Abstract
Currently available European Alpine Altitude Climate Treatment (AACT) programs combine the physical characteristics of altitude with the avoidance of environmental triggers in the alpine climate and a personalized multidisciplinary pulmonary rehabilitation approach. The reduced barometric pressure, oxygen pressure, and air density, the relatively low temperature and humidity, and the increased UV radiation at moderate altitude induce several physiological and immunological adaptation responses. The environmental characteristics of the alpine climate include reduced aeroallergens such as house dust mites (HDM), pollen, fungi, and less air pollution. These combined factors seem to have immunomodulatory effects controlling pathogenic inflammatory responses and favoring less neuro‐immune stress in patients with different asthma phenotypes. The extensive multidisciplinary treatment program may further contribute to the observed clinical improvement by AACT in asthma control and quality of life, fewer exacerbations and hospitalizations, reduced need for oral corticosteroids (OCS), improved lung function, decreased airway hyperresponsiveness (AHR), improved exercise tolerance, and improved sinonasal outcomes. Based on observational studies and expert opinion, AACT represents a valuable therapy for those patients irrespective of their asthma phenotype, who cannot achieve optimal control of their complex condition despite all the advances in medical science and treatment according to guidelines, and therefore run the risk of falling into a downward spiral of loss of physical and mental health. In the light of the observed rapid decrease in inflammation and immunomodulatory effects, AACT can be considered as a natural treatment that targets biological pathways.
Keywords: altitude, asthma, climate, environment, pulmonary rehabilitation
Short abstract

Abbreviations
- 6MWD
6‐minute walking distance
- AACT
alpine altitude climate treatment
- ACQ
asthma control questionnaire
- AHR
airway hyperresponsiveness
- AQLQ
asthma quality of life questionnaire
- FeNO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume during the first second
- FVC
forced vital capacity
- HDM
house dust mite
- HIF‐1a
hypoxia‐inducible factor 1a
- ILC
innate lymphoid cells
- ISWT
incremental shuttle walk test
- m
meters above sea level
- MCID
minimal clinically important difference
- NK
natural killer cells
- OCS
oral corticosteroids
- PAQLQ
pediatric asthma quality of life questionnaire
- PD20
the provocative dose of methacholine that results in a 20% fall in FEV1
- QoL
quality of life
- SMD
standardized mean difference
- Th1
T helper cells type 1
- Th2
T helper cells type 2
- TRAP
traffic‐related air pollution
- Tregs
regulatory T cells
- UVR
ultraviolet radiation
1. INTRODUCTION
A significant proportion of patients with asthma remains uncontrolled, despite well‐designed and accepted guidelines, effective medication and the increasing availability of new therapies, such as biologicals. Suboptimal asthma control has a major impact on disease burden, quality of life, and healthcare costs. Proper identification of treatable traits, follow‐up on medication adherence, maintenance of daily physical activity, avoidance of indoor and outdoor allergens, air pollution and occupational exposure, and non‐pharmacological add‐on therapies facilitate achieving asthma control. If still uncontrolled, referral to a severe asthma clinic to assess the asthma endotype/phenotype and suitability of additional targeted treatment with bronchial thermoplasty or biologicals is recommended. 1 , 2 , 3 , 4
Alpine altitude climate treatment (AACT) has been applied for more than a century in the treatment of pulmonary diseases like asthma, and more recently for allergic diseases such as atopic dermatitis and chronic rhinitis/rhinosinusitis. 5 , 6 , 7 The European altitude clinics for patients with tuberculosis were built in 19th century in the clean alpine environment in close proximity to green space and forests. Currently available European AACT programs combine the avoidance of environmental triggers in the alpine climate and the physical characteristics of altitude with a personalized multidisciplinary pulmonary rehabilitation approach. The environmental mechanisms supporting the benefit provided by AACT, including the influence of altitude characteristics and the alpine climate, are not yet fully understood. In the first observations of the health benefits for patients with asthma, the mountain climate, the clear air, the radiant sun, and the absence of harmful factors were hypothesized to be protective and healing. With the aim of understanding the underlying mechanisms, the relationship of clinical symptoms with altitude was systematically studied, but rarely in a randomized controlled study. The house dust mite (HDM) was discovered as the main allergic component in environmental house dust. Allergen avoidance was thought to be the main mechanism of effect during AACT. 8 Recent studies also showed improvement in inflammatory and immunological markers in patients with different asthma phenotypes, including non‐atopic, suggesting that immunomodulatory changes apart from allergen avoidance may contribute to the clinical improvement with AACT. 9 , 10 , 11 , 12 Furthermore, many observational studies have shown that AACT also improves clinical and functional parameters in asthma. 5 , 6 , 7 However, it is not yet understood which mechanisms contribute to the observed effects or which factor contributes the most. (Table 1) The EAACI Task Force on AACT aims to describe the physical and environmental characteristics of moderate altitude in the alpine climate, its immunomodulatory effects, its multidisciplinary pulmonary rehabilitation approach, and its clinical impact on uncontrolled and severe asthma and to advice, which patients might profit most from AACT.
Key messages.
A significant proportion of patients with asthma remains uncontrolled, despite increasing availability of biologicals.
European AACT programs combine the avoidance of environmental triggers in the alpine climate and the physical characteristics of altitude with a personalized multidisciplinary pulmonary rehabilitation approach.
TABLE 1.
Summary of observed effects and open questions
| Observed effects | Open questions |
|---|---|
|
Observational studies of AACT show improvement in
|
|
Abbreviations: AACT: alpine altitude climate treatment, 6MWD: 6‐min walking distance, ISWT: incremental shuttle walk test, m: meters above sea level, and OCS: oral corticosteroids
2. METHODS
Two experts in the field reviewed the literature for each of the following topics: physical characteristics of altitude, environmental characteristics of the alpine climate, immunological outcomes, clinical outcomes adults, and clinical outcomes children. The search was performed using Medline, Embase, and Cochrane databases including papers published before April 24, 2021. Search terms are listed in Table S1. We focused on studies reporting treatment provided in Europe. Because of the lack of randomized trials assessing the effectiveness of AACT, we did not use GRADE to provide scientific recommendations, but we decided on an expert‐based consensus approach resulting in position statements. Quality assessment of non‐randomized interventional studies was done according to the National Institutes of Health (NIH) Study quality assessment tool for before–after (pre–post) studies with no control group, available at https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools. Items 11 and 12 of this tool were not applicable to any of the included studies and therefore replaced by the question whether all measurements from the protocol were reported to further assess reporting bias.
3. ALTITUDE AND PHYSIOLOGICAL CHANGES
Based on the effects of altitude on human physiology and well‐being in healthy individuals, altitude has been divided into low (<1200 m), moderate (1200 m–2500 m), high (2500 m–3500 m), very high (3500–5800 m), and extreme (>5800 m). 13 Other definitions consider moderate altitude up to 3000 m. 14 Physiological changes induced by hypobaric hypoxia gradually increase with incrementing altitude and are usually mild up to an altitude of 1500 m. 15 , 16 Acute mountain sickness (AMS) is the most common form of acute altitude illness and typically occurs in unacclimatized persons ascending to altitudes >2500 m, although it can develop at lower altitudes in highly susceptible individuals. There are large interindividual differences in susceptibility to effects of hypobaric hypoxia. 17
At 3000 m, the inspired partial pressure of oxygen is reduced to 100 mmHg (13.3 kPa), the alveolar oxygen pressure is estimated to be 70 mmHg (9.3 kPa), which means an arterial oxygen pressure of about 60–63 mmHg (8 kPa) and the compensatory responses to hypobaric hypoxia become increasingly evident (Table S2). 3000 m is the altitude above which many of the physiological responses that represent challenges for the human body (i.e., hypoxic ventilatory response and hypoxic pulmonary vasoconstriction) 18 , 19 start developing, thereby imposing an increased workload on the cardiovascular and respiratory systems. The effects of hypobaric hypoxia are very mild up to 1200 m–1500 m, may become perceptible at around 1800 m, and start to affect an increasing percentage of the population above 2500–3000 m. Most altitude clinics in the Alps are located between 1200 m and 2500 m, where hypoxia does not excessively stress the body as the reduction of pO2 is between 15% and 20%. Thus, acute mountain sickness is an unlikely risk for most asthma patients residing at sea level, who are treated at European altitude clinics. Very seldom therapy‐resistant hypertension develops, leading to a return to sea level.
Key messages.
There are large interindividual differences in susceptibility to effects of hypobaric hypoxia.
Most European altitude clinics are located in the Alps between 1200 m and 2500 m, where hypoxia does not excessively stress the body.
4. EFFECTS OF ALTITUDE ON LUNG PHYSIOLOGY, ADAPTATION, AND IMMUNE RESPONSE
Several physical characteristics change with increasing altitude, such as barometric pressure and inspiratory oxygen pressure, air density, relative temperature, and humidity. (Figure 1) These changes induce several physiological and immunological adaptation responses. 20 , 21 , 22 Rapid onset physiological adaptive responses such as hyperventilation and increased heart rate in the acclimatization process arriving at moderate/high altitude aim at increasing the efficiency of oxygen uptake, transport, and delivery to cells in various ways. 23 With longer‐term altitude exposure, there is an increase in hemoglobin concentration, mitochondrial oxidative capacity, and capillary density which improve physical fitness and performance. 24 , 25 , 26 , 27 Altitude training is frequently used by competitive athletes to improve sea level performance. The reduced air density at altitude improves airflow and reduces airway resistance, which makes exhalation easier and reduces dynamic hyperinflation. 26 , 28 This may also be a useful setting for more easily engaging asthmatic patients in various physical activities at moderate altitude. This contrasts with what happens at sea level, where the higher air density may hinder effective physical training for patients. However, there are some limits to the benefits of altitude on physical activity. Above 2000 m, the reduction of maximal oxygen consumption negatively affects performance. At high altitude (i.e., >3000 m), where the ventilatory response, the cold and dry weather are more pronounced, the hyperventilation of cold air, especially during exercise, has a potential for airways dehydration, which can subsequently trigger bronchospasm.
FIGURE 1.
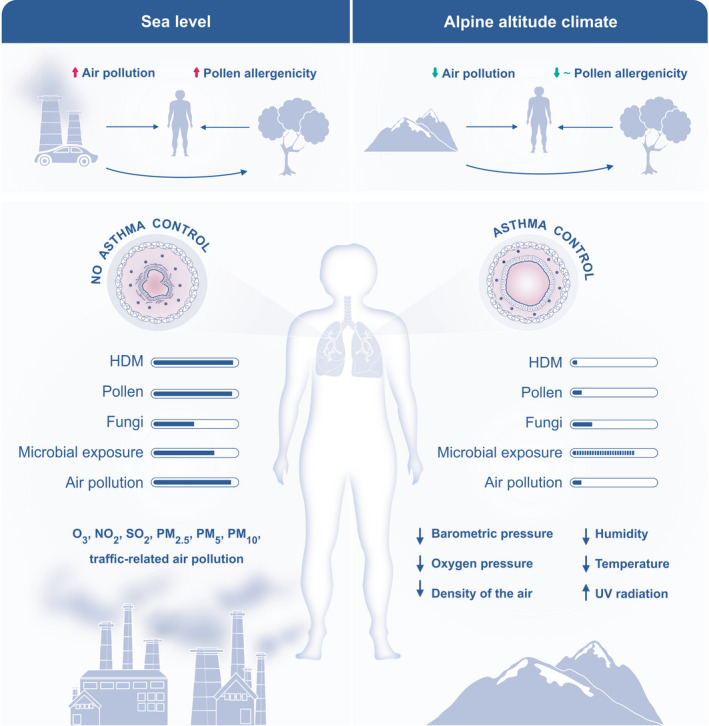
Overview of physical and environmental characteristics of the alpine altitude climate compared to sea level. Several physical characteristics change with increasing altitude, such as barometric pressure and inspiratory oxygen pressure, air density, relative temperature, and humidity. Environmental characteristics vary in different climate zones, because of factors like air pollution and climate change. The environmental characteristics of the alpine climate include reduced aeroallergen burden regarding HDM, pollen, fungi, air pollution, and different microbial exposure. HDM, house dust mite
Immunological adaptation responses may result in either immune stimulation or immune suppression. Studies at high altitude show varying degrees of immune system modulation. Healthy individuals demonstrate an increase of total white blood cells accompanied by a decrease of T helper cells (CD4+ T cells), whereas cytotoxic memory T cells (CD8+T cells) remain unchanged (Figure 2). 29 , 30 The decrease of CD4+T cells is mainly due to a decrease of type 1 T helper (Th1) cells. CD4+ T cells and CD8+T cells are more activated, and they respond more rapidly after recall antigen and mitogen stimulation in vitro. B cells and natural killer (NK) cells increase in healthy individuals ascending to high altitude. 29 , 30 Studies in animals support the observed decreases in Th1 cells and additionally demonstrate the migration of Th2 cells from the organs and periphery to the bone marrow. 31 , 32 , 33
FIGURE 2.
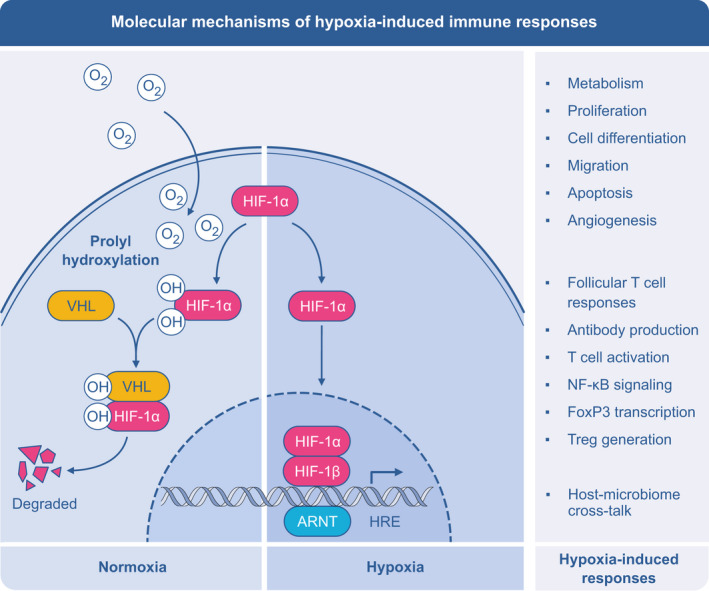
Central role of hypoxia‐inducible factor 1a (HIF‐1α) in hypoxia‐induced immune responses. HIF‐1 is expressed in a wide array of immune and tissue‐resident cells including eosinophils, dendritic cells, macrophages, neutrophils, T cells, B cells, ILCs, NK cells, epithelium, and endothelium. In normoxia, HIF‐1α is hydroxylated, which facilitates HIFα binding to the von Hippel‐Lindau (VHL) E3 ubiquitin ligase complex, leading to fast ubiquitination and proteasomal degradation. During hypoxia, hydroxylation is inhibited by the absence of oxygen, which leads to HIFα stabilization and activation. Once stabilized, HIF‐1α subunit is translocated to the nucleus, where it forms a complex with HIF‐1β and other coactivators, and binds to the consensus hypoxia response elements (HRE) within target genes, involved in a large type of processes, as cellular metabolism, proliferation, differentiation, cell survival, migration, apoptosis, and angiogenesis in cell‐specific manner. ARNT, arylhydrocarbon receptor nuclear translocator
The master regulator of hypoxic conditions, which is not only present in response to altitude but also locally in the tissue, is the hypoxia‐inducible factor 1a (HIF‐1α). HIF‐1α is expressed in a wide array of immune and tissue‐resident cells including eosinophils, dendritic cells, macrophages, neutrophils, T cells, B cells, innate lymphoid cells (ILCs), and NK cells, as well as epithelial and endothelial cells. 34 (Figure 2) Hypoxia influences chemokine responses, which can be a mechanism of a selective recruitment of the T‐cell subsets and also other cells to the specific tissues, such as lungs. HIF‐1α promotes transcription of FOXP3 and generation of Treg cells, which may lead to the resolution of inflammation. 35 , 36 Hypoxia also alters function of the antigen‐presenting cells, especially macrophages and their capacity to stimulate T cells. 37 Finally, HIF‐1a is a central player in maintaining gut‐lung axis of microbiome homeostasis, which has been repeatedly shown to impact on asthma and allergy prevalence, phenotype, and response to treatment. 38 , 39
At moderate altitudes, the beneficial physiological and immunomodulatory effects prevail 21 , 40 due to the subtle, yet systematic, changes in gene expression, protein translation, cell signaling followed by erythropoietic, metabolic, immunological, and other physiological adaptation responses. 20 , 41 , 42
Key messages.
Reduced barometric pressure and inspiratory pressure, reduced air density, relative low temperature and humidity, and increased UV radiation at moderate altitude induce several physiological and immunological adaptation responses.
