8140(91)90080-Z. [DOI] [PubMed] [Google Scholar]
- 29. O'Donoghue JA, Bardies M, Wheldon TE, Sgouros G. Relationships between tumor size and curability for uniformly targeted therapy with beta‐emitting radionuclides. J Nucl Med. 1995;36(10):1902‐1912. [PubMed] [Google Scholar]
- 30. Kunikowska J, Królicki L, Hubalewska‐Dydejczyk A, Mikołajczak R, Sowa‐Staszczak A, Pawlak D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90 Y‐DOTATATE and tandem 90 Y/177 Lu‐DOTATATE: which is a better therapy option? Eur J Nucl Med Mol Imaging. 2011;38(10):1788‐1797. 10.1007/s00259-011-1833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43:260. 10.1039/c3cs60304k. [DOI] [PubMed] [Google Scholar]
- 32. Apelgot S, Coppey J, Gaudemer A, et al. Similar lethal effect in mammalian cells for two radioisotopes of copper with different decay schemes, 64cu and 67cu. Int J Radiat Biol. 1989;55(3):365‐384. 10.1080/09553008914550421. [DOI] [PubMed] [Google Scholar]
- 33. Connett JM, Anderson CJ, Guo LW, et al. Radioimmunotherapy with a 64Cu‐labeled monoclonal antibody: a comparison with 67Cu. Proc Natl Acad Sci USA. 1996;93(13):6814‐6818. 10.1073/pnas.93.13.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mou Liliana, Pupillo Gaia, Petra Martini MP, INFN patent no. WO 2019/220224 A1, International Publication Date: 21 November 2019; International Application Number: PCT/IB2019/052609; Title: “A method and a target for the production of 67Cu.”
- 35. ICRP , 1988. Radiation dose to patients from radiopharmaceuticals. ICRP Publication 53. Ann. ICRP 18(1‐4). [DOI] [PubMed] [Google Scholar]
- 36. Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second‐generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023‐1027. http://jnm.snmjournals.org/content/46/6/1023.full.pdf. [PubMed] [Google Scholar]
- 37. ISOTOPIA, Medical Isotope Browser . IAEA nuclear data section. Accessed February 18, 2021. https://www‐nds.iaea.org/relnsd/isotopia/isotopia.html
- 38. IAEA 2019 , Recommened nuclear cross section for therapeutic radionuclides. Accessed June 18, 2021. https://www‐nds.iaea.org/medical/therapeutic_2019.html
- 39. EXFOR: experimental nuclear reaction data. Accessed February 18, 2021. https://www‐nds.iaea.org/exfor/exfor.htm
- 40. TENDL‐2017 . TALYS‐based evaluated nuclear data library. Accessed February 18, 2021. https://tendl.web.psi.ch/tendl_2017/tendl2017.html
- 41. Szelecsényi F, Kovács Z & Suzuki K et al. Production possibility of 61 Cu using proton induced nuclear reactions on zinc for PET studies. Journal of Radioanalytical and Nuclear Chemistry 263; 2005: 539‐546.
- 42. Stabin M, Farmer A. OLINDA/EXM 2.0: the new generation dosimetry modeling code. J Nucl Med. 2012;53(supplement 1):585. http://jnm.snmjournals.org/content/53/supplement_1/585.abstract?sid=61dd62a5‐0bc2‐4a78‐ae1d‐8406ad9619b1. [Google Scholar]
- 43. Stabin M, Siegel JA. Radar dose estimate report: a compendium of radiopharmaceutical dose estimates based on Olinda/Exm Version 2.0. J Nucl Med. 2017;59(1):154‐160. 10.2967/jnumed.117.196261. [DOI] [PubMed] [Google Scholar]
- 44. Stabin MG, Xu XG, Emmons MA, Segars WP, Shi C, Fernald MJ. RADAR reference adult, pediatric, and pregnant female phantom series for internal and external dosimetry. J Nucl Med. 2012;53(11):1807‐1813. 10.2967/jnumed.112.106138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. ICRP 2002 . Basic anatomical and physiological data for use in radiological protection reference values. ICRP Publication 89. Ann. ICRP 2002;32(3‐4). 10.1016/0146-6453(81)90127-5 [DOI] [PubMed] [Google Scholar]
- 46. ICRP 1977 . Recommendations of the ICRP. ICRP Publication 26. Ann. ICRP 1977;1(3). 10.1177/ANIB_1_3 [DOI] [Google Scholar]
- 47. ICRP 1991 . 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann. ICRP 1991;21(1‐3). http://www.icrp.org/publication.asp?id=ICRP Publication 60 [PubMed] [Google Scholar]
- 48. ICRP 2007 . The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 2007;37(2‐4). 10.1177/ANIB_37_2-4 [DOI] [PubMed] [Google Scholar]
