Abstract
The Wound Healing Foundation (WHF) recognised a need for an unbiased consensus on the best treatment of chronic wounds. A panel of 13 experts were invited to a virtual meeting which took place on 27 March 2021. The proceedings were organised in the sub‐sections diagnosis, debridement, infection control, dressings, grafting, pain management, oxygen treatment, outcomes and future needs. Eighty percent or better concurrence among the panellists was considered a consensus. A large number of critical questions were discussed and agreed upon. Important takeaways included that wound care needs to be simplified to a point that it can be delivered by the patient or the patient's family. Another one was that telemonitoring, which has proved very useful during the COVID‐19 pandemic, can help reduce the frequency of interventions by a visiting nurse or a wound care center. Defining patient expectations is critical to designing a successful treatment. Patient outcomes might include wound specific outcomes such as time to heal, wound size reduction, as well as improvement in quality of life. For those patients with expectations of healing, an aggressive approach to achieve that goal is recommended. When healing is not an expectation, such as in patients receiving palliative wound care, outcomes might include pain reduction, exudate management, odour management and/or other quality of life benefits to wound care.
Keywords: arterial, chronic, pressure, treatment consensus, venous, wounds
Abbreviations
- ABI
ankle/brachial index
- CMS
Centers for Medicare and Medicaid Services
- CTPs
cell‐ and/or tissue‐based products
- DACC
dialkylcarbamoyl chloride
- DFUs
diabetic foot ulcers
- DVTs
deep vein thromboses
- FDA
United States Food and Drug Administration
- HBOT
hyperbaric oxygen therapy
- NPWT
negative pressure wound therapy
- OCT
optical coherence tomography
- QOL
quality of life
- PCR
polymerase chain reaction
- SPP
skin perfusion pressure
- TcPO2
transcutaneous oxygen measurement
- TCC
total contact cast
- TI
therapeutic index
- VNA
visiting nurse association
- WHF
Wound Healing Foundation
- WVTR
water vapour transmission rates
1. INTRODUCTION
The Consensus Panel on Chronic Wounds was convened virtually on 27 March 2021. The members of the panel, all with extensive experience in clinical and research areas pertaining to wound healing, have summarised their conclusions in this article. The Consensus Panel Members were invited by the five editors (the five first listed authors who are all Wound Healing Foundation Board Members). All consensus statements were based on 80% or higher concurrence.
Chronic wounds create major problems for patients. They are painful and tender. Wounds often drain and emit odours, and applied dressings are often conspicuous. Unprotected by an intact skin envelope, these wounds can be retraumatised and progress in size. They may develop an infection leading to sepsis and amputation.
The incidence of chronic wounds is high and increasing. Venous ulcers, active or healed, are present in 1% of the population in the United States. 1 Pressure sores will occur in 0.75% of the population. 2 Of the 30 M+ patients with diabetes in the United States, approximately 1 M will develop a foot ulcer annually, and 6–7 M will over their lifetimes. 3 The incidence of chronic wounds developing from acute wounds is not known. Efforts at analysing their incidence and prevalence is ongoing. 4
The greatest need today in the treatment of chronic wounds is consensus‐based knowledge vetted by practical experience and backed up by scientific evidence, which is easily communicated and available to all wound care practitioners. This publication is organised in treatment categories to make it as universally applicable to various chronic wounds as possible. In this way, the titles of the sections can be used as a check list.
One takeaway from this consensus panel was that wound care needs to be simplified to a point where it can be delivered mainly by the patient and his/her family. The current rapid development and integration of telemonitoring spurred by the global COVID‐19 pandemic may also help reduce the frequency of interventions by a visiting nurse or a wound care center. This would also extend currently available resources to manage a constantly increasing number of patients with chronic wounds.
2. METHODS
A group of five members of the WHF (the editors/five first listed authors) defined the topic and outlined its various parts. The participants to be invited were then selected. Selection criteria included: nationally recognised expertise in chronic wound care and research, specialty diversity (seven clinical and two research specialties were represented) (dermatology, general surgery, vascular surgery, paediatric surgery, plastic surgery, podiatry, nursing and wound healing research), practice setting diversity (academic hospital, private hospital, wound care clinic) and geographic diversity. Even if 12 of the 13 panel members were based in the United States, many foreign publications were reviewed and most panel members participate frequently in international meetings. The conclusions of the panel should therefore be of interest to wound care practitioners outside the United States. A very large number of topics and issues were raised to the panel members before and during the proceedings. These were discussed and statements were formulated. The group unanimously agreed on the vast majority of statements. If there was any dissent, a formal vote took place. Consensus on each statement was defined as 80% or better concurrence among the panel members. In a few cases, the statements were reformulated to reach consensus.