The master regulator of hypoxic physiologic responses is the hypoxia‐inducible factor 1a (HIF‐1a) which is expressed in a wide array of immune and tissue‐resident cells.
Studies at high altitude show varying degrees of hypoxia‐related immune modulation which likely already start at moderate altitude.
5. UV RADIATION AT MODERATE ALTITUDE
With every 1000 meters increase in altitude, the amount of ultraviolet radiation (UVR) increases by 10% to 12%. 43 Several other factors modulate the amount of UVR, such as the height of the sun in the sky, latitude, clouds, thickness of the ozone layer, and ground reflection. 43 Irradiation of the skin with UVR, both UVA and UVB, leads to cutaneous synthesis of vitaminD3. The prevalence of vitamin D deficiency is currently relatively high in Europe. 44 In addition, vitamin D deficiency may contribute to asthma severity. 45 An increase in serum vitamin D levels was observed in 73 patients with ankylosing spondylitis who stayed at moderate altitude for 3 weeks during spring. 46 It is possible that increased UVR exposure at moderate altitude may improve serum vitamin D levels in asthmatic patients, but this should be tested in future, well‐controlled, studies. 47 UVR and vitamin D share common pathways of innate immune activation primarily via antimicrobial peptide production, stimulation of Toll‐like receptors, and increase of pro‐inflammatory cytokine production. The same synergic action leads to adaptive immune suppression through regulation of actions of lymphocytes, mast cells, and antigen‐presenting cells to dampen excessive inflammatory responses. 48
6. ENVIRONMENTAL CHARACTERISTICS OF THE ALPINE CLIMATE
Different altitudes harbor different climatic, flora, and fauna conditions. Environmental characteristics vary in different climate zones, because of factors like climate change and air pollution, but also regarding exposure to terpenes and aeroallergens. 49 (Figure 1) Forest trees emit a large variety of biogenic volatile organic compounds, complex mixtures containing up to 200 different compounds. In forested areas, substantial terpene emission levels are released to the atmosphere where they exert biological activities and may contribute toward reducing respiratory inflammatory processes. 50
Moderate altitude also seems to be associated with a lower aeroallergen burden. Various studies have shown that, especially in uncontrolled asthma, environmental exposure to aeroallergens is important in disease exacerbations and symptoms. 51 , 52 , 53 Sensitization to HDM has been associated with asthma in several studies. Treatments targeting environmental exposure have been investigated, but conclusive evidence from randomized studies is scarce. The results of single‐strategy avoidance, for example HDM allergens, demonstrated a lower efficacy than expected. 54 , 55 The “multiple hit” hypothesis for the origins of severe airway disease suggests that identification and removal of multiple inflammatory stimuli may delay progression of the underlying airway disease. 56 The home environment including exposure to allergens, molds, air pollution, passive smoking, or stress can be regarded as continuous multiple‐hit exposure. Personalized avoidance of the most important allergens showed better results than single intervention strategies in patients with severe disease. 57 , 58 , 59 The integrated reduction of exposure during AACT may contribute toward reducing airways inflammation.
6.1. House dust mites
HDM allergens have been isolated from mite feces and are highly prevalent in dust reservoirs in the domestic environment, including bedding, carpets, sofas, soft toys, and clothing. 60 , 61 Differences in the geographic distributions of HDM species are most likely influenced by abiotic factors such as humidity, temperature, way of living including furniture, carpets, and available food. 62 There are several sampling techniques to assess residential exposure to HDM, collecting either airborne dust or settled dust. Vacuum cleaning a square meter of settled dust from a carpet or mattress is the most commonly used method. Earlier studies identified and counted extracted mites from the dust samples with a microscope. Currently, measurement of allergenic content of dust has replaced mite identification and counting, because it is more amenable to standardization, less time‐consuming and more closely related to clinical impact.
Generally, HDM counts and HDM allergen content are lower when altitude increases. 8 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 Table S3 summarizes studies comparing HDM counts and HDM allergen content at different altitudes. In tropical or sub‐tropical areas, this decrease was not observed. 69 , 71 , 72 , 73 Mites require a high ambient air humidity to prevent excessive water loss. At lower temperatures, development and reproduction of HDM need more time compared to higher temperatures, which may contribute to the lower HDM concentrations at moderate non‐tropical altitude. 74 However, a recent Austrian study showed similar HDM‐allergen Der p 1 levels at different altitudes, while Der f 1 concentrations were significantly lower at high altitude. 75 In this study, mite‐allergen content in areas below 1500 m was very low, corresponding to a mean Der p 1 concentration less than 0.5 µg/g dust and Der f 1 <2 µg/g dust, which might explain the lack of difference with areas in higher altitudes. The authors speculate that current building construction including better insulation, heating, and glazing, resulting in similar indoor temperature across different altitudes could partly account for their results.
6.2. Pollen
Grass and tree pollen counts are lower at altitude than at sea level. 76 , 77 , 78 There is a general delay in flowering with increasing altitude, resulting in a shorter pollen season. 79 Personal thresholds for clinically relevant pollen concentrations in the air may vary among patients, but are relevant to estimate the impact of reduced exposure. There is scarce data on pollen measurement or pollen allergenicity at various altitudes directly connected to allergic rhinitis, conjunctivitis, or asthma. A recent study in pollen allergic patients showed that allergic symptoms decreased significantly within hours after arriving at an altitude of 2565 m and remained low several days after returning to 490 m. 78
6.3. Fungi
Fungi such as Aspergillus and Alternaria are less often recognized as a cause of respiratory allergy compared to pollen. 80 However, exposure to fungal spores (Penicillium, Aspergillus, Cladosporium, and Alternaria species) frequently induces respiratory allergy symptoms including acute respiratory failure or exacerbates current asthma symptoms in children and adults. 81 , 82 , 83
Little is known about the presence or allergenicity of fungal spores at moderate altitude. There is variability in the amount of Cladosporium and Alternaria alternata spores in outdoor air, depending on geographical location, relative humidity, and temperature. 84 , 85 , 86 Furthermore, high and low potency spores have been recorded, with large intra‐seasonal fluctuations, related to source area and species diversity. 87 The highest Alternaria spore allergenicity was observed in the season with the warmest temperatures, driest conditions, and the highest levels of air pollution (ozone, PM10, and sulfur dioxide). 88 Most likely, there are less fungal spores at moderate altitude because of the low relative humidity and temperature. 81
6.4. Air pollution
The existing European altitude clinics are located in areas with low outdoor air pollution. Outdoor air pollution is a mixture of gaseous pollutants (ozone, nitrogen dioxide, and sulfur dioxide), particulate matter with different sizes (PM2.5, PM5, PM10), and traffic‐related air pollution (TRAP). Urbanization, industrialization, and TRAP determine the level of air pollution, together with weather and landscape influences, resulting in less air pollution at alpine altitude villages. 89 However, in certain alpine settlements air pollution due to traffic and heating, remains a point of concern. Studies have linked asthma exacerbations with exposure to TRAP, identifying specific pollutants that can induce airway inflammation and airway hyperresponsiveness. 53 , 90 Four main mechanisms have been postulated to explain the link between air pollution and asthma exacerbations: oxidative stress and damage, inflammatory‐immune responses, airway remodeling, and enhancement of respiratory sensitization to aeroallergens. 53 , 91 The exact mechanisms by which pollutants induce these effects are not completely clear.
Apart from the direct effect of air pollution on human health, there is an indirect effect through the interaction with the allergenicity of pollen and fungal spores. Air pollution can alter the immune‐stimulatory potential of pollen: either by making the pollen more allergenic or by aggravating allergic symptoms in already sensitized individuals. 92 Fungal spores are also impacted by environmental changes. 93
6.5. Microbial exposure
Several studies have demonstrated differences in the airway, nasal and gut microbiome between asthmatic patients and healthy subjects. 94 , 95 , 96 The different microbial communities (environmental, gut, airways, nose) are able to interact with each other. 97 Furthermore, microbial communities are able to regulate immune function and may impact asthma development and severity. 98 During AACT a significant increase in microbial diversity on the skin of children with difficult to treat atopic dermatitis and asthma was observed, whereas such alpha diversity did not change in a control group treated at sea level. 99 This suggests that microbiome composition may be influenced by the alpine environment or by the moderate altitude. However, it is unclear how much time in a different environment would be needed to realize a sustained change in microbiome. Whether AACT‐induced microbiome changes could play a role in the observed improvements in inflammation in asthma patients remains to be sorted out in future studies.
Key message.
The environmental characteristics of the alpine climate include a reduced aeroallergen burden regarding HDM, pollen, fungi but also air pollution and different microbial exposure.
7. EFFECTS OF ENVIRONMENTAL CHARACTERISTICS OF THE ALPINE CLIMATE ON IMMUNE RESPONSE IN ASTHMA
Observational studies have shown that periods of lower exposure to allergens during AACT may result in significant decreases in antigen‐induced basophil histamine release, total IgE, and specific IgE of asthmatic patients. (Table 2 Immunological outcomes, Figure 3) After allergen re‐exposure, this effect is reversed, as specific antigen‐induced basophil histamine release and serum IgE increase again. 65 , 100 , 101 Similarly, blood and sputum eosinophils significantly decreased after AACT (Tables 2, 3B, and 4B). Serum eosinophil cationic protein (ECP) and eosinophil protein X (EPX) and urinary EPX significantly decreased after AACT and increased again after return to the home environment. 102 , 103 , 104 , 105 , 106 , 107 Cysteinyl leukotrienes (Cys‐LTs) mean level, 8‐Isoprostane level, and nitrites were significantly reduced by AACT in both induced sputum and exhaled breath condensate 103 , 108 , 109 An earlier study demonstrated a decrease in monocyte activation and in the frequency of CRTH2+ (Th2) cells in peripheral blood of severe asthmatic patients who were treated with AACT at 1600 meters for at least 3 weeks. 9 Increased purinergic G protein‐coupled receptor (P2Y1) activity was reported at high altitude together with downregulation of the P2Y12 pathway through increased vasodilator‐stimulated phospho‐protein (VASP) phosphorylation. 110 This modification might be of special interest for the non‐T2 asthma endotype, especially for the sub‐endotype with a strong signal of the inflammasome pathway activation. 111 Furthermore, some studies have shown that there may be a differential impact of altitude on immunological outcomes in different phenotypes of asthma. In patients with eosinophilic allergic asthma, moderate altitude exposure decreased total and activated blood eosinophils, type 2 innate lymphoid cells (ILC2), as well as CRTH2+ CD4 and CD8 T cells. These effects were either absent or less evident in patients with eosinophilic non‐allergic asthma, non‐eosinophilic non‐allergic asthma, and healthy controls. Interestingly, the frequency of CRTH2+ Treg cells, of more inflammatory phenotype than CRTH2− Tregs, decreased in all asthma phenotypes, but not in controls. 11 Reduction of serum IL‐13 has been reported after 3 weeks of AACT, in adults and in children. 11 , 12 In children with allergic asthma, a decrease in serum IL‐10 has been observed, whereas in adult patients with eosinophilic asthma serum IL‐5 also decreased. 11 , 12 This suggests that the type 2 inflammation, represented by ILC2 and Th2 cells and eosinophils, is reduced at moderate altitude in patients with allergic asthma. In addition, altitude is restoring the suppressive and regulatory phenotype of Tregs in all asthma phenotypes. 11 Reduced macrophage phagocytic activity and reduced eosinophil recruitment to the airways after AACT have also recently been proposed as a possible mechanism contributing to clinical improvement in children with mild asthma. 112
Key messages.
There may be a differential beneficial impact of altitude on immunological outcomes in different phenotypes of asthma.
Type 2 inflammation is reduced in patients with allergic asthma
Altitude is restoring the suppressive and regulatory phenotype of Tregs in all asthma phenotypes
Lower exposure to allergens results in significant decreases in antigen‐induced basophil histamine release, total IgE, and specific IgE.
TABLE 2.
Immunological results of AACT: total IgE, specific IgE, sputum eosinophils
| Reference | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change |
|---|---|---|---|---|---|
| Vervloet D et al (1982) a | N = 42 | 9 months | Children with positive intradermal skin tests to dermatophagoides pteronyssinus and domestic dust | T0 Sep – T3 June |
Total IgE geometric mean 1047 U/ml to 603 U/ml (p < .001) Specific IgE to dermatophagoides, to domestic dust, and to grass pollen dropped during the stay (p < .001). IgG mg/100 ml mean (SEM) 1521 (105) to 1482 (61), IgA mean (SEM) 232 (21) to 251 (23) and IgM mean (SEM) 188 (19) to 174 (19) |
| Piacentini G et al (1993) a | N = 20 | 3 months | Children with allergic asthma | T0 Sep – T1 Oct – T2 Dec – T3 Jan |
sIgE kU/L to Dpt mean ± SEM T0:58.485 ± 7.059 kU/L T1:45.067 ± 5.711 T2:48.722 ± 8.093 kU/L T3: 62.629 ± 8.055 kU/L |
| Boner AL et al (1993) CEA | N = 12 | 9 months | Asthmatic atopic children (sensitized to HDM) | T3 June – T0 Sep – T1 Dec |
Total IgE IU/ml mean ± SEM 477.28 ± 1375 to 680.65 ± 151.4 to 472.56 ± 99.26 Dpt IgE IU/ml mean ± SEM 48.27 ± 8.97 to 57.46 ± 11.64 to 48.28 ± 10.59 Df IgE IU/ml mean ± SEM T3 21.79 ± 4.58 to 33.35 ± 7.71 to 25.2 ± 5.57 |
| Peroni DG et al (1994) | N = 22 | 9 months | Asthmatic children allergic to HDM | Oct – Jan – June – Sep |
Total IgE IU/ml mean (SD) 886 (800), 585 (434), 463 (350), 877 (701) HDM specific IgE IU/ml mean ± SD 35 ± 6.6, 332. ± 6.8, ±29.6 ± 6.7, 25.6 ± 8.5 |
| Christie PE et al (1995) | N = 14 | At least 1 month | Adolescents with mild atopic asthma and HDM sensitization |
(1) Baseline – 3 weeks AACT (2) Baseline – 2 weeks sea level – return to AACT |
Total IgE kU/L Mean (SEM) AACT 1400 (517) to 1627 (799) Sea level 1794 (631) to 2073 (796) |
| van Velzen E et al (1996) | N = 16 | 1 month | Children with allergic asthma | Before–after AACT |
Total IgE kU/L mean (SE) 961.61(512.82) to 957.19(475.66) |
| Piacentini GL et al (2011) | N = 14 | 6 months | Children with mild‐to‐moderate asthma, sensitized to HDM or grass | T0 Sep – T1 Dec – T2 Jan – T3 Mar |
Specific IgE kU/L: T0 113.89 to T1 99.52 kU/L to T3 92.83 kU/L Specific IgG4: No significant variations were found IgG4/IgE mg/kU: T0 59.6 to T1 76.75 to T3 86.84 |
| Rijssenbeek‐Nouwens LH et al (2012) | N = 137 | 12 weeks | Adults with severe refractory asthma, HDM sensitized (n = 68), non‐HDM‐sensitized (n = 69) | Before– after AACT |
Total IgE kU/L mean (SD): 376 (7–5000) to 245 (6–4682) (p = .003) N = 43 Total IgE kU/L mean (SD): 94 (5–1781) to 58 (5–1961) (p = .039) N = 36 |
| Basler L et al (2020) |
RCT AACT n = 24 adults Control group at low altitude n = 25 adults |
3 weeks FU: 3 m |
Adults with asthma (ACQ >0.75) | Before–after AACT |
Total IgE baseline only AACT 350 ± 445 Control group 267 ± 365 sIgE mite kUA/L AACT 23.2 (25) to 19 (18) mean change (95% CI) −4.2 (−11.3 to 2.9) Control group 37.7 (59.2) to 27.5 (53) mean change (95% CI)−10.2 (−18.9 to −1.4) sIgE pollen kUA/L AACT 14.9 (25.2) to 12.6 (22.1) mean change (95% CI) −2.31 (−5.4 to 0.79)kU/L Control group 21.5 (30.9) to 16.5 (27.8) mean change (95% CI) −4.95 (−9.69 to −0.21) |
| Piacentini G et al a (1993) | N = 20 | 3 months | Children with allergic asthma | T0 Sep – T1 Oct – T2 Dec – T3 Jan |
Spontaneous histamine release mean ± SEM T0:6.08% ± 0.51% T1:6.77% ± 0.54% T2:6.2% ± 0.56% T3:5.03% ± 0.57% antigen‐induced histamine release mean ± SEM T0: 34.2% ± 4.7% T1:22.76% ± 4.0% T2:22.9% ± 3.2% T3:33.3% ± 5.8% |
| Boner AL et al (1993) a Allergy | N = 23 | 9 months (Sep – June) | Moderately severe HDM allergic asthmatic children | T0 Sep – T2 Mar – T3 June |
Sputum eosinophils % mean ± SEM T0 14.4.3 ± 3.32 to T2 7.43 ± 2.11 to T3 5.97 ± 1.79 |
| Piacentini GL et al (1996) a | N = 16 | 3 months | Asthmatic children allergic to HDM | T0 Sep – T1 Dec | Sputum eosinophils % median (Q1, Q3) of 14.02 (3.34, 28.24) to 2.08 (0, 7.4) (p < .01) |
| Piacentini GL et al (1998) a | N = 10 | 3 months | Children with a history of bronchial asthma and positive SPT to HDM | T0 Sep – T1 Dec – T2 Jan |
Sputum eosinophils % T0 1(0;5.25) to T1 0(0,1.5) (p < .05) to T2 1.5 (0;3) (NS) Airway epithelial cells (%) in sputum T0: 3.50 (0.50;6.98) T1 0 (0;0.5) (p = .012) T2 3.9(1.50;6) (p = .027) |
| Grootendorst DC et al (2001) |
AACT: N = 10 Sea level: N = 8 |
10 weeks FU:6w |
Atopic adolescents sensitized for HDM |
(1) Baseline – 4 w AACT – 8 w AACT – 6 w FU (2) Control group: 0 w 8 w 16 w |
Sputum eosinophils % AACT 3.1 (0–5.6), 2.2 (0–8.6), 1.7 (0–9.4), 3.0 (0–6.0) Control group 3.0 (0–10.8), 1.6 (0–2.2), 2.6 (0–6.4) |
| Peroni DG et al (2001) a | N = 15 | 3 months | HDM sensitized children with asthma (6–14 years of age) | T0 Sep – T1 Dec – T2 Jan |
Sputum eosinophils % T0: 9.0% ± 2.9% T1: 3.2% ± 0.9% T2: 5.9% ± 1.6% |
| Bodini A et al (2004) a | N = 12 | 3 months | Asthmatic children sensitized to HDM | T0 Sep – T1 Dec | Sputum eosinophils % T0 8.5 ± 1.1% to T1 3.5 ± 0.4% (p = .011) |
| Kulkarni N et al (2018) a | N = 62 | 3 weeks | Children with mild‐to‐moderate asthma (77% GINA 1 and 2) | Before–after | Sputum eosinophils % 3 (0–52) to 1 (0–30) p = .001 |
Abbreviations: FU, follow‐up; HDM, house dust mite.