- 49. Vaziri B, Wu H, Dhawan AP, et al. MIRD pamphlet No. 25: mIRDcell V2.0 software tool for dosimetric analysis of biologic response of multicellular populations in collaboration with the SNMMI MIRD committee. J Nucl Med. 2014;55:1557‐1564. 10.2967/jnumed.113.131037. [DOI] [PubMed] [Google Scholar]
- 50. Shashni B, Ariyasu S, Takeda R, et al. Size‐Based Differentiation of Cancer and Normal Cells by a Particle Size Analyzer Assisted by a Cell‐Recognition PC Software. Vol 41(4); 2018:487‐503. [DOI] [PubMed] [Google Scholar]
- 51. Huber MD, Gerace L. The size‐wise nucleus: nuclear volume control in eukaryotes. J Cell Biol. 2007;179(4):583‐584. 10.1083/jcb.200710156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saga R, Matsuya Y, Takahashi R, Hasegawa K, Date H, Hosokawa Y. Analysis of the high‐dose‐range radioresistance of prostate cancer cells, including cancer stem cells, based on a stochastic model. J Radiat Res. 2019;60(3):298‐307. 10.1093/jrr/rrz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stabin MG. Health concerns related to radiation exposure of the female nuclear medicine patient. Environ Health Perspect. 1997;105(suppl 6):1403‐1409. 10.1289/ehp.97105s61403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Nardo L, Ferro‐Flores G, Bolzati C, Esposito J, Meléndez‐Alafort L. Radiation effective dose assessment of [51Mn]‐ and [52Mn]‐chloride. Appl Radiat Isot. 2019;153(July):108805. 10.1016/j.apradiso.2019.108805. [DOI] [PubMed] [Google Scholar]
- 55. ICRP 2008 . Nuclear decay data for dosimetric calculations. ICRP Publication 107. Ann. ICRP 2008;38(3). 10.1177/ANIB_38_3 [DOI] [PubMed] [Google Scholar]
- 56. Stabin MG, Konijnenberg MW. Re‐evaluation of absorbed fractions for photons and electrons in spheres of various sizes. J Nucl Med. 2000;41(1):149‐160. [PubMed] [Google Scholar]
- 57. Avila‐Rodriguez MAA, Rios C, Carrasco‐Hernandez J, et al. Biodistribution and radiation dosimetry of [ 64 Cu]copper dichloride: first‐in‐human study in healthy volunteers. EJNMMI Res. 2017;7(1):98. 10.1186/s13550-017-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Piccardo A, Paparo F, Puntoni M, et al. CuCl 2 PET/CT in prostate cancer relapse. J Nucl Med. 2018;59:444‐451. 10.2967/jnumed.117.195628. [DOI] [PubMed] [Google Scholar]
- 59. Panichelli P, Villano C, Cistaro A, et al. Imaging of brain tumors with copper‐64 chloride: early experience and results. Cancer Biother Radiopharm. 2016;31(5):159‐167. 10.1089/cbr.2016.2028. [DOI] [PubMed] [Google Scholar]
- 60. Khawar A, Eppard E, Sinnes JP, et al. Prediction of normal organ absorbed doses for [177Lu]Lu‐PSMA‐617 using [44Sc]Sc‐PSMA‐617 pharmacokinetics in patients with metastatic castration resistant prostate carcinoma. Clin Nucl Med. 2018;43(7):486‐491. 10.1097/RLU.0000000000002102. [DOI] [PubMed] [Google Scholar]
- 61. Kratochwil C, Fendler WP, Eiber M, et al. EANM procedure guidelines for radionuclide therapy with 177Lu‐labelled PSMA‐ligands (177Lu‐PSMA‐RLT). Eur J Nucl Med Mol Imaging. 2019;46(12):2536‐2544. 10.1007/s00259-019-04485-3. [DOI] [PubMed] [Google Scholar]
- 62. Howell RW, Bishayee A, Bystander Effects Caused by Nonuniform Distributions of DNA‐Incorporated 125 I. [DOI] [PMC free article] [PubMed]
- 63. Beaino W, Guo Y, Chang AJ, Anderson CJ. Roles of Atox1 and p53 in the trafficking of copper‐64 to tumor cell nuclei: implications for cancer therapy. JBIC J Biol Inorg Chem. 2014;19(3):427‐438. 10.1007/s00775-013-1087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Strauch BM, Niemand RK, Winkelbeiner NL, Hartwig A. Comparison between micro‐ and nanosized copper oxide and water soluble copper chloride: interrelationship between intracellular copper concentrations, oxidative stress and DNA damage response in human lung cells. Part Fibre Toxicol. 2017;14(1). 10.1186/s12989-017-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. NuDat 2.8 database . Accessed March 22, 2021. https://www.nndc.bnl.gov/nudat2/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