Each part of the topic was presented by one of the three people who had been assigned to this part. All presentations were recorded, transcribed and edited into a draft manuscript.
The expenses (personnel, IT, recording, transcription and outside editor) were covered by unrestricted donations (from Medline, Convatec, David Zamierowski, MD and Chromologic). No editor or panel member received any payment for their participation.
3. DIAGNOSIS
A cutaneous wound may be defined as any break in the structural and functional integrity of the skin. It is paramount, however, to remember that wounds occur in people. This helps us focus on the true target – a patient with a wound – not only the wound itself. Determining when that wound has failed to heal for long enough to be deemed chronic has been a subject of debate 5 with time courses ranging from weeks to months. When the four normal phases of wound healing (haemostasis, inflammation, proliferation and remodelling) fail to follow this orderly progression of events to complete coverage, the wound stalls (frequently in the inflammatory phase). Considering previous studies, this consensus panel agreed that when standard of care has been applied to a wound with failure to progress towards healing within 4 weeks, that wound should be considered chronic. 6 , 7 , 8 , 9 A time‐related assessment has also been applied to the process of healing of a wound that is diagnosed as chronic. If during 4 weeks of standard of care, the wound surface area is reduced by 50%, it is likely to heal on the same treatment in 12 weeks. If less than a 50% reduction occurs, it is unlikely to heal on this treatment and a reassessment and change of treatment should be considered. 6 , 9 , 10
Approaching the diagnosis of wounds may be simplified by first considering it from a systemic perspective, proceeding then to regional causes, then lastly to local causes. Systemic illness most relevant to wound healing is diabetes mellitus. The patient with a chronic wound who has diabetes will usually be aware of this diagnosis and be taking medication for it. Prediabetes can be diagnosed with an HbA1C test and a value over 5.7 indicates prediabetes. 11 The implications diabetes has for wound healing are myriad and complex but include impaired oxygen delivery to the wound via stenotic inflow arteries (also a regional aetiology), decreased ability to ward off infection via impaired neutrophil diapedesis, phagocytosis and intracellular killing of bacteria. 12
Malnutrition is another systemic setting for impaired wound healing, and occurs when the body is deprived of vitamins, minerals and other nutrients it needs to maintain healthy tissue and organ functions. 13 It occurs in both undernourished and over‐nourished individuals. Normal collagen synthesis, required for new extracellular matrix formation, as an example, requires adequate protein intake, cofactors such as Vitamins A and C, and zinc. 14 We diagnose protein synthetic function in a patient by measuring serum albumin, retinol‐binding protein, prealbumin, transferrin, creatinine and blood urea nitrogen. Long‐term malnutrition is best assessed using albumin because of its longer half‐life. 15
Connective tissue diseases include diagnoses such as rheumatoid arthritis, scleroderma, lupus, polyangiitis and more. When one of these diagnoses is being considered, the workup should be done by a specialist in this area. Age and certain medications act synergistically with these conditions to increase the incidence of chronic wounds. 16 Occasionally, therapies designed to treat these conditions, such as anti‐tumour necrosis factor drugs, may impair wound healing and contribute to chronicity. 17 Another example of this would be for patients on chronic anticoagulation for artificial heart valves or blood clots experiencing Warfarin‐associated skin necrosis. 18
Regional contributors to chronicity of wounds may be neuropathic, arterial, venous or lymphatic or combinations of these. Arterial symptoms include claudication and rest or night pain, or pain when a lower extremity is elevated. Key items to elicit during history‐taking include previous ulcerations or operative interventions on the arterial tree. 19 Positive smoking status and the presence of diabetes markedly elevate risk. 20 Assessment of blood supply and peripheral arterial disease rest both on physical examination findings as well as laboratory assessments. Colour, blanching and capillary refill, hair presence, and warmth all may indicate adequate circulation, as does the presence of distal pulses (dorsalis pedis and posterior tibial flow into the foot). Parameters such as an ankle/brachial index (ABI) above 0.8 or toe pressures above 30 mm Hg favour healing. 21 Transcutaneous oxygen measurement (TcPO2) of skin perfusion pressure (SPP) can be done to measure skin perfusion near the wound. Vascular laboratories perform duplex ultrasound which can give additional information on venous outflow (see below) as well as assessing the compressibility/elasticity of the larger arteries of the legs.