Children were admitted to the clinics in Briançon and Misurina for an entire school year. Admission is usually in September (T0 the period of allergen avoidance starts), children go home for a 2 or 3 weeks Christmas holiday (T1 re‐exposure to allergens starts) and return to the clinic from January (T2 allergen avoidance continues) until June (T3 end of school year).
FIGURE 3.
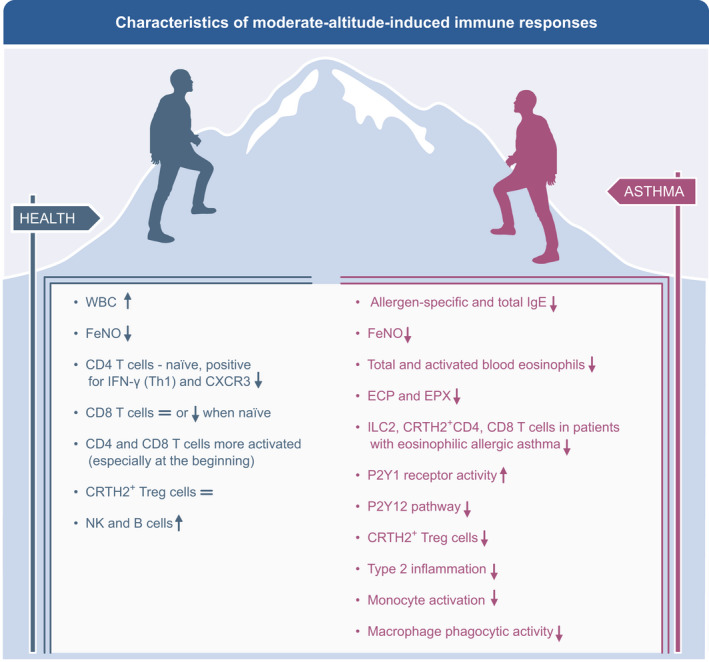
Characteristics of moderate altitude‐induced immunomodulatory responses in healthy persons and asthma patients resulting in decreased inflammation. The moderate altitude environment leads to several immunomodulatory responses including a reduction of type 2 inflammation and restoring of the suppressive and regulatory phenotype of Tregs in all asthma phenotypes. Lower exposure to allergens leads to decreased antigen‐induced basophil histamine release, total IgE, and specific IgE. WBC, White blood cells; CRTH2, cytotoxic regulatory T helper 2 cells; NK, natural killer; ILC, innate lymphoid cell; ECP, eosinophil cationic protein; EPX, eosinophil protein X; CXCR3, C‐X‐C Motif Chemokine Receptor 3; FeNO, fraction of exhaled nitric oxide
TABLE 3.
(A) Outcomes after AACT (asthma control, asthma‐related quality of life). (B) Outcomes after AACT (FeNO, blood eosinophils). (C) Outcomes after AACT (medical consumption, exacerbation rate, OCS use). (D) Outcomes after AACT (lung function). (E) Outcomes after AACT (Upper airways symptoms). (F) Outcomes after AACT (exercise capacity)
| Reference | Study design | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change | Outcome, observed change |
|---|---|---|---|---|---|---|---|
| A | |||||||
| van der Schoot TA et al (1993) | Observational study | N = 147 | 3 months | Nonspecific chronic lung disease: asthma and COPD | Baseline – end of AACT – 6 m FU – 12 m FU |
Quality of life:
|
|
| Rijssenbeek‐Nouwens, LH et al (2012) | Observational study | N = 137 | 12 weeks | Severe refractory asthma, HDM sensitized (n = 68) | Baseline – end of AACT | ACQ: 3.0 (1.0) to 1.6 (1.2) (p < .001) | AQLQ: 4.0 (0.9) to 5.6 (1.0) (p < .001) |
| Severe refractory asthma, non‐HDM‐sensitized (n = 69) | ACQ: 3.3 (1.0) to 1.8 (1.0) (p < .001) | AQLQ: 3.8 (0.9) to 5.3 (1.1) (p < .001) | |||||
| Müller A et al (2018) | Observational study | N = 522 |
3 weeks FU: 6 months |
Unknown | End of AACT – monthly – 6 m FU | ACT mean: 19.86 to 19.2 to 17.16 to 15.65 to 13.99 to 13.2 (p < .001) | |
| Fieten KB et al (2019) | Observational study | N = 101 |
12 weeks FU: 12 months |
Severe asthma | Baseline – End of AACT – 12 m FU | ACQ median (IQR): 3.0 (1.4) to 1.0 (1.5) to 2.3 (2.0) (12 months FU) (p < .000) | AQLQ median (IQR): 4.0 (1.2) to 6.0 (1.1) to 5.0 (1.6) 12 months FU (p < .000) |
| Saxer S et al (2019) | RCT |
AACT (n = 25) Control group at low altitude (n = 25) |
3 weeks FU: 3 months |
Adults with asthma (ACQ >0.75) | Baseline – End of AACT – 3 m FU |
ACQ median (quartiles):
Between group difference of change from baseline to 3 months median (95% CI) 0.4 (−0.4 to 1.1) |
AQLQ median (quartiles):
Between group difference of change from baseline to 3 months median (95% CI) −0.5 (−1.6 to 0.3) |
| de Nijs SB et al (2020) | Prospective comparative study |
AACT (n = 93) Control group at sea level (n = 45) |
12 weeks FU: 12 months |
Severe asthma | Baseline – End of AACT – 12m FU |
ACQ:
Between group difference at 12 months coefficient (SE) −0.87 (0.20) (p < .001) |
AQLQ:
Between group difference at 12 months coefficient (SE) 0.82 (0.23) (p < .001) |
| Reference | Study design | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change, and mean difference from baseline | Outcome, observed change |
|---|---|---|---|---|---|---|---|
| B | |||||||
| Karagiannidis C et al (2006) | Observational study | N = 72 | 3 weeks | Moderate allergic asthma | Baseline – end of AACT |
FeNO ppm (mean): moderate allergic asthma: 56.95 to 27.29 (p < .01) moderate intrinsic asthma: 38.16 to 25.99 (p < .05) severe allergic asthma: 76.12 to 38.59 (p < .01) severe intrinsic asthma: 113.8 to 60.41 (p < .01) |
|
| Moderate intrinsic asthma | |||||||
| Severe allergic asthma | |||||||
| Severe intrinsic asthma | |||||||
| Rijssenbeek‐Nouwens, LH et al (2012) | Observational study | N = 137 | 12 weeks | Severe refractory asthma, HDM sensitized (n = 68) | Baseline – end of AACT | FeNO ppb median (range): 27.6 (5–209) to 18.4 (3–70) (p < .001) | Blood eosinophils 109/L median (range): 235 (0–1050) to 210 (50–570) (p = .033) |
| Severe refractory asthma, non‐HDM‐sensitized (n = 69) | Baseline – end of AACT | FeNO ppb median (range): 16 (5–224) to 16 (1–61) (p = .058) | Blood eosinophils 109/L median (range): 200 (0–880) to 200 (0–630) (p = .207) | ||||
| Basler L et al (2020) | RCT |
AACT (n = 24) Control group at low altitude (n = 25) |
3 weeks | Adults with asthma (ACQ >0.75) | Baseline – end of AACT |
FeNO ppb AACT: 69 (56) to 37 (23), mean difference (95% CI) −31 (−50 to −13) (p < .01) Control group: 48 (33) to 39 (25), mean difference (95% CI) −8 (−24 to 8) (p > .05) Between group difference mean (95% CI) −31 (−50 to −13) |
Blood eosinophils 109/L AACT: 0.46 (0.26) to 0.31 (0.18), mean difference (95% CI) −0.17 (−0.26 to −0.08) (p < .01) Control group: 0.43 (0.22) to 0.36 (0.24), mean difference (95% CI) −0.07 (−0.16 to 0.02) (p >.05) Between group difference mean (95% CI) 0.10 (−0.02 to 0.22) |
| Reference | Study design | Study population (n) | Treatment duration | Study measurements | Asthma characteristics | Outcome, observed change | Outcome, observed change |
|---|---|---|---|---|---|---|---|
| C | |||||||
| Hlinka V et al (1992) | Observational study | N = 1725 | 3 weeks | Baseline – end of AACT | Unknown |
Reduction in asthma medication
|
|
| Speelberg B et al (1992) | Observational study | N = 34 | 3 months | Baseline – end of AACT | Asthma (n = 34) | OCS use mg/day 2.9 (6.0) to 1.4 (1.4) | |
| van der Schoot TA et al (1993) | Observational study | N = 147 | 3 months | Year before ‐ year after AACT | Nonspecific chronic lung disease: asthma and COPD |
Medical consumption:
|
|
| Rijssenbeek‐Nouwens, LH et al (2012) | Observational study | N = 137 | 12 weeks | Baseline – end of AACT | Severe refractory asthma, HDM sensitized (n = 68) |
OCS maintenance (%) 43% to 22% (p < .001) |
|
| Baseline – end of AACT | Severe refractory asthma, non‐HDM‐sensitized (n = 69) |
OCS maintenance (%) 59% to 38% (p < .001) |
|||||
| Fieten KB et al (2019) | Observational study | N = 101 |
12 weeks FU: 12 months |
Year before ‐ year after AACT | Severe asthma |
OCS maintenance (%) 33% to 34% (p = 1.0) OCS use mg/day median (IQR): 2.9 (6.0) to 1.4 (1.4) (p = .684) |
Exacerbation rate median (IQR) 3 (4) to 2 (3) (p = .000) % of hospitalized patients 50% to 32% (p = .010) |
| de Nijs SB et al (2020) | Prospective comparative study |
AACT (n = 93) Control group at sea level (n = 45) |
12 weeks FU: 12 months |
Baseline – end of AACT – 1 year FU | Severe asthma |
OCS maintenance (%) AACT: 52% to 22% to 30% (p < .001)
OCS use mg/day
|
| Reference | Study design | Study population (n) | Treatment duration | Study measurements | Asthma characteristics | Outcome, observed change |
|---|---|---|---|---|---|---|
| D | ||||||
| Dubileĭ V et al (1973) | Observational study | N = 150 | 8 weeks | Baseline – end of AACT | Mild asthma (n = 45) | FEV1/FVC: 71.9 (7.4) to 88.4 (2.7) |
| Moderate asthma (n = 35) | FEV1/FVC: 68.7 (10.0) to 80.4 (3.3) | |||||
| Severe asthma (n = 15) | FEV1/FVC: 64.0 (5.3) to 71.4 (7.1) | |||||
| Kolesár J et al (1977) | Observational study | N = 15 | 2 weeks | Baseline – end of AACT | Unknown | FEV1%pred: 67.1 (6.6) to 69.9 (7.3) |
| Bobokhozhdaev O et al (1990) | Observational study | N = 44 | 4 weeks | Baseline – end of AACT | Atopic asthma (n = 20) | FEV1/FVC: 54.3 (10.3) to 63.2 (17.4) |
| Non‐atopic asthma (n = 24) | FEV1/FVC: 54.1 (10.3) to 75.1 (23.5) | |||||
| Brimkulov N et al (1991) | Observational study | N = 132 | 4 weeks | Baseline – end of AACT | Mild asthma (n = 38) | FEV1%pred: 74.9 (5.5) to 86.4 (7.4) |
| Moderate asthma (n = 94) | FEV1%pred:64.5 (17.2) to 73.3 (18.0) | |||||
| Speelberg B et al (1992) | Observational study | N = 34 | 3 months | Baseline – end of AACT | Asthma (n = 34) |
FEV1%pred: 67 (18) to 66 (21) before salbutamol FEV1%pred: 92 (21) to 95 (19) after salbutamol |
| Rijssenbeek‐Nouwens, LH et al (2012) | Observational study | N = 137 | 12 weeks | Baseline – end of AACT | Severe refractory asthma, HDM sensitized (n = 68) | Post‐bronchodilator FEV1%pred: 88 (20) to 94 (20) (p = .001) |
| Severe refractory asthma, non‐HDM‐sensitized (n = 69) | Post‐bronchodilator FEV1%pred: 86 (26) to 93 (23) (p = .004) | |||||
| Müller A et al (2018) | Observational study | N = 522 | 3 weeks | Baseline – end of AACT | Unknown |
FEV1%: 72.2 (23.6) to 78.3 (22.7) (p < .001) FEV1 /VC: 88.6 (17.3) to 91.8 (15.1) (p < .001) |
| Saxer S et al (2019) | RCT |
AACT (n = 25) Control group at low altitude (n = 25) |
Treatment duration: 3 weeks FU: 3 months |
Baseline – end of AACT – 3 m FU | Adults with asthma (ACQ >0.75) |
|
| de Nijs SB et al (2020) | Prospective comparative study |
AACT (n = 93) Control group at sea level (n = 45) |
12 weeks FU: 12 months |
Baseline – end of AACT – 12m FU | Severe asthma |
FEV1% pred:
|
| Reference | Study design | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change |
|---|---|---|---|---|---|---|
| E | ||||||
| Rijssenbeek‐Nouwens, LH et al (2012) | Observational study | N = 137 | 12 weeks | Severe refractory asthma, HDM sensitized (n = 68) | Baseline – end of AACT | SNOT−20 score: 2.2 (0.8) to 1.5 (1.1) (p < .001) |
| Severe refractory asthma, non‐HDM‐sensitized (n = 69) | SNOT‐20 score: 2.2 (0.8) to 1.6 (1.0) (p < .001) | |||||
| de Nijs SB et al (2020) | Prospective comparative study |
AACT (n = 93) Control group at sea level (n = 45) |
12 weeks FU: 12 months |
Severe asthma | Baseline – end of AACT |
SNOT‐22 score:
|
| Reference | Study design | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change |
|---|---|---|---|---|---|---|
| F | ||||||
| Bijl D et al (1994) | Observational study | N = 62 | 10 weeks | Nonspecific chronic lung disease: asthma and COPD | Baseline – end of AACT – 12 m FU |
VO2max L/min 1.4 (0.4) to 1.9 (0.6) to 1.8 (0.6) (p < .001) |
| Rijssenbeek‐Nouwens, LH et al (2012) | Observational study | N = 137 | 12 weeks | Severe refractory asthma, HDM sensitized (n = 68) | Baseline – end of AACT | 6MWD (meters): 516 (178) to 636 (219) (p < .001) |
| Severe refractory asthma, non‐HDM‐sensitized (n = 69) | 6MWD (meters): 430 (182) to 575 (197) (p < .001) | |||||
| Müller A et al (2018) | Observational study | N = 522 | 3 weeks | Unknown | Baseline – end of AACT | 6MWD (meters): 379.8 (106.6) to 456.98 (111) (p < .001) |
| Saxer S et al (2019) | RCT |
AACT (n = 25) Control group at low altitude (n = 25) |
3 weeks FU: 3 months |
Adults with asthma (ACQ >0.75) | Baseline – end of AACT – 3 m FU |
6MWD (meters) AACT: 536 (491;571) to 571 (522;624) to 570 (517;607) (p < .01) Control group: 490 (452;510) to 521 (493;549) to 504 (486;536) (p < .05) Sit‐to‐stand repetitions AACT: 25 (23–28) to 36 (30–40) to 30 (27–39) (p < .001) Control group: 21 (19–24) to 24 (21–26) to 25 (22–28) (p < .01) |
| de Nijs SB et al (2020) | Prospective comparative study |
AACT (n = 93) Control group at sea level (n = 45) |
12 weeks FU: 12 months |
Severe asthma | Baseline – end of AACT – 12 m FU |
ISWT (m):
|
Data are presented as mean (SD), unless otherwise stated.
Abbreviations: %pred, percentage predicted; 6MWD, 6‐min walking distance; ACQ, asthma control questionnaire; AQLQ, asthma‐related quality of life questionnaire; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume during the first second; FU, follow‐up; FVC, forced vital capacity; HDM, house dust mite; ISWT, incremental shuttle walk test; OCS, oral corticosteroids; SNOT, sinonasal outcome test; VC, vital capacity; VO2max, maximal oxygen consumption.
TABLE 4.
(A) Pediatric outcomes after AACT: asthma control and asthma‐related quality of life. (B) Pediatric outcomes after AACT: Blood eosinophils and FeNO. (C) Pediatric outcomes after AACT: Lung function. (D) Pediatric outcomes after AACT: AHR
| Reference | Study design | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change |
|---|---|---|---|---|---|---|
| A | ||||||
| Boner AL et al (1985) a | Observational study | N = 14 | 9 months | Allergic bronchial asthma | T0 Sep – T3 June |
Decreased requirement for drugs Inhaled steroids discontinued |
| Grootendorst DC et al (2001) | Observational parallel group study |
AACT: n = 10 Control group: n = 8 |
10 weeks FU: 6 weeks |
Atopic adolescents sensitized for HDM |
(1) Baseline – end of AACT – 6 w FU (2) Control group: 0 w 8 w 16 w |
Asthma‐related quality of life median (range) AACT: 5.0 (4.0–6.6) to 6.6 (6.1–7.0) to 6.5 (6.2–7.0) Control group: 4.8 (2.7–6.2) to 5.2 (2.9–6.4) to 5.9 (2.2–6.6) |
| van de Griendt EJ et al (2014) | Observational study | N = 43 | 10 weeks | Moderate‐to‐severe asthma, 74% PSA problematic severe asthma | Baseline – end of AACT |
Asthma control test 6.5 (1.7) to 9.7 (1.7) (p < .001) Asthma‐related quality of life 4.8 (1.2) to 6.2 (0.76) (p < .001) |
| Quignon P et al (2021) a | Observational study | N = 67 | 9 months | Children with severe bronchial asthma for more than 2 years, atopic or non‐atopic | T0 admission (Sep) – T1 (Dec) – T2 15 days home (Jan) – T3 end of school year (June) |
Assessment of asthma control % well controlled: T0 36%, T1 66%, T2 15%, T3 88% Asthma quality of life T0 113 ± 26, T1 121 ± 25, T2 121 ± 24, T3 128 ± 23 (p < .05) |
| Reference | Study design | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change |
|---|---|---|---|---|---|---|
| B | ||||||
| Boner AL et al (1993) CEA | Observational study | N = 12 | 9 months | Allergic asthmatic children sensitized to HDM | T3 June – T0 Sep – T1 Dec |
Blood eosinophils 106/L (mean ± SEM) 215 ± 56, 159 ± 51, 81 ± 13 |
| Boner AL et al (1993) Allergy | Observational study | N = 23 | 9 months | Moderately severe HDM allergic asthmatic children | T0 Sep – T2 Mar – T3 June |
Blood eosinophil (%) (mean ± SEM) T0: 4.23 ± 0.56, T2: 3.4 ± 0.36, T3: 6.47 ± 0.63 |
| Simon HU et al (1994) | Observational study | N = 14 | 5 weeks | Asthmatic children allergic to HDM | Baseline – end of AACT |
Blood eosinophils 106/ml 0.5 to 0.3 |
| Christie PE et al (1995) | RCT | N = 14 | At least 1 month | Adolescents with mild atopic asthma and HDM sensitization |
(1) Baseline – 3 weeks AACT (2) Baseline – 2 weeks sea level – return to AACT |
Blood eosinophils 106/L mean (SE) AACT: 520 (127) to 411 (837) Sea level: 237 (60) to 328 (102) |
| van Velzen E et al (1996) | Observational study | N = 16 | 1 month | Children with allergic asthma | Baseline – end of AACT |
Blood eosinophil counts geometric mean (SE) 372.39 (87.04)/mm3 to 233.35 (67.83)/mm3 (p < 0–01; 95% CI 0.060–0.346) |
| Grootendorst DC et al (2001) | Observational parallel group study |
AACT: n = 10 Control group: n = 8 |
10 weeks FU: 6 w | Atopic adolescents sensitized for HDM |
(1) Baseline – 4 w AACT – 8 w AACT – 6 w FU (2) Control group: 0 w 8 w 16 w |
Blood eosinophils 106/L (geometric mean, range) AACT: 326.4 (31–1316) to 193.8 (88–918) to 173.6 (75–544) to 306.7 (100–840) Control group: 353.2 (200–1045), 288.3 (200–374), 246.7(143–517) |
| Piacentini GL et al (1999) | Observational study | N = 20 | 2 months | Asthmatic children (6–15 years) sensitized to HDM | T0 Sep – T1 2 w – T2 Dec – T3 Jan |
FeNO ppb (geometric mean) T0: 16.3 T1:7.225 T2:7.77 T3:16.85 |
| Peroni DG et al (2002) | Observational study | N = 18 | 9 months (2 weeks at home) | Moderate‐to‐severe asthma, sensitized to HDM | T0 Sep – T1 Dec – T2 Jan – T3 June |
FeNO (ppb) (mean ± SEM) T0: 21.3 ± 3.9 T1: 11.9 ± 1.7 T2:12.5 ± 2.6 T3: 13.2 ± 2.0 |
| Huss‐Marp J et al (2007) | Observational study | N = 311 | 4–6 weeks | Children with increased FENO values at admission (>17 ppb) | Baseline – end of AACT |
FeNO (ppb) geometric mean ±95% CI 38.5 ± 22.8 ppb to 20.1 ± 13.2 ppb (p < .001) |
| Piacentini GL et al (2011) | Observational study | N = 14 | 6 months | Mild‐to‐moderate asthma, sensitized to HDM or grass | T0 Sep – T1 Dec – T2 Jan – T3 Mar |
FeNO (ppb) mean T0: 26.30 T1: 17.43 T2: 27.40 T3: 15.11 (p=.01) |
| van de Griendt EJ et al (2014) | Observational study | N = 43 | 10 weeks | Moderate‐to‐severe asthma, 74% PSA problematic severe asthma | Baseline – end of AACT |
FeNO (ppb) 39.8 (26.4) to 15.2 (7.6) (p < .001) |
| Bersuch, E et al (2017) | Observational study | N = 344 | 3 weeks | Controlled, partly controlled, and uncontrolled asthma | Baseline – end of AACT |
FeNO (ppb) median Controlled: 27.6–15.8 (12 ppb reduction) (p < .001), Partly controlled: 41.8–14.9 (27 ppb reduction) (p < .001) Uncontrolled: 49.9–13 (36 ppb reduction) (p < .001) |
| Kulkarni N et al (2018) | Observational study | N = 62 | 3 weeks | Children with mild‐to‐moderate asthma (77% GINA 1 and 2) | Baseline – 3 w AACT |
FeNO (ppb) median (range) 18.85 (2.6–79.3) to 11.5 (2.1–52.2) (p < .001) |
| Quignon P et al (2021) | Observational study | N = 67 | 9 months | Children with severe bronchial asthma for more than 2 years, atopic or non‐atopic | T0 Sep – T1 Dec – T2 Jan – T3 June | FeNO (ppb) 30 – 18 – 24 – 20 (p < .05) |
| Reference | Study design | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change |
|---|---|---|---|---|---|---|
| C | ||||||
| Boner AL et al (1985) a | Observational study | N = 14 | 9 months | Allergic bronchial asthma | T0 Sep – T3 June | Improved pulmonary function |
| Simon HU et al (1994) | Observational study | N = 14 | 5 weeks | Asthmatic children allergic to HDM | Day 2 – Day 21 – Day 35 |
Lung function at rest mean (SD) FEV1 (% pred) 95.1 (9.8) to 98.3 (10.7) to 104 (14.7) PEF (% pred) 96.6 (20.8) to 99.2 (18.5) to 101 (15.4) PEF variability 17.1 (13.3) to 10.7 (6.3) to 9.5 (5.4) RV/TLC (%) 185 (28) to 171 (20) to 168 (19) FEF50 (% pred) 75.4 (5.2) to 81.5 (6.3) to 79.6 (8.7) |
| Christie PE et al (1995) | RCT | N = 14 | At least 1 month | Adolescents with mild atopic asthma and HDM sensitization |
(1) Baseline – 3 weeks AACT (2) Baseline – 2 weeks sea level – return to AACT |
FEV1 (l) (mean ± SEM) AACT 2.9 ± 0.3 and 2.9 ± 0.4 (p = .35) Sea level 3.0 ± 0.3 to 2.8 ± 0.3 (p = .04) |
| Valletta EA et al (1995) a | Observational study | N = 12 | 3 months | Children with mild‐to‐moderate asthma and house dust mite allergy | Oct – Dec – Jan | %PEF variability (median): 10.6 – 8.1 – 10.4 |
| Benckhuijsen J et al (1996) | Observational study | N = 13 | 1 month | Asthmatic children with allergy to HDM | Before–after | FEV1 (% pred) (mean ± SEM) 97.6 ± 6.3–101 ± 5.5 |
| van Velzen E et al (1996) | Observational study | N = 16 | 1 month | Children with allergic asthma | Before–after |
FEV1 (% pred) mean (SE) 92.1 (5.8) to 97 (5.1) PEF variability % mean (SE) 12.1 (2.5) to 7.2 (1.2) (p < .01) |
| Valletta EA et al (1997) a | Observational study | N = 14 | 3 months | Children with mild‐moderate asthma, allergic to HDM | T0 Sep – T2 Dec |
FEV1%mean (SD): 82 (7) to 86 (6) (p = .05). Mean difference (95% CI): 4% (0–8) FEF25–75%pred mean (SD) 61 (12) to 68 (11) (p = .005). Mean difference (95% CI) 7.4% (2.7–12.2) PEF % mean (SD): 95 (16) to 103 (13) (p = .002) Mean difference (95% CI): 8.2 (3.5–13) |
| Piacentini GL et al (1999) a | Observational study | N = 20 | 2 months | Asthmatic children (6–15 years) sensitized to HDM | T0 Sep – T1 2 w – T2 Dec – T3 Jan | FEV1 L/min: T0 2.006 ± 0.42 to T1 2.0075 ± 0.43 to T2 2.112 ± 0.63 to T3 2.496 ± 0.63 |
| Grootendorst DC et al (2001) | Observational parallel group study |
AACT: n = 10 Control group: n = 8 |
10 weeks FU: 6 w |
Atopic adolescents sensitized for HDM |
(1) Baseline – 4 w AACT – 8 w AACT – 6 w FU (2) Control group: 0 w 8 w 16 w |
FEV1(%) mean (SEM) AACT: 85.6 (4.5) to 94.8(4.2) to 92.6 (4.0) Control group: 86.6 (6.8) to 93.3 (4.2) to 92.5 (4.0) |
| Peroni DG et al (2001) a | Observational study | N = 15 | 3 months | HDM–sensitized children with asthma (6–14 years of age) | T0 Sep – T1 Dec – T2 Jan |
FEV1%pred median (Q1–Q3) T0 103% (99%–108%) to T1 106% (98%–110%) to T2 107% (99%–115%) RV mean (SEM) T0 117.6% (8.6%) to T1 98.0% (3.8%) to T2 133.3% (20.8%) FEF25‐75%pred median (Q1–Q3) T0 84% (67%–93%) to T1 92% (75%–100%) to T2 94% (78%–113%) |
| Peroni DG et al (2002) a | Observational study | N = 18 | 9 months (2 weeks at home) | Moderate‐to‐severe asthma, sensitized to HDM | T0 Sep – T1 Dec – T2 Jan –T3 June |
FEV1(%) (mean ± SEM) T0:100.5 ± 3.6 T1:102.0 ± 2.9 T2:105.1 ± 2.6 T3:100.5 ± 2.3 FEF25–75(%) (mean ± SEM) T0:81.5 ± 5.9 T1:91.3 ± 5.1 T2:94.4 ± 5.8 T3:93.2 ± 5.9 RV (%) T0:117.7 ± 7.7 T1:96.5 ± 3.2 T2: 126.2 ± 17.2 T3:91.1 ± 6.0 |
| Petermann F et al (2004) | Observational study | N = 60 | 4 weeks | Moderate asthma with or without HDM sensitization | Before–after | FEV1%pred 95.27% to 99.4% |
| Straub DA et al (2004) | Observational study | N = 48 | 4 weeks | Asthmatic children with a normal FEV1 and positive skin prick test for HDM | Before–after |
FEV1 (% pred) mean (range) Arrival: 104.5% (80–154%) Departure: 105% (78–168%) |
| Piacentini GL et al (2011) a | Observational study | N = 14 | 6 months | Mild‐to‐moderate asthma, sensitized to HDM or grass | T0 Sep – T1 Dec – T2 Jan – T3 Mar |
FEV1%pred T0: 85 T3:92.75 FEV1/FVC %pred T0: 89.5% T3:92.25% FEF25‐75 T0:66.7% T3:76.87% |
| van de Griendt EJ et al (2014) | Observational study | N = 43 | 10 weeks | Moderate‐to‐severe asthma, 74% PSA problematic severe asthma | Before–after |
FEV1%pred mean (SD) 105.1(14.7) to 108.1(13.9) FEV1/VC %pred mean (SD) 115.3(16.4) to 111.5(12.1) |
| Bersuch, E et al (2017) | Observational study | N = 344 | 3 weeks | Controlled, partly controlled, and uncontrolled asthma | Before–after |
FEV1%pred Controlled: 96.8%–99.1%, Partly controlled: 94.2%–96.0%, Uncontrolled: 89.4%–97.1% MEF25%pred Controlled: 93.6%–95.3%, Partly controlled: 85.9%–85.7%, Uncontrolled: 75.7%–85.6% MEF75%pred Controlled: 93.1%–98.6%, Partly controlled: 88.7%–92%, Uncontrolled: 76.5%–89.2% |
| Kulkarni N et al (2018) a | Observational study | N = 62 | 3 weeks | Children with mild‐to‐moderate asthma (77% GINA 1 and 2) | Baseline – 3 w AACT |
FEV1%pred mean (SE) 109.5 (2.4) to 116.0 (2.04) p = .67 FVC %pred mean (SE) 105.8 (1.81) to 105.6 (1.89) p = .91 FEV1/FVC ratio %pred mean (SE) 103.1 (1.29) to 101.8 (1.3) p = .56 FEF25‐75%pred mean (SE) 105.3 (4.38) 102.9 (4.01) p = .72 |
| Quignon P et al (2021) a | Observational study | N = 67 | 9 months | Children with severe bronchial asthma for more than 2 years, atopic or non‐atopic | T0 Sep – T1 Dec – T2 Jan – T3 June |
PEF 322 ± 97, 362 ± 101, 330 ± 94, 362 ± 98 FEV1 2.6 ± 0.7, 2.64 ± 0.8, 2.7 ± 0.8, 2.64 ± 0.7 |
| Reference | Study design | Study population (n) | Treatment duration | Asthma characteristics | Study measurements | Outcome, observed change |
|---|---|---|---|---|---|---|
| D | ||||||
| Boner AL et al (1985) a | Observational study | N = 14 | 9 months | Allergic bronchial asthma | T0 Sep – T3 June | Decreased nonspecific hyperreactivity |
| Boner AL et al (1985) a | Observational study | N = 23 | 3 months | Allergic bronchial asthma | T0 Sep – T1 Dec |
Only individual values reported Exercise challenge: 10 no response 7 isolated immediate bronchospasm 6 biphasic responses with immediate bronchospasm followed 4–10 h later by a late reaction sustained for at least 2 h. |
| Boner AL et al (1993) CEA a | Observational study | N = 12 | 9 months | Allergic asthmatic children sensitized to HDM | T3 June – T0 Sep – T1 Dec |
Histamine PC20 FEV1 µg/ml (mean ± SEM) 2.89 ± 0.91, 5.54 ± 2.51, 6.66 ± 2.34 |
| Boner AL et al (1993) Allergy a | Observational study | N = 23 | 9 months | Moderately severe HDM allergic asthmatic children | T0 Sep – T2 Mar – T3 June |
Methacholine PC20‐FEV1 mg/ml (mean ± SEM) T0 5.20 ± 1.72 to T2 7.43 ± 2.11 to T3 5.97 ± 1.79 |
| Piacentini G et al (1993) a | Observational study | N = 20 | 3 months (Sep – Dec) | Children with allergic asthma | T0: Admission, T1 after 40 days, T2 after 80 days of allergen avoidance, T3 after 15 days of allergen exposure at sea level. |
Methacholine PC20 mg/ml (mean ± SEM) T0 3.27 ± 0.95 to T1 6.76 ± 1.869 to T2 7.387 ± 2.115 to T3 7.136 ± 3.322 |
| Peroni DG et al (1994) a | Observational study | N = 45 | 9 months |
asthmatic children allergic to HDM 22 children (methacholine) 23 children (histamine, HDM) |
Oct – Jan – June – Sep (1) Oct – Mar – June (2) |
(1) methacholine PD20‐FEV1 µg/ml (mean ± SD) 124 ± 213, 463 ± 612, 589 ± 664, 140 ± 125 (1) PEF decrease after exercise (%) mean ± SD 27.8 ± 20.8, 18.8 ± 15.9, 14.2 ± 12.5, 27.9 ± 12.1 (2) histamine PD20‐FEV1 µg/ml mean ± SD 128.8 ± 30.5, 380 ± 47.8, 275.4 ± 34.3 (2) HDM PD20‐FEV1 AUR/ml mean ± SD 47.86 ± 2.81, 95.49 ± 3.31, 117.4 ± 2.81 |
| Christie PE et al (1995) | RCT | N = 14 | At least 1 month | Adolescents with mild atopic asthma and HDM sensitization |
(1) Baseline – 3 weeks AACT (2) Baseline – 2 weeks sea level – return to AACT |
histamine PC20 mg/ml (geometric mean, range) AACT 1.5 (0.3–22) to 1.5 (0.3–32) (p = .89) Sea level 1.7 (0.3–16.4) to 0.9 (0.1–5.2) (p = .04) (n = 5) |
| Peroni DG et al (1995) | RCT | N = 23 | 12 months | Dermatophagoides pteronyssinus (Dpt)‐sensitive asthmatic children aged 7–14 years | Enrollment, after 6 months, after 12 months of treatment |
IT during AACT histamine PD20 mg/ml (median, interquartile range) 1.87 (1.29–4.76) to 3.80 (1.43–13.6) to 3.79 (2.16–8.44) IT group 1.62 (0.55–2.45) to 2.59 (2.15–4.28) to 2.63 (1.35–4.8) placebo |
| Valletta EA et al (1995) a | Observational study | N = 12 | 3 months |
Children with mild‐to‐moderate asthma and house dust mite allergy |
Oct – Dec – Jan | Methacholine PC20 µg/ml (median): 2.4 – 3.1 – 2 |
| Benckhuijsen J et al (1996) | Observational study | N = 13 | 1 month | Asthmatic children with allergy to HDM | Before–after |
Methacholine PC20 mg/ml (mean ± SEM) 0.4 ± 0.69–0.51 ± 0.99 AMP PC20 mg/ml (mean ± SEM) 4.87 ± 32.76–21.63 ± 31.58 %decrease FEV1 after exercise (mean ± SEM) 26.6 ± 5.7–20.9 ± 5.5 |
| Piacentini GL et al (1996) a | Observational study | N = 16 | 3 months | Asthmatic children allergic to HDM | T0 Sep – T1 Dec |
Methacholine PC20 mg/ml median (lower, upper quartile: Q1, Q3) T0: 1.17 (0.74, 4.75) mg/ml T1: 3.5 (1.18, 8.87) mg/ml (p = .02) |
| van Velzen E et al (1996) | Observational study | N = 16 | 1 month | Children with allergic asthma | Before–after |
Bronchial hyperresponsiveness Methacholine PC20 mg/ml geometric mean ± SE 0.48 ± 0.56 to 0.62 ± 0.77 AMP PC20 mg/ml geometric mean ± SE 6.21 ± 26.72 to 25.78 ± 25.6 |
| Piacentini GL et al (1998) a | Observational study | N = 10 | 3 months | Children with a history of bronchial asthma and positive SPT to HDM | T0 Sep – T1 Dec – T2 Jan |
Bronchial hyperresponsiveness to methacholine PC20 mg/ml median (Q1;Q3) T0: 2.75 (1.53;7.5) T1: 3.25 (1.65;15.25) (p = .038) T2: 5.25 (1.68;14.5) mg/ml (NS) |
Data are presented as mean (SD), unless otherwise stated.