Venous disease manifests as swelling and oedema of the legs. It occurs in patients often with a positive family history of the same. 22 Venous engorgement may result in reflux of a standing column of blood when the delicate cusps of valves within veins no longer coapt, leading to extravasation of blood into the interstitium and stranding of red cells in an extra circulatory location. The iron of the haemoglobin molecule is toxic and leads to hemosiderosis and ulceration of the skin. 23 It is more frequent in patients with multiple gestations, vocations that require standing or deep vein thromboses (DVTs). 24 The skin surrounding the ulcer often has a brawny induration that presages actual breakdown. Venous reflux may be diagnosed via duplex ultrasound with careful physical examination of varicosities. It frequently coexists with arterial insufficiency, leading to a mixed aetiology of ulcers, which may cause diagnostic confusion.
Another regional contributor to skin breakdown is lymphatic obstruction, sometimes leading to massive extremity oedema. It too may co‐exist with venous disease, known as phlebo‐lymphedema. 25 In this situation, the venous hypertension leads to overwhelming of the lymphatic system and may respond to appropriate therapies for the venous engorgement. Other aetiologies for lymphedema may be from parasitic infections seen more commonly in the Third World, surgical lymphadenectomies in cancer staging or therapeutic extirpation and radiation of lymphatic regional beds in the groin or axilla. It also exists in spontaneous cases or congenitally. 26
Local causes of wounds include unremitting pressure, trauma, non‐healing burns, infection (including osteomyelitis), autoimmune conditions, especially manifested in the lower extremities such as pyoderma gangrenosum, the presence of orthopaedic hardware or other foreign bodies which may be evident with plain x‐rays or computerized tomography (CT) scans and vasculitis. A diagnosis of pyoderma may require a biopsy including the wound edge and alerting the dermatopathologist to this as a diagnostic concern to look specifically for dermal infiltrates of inflammatory cells and perivascular lymphocytic infiltrates. Osteomyelitis may be diagnosed on physical examination by probing directly to bone without periosteum, and/or the presence of mushy and necrotic bone. Biopsy of bone with a sterile rongeur may confirm the diagnosis as well as provide the identification and sensitivity of bacteria to antibiotic therapy. DNA identification may be the only accurate method of such bacterial identification (see Section 4 on wound infections). CT scans and magnetic resonance imaging (MRI) scans may also assist in the diagnosis of osteomyelitis though MRI may overcall bony involvement. Very long‐standing wounds, of many years' duration may necessitate biopsy of the normal skin/wound interface to rule out Marjolin's ulceration, a particularly aggressive form of squamous cell cancer. 27
The accurate diagnosis of wounds should proceed in orderly fashion, ruling in or out systemic and regional aetiologies first. Only then are local factors considered. Sophisticated techniques such as PCR to assess bioburden are becoming more readily available and may aid in accuracy of diagnosis. Last, and far more rare, are factitious (self‐induced) wounds which may persist into chronicity due to some perceived secondary gain or obsessive–compulsive behaviour on the part of the patient.
4. DEBRIDEMENT
Debridement is the removal of non‐viable wound components. In addition to dead tissue, it includes necrotic material, slough and biofilm. The general goal of debridement is to use the most effective means (with the fewest side effects) that can be carried out at the least complex site of service. 28 The diagram in Figure 1 shows the various techniques of debridement, and ranks them in terms of complexity.
FIGURE 1.
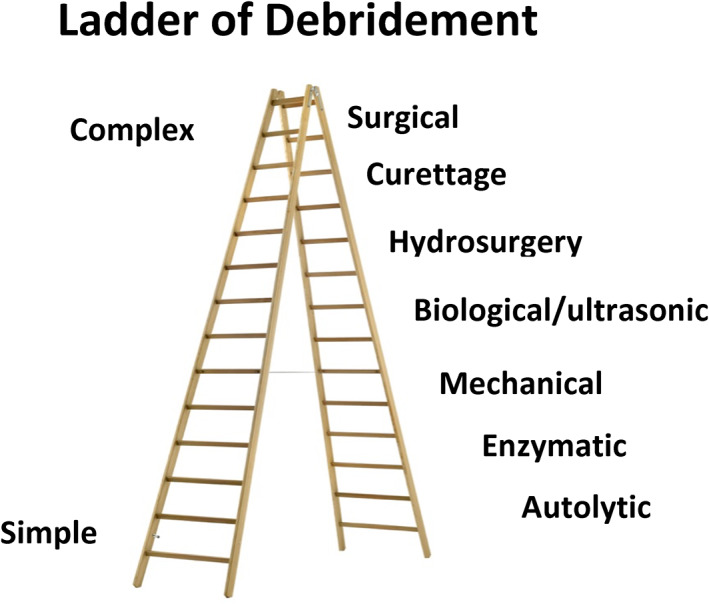
Ladder of complexity of debridement. The bottom rungs are the least complex methods and require the fewest resources
There was unanimous consensus among the panel members ‘that early aggressive, initial and serial debridement, is a cornerstone of wound care. Sharp debridement is the gold standard but the method by which debridement may occur may be altered by patient and wound characteristics, as well as cost and convenience’.