Abbreviations: %pred, percentage predicted; AMP, adenosine monophosphate; FEF, forced expiratory flow; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume during the first second; FVC, forced vital capacity; HDM, house dust mite; MEF, maximum expiratory flow; PC20, concentration of inhaled substance that provokes a 20% decrease in the FEV1; PEF, peak expiratory flow; RV, residual volume.
Children were admitted to the clinics in Briançon and Misurina for an entire school year. Admission is usually in September (T0 the period of allergen avoidance starts), children go home for a 2 or 3 weeks Christmas holiday (T1 re‐exposure to allergens starts) and return to the clinic from January (T2 allergen avoidance continues) until June (T3 end of school year).
8. MULTIDISCIPLINARY PULMONARY REHABILITATION FOR SEVERE ASTHMA
The international guidelines recommend a stepwise multidisciplinary approach for asthma management with the aim to achieve optimal disease control. 1 , 2 Several multidisciplinary treatment programs have been described, but were mostly observational without a control group. These programs reported significant improvement in asthma control, quality of life, exercise capacity, and emotional well‐being. 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 A framework for systematic patient assessment is provided by the concept of treatable traits, divided into pulmonary, extrapulmonary, and behavioral/lifestyle factors. 3 Avoidance of environmental triggers, assessment of treatable traits, treatment of modifiable risk factors and comorbidities, non‐pharmacological strategies, identifying possible obstacles to adherence, checking inhaler technique, education, and skills training have been proposed to manage uncontrolled and severe asthma. 121 , 122 , 123 , 124 Currently available European AACT programs follow such asthma management strategy by combining the physical characteristics of altitude with the avoidance of environmental triggers in the alpine climate and a personalized multidisciplinary pulmonary rehabilitation approach. Most AACT programs contain an extensive patient assessment, psychological and behavioral interventions, personalized exercise programs, extensive patient education, and personalized action plans for use after the end of treatment (Table S6). The content of these programs is not unique and may be used in pulmonary rehabilitation programs anywhere. For some patients, AACT is not suitable because of severe home sickness or because of personal reasons that prevent them from being away from home for a longer period of time. During AACT, regular pharmacological treatment according to treatment guidelines continues. There is no indication that its efficacy would be different at moderate altitudes, but there are no studies comparing the efficacy of bronchodilators or inhaled corticosteroids with different types of inhalers at altitude. However, the specific effectiveness of AACT may lie in the observed rapid decrease in inflammation, either because of fewer environmental triggers in the alpine climate or physical altitude climate factors and their immunomodulatory effects, or because of other yet unknown mechanisms. Medical treatment is optimized during AACT according to the most recent treatment standards. Most patients improve their asthma control, resulting in less need for SABA and optimized ICS use during AACT. Because patients improve their asthma control, there are more possibilities for exercise compared to the home environment. Routine physical exercise results in improved aerobic fitness, improved lung function parameters, reduced exercise‐induced bronchoconstriction, and a further decrease in asthma symptoms. Improved asthma control supports the relevance of behavioral, environmental, and stress factors for future disease control and endorses self‐management, even after AACT.
Psychological and behavioral interventions are major components of most pulmonary alpine climate treatment programs and are aimed at reducing psychological stress, obtaining appropriate adherence to treatment, as well as helping patients to cope with a chronic disease and deal with functional limitations in daily life. 125 Recent studies have supported the link between asthma (severity, control), psychological aspects (subjective perception, coping style), and mental health (anxiety, depression). 126 , 127 , 128 Psychological comorbidities are frequently present in patients with difficult to control disease. 129 , 130 Furthermore, perceived chronic or toxic stress at several levels has been associated with asthma incidence and asthma morbidity. 131 , 132 , 133 Reducing psychological stress may also affect neuro‐immune mediators resulting in reduced airway inflammation and asthma exacerbations. 134 During AACT, patients avoid those psychosocial stress factors that are continually present in their usual everyday life by being away from home, work environment, or school, and receive psychological assessment and treatment of stress‐related factors. This may lower the threshold to accept psychological help for patients, because psychological intervention is a standard component of the treatment program.
Asthma requires self‐management over a long period of time, and low or inadequate health literacy has been associated with poor longitudinal asthma outcomes, increased emergency department utilization, increased symptoms, and significant adverse outcomes in adults with asthma. 135 Asthma education centered around a written action plan includes individualized self‐management instructions to help maintain disease control, how to respond to exacerbations and when to seek medical help, leading to improved disease control and is described in treatment guidelines. 1 , 136 After AACT, personal action plans should be integrated into the patient's own environment and healthcare setting. They also guarantee continuity in asthma care when the patient returns to the referring physician. The use of Internet‐based self‐management applications in the home situation contributes toward sustaining improved asthma control after AACT. 137
Key messages.
Most AACT programs contain an extensive patient assessment, psychological and behavioral interventions, personalized exercise programs, extensive patient education, and personalized action plans for use after the end of treatment.
The specific effectiveness of AACT may lie in the observed rapid decrease in inflammation, either because of fewer environmental triggers in the alpine climate or the physical altitude climate factors and its immunomodulatory effects or other yet unknown mechanisms.
9. CLINICAL IMPACT OF ALPINE ALTITUDE CLIMATE TREATMENT IN ADULTS
Several European observational studies describe AACT in adults with severe or uncontrolled asthma. (Figure 4). There is great heterogeneity in inclusion criteria, resulting in different study populations regarding asthma phenotypes, but also baseline therapy, treatment duration, altitude, and outcome measures (Table S4). Quality assessment demonstrated sufficient quality for most studies while taking the non‐randomized observational study design into account (Table S5). Because the effectiveness of AACT has not been assessed in a randomized controlled trial, there is no consensus on its optimal duration or cost‐effectiveness. One randomized controlled trial outside Europe (Kyrgyzstan) compared rehabilitation at 700 m with 3100 m for patients with uncontrolled asthma and found no differences between the groups. 138 , 139 The effects of AACT on various outcomes such as asthma control and quality of life, exacerbation rate and hospitalizations, oral corticosteroid (OCS) reduction, lung function parameters, upper airways symptoms, and exercise tolerance are described in Table 3A–F. Up to 12 months after the end of AACT, observational studies demonstrate increased exercise tolerance, increased asthma control and asthma‐related quality of life, decreased use of oral corticosteroids, decreased number of outpatient consultations, decreased number and duration of hospital admissions, and decreased numbers of exacerbations. 140 , 141 , 142 , 143
FIGURE 4.
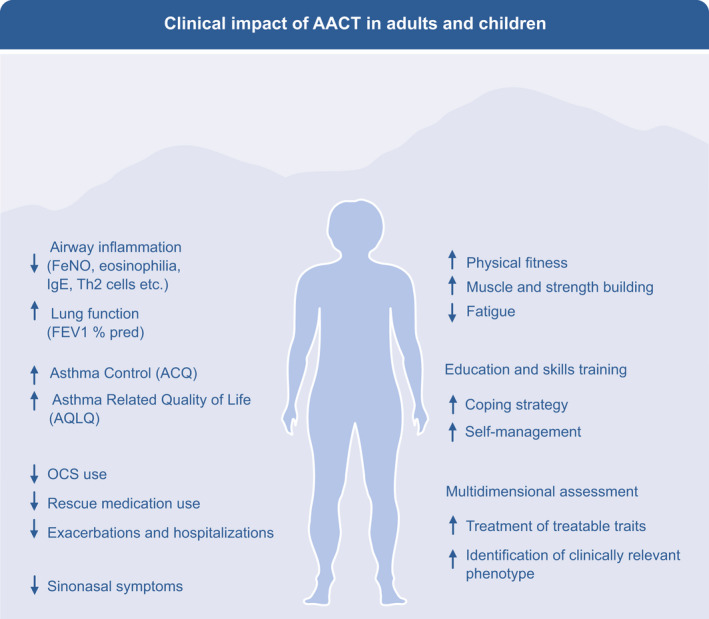
Clinical impact of alpine altitude climate treatment in adults and children. The downregulation of the airway inflammation and the immune system during AACT results in improved asthma control and quality of life, leading to reduction of OCS and rescue medication, less exacerbations and hospitalizations. With a better asthma control, patients can improve their physical fitness, fatigue, coping strategy, and self‐management during the pulmonary rehabilitation including assessment of the treatable traits and asthma phenotype. AACT, alpine altitude climate treatment; OCS, oral corticosteroids
9.1. Impact on Asthma control and quality of life
The degree of asthma control (ACQ—Asthma Control Questionnaire) and quality of life (AQLQ – Asthma Quality of Life Questionnaire) are often used as outcome measures (Table 3A), with a validated minimal clinical important difference (MCID) in score of 0.5. 144 , 145 In an observational study, Rijssenbeek examined (n = 137) severe asthma patients and observed statistically significant and clinically relevant improvements in ACQ and AQLQ after 12 weeks of ACT in both allergic (n = 92) and non‐allergic (n = 45) patients, while background asthma medication was being tapered including OCS. 10 Fieten showed statistically significant and clinically relevant sustained improvements in ACQ and AQLQ at 12 months follow‐up compared to baseline in a follow‐up study from the same cohort. 142 In a prospective comparative study, de Nijs showed that after the 12‐week treatment period, the group of patients treated with AACT (n = 93) had a significantly greater improvement in ACQ score and in AQLQ score relative to patients treated at sea level (n = 45). 143 After a year of follow‐up, the AACT group had a significantly greater improvement in ACQ score and in AQLQ score compared to the sea level group. These studies were conducted more than 5 years apart, but show remarkably similar results in a relatively large well‐defined patient group with severe asthma, supporting the direction of effect. 10 , 142 , 143 Furthermore, correlations between improvement in asthma control and changes in immunoinflammatory parameters namely eosinophils, T‐cell subsets, and ILC2s have been observed. 9 , 11
9.2. Impact on FeNO
A significant reduction of FeNO during AACT has been reported in several studies, starting from the first day of arrival at altitude (Table 3B). One study differentiated between asthma phenotypes and observed significant reductions in the eosinophilic and non‐eosinophilic allergic asthma phenotype, but not in the non‐eosinophilic non‐allergic asthma phenotype. 11 Another study reported decreased FeNO in patients with allergic and non‐allergic, moderate and severe asthma, after 3‐week treatment at an alpine clinic (1560 m). 9 A report involving 68 HDM‐sensitized and 69 non‐HDM‐sensitized asthmatic patients only found a reduction in FeNO in the sensitized patients, after 12‐week treatment at altitude. 10 Finally, a randomized, parallel group trial showed a greater reduction in FeNO in atopic and non‐atopic asthma patients (92% atopic) who were treated for 3 weeks at high altitude (3100 m) than in those treated at low altitude (700 m), with a median between group difference (95% CI) of −18 ppb (−36 to 0). 139
9.3. Impact on exacerbation rate and hospitalizations
Exacerbations are a prominent feature of both poorly controlled and severe asthma. Several studies describe a decrease in number and duration of hospital admissions and less frequent exacerbations up to 1 year after AACT (Table 3C). 141 , 142 , 143
9.4. Impact on oral corticosteroid (OCS) use
A reduction in OCS use is of great clinical relevance because of the complications of its long‐term use. 146 Several studies observed a reduction in OCS use after AACT treatment, either complete discontinuation of maintenance OCS use or a significant decrease in the mean daily dose (Table 3C). 10 , 141 , 147 Comparing 12 months before with 12 months after AACT, there was no change in need for or amount of maintenance oral corticosteroids. 142 However compared with patients treated at sea level, there were still 46% fewer OCS‐dependent patients in the AACT‐treated group, with a mean dose reduction from 18.3 mg to 7.5 mg per day (p < .001) after 12 months follow‐up. 143
9.5. Impact on lung function outcomes
The minimal clinically important difference (MCID) in FEV1 has not been validated for asthma, but improvements of 100–200 ml in FEV1 are likely to be clinically relevant. 148 In a systematic review and meta‐analysis, Vinnikov included 21 studies published between 1973 and 2013, and analyzed changes in FEV1, FEV1/FVC ratio or peak expiratory flow rate (PEF) as outcomes (Table 3D). 7 Asthma severity varied from mild to severe, altitude ranged from 1560 m to >4000 m, and duration of treatment ranged from 2 to 12 weeks. Despite the heterogeneity observed across studies, 93% of the studies reported lung function improvement with an overall pooled standardized mean difference (SMD) of 0.53 (95% CI 0.43–0.62), with a 0.50 change in SMD having been defined as a meaningful clinical difference. Efficacy was demonstrated for adults but not clearly for children, and treatment for >4 weeks was associated with a marginally greater effect size than shorter treatment. 7
9.6. Impact on upper airways symptoms
The close pathophysiological relationship between upper and lower airways results in associations between asthma and upper airway diseases such as allergic rhinitis, chronic rhinosinusitis with or without nasal polyps. 146 In patients with severe asthma, sinonasal involvement is almost 100%. 149 AACT has also been shown to result in improvements in sinonasal symptoms in patients with severe asthma (Table 3E). 10 , 143 In fact, AACT has recently been suggested as treatment for allergic rhinitis and chronic rhinosinusitis as well. 150
9.7. Impact on exercise capacity
Various observational studies have shown that AACT in asthmatic patients may result in improved exercise capacity (Table 3F). In an observational study, Bobokhozhadaev described a higher exercise tolerance after a 4‐week treatment at 1960 m in 96 adults with asthma, 151 and another study showed increased exercise tolerance (VO2max) after a 10 weeks AACT program involving regular physical exercise at 1560 m, in patients with chronic obstructive lung diseases, including asthma. 140 Rijssenbeek et al also reported a clinically relevant improvement in 6‐MWD (6‐min walking distance) after 12 weeks of treatment at 1560 m (Davos, Switzerland). 10 De Nijs described a clinically relevant improvement in exercise tolerance (distance during incremental shuttle walk test (ISWT)) after 12 weeks of treatment at 1560 m, and this improvement lasted up to 12 months. 143 Saxer reported an increased exercise capacity after pulmonary rehabilitation both at 700 m and 3100 m, with an increased 6MWD in both groups but significantly more sit to stand repetitions in the HA group. 138
10. CLINICAL IMPACT OF ALPINE ALTITUDE CLIMATE TREATMENT IN CHILDREN
The effects of AACT on different outcome measures in children with asthma are described in Table 4. Studies were observational and have mainly assessed outcomes regarding asthma control and quality of life, lung function, and AHR. Other outcomes related to exacerbation rates and hospitalizations, exercise tolerance, upper airways symptoms, and immunological outcomes were not reported. Quality assessment demonstrated sufficient quality for most studies while taking the non‐randomized observational study design into account (Table S5). No randomized trials have been done.