A recent publication 29 updates the various aspects of wound bed preparation including debridement. Surgical debridement usually requires anaesthesia and is done with scalpel, curettage or tangential hydrosurgery. If indicated, debridement may be performed upon initial presentation to a wound care specialist and as needed to promote closure. 30 , 31 Initial assessment for ischemia is critical prior to debridement since in ischemic wounds, surgical debridement may cause extension of the necrosis. 32 , 33 Bleeding requiring electrocautery may create a thermal injury which can extend the tissue injury. 28 The majority of chronic wounds likely will benefit from serial surgical debriding to facilitate either secondary closure or healing with grafting or tissue transfer. 28 The degree of debridement should generally begin with early substantial surgical debridement followed by maintenance debridement. 34 Hydrosurgery may be particularly advantageous when tissue is friable or when tangential excision is required as in burns or large wounds with a thin layer of non‐viable tissue. 28 , 35 This may be followed by additional debridement requiring methods/resources further down the ladder. The consensus panel recommends initiating debridement as early as possible, with weekly debridement initially to optimise outcomes, 28 by removing debris and reducing bioburden (see Section 4 on infection control).
Mechanical debridement may utilise hydro‐jet, ultrasound, pulsatile lavage, gauze abrasion or wet‐to‐dry dressings. It can often be done without local anaesthesia and usually does not cause significant bleeding though care must be exercised if the patient is on systemic anticoagulation. All of these are effective, and the choice is usually determined by the practitioner's experience. 28
Enzymatic debridement entails the application of chemicals or enzymes that degrade components of necrotic tissue. Bromelain and collagenase are most commonly used. 36 Pain is usually minor and if the treated area is small, there is very little risk of propagating infection. 28 The treated wound bed may need additional surgical preparation if grafting is being used to attain wound closure.
It is common practice to use surgical debridement at the first or second visits followed by enzymatic debridement as an outpatient. It is important to remove all dead and dying components in the wound. A wound culture, either using traditional methods or more recent molecular microbiological methods can be used to verify the removal of heavily contaminated tissue. 37 The adequacy of removal of devitalised tissue may not be readily apparent and one often has to give the wound surface a few days before ascertaining if debridement was adequate to promote the growth of healthy granulation tissue.
The ladder of debridement roughly parallels cost and efficacy as well, with wound beds being demonstrably cleaner and more vascular after surgical debridement. This will enhance surgical outcomes. 28 But this may require in‐patient hospitalisation and general anaesthesia and may be more expensive than outpatient visits. However, an initial thorough surgical debridement may reduce the need for serial outpatient debridement. Ultrasound units are expensive and require sterile saline as the medium to propagate the sound waves, which may pose a risk of aerosolisation. Reusable handpieces may reduce cost if sterile processing is available. Choices are usually based on local experience and supply chains/costs.
Future debridement therapies should consider marrying companion diagnostics with debridement to assist the clinician in identifying marginal tissue viability as well as wound preparation. This type of biomarker‐based debridement has the potential to identify viable tissue with “receptive receptors”. 38 Furthermore, we believe that innovation efforts might help to further drive care out of the hospital and towards outpatient and home settings. 28
5. INFECTION CONTROL
All chronic wounds should be assumed to be contaminated or infected with bacteria. The level of wound bioburden is an essential factor in determining if a wound can heal. Reducing the bioburden in every wound is a critical component of wound bed preparation and individualising treatment of each wound. 38 , 39
Not all surface bacteria result in infection or even necessarily contribute to the chronicity of the wound. Planktonic bacteria are not bound together and necessarily anchored to the surface of the wound, as is the case with bacteria associated together as a biofilm. 40 Standard microbiological swabbing for culture and sensitivity of wound bacteria may thus result in identification of a species that is not pathogenic and thus treating for it may not aid healing. 41 Biofilms contribute to the bioburden of that wound, and the level of wound bioburden is an essential factor in determining if the wound can heal. 38 Reducing the bioburden in every wound is a critical component of wound bed preparation and individualising treatment of each wound.