10.1. Impact on asthma control and asthma‐related quality of life
A study involving 18 atopic adolescent asthmatics on regular inhaled treatment showed that quality of life (PAQLQ—Pediatric Asthma Quality of Life Questionnaire) improved significantly after 10 weeks of treatment at moderate altitude, an effect which was seen up to 6 weeks after the end of treatment, and less in a control group matched for asthma severity. 152 Another observational study in 32 children with problematic severe asthma, demonstrated improvement in PAQLQ after 10 weeks of AACT, associated with concomitant reduction of inhaled and oral steroids. 153
10.2. Impact on FeNO
Several studies involving asthmatic children have shown a significant decrease in FeNO. 12 , 153 , 154 , 155 , 156 , 157 FeNO presented a significant reduction observed after the first 2 weeks of altitude treatment with an improvement which was faster than that showed by lung function tests (i.e., FEV1). 106
10.3. Impact on OCS use
Discontinuation of oral corticosteroids has been reported in 3 children after 10 weeks of AACT. 152 , 153
10.4. Impact on lung function outcomes
Several studies assessed lung function before and after AACT. A systematic review and meta‐analysis including 3 randomized control trials, 2 clinical‐controlled trials, and 15 single‐arm studies concluded that although there was a lack of significant improvement in FEV1 and PD20, still a trend for improvement could be observed. 6 Over a 2‐week period at 1560 m (Davos, Switzerland), FEV1, MEF25, and MEF75 significantly improved in 124 asthmatic children and adolescents. 155 Similar results were observed in a group of 60 10‐ to 16‐year‐old asthmatic children, after a 4‐week long treatment at altitude, together with a decrease in indices of hyperinflation and improvement in FEV1 after exercise. 105 However, 2 weeks after returning to the Netherlands, a significant reduction in FEV1 was observed in a group of 8 adolescents after staying at least 1 month in Davos. 158 Another study from Davos reported normal pulmonary function at admission and at discharge. 153 A follow‐up study of pulmonary function carried out in 15 HDM‐sensitized 6‐ to 14‐year‐old asthmatic children who stayed at Misurina, Italy (1752 m) for 12 weeks and then returned home, at sea level, for 2 weeks, demonstrated variations in residual volume, a marker of air trapping, that were related to mite‐exposure variations, while there were no significant changes in spirometric parameters. 159 In another study by the same authors, in 18 HDM‐sensitized allergic asthmatic children, after 3 months of AACT, residual volume showed a significant decrease with subsequent increase after 3 weeks of allergen re‐exposure at home. Residual volume decreased again after the following 5 months at altitude. 154 Flow‐volume indices were also investigated in children with mild‐moderate asthma for 12 weeks, resulting in significant improvement of both mean forced expiratory flow FEF25‐75%predicted and PEF% predicted, but marginal changes in FEV1. 160
10.5. Impact on specific and nonspecific airway hyperresponsiveness (AHR)
Various studies have shown that AACT decreases nonspecific AHR, delays the time to onset of allergen‐induced bronchial reactions, and enhances the threshold to bronchial allergen challenge in asthmatic children. After 3 and 9 months of AACT, exercise‐induced and methacholine PD20 (the provocative dose that results in a 20% fall in FEV1) increased, but decreased after 3 months at sea level. 161 Histamine PD20 and HDM PD20 significantly increased after 6 and 9 months. 162 , 163 , 164 One month of AACT could not prevent a significant reduction of histamine PC20 in adolescents with atopic asthma after 2 weeks in the Netherlands. 158 Other studies did not report an improvement in nonspecific AHR to methacholine, but a progressive increase in PD20 for adenosine 5′‐monophospate (AMP) and a reduction in exercise‐induced airway narrowing have been reported. 102 , 103 , 165
Key messages.
Several European observational studies describe AACT in adults and children with severe or uncontrolled asthma, but there is a lack of randomized trials or studies with a control group.
AACT improves various outcomes such as asthma control and quality of life, exacerbation rate and hospitalizations, oral corticosteroid reduction, lung function parameters, upper airways symptoms, and exercise tolerance in adults and children.
11. WHICH PATIENTS MAY BENEFIT FROM AACT
From a clinical point of view, in patients with persistent uncontrolled asthma despite maximum treatment according to guidelines, AACT should be considered as a suitable treatment option. (Figure 5) These may be patients who do not respond to biologicals, who experience impaired quality of life, are oral steroid dependent, have frequent exacerbations and hospitalizations, severely impaired lung function, marked upper airway symptoms, or decreased exercise tolerance. Importantly, similar improvement in asthma control has been observed in sensitized and non‐sensitized patients. 10 From an immunological point of view, the type 2 immune response, which is a significant driver of asthma pathology in eosinophilic and allergic asthma, is reduced during AACT. It is not known whether simultaneous use of biologicals targeting the IL‐4 and IL‐13 pathway or the IL‐5 pathway during AACT further impacts the observed immune modulation. Non‐eosinophilic non‐allergic patients also showed decreased blood lymphocytes, improved lung function, and decreased lung inflammation after AACT. 11 Previously, no patient characteristics could predict significant improvement in AQLQ or FEV1 after AACT. 166 These findings suggest that AACT is a treatment option irrespective of asthma phenotype. The specific effectiveness of AACT may lie in the observed rapid decrease in inflammation and immunomodulatory downregulation which enables the regain of asthma control as the basis for further improvement during multidisciplinary pulmonary rehabilitation. Further basic immunological and clinical research will be necessary to explore the opportunities of AACT as a natural treatment that targets biological pathways, and to further define which asthma phenotypes will benefit most from AACT and which patients will retain the effect most efficiently in their home environment. Randomized trials could compare AACT to other pulmonary rehabilitation programs, assess its cost‐effectiveness, and identify which factor is the most contributing to the observed treatment effect. The use of European severe asthma registries that regularly collect data on patients with severe and uncontrolled asthma could also help to better understand the added value of AACT and the variation in treatment of severe asthma across Europe.
Key message.
AACT can be considered a suitable treatment option for patients with a persistent uncontrolled asthma despite maximum treatment according to guidelines.
FIGURE 5.
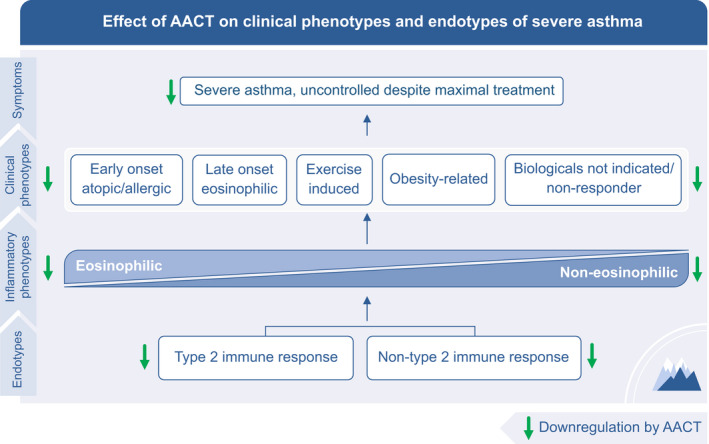
Effect of alpine altitude climate treatment on clinical and inflammatory phenotypes and endotypes of severe asthma. In patients with severe uncontrolled asthma despite maximal medical treatment, AACT offers an opportunity to regain asthma control, irrespective of the clinical or inflammatory phenotype and endotype
12. CONCLUSION AND PERSPECTIVES
Alpine altitude climate treatment takes advantage of the physical characteristics of moderate altitude and the favorable environmental characteristics of the alpine climate and combines this with personalized integrative multidisciplinary pulmonary rehabilitation approaches in an inpatient setting. These factors seem to contribute to the immunomodulatory effects reducing inflammatory responses and neuro‐immune stress in patients with different asthma phenotypes.
Based on the available observational studies and expert opinion, AACT is a therapy for those asthma patients who, despite all the advances in medical science, still cannot achieve optimal control of their complex condition and therefore run the risk of falling into a downward spiral of loss of physical and mental health.
Well‐designed and standardized randomized trials are needed in the future to corroborate the effects of AACT on clinical outcomes and to assess which factors mainly contribute to clinical improvements, which molecular mechanisms underlie successful AACT, and which asthma phenotypes profit more from AACT.
Core message.
The combination of altitude‐related physical and alpine climate‐related environmental characteristics and multidisciplinary personalized treatment during pulmonary rehabilitation support decreased inflammation and immune modulation in patients with uncontrolled asthma of all phenotypes. AACT can be seen as a natural treatment that targets biological pathways.
CONFLICT OF INTEREST
CA has received research grants from the Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, European Commission Horizon's 2020 Framework Programme “CURE,” Novartis Research Institutes, GlaxoSmithKline and AstraZeneca. He took part in the advisory board and received research grants from GlaxoSmithKline, Sanofi/Regeneron, SciBase, and Novartis. He is the Editor‐in‐Chief of Allergy. IA has received payments from Sanofi, Chiesi, Novartis, AZN, Mylan and participated on advisory boards from Sanofi, Chiesi, Novartis, AZN. She is Associate Editor of Allergy and CTA. MS has received research grants from the Swiss National Science Foundation, GlaxoSmithKline, Novartis, and AstraZeneca speaker fee. MC is currently employed by Roche, with no relation to this work. GJB received research grants from AstraZeneca and received payments for consultations and/or speaking at conferences from Novartis, Sanofi, GSK, AstraZeneca, ALK, TEVA, and Chiesi. AC is health director at the Institute Pio XII, Misurina, Italy since June 1, 2020. GP serves on the scientific advisory board to the owner of the Institute Pio XII, Misurina since the beginning of 2020 and received payments for advisory board participation and or speaker fees from GlaxoSmithKline, Novartis, Sanofi, Regeneron, Chiesi, Omron, NOOS, Angelini, Recordati, Pediatrica, OMPharma, MSD. AtB received research grants from AstraZeneca, GSK, TEVA and received payments for consultations and/or speaking at conferences from AstraZeneca, GSK, Novartis, SanofiGenzyme, and TEVA. SS is currently employed by Galenus Health, with no relation to this work. The other authors declare they have no conflict of interest.
Supporting information
Tab S1‐S6
ACKNOWLEDGEMENTS
We want to acknowledge Prof. Dr. Elisabeth Bel for critical reading of the manuscript. Open Access Funding provided by Universitat Zurich. [Correction added on 14‐May‐2022, after first online publication: CSAL funding statement has been added.]
Fieten KB, Drijver‐Messelink MT, Cogo A, et al. Alpine altitude climate treatment for severe and uncontrolled asthma: An EAACI position paper. Allergy. 2022;77:1991–2024. doi: 10.1111/all.15242
L. H. M. Rijssenbeek‐Nouwens and Cezmi A. Akdis should be considered joint senior author.
Funding information
This work was funded by the European Academy of Allergy and Clinical Immunology.
REFERENCES
- 1. Global strategy for asthma management and prevention . 2020. www.ginasthma.org. Accessed June 24, 2020.
- 2. Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55:1900588. [DOI] [PubMed] [Google Scholar]
- 3. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47:410‐419. [DOI] [PubMed] [Google Scholar]
- 4. Agache I, Akdis C, Akdis M, et al. EAACI biologicals guidelines‐recommendations for severe asthma. Allergy. 2020;76(1):14‐44. [DOI] [PubMed] [Google Scholar]
- 5. Rijssenbeek‐Nouwens LH, Bel EH. High‐altitude treatment: a therapeutic option for patients with severe, refractory asthma? Clin Exp Allergy. 2011;41:775‐782. [DOI] [PubMed] [Google Scholar]
- 6. Massimo T, Blank C, Strasser B, Schobersberger W. Does climate therapy at moderate altitudes improve pulmonary function in asthma patients? A systematic review. Sleep Breath. 2014;18:195‐206. [DOI] [PubMed] [Google Scholar]
- 7. Vinnikov D, Khafagy A, Blanc PD, Brimkulov N, Steinmaus C. High‐altitude alpine therapy and lung function in asthma: systematic review and meta‐analysis. ERJ Open Research. 2016;2(2):00097‐2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voorhorst R, Spieksma FTM, Varekamp H. House‐Dust Atopy and the House‐Dust Mite Dermatophagoides pteronyssinus. Stafleu. 1969. [Google Scholar]
- 9. Karagiannidis C, Hense G, Rueckert B, et al. High‐altitude climate therapy reduces local airway inflammation and modulates lymphocyte activation. Scand J Immunol. 2006;63:304‐310. [DOI] [PubMed] [Google Scholar]
- 10. Rijssenbeek‐Nouwens LH, Fieten KB, Bron AO, Hashimoto S, Bel EH, Weersink EJ. High‐altitude treatment in atopic and nonatopic patients with severe asthma. Eur Respir J. 2012;40:1374‐1380. [DOI] [PubMed] [Google Scholar]
- 11. Boonpiyathad T, Capova G, Duchna HW, et al. Impact of high‐altitude therapy on type‐2 immune responses in asthma patients. Allergy. 2020;75:84‐94. [DOI] [PubMed] [Google Scholar]
- 12. Quignon P, da Mata P, Faraj F, et al. Altitude healing effect in severe asthmatic children. Respir Med Res. 2020;79:100810. [DOI] [PubMed] [Google Scholar]
- 13. Pollard AJ, Murdoch DR. The High Altitude Medicine Handbook. Radcliffe Publishing; 2003. [Google Scholar]
- 14. Bartsch P, Saltin B. General introduction to altitude adaptation and mountain sickness. Scand J Med Sci Sports. 2008;18(Suppl 1):1‐10. [DOI] [PubMed] [Google Scholar]
- 15. Milledge J, West J, Ward M. High Altitude Medicine and Physiology. London Chapman & Hall Medical; 1995. [Google Scholar]
- 16. Luks AM, Swenson ER. Travel to high altitude with pre‐existing lung disease. Eur Respir J. 2007;29:770‐792. [DOI] [PubMed] [Google Scholar]
- 17. Luks AM, Swenson ER, Bärtsch P. Acute high‐altitude sickness. Eur Respir Rev. 2017;26(143):160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peacock AJ. ABC of oxygen: oxygen at high altitude. BMJ. 1998;317:1063‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390‐403. [DOI] [PubMed] [Google Scholar]
- 20. Dünnwald T, Gatterer H, Faulhaber M, Arvandi M, Schobersberger W. Body composition and body weight changes at different altitude levels: a systematic review and meta‐analysis. Front Physiol. 2019;10:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grissom CK, Jones BE. Respiratory health benefits and risks of living at moderate altitude. High Alt Med Biol. 2017;19:109‐115. [DOI] [PubMed] [Google Scholar]
- 22. Azad P, Stobdan T, Zhou D, et al. High‐altitude adaptation in humans: from genomics to integrative physiology. J Mol Med (Berl). 2017;95:1269‐1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulrich S, Schneider SR, Bloch KE. Effect of hypoxia and hyperoxia on exercise performance in healthy individuals and in patients with pulmonary hypertension: a systematic review. J Appl Physiol. 2017;123:1657‐1670. [DOI] [PubMed] [Google Scholar]
- 24. Hamlin MJ, Hopkins WG, Hollings SC. Effects of altitude on performance of elite track‐and‐field athletes. Int J Sports Physiol Perform. 2015;10:881. [DOI] [PubMed] [Google Scholar]
- 25. Brothers MD, Wilber RL, Byrnes WC. Physical fitness and hematological changes during acclimatization to moderate altitude: a retrospective study. High Alt Med Biol. 2007;8:213‐224. [DOI] [PubMed] [Google Scholar]
- 26. Maio DA, Farhi LE. Effect of gas density on mechanics of breathing. J Appl Physiol. 1967;23:687‐693. [DOI] [PubMed] [Google Scholar]
- 27. Staub K, Haeusler M, Bender N, et al. Hemoglobin concentration of young men at residential altitudes between 200 and 2000 m mirrors Switzerland's topography. Blood. 2020;135:1066‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas PS, Harding RM, Milledge JS. Peak expiratory flow at altitude. Thorax. 1990;45:620‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Facco M, Zilli C, Siviero M, et al. Modulation of immune response by the acute and chronic exposure to high altitude. Med Sci Sports Exerc. 2005;37:768‐774. [DOI] [PubMed] [Google Scholar]
- 30. Feuerecker M, Crucian BE, Quintens R, et al. Immune sensitization during 1 year in the Antarctic high‐altitude Concordia Environment. Allergy. 2019;74:64‐77. [DOI] [PubMed] [Google Scholar]
- 31. Solak HM, Yanchukov A, Colak F, et al. Altitudinal effects on innate immune response of a subterranean rodent. Zoolog Sci. 2020;37:31‐41. [DOI] [PubMed] [Google Scholar]
- 32. Thiersch M, Swenson ER. High altitude and cancer mortality. High Alt Med Biol. 2018;19:116‐123. [DOI] [PubMed] [Google Scholar]
- 33. Li P, Zheng SJ, Jiang CH, et al. Th2 lymphocytes migrating to the bone marrow under high‐altitude hypoxia promote erythropoiesis via activin A and interleukin‐9. Exp Hematol. 2014;42:804‐815. [DOI] [PubMed] [Google Scholar]
- 34. Krzywinska E, Stockmann C. Hypoxia, metabolism and immune cell function. Biomedicines. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ben‐Shoshan J, Maysel‐Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T‐cell homeostasis via hypoxia‐inducible factor‐1alpha. Eur J Immunol. 2008;38:2412‐2418. [DOI] [PubMed] [Google Scholar]
- 36. Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia‐inducible factor‐1 alpha‐dependent induction of FoxP3 drives regulatory T‐cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109:E2784‐E2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pathria P, Louis TL, Varner JA. Targeting tumor‐associated macrophages in cancer. Trends Immunol. 2019;40:310‐327. [DOI] [PubMed] [Google Scholar]
- 38. Kumar T, Pandey R, Chauhan NS. Hypoxia inducible factor‐1α: the curator of gut homeostasis. Front Cell Infect Microbiol. 2020;10:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52:241‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vargas MH, Becerril‐Angeles M, Medina‐Reyes IS, Rascon‐Pacheco RA. Altitude above 1500m is a major determinant of asthma incidence. An ecological study. Respir Med. 2018;135:1‐7. [DOI] [PubMed] [Google Scholar]
- 41. Torlasco C, Bilo G, Giuliano A, et al. Effects of acute exposure to moderate altitude on blood pressure and sleep breathing patterns. Int J Cardiol. 2020;301:173‐179. [DOI] [PubMed] [Google Scholar]
- 42. Woolcott OO, Ader M, Bergman RN. Glucose homeostasis during short‐term and prolonged exposure to high altitudes. Endocr Rev. 2015;36:149‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. UV and health . www.who.int/uv/uv_and_health. Accessed June 24 2020.
- 44. Cashman KD, Dowling KG, Skrabakova Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morgan KA, Mann EH, Young AR, Hawrylowicz CM. ASTHMA ‐ comparing the impact of vitamin D versus UVR on clinical and immune parameters. Photochem Photobiol Sci. 2017;16:399‐410. [DOI] [PubMed] [Google Scholar]
- 46. Falkenbach A, Tripathi R, Sedlmeyer A, Staudinger M, Herold M. Serum 25‐hydroxyvitamin D and parathyroid hormone in patients with ankylosing spondylitis before and after a three‐week rehabilitation treatment at high altitude during winter and spring. Wien Klin Wochenschr. 2001;113:328‐332. [PubMed] [Google Scholar]
- 47. Abhimanyu A, Coussens AK. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16:314‐338. [DOI] [PubMed] [Google Scholar]
- 48. Pfeffer PE, Hawrylowicz CM. Vitamin D in asthma: mechanisms of action and considerations for clinical trials. Chest. 2018;153:1229‐1239. [DOI] [PubMed] [Google Scholar]
- 49. Eguiluz‐Gracia I, Mathioudakis AG, Bartel S, et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy. 2020;75:2170‐2184. [DOI] [PubMed] [Google Scholar]
- 50. Kim T, Song B, Cho KS, Lee IS. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int J Mol Sci. 2020;21(6):2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tovey E, Ferro A. Time for new methods for avoidance of house dust mite and other allergens. Curr Allergy Asthma Rep. 2012;12:465‐477. [DOI] [PubMed] [Google Scholar]
- 52. Gautier C, Charpin D. Environmental triggers and avoidance in the management of asthma. J Asthma Allergy. 2017;10:47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581‐1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilson JM, Platts‐Mills TAE. Home environmental interventions for house dust mite. J Allergy Clin Immunol Pract. 2018;6:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rijssenbeek‐Nouwens LH, Oosting AJ, de Bruin‐Weller MS, Bregman I, de Monchy JG, Postma DS. Clinical evaluation of the effect of anti‐allergic mattress covers in patients with moderate to severe asthma and house dust mite allergy: a randomised double blind placebo controlled study. Thorax. 2002;57:784‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pavord ID, Birring SS, Berry M, Green RH, Brightling CE, Wardlaw AJ. Multiple inflammatory hits and the pathogenesis of severe airway disease. Eur Respir J. 2006;27:884‐888. [DOI] [PubMed] [Google Scholar]
- 57. Krieger J, Jacobs DE, Ashley PJ, et al. Housing interventions and control of asthma‐related indoor biologic agents: a review of the evidence. J Public Health Manag Pract. 2010;16:S11‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home‐based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068‐1080. [DOI] [PubMed] [Google Scholar]
- 59. Bobb C, Ritz T, Rowlands G, Griffiths C. Effects of allergen and trigger factor avoidance advice in primary care on asthma control: a randomized‐controlled trial. Clin Exp Allergy. 2010;40:143‐152. [DOI] [PubMed] [Google Scholar]
- 60. Platts‐Mills TA, Heymann PW, Chapman MD, Hayden ML, Wilkins SR. Cross‐reacting and species‐specific determinants on a major allergen from Dermatophagoides pteronyssinus and D. farinae: development of a radioimmunoassay for antigen P1 equivalent in house dust and dust mite extracts. J Allergy Clin Immunol. 1986;78:398‐407. [DOI] [PubMed] [Google Scholar]
- 61. Sidenius KE, Hallas TE, Brygge T, Poulsen LK, Mosbech H. House dust mites and their allergens at selected locations in the homes of house dust mite‐allergic patients. Clin Exp Allergy. 2002;32:1299‐1304. [DOI] [PubMed] [Google Scholar]
- 62. Colloff MJ. Dust Mites. Springer Netherlands; 2009. [Google Scholar]
- 63. Spieksma FT, Zuidema P, Leupen MJ. High altitude and house‐dust mites. Br Med J. 1971;1:82‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gomez MS, Portus M. Factors influencing the house dust mite population IV. Altitude. Allergol Immunopathol. 1981;9:123‐130. [PubMed] [Google Scholar]
- 65. Vervloet D, Penaud A, Razzouk H, et al. Altitude and house dust mites. J Allergy Clin Immunol. 1982;69:290‐296. [DOI] [PubMed] [Google Scholar]
- 66. Budak SON. Acar fauna of the house‐dust in Aegean Region. Acta Parasitol Turcica. 1988;12:47‐53. [Google Scholar]
- 67. Charpin D, Birnbaum J, Haddi E, et al. Altitude and allergy to house‐dust mites. A paradigm of the influence of environmental exposure on allergic sensitization. Am Rev Respir Dis. 1991;143:983‐986. [DOI] [PubMed] [Google Scholar]
- 68. de Andrade AD, Bartal M, Birnbaum J, Lanteaume A, Charpin D, Vervloet D. House dust mite allergen content in two areas with large differences in relative humidity. Ann Allergy Asthma Immunol. 1995;74:314‐316. [PubMed] [Google Scholar]
- 69. Valdivieso R, Estupinan M, Acosta ME. Asthma and its relation with Dermatophagoides pteronyssinus and Dermatophagoides farinae in Andean altitudes (Quito, Ecuador). J Investig Allergol Clin Immunol. 1997;7:46‐50. [PubMed] [Google Scholar]
- 70. Stemeseder T, Schweidler B, Doppler P, et al. Exposure to indoor allergens in different residential settings and its influence on IgE sensitization in a geographically confined Austrian Cohort. PLoS One. 2017;12:e0168686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Valdivieso R, Iraola V, Pinto H. Presence of domestic mites at an extremely high altitude (4800 m) in Andean Ecuador. J Investig Allergol Clin Immunol. 2009;19:323‐324. [PubMed] [Google Scholar]
- 72. Ciftci IH, Cetinkaya Z, Atambay M, Kiyildi N, Aycan OM, Daldal N. House dust mite fauna in western Anatolia, Turkey. Korean J Parasitol. 2006;44:259‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Duenas‐Meza E, Torres‐Duque CA, Correa‐Vera E, et al. High prevalence of house dust mite sensitization in children with severe asthma living at high altitude in a tropical country. Pediatr Pulmonol. 2018;53:1356‐1361. [DOI] [PubMed] [Google Scholar]
- 74. Hart BJ. Life cycle and reproduction of house‐dust mites: environmental factors influencing mite populations. Allergy. 1998;53:13‐17. [DOI] [PubMed] [Google Scholar]
- 75. Grafetstatter C, Prossegger J, Braunschmid H, et al. No concentration decrease of house dust mite allergens with rising altitude in alpine regions. Allergy Asthma Immunol Res. 2016;8:312‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Michel FB, Cour P, Quet L, Marty JP. Qualitative and quantitative comparison of pollen calendars for plain and mountain areas. Clin Allergy. 1976;6:383‐393. [DOI] [PubMed] [Google Scholar]
- 77. Charpin D, Hughes B, Mallea M, Sutra JP, Balansard G, Vervloet D. Seasonal allergic symptoms and their relation to pollen exposure in south‐east France. Clin Exp Allergy. 1993;23:435‐439. [DOI] [PubMed] [Google Scholar]
- 78. Damialis A, Häring F, Gökkaya M, et al. Human exposure to airborne pollen and relationships with symptoms and immune responses: indoors versus outdoors, circadian patterns and meteorological effects in alpine and urban environments. Sci Total Environ. 2019;653:190‐199. [DOI] [PubMed] [Google Scholar]
- 79. Cornelius C, Estrella N, Franz H, Menzel A. Linking altitudinal gradients and temperature responses of plant phenology in the Bavarian Alps. Plant Biol. 2013;15(Suppl 1):57‐69. [DOI] [PubMed] [Google Scholar]
- 80. Crameri R, Weichel M, Fluckiger S, Glaser AG, Rhyner C. Fungal allergies: a yet unsolved problem. Chem Immunol Allergy. 2006;91:121‐133. [DOI] [PubMed] [Google Scholar]
- 81. Sharpe RA, Bearman N, Thornton CR, Husk K, Osborne NJ. Indoor fungal diversity and asthma: a meta‐analysis and systematic review of risk factors. J Allergy Clin Immunol. 2015;135:110‐122. [DOI] [PubMed] [Google Scholar]
- 82. Dales RE, Cakmak S, Burnett RT, Judek S, Coates F, Brook JR. Influence of ambient fungal spores on emergency visits for asthma to a regional children's hospital. Am J Respir Crit Care Med. 2000;162:2087‐2090. [DOI] [PubMed] [Google Scholar]
- 83. Bush RK, Prochnau JJ. Alternaria‐induced asthma. J Allergy Clin Immunol. 2004;113:227‐234. [DOI] [PubMed] [Google Scholar]
- 84. Kasprzyk I, Rodinkova V, Sauliene I, et al. Air pollution by allergenic spores of the genus Alternaria in the air of central and eastern Europe. Environ Sci Pollut Res Int. 2015;22:9260‐9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grinn‐Gofron A, Nowosad J, Bosiacka B, et al. Airborne Alternaria and Cladosporium fungal spores in Europe: forecasting possibilities and relationships with meteorological parameters. Sci Total Environ. 2019;653:938‐946. [DOI] [PubMed] [Google Scholar]
- 86. Erkara IP, Asan A, Yilmaz V, Pehlivan S, Okten SS. Airborne Alternaria and Cladosporium species and relationship with meteorological conditions in Eskisehir City, Turkey. Environ Monit Assess. 2008;144:31‐41. [DOI] [PubMed] [Google Scholar]
- 87. Grewling L, Nowak M, Szymanska A, Kostecki L, Bogawski P. Temporal variability in the allergenicity of airborne Alternaria spores. Med Mycol. 2019;57:403‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lang‐Yona N, Shuster‐Meiseles T, Mazar Y, Yarden O, Rudich Y. Impact of urban air pollution on the allergenicity of Aspergillus fumigatus conidia: outdoor exposure study supported by laboratory experiments. Sci Total Environ. 2016;541:365‐371. [DOI] [PubMed] [Google Scholar]
- 89. Downs SH, Schindler C, Liu LJ, et al. Reduced exposure to PM10 and attenuated age‐related decline in lung function. N Engl J Med. 2007;357:2338‐2347. [DOI] [PubMed] [Google Scholar]
- 90. Seltzer J, Bigby BG, Stulbarg M, et al. O3‐induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol. 1986;60:1321‐1326. [DOI] [PubMed] [Google Scholar]
- 91. Gowers AM, Cullinan P, Ayres JG, et al. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology. 2012;17:887‐898. [DOI] [PubMed] [Google Scholar]
- 92. Zhao F, Durner J, Winkler JB, et al. Pollen of common ragweed (Ambrosia artemisiifolia L.): illumina‐based de novo sequencing and differential transcript expression upon elevated NO(2)/O(3). Environ Pollut. 1987;2017(224):503‐514. [DOI] [PubMed] [Google Scholar]
- 93. Lang‐Yona N, Levin Y, Dannemiller KC, Yarden O, Peccia J, Rudich Y. Changes in atmospheric CO2 influence the allergenicity of Aspergillus fumigatus. Glob Change Biol. 2013;19:2381‐2388. [DOI] [PubMed] [Google Scholar]
- 94. Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135:25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fazlollahi M, Lee TD, Andrade J, et al. The nasal microbiome in asthma. J Allergy Clin Immunol. 2018;142:834‐843 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Michalovich D, Rodriguez‐Perez N, Smolinska S, et al. Obesity and disease severity magnify disturbed microbiome‐immune interactions in asthma patients. Nat Commun. 2019;10:5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Frati F, Salvatori C, Incorvaia C, et al. The role of the microbiome in asthma: the Gut(‐)Lung axis. Int J Mol Sci. 2018;20(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Carr TF, Alkatib R, Kraft M. Microbiome in mechanisms of asthma. Clin Chest Med. 2019;40:87‐96. [DOI] [PubMed] [Google Scholar]
- 99. van Mierlo MMF, Totté JEE, Fieten KB, et al. The influence of treatment in alpine and moderate maritime climate on the composition of the skin microbiome in patients with difficult to treat atopic dermatitis. Clin Exp Allergy. 2019;49:1437‐1445. [DOI] [PubMed] [Google Scholar]
- 100. Piacentini GL, Martinati L, Fornari A, et al. Antigen avoidance in a mountain environment: influence on basophil releasability in children with allergic asthma. J Allergy Clin Immunol. 1993;92:644‐650. [DOI] [PubMed] [Google Scholar]
- 101. Piacentini GL, Guerresi S, Kantar A, et al. A comparison between IgE and IgG4 as markers of allergy in children: an experimental trial in a model of natural antigen avoidance. Int J Immunopathol Pharmacol. 2011;24:1049‐1056. [DOI] [PubMed] [Google Scholar]
- 102. van Velzen E, Van Den Bos JW, Benckhuijsen JA, van Essel T, de Bruijn R, Aalbers R. Effect of allergen avoidance at high altitude on direct and indirect bronchial hyperresponsiveness and markers of inflammation in children with allergic asthma. Thorax. 1996;51:582‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Boner AL, Peroni DG, Piacentini GL, Venge P. Influence of allergen avoidance at high altitude on serum markers of eosinophil activation in children with allergic asthma. Clin Exp Allergy. 1993;23:1021‐1026. [DOI] [PubMed] [Google Scholar]
- 104. Piacentini GL, Martinati L, Mingoni S, Boner AL. Influence of allergen avoidance on the eosinophil phase of airway inflammation in children with allergic asthma. J Allergy Clin Immunol. 1996;97:1079‐1084. [DOI] [PubMed] [Google Scholar]
- 105. Petermann F, Gulyas AF, Niebank K, Warschburger P. Effects of allergen avoidance at high altitude on children with asthma or atopic dermatitis. Pediatr Asthma Allergy Immunol. 2004;17:15‐24. [Google Scholar]
- 106. Piacentini GL, Bodini A, Costella S, et al. Allergen avoidance is associated with a fall in exhaled nitric oxide in asthmatic children. J Allergy Clin Immunol. 1999;104:1323‐1324. [DOI] [PubMed] [Google Scholar]
- 107. Piacentini GL, Peterson C, Peroni DG, Bodini A, Boner AL. Allergen avoidance at high altitude and urinary eosinophil protein X. J Allergy Clin Immunol. 1999;104:243‐244. [DOI] [PubMed] [Google Scholar]
- 108. Bodini A, Peroni D, Vicentini L, et al. Exhaled breath condensate eicosanoids and sputum eosinophils in asthmatic children: a pilot study. Pediatr Allergy Immunol. 2004;15:26‐31. [DOI] [PubMed] [Google Scholar]
- 109. Straub DA, Ehmann R, Hall GL, et al. Correlation of nitrites in breath condensates and lung function in asthmatic children. Pediatr Allergy Immunol. 2004;15:20‐25. [DOI] [PubMed] [Google Scholar]
- 110. Paterson GG, Young JM, Willson JA, et al. Hypoxia modulates platelet purinergic signalling pathways. Thromb Haemost. 2020;120:253‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Invest. 2019;129:1493‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kulkarni N, Kantar A, Costella S, et al. Macrophage phagocytosis and allergen avoidance in children with asthma. Front Pediatr. 2018;6:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lingner H, Ernst S, Grobetahennig A, et al. Asthma control and health‐related quality of life one year after inpatient pulmonary rehabilitation: the ProKAR study. J Asthma. 2015;52:614‐621. [DOI] [PubMed] [Google Scholar]
- 114. Trevor JL, Bhatt SP, Wells JM, et al. Benefits of completing pulmonary rehabilitation in patients with asthma. J Asthma. 2015;52:969‐973. [DOI] [PubMed] [Google Scholar]
- 115. Candemir I, Ergun P, Kaymaz D. Efficacy of a multidisciplinary pulmonary rehabilitation outpatient program on exacerbations in overweight and obese patients with asthma. Wien Klin Wochenschr. 2017;129:655‐664. [DOI] [PubMed] [Google Scholar]
- 116. Bratton DL, Price M, Gavin L, et al. Impact of a multidisciplinary day program on disease and healthcare costs in children and adolescents with severe asthma: a two‐year follow‐up study. Pediatr Pulmonol. 2001;31:177‐189. [DOI] [PubMed] [Google Scholar]
- 117. Chan DS, Callahan CW, Moreno C. Multidisciplinary education and management program for children with asthma. Am J Health Syst Pharm. 2001;58:1413‐1417. [DOI] [PubMed] [Google Scholar]
- 118. Cook J, Beresford F, Fainardi V, et al. Managing the pediatric patient with refractory asthma: a multidisciplinary approach. J Asthma Allergy. 2017;10:123‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sahin H, Naz I. Comparing the effect of pulmonary rehabilitation in patients with uncontrolled and partially controlled asthma. J Asthma. 2018;56:87‐94. [DOI] [PubMed] [Google Scholar]
- 120. Zampogna E, Zappa M, Spanevello A, Visca D. Pulmonary rehabilitation and asthma. Front Pharmacol. 2020;11:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24:37‐47. [DOI] [PubMed] [Google Scholar]
- 122. Clark VL, Gibson PG, Genn G, Hiles SA, Pavord ID, McDonald VM. Multidimensional assessment of severe asthma: a systematic review and meta‐analysis. Respirology. 2017;22:1262‐1275. [DOI] [PubMed] [Google Scholar]
- 123. van der Meer AN, Pasma H, Kempenaar‐Okkema W, et al. A 1‐day visit in a severe asthma centre: effect on asthma control, quality of life and healthcare use. Eur Respir J. 2016;48:726‐733. [DOI] [PubMed] [Google Scholar]
- 124. Price DB, Roman‐Rodriguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5:1071‐1081 e9. [DOI] [PubMed] [Google Scholar]
- 125. Smith JR, Mugford M, Holland R, Noble MJ, Harrison BD. Psycho‐educational interventions for adults with severe or difficult asthma: a systematic review. J Asthma. 2007;44:219‐241. [DOI] [PubMed] [Google Scholar]
- 126. Baiardini I, Sicuro F, Balbi F, Canonica GW, Braido F. Psychological aspects in asthma: do psychological factors affect asthma management? Asthma Res Pract. 2015;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ritz T, Bobb C, Griffiths C. Predicting asthma control: the role of psychological triggers. Allergy Asthma Proc. 2014;35:390‐397. [DOI] [PubMed] [Google Scholar]
- 128. Smith HE, Jones CJ. Psychological interventions in asthma. Curr Treat Options Allergy. 2015;2:155‐168. [Google Scholar]
- 129. Lavoie KL, Cartier A, Labrecque M, et al. Are psychiatric disorders associated with worse asthma control and quality of life in asthma patients? Respir Med. 2005;99:1249‐1257. [DOI] [PubMed] [Google Scholar]
- 130. Tay TR, Hew M. Comorbid, "treatable traits" in difficult asthma: current evidence and clinical evaluation. Allergy. 2018;73:1369‐1382. [DOI] [PubMed] [Google Scholar]
- 131. Rod NH, Kristensen TS, Lange P, Prescott E, Diderichsen F. Perceived stress and risk of adult‐onset asthma and other atopic disorders: a longitudinal cohort study. Allergy. 2012;67:1408‐1414. [DOI] [PubMed] [Google Scholar]
- 132. Yonas MA, Lange NE, Celedon JC. Psychosocial stress and asthma morbidity. Curr Opin Allergy Clin Immunol. 2012;12:202‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Barnthouse M, Jones BL. The impact of environmental chronic and toxic stress on asthma. Clin Rev Allergy Immunol. 2019;57:427‐438. [DOI] [PubMed] [Google Scholar]
- 134. Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21:993‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mancuso CA, Rincon M. Impact of health literacy on longitudinal asthma outcomes. J Gen Intern Med. 2006;21:813‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gibson PG, Powell H. Written action plans for asthma: an evidence‐based review of the key components. Thorax. 2004;59:94‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Beerthuizen T, Rijssenbeek‐Nouwens LH, van Koppen SM, Khusial RJ, Snoeck‐Stroband JB, Sont JK. Internet‐based self‐management support after high‐altitude climate treatment for severe asthma: randomized controlled trial. J Med Internet Res. 2020;22:e13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Saxer S, Schneider SR, Appenzeller P, et al. Asthma rehabilitation at high vs. low altitude: randomized parallel‐group trial. BMC Pulm Med. 2019;19:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Basler L, Saxer S, Schneider SR, et al. Asthma rehabilitation at high vs. low altitude and its impact on exhaled nitric oxide and sensitization patterns: Randomized parallel‐group trial. Respir Med. 2020;170:106040. [DOI] [PubMed] [Google Scholar]
- 140. Bijl D, Speelberg B, Folgering HT. Pulmonary rehabilitation at moderate altitude: a 1‐year follow‐up. Neth J Med. 1994;45:154‐161. [PubMed] [Google Scholar]
- 141. van der Schoot TA, de Weerdt I, Kaptein AA, Dekker FW, Deenen TA, Speelberg B. Favorable effects of a stay in the Dutch Asthma Center Davos on medical consumption and quality of life in COPD patients. Ned Tijdschr Geneeskd. 1993;137:197‐201. [PubMed] [Google Scholar]
- 142. Fieten KB, Rijssenbeek‐Nouwens LH, Hashimoto S, Bel EH, Weersink EJ. Less exacerbations and sustained asthma control 12 months after high altitude climate treatment for severe asthma. Allergy. 2019;74:628‐630. [DOI] [PubMed] [Google Scholar]
- 143. de Nijs SB, Krop EJM, Portengen L, et al. Effectiveness of pulmonary rehabilitation at high‐altitude compared to sea‐level in adults with severe refractory asthma. Respir Med. 2020;171:106123. [DOI] [PubMed] [Google Scholar]
- 144. Juniper EF, Bousquet J, Abetz L, Bateman ED, Committee G. Identifying ‘well‐controlled’ and ‘not well‐controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616‐621. [DOI] [PubMed] [Google Scholar]
- 145. Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999;14:32‐38. [DOI] [PubMed] [Google Scholar]
- 146. Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long‐term oral corticosteroid therapy and its side‐effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52:1800703. [DOI] [PubMed] [Google Scholar]
- 147. Speelberg B, Folgering HT, Sterk PJ, van Herwaarden CL. Lung function of adult patients with bronchial asthma or chronic obstructive lung disease prior to and following a 3‐month‐stay in the Dutch Asthma Center in Davos. Ned Tijdschr Geneeskd. 1992;136:469‐473. [PubMed] [Google Scholar]
- 148. Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129:S65‐S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Braunstahl GJ. Chronic rhinosinusitis, nasal polyposis and asthma: the united airways concept reconsidered? Clin Exp Allergy. 2011;41:1341‐1343. [DOI] [PubMed] [Google Scholar]
- 150. Kang G. High‐altitude treatment in rhinitis patients. Am J Rhinol Allergy. 2020;34:141‐142. [DOI] [PubMed] [Google Scholar]
- 151. Bobokhozhdaev OI, Shirinskii VS. The efficacy of treating bronchial asthma patients at Khodzhaobigarm health resort. Vopr Kurortol Fizioter Lech Fiz Kult. 1990;6:21‐26. [PubMed] [Google Scholar]
- 152. Grootendorst DC, Dahlen SE, Van Den Bos JW, et al. Benefits of high altitude allergen avoidance in atopic adolescents with moderate to severe asthma, over and above treatment with high dose inhaled steroids. Clin Exp Allergy. 2001;31:400‐408. [DOI] [PubMed] [Google Scholar]
- 153. van de Griendt EJ, Verkleij M, Douwes JM, van Aalderen WM, Geenen R. Problematic severe asthma in children treated at high altitude: tapering the dose while improving control. J Asthma. 2014;51:315‐319. [DOI] [PubMed] [Google Scholar]
- 154. Peroni DG, Piacentini GL, Costella S, et al. Mite avoidance can reduce air trapping and airway inflammation in allergic asthmatic children. Clin Exp Allergy. 2002;32:850‐855. [DOI] [PubMed] [Google Scholar]
- 155. Bersuch E, Graf F, Renner ED, et al. Lung function improvement and airways inflammation reduction in asthmatic children after a rehabilitation program at moderate altitude. Pediatr Allergy Immunol. 2017;28:768‐775. [DOI] [PubMed] [Google Scholar]
- 156. Huss‐Marp J, Kramer U, Eberlein B, et al. Reduced exhaled nitric oxide values in children with asthma after inpatient rehabilitation at high altitude. J Allergy Clin Immunol. 2007;120:471‐472. [DOI] [PubMed] [Google Scholar]
- 157. Fieten KB, Schappin R, Zijlstra WT, et al. Effectiveness of alpine climate treatment for children with difficult to treat atopic dermatitis: Results of a pragmatic randomized controlled trial (DAVOS trial). Clin Exp Allergy. 2018;48:186‐195. [DOI] [PubMed] [Google Scholar]
- 158. Christie PE, Yntema JL, Tagari P, Ysselstijn H, Ford‐Hutchinson AW, Lee TH. Effect of altitude on urinary leukotriene (LT) E4 excretion and airway responsiveness to histamine in children with atopic asthma. Eur Respir J. 1995;8:357‐363. [DOI] [PubMed] [Google Scholar]
- 159. Peroni DG, Piacentini GL, Vicentini L, Costella S, Pietrobelli A, Boner AL. Effective allergen avoidance reduces residual volume and sputum eosinophils in children with asthma. J Allergy Clin Immunol. 2001;108:308. [DOI] [PubMed] [Google Scholar]
- 160. Valletta EA, Piacentini GL, Del Col G, Boner AL. FEF25‐75 as a marker of airway obstruction in asthmatic children during reduced mite exposure at high altitude. J Asthma. 1997;34:127‐131. [DOI] [PubMed] [Google Scholar]
- 161. Valletta EA, Comis A, Del Col G, Spezia E, Boner AL. Peak expiratory flow variation and bronchial hyperresponsiveness in asthmatic children during periods of antigen avoidance and reexposure. Allergy. 1995;50:366‐369. [DOI] [PubMed] [Google Scholar]
- 162. Boner A, Niero E, Antolini I, Warner JO. Biphasic (early and late) asthmatic responses to exercise in children with severe asthma, resident at high altitude. Eur J Pediatr. 1985;144:164‐166. [DOI] [PubMed] [Google Scholar]
- 163. Peroni DG, Boner AL, Vallone G, Antolini I, Warner JO. Effective allergen avoidance at high altitude reduces allergen‐induced bronchial hyperresponsiveness. Am J Respir Crit Care Med. 1994;149:1442‐1446. [DOI] [PubMed] [Google Scholar]
- 164. Piacentini GL, Peroni DG, Vicentini L, Benedetti M, Spezia E, Martinati LC. Beta‐2 agonists, exposure to allergens and bronchial hyperreactivity in children with allergic asthma. Pediatr Med Chir. 1995;17:515‐517. [PubMed] [Google Scholar]
- 165. Benckhuijsen J, van den Bos JW, van Velzen E, de Bruijn R, Aalbers R. Differences in the effect of allergen avoidance on bronchial hyperresponsiveness as measured by methacholine, adenosine 5'‐monophosphate, and exercise in asthmatic children. Pediatr Pulmonol. 1996;22:147‐153. [DOI] [PubMed] [Google Scholar]
- 166. Hashimoto S, Rijssenbeek‐Nouwens LH, Fieten KB, Weersink EJ, Bel EH. Predictors of benefit from high‐altitude climate therapy in adults with severe asthma. Neth J Med. 2018;76:218‐225. [PubMed] [Google Scholar]
- 167. Brimkulov NN. The alpine climatotherapy of bronchial asthma patients. Ter Arkh. 1991;63:25‐30. [PubMed] [Google Scholar]
- 168. Müller A, Balatoni I, Csernoch L, et al. Quality of life of asthmatic patients after complex rehabilitation treatment. Orv Hetil. 2018;159:1103‐1112. [DOI] [PubMed] [Google Scholar]
- 169. Dubileĭ VV, Shogentsukova EA. Experience in treating bronchial asthma under the conditions of the Mount El'brus area. Vopr Kurortol Fizioter Lech Fiz Kult. 1973;38:136‐140. [PubMed] [Google Scholar]
- 170. Kolesár J, Eisner J, Michalicka D, Slavka V. Influence of different altitudes on respiratory changes in patients with bronchial asthma. Fysiatr Reumatol Vestn. 1977;55:268‐274. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1‐S6