5.1. Diagnostic tests
DNA identification of bacteria should be the preferred option when available. It requires specific sampling/transport kits. DNA identification identifies close to 100% of dominant bacterial species but is less quantitative than swab cultures. 38 Deep swab culturing detects 20% of all bacteria in the wound compared to DNA identification, but is semiquantitative and most practitioners are already adept in procuring proper samples and interpreting the result of the culture. Using 16Sr RNA sequencing, swabs collected using the Levine technique recovered higher relative abundance of known and potential pathogen genera when compared to tissue biopsies collected in the same diabetic foot ulcers (DFUs). 42
As DNA identification becomes more readily available, deep swabbing is likely to be supplanted. Diagnostic tests to identify the bacteria responsible for biofilm‐associated infections is challenging due to the dense and impervious glycocalyx matrix secreted by the biofilm. DNA identification of bacteria elaborating the biofilm and localisation of extent/presence of biofilm using violet light or wound biofilm blots 1 may assist in treatment. 43 , 44 Occasionally, punch biopsies must be taken to rule out specific organisms such as chlamydia and acid‐fast bacilli or a malignancy, as when hyper‐granulation tissue in an older person persists on optimal treatment. Quantitative cultures of bacteria in wound tissue is a great research tool, and of historic importance in defining burn wound sepsis, but is no longer practical since most labs have abandoned the practice. 45
5.2. Treatment
Wound debridement (see Section 3) is a necessary part of treating the infective component of chronic wounds and should precede any antibiotic therapy. Systemic antibiotics (parenteral or oral) are given for a clinically invasive infection, a diagnosis of haemolytic streptococci and diagnosed osteomyelitis. The first two require 10 days of systemic antibiotics, while osteomyelitis requires treatment for 6 weeks or more. Exposed, contaminated bone in a common pressure sore should be treated with surgical debridement and a short (10 days) course of systemic antibiotics. It should not be referred to as osteomyelitis unless bony destruction has occurred. 45
Topical antimicrobials will reduce contamination in a wound but are not powerful enough to eradicate an invasive infection. In general, they should not be used for more than 2 weeks. Many dressings incorporate nanocrystalline silver as an anti‐infective. Most show efficacy in laboratory assays, but clinical effectiveness is less clear. 46 In addition, some are relatively cytotoxic. Sustained release iodine is effective with low toxicity, but has a high incidence of sensitivity reactions. It has been shown to be effective against some biofilms. 46 , 47 , 48 Hypochlorous acid combines efficacy with relatively low cytotoxicity. Studies have shown complete antibiotic activity in vitro against Pseudomonas, Staphylococcus aureus and Candida albicans with permissive effects on keratinocyte and fibroblast migration and replication rates. 38 , 49 Dialkylcarbamoyl chloride (DACC) impregnated dressings are highly hydrophobic and irreversibly bind bacteria at the wound surface, trapping them in the dressing, thus reducing the number of organisms in the wound. 38
Topical antibiotics are attractive due to low cytotoxicity in high concentration. Topical delivery can provide very high concentrations with relatively little systemic absorption and potential toxicity. Minocycline and gentamycin are the only systemic antibiotics that are cleared by the United States Food and Drug Administration (FDA) for the topical treatment of infected wounds. Both can be delivered in concentrations of up to 100× minimum inhibitory concentration (MIC). Minocycline is very effective against many Gram+ bacteria and in high concentrations against many Gram− bacteria including Pseudomonas. It also has an independent anti‐inflammatory effect due to inhibition of metalloproteases. Treatment should be limited to 2 weeks due to concerns about inducing antibiotic resistance. 38 , 49 Several antibiotics are approved for topical treatment of ocular and periocular wounds and for treatment of external otitis. 48 , 49 Over‐the‐counter antibiotic ointments like bacitracin and Neosporin may be useful short‐term adjuncts, but up to 2% of adults now have hypersensitivity reactions to bacitracin due to injudicious and prolonged exposures. 38
Treatment of biofilm merits separate mention. Diagnosis has been covered, but once it has been ascertained biofilm from a specific bacterial species or combination is present, debridement is mandated followed by additional surgical debridement at follow‐up visits until contamination in the wound has been reduced to an acceptable level. Debridement degrades biofilm structures thus exposing constituent bacteria which makes treating agents more effective as the biofilm regrows. 41 After debridement the wound is treated with an antimicrobial such as hypochlorous acid, cadexomer iodine, Ag+ and others. Washing and scrubbing the wound will also decrease biofilm presence. 47 , 48
5.3. Therapeutic index: antimicrobial effectiveness versus cytotoxicity
Using topical antimicrobials to reduce bacterial (and fungal) bioburden in chronic wounds to levels that do not impair healing is based on the principle that the topical antimicrobial treatments can effectively kill the planktonic and biofilm bacteria without killing an unacceptable amount of wound cells (fibroblasts, keratinocytes, vascular endothelial cells) that are required to actually heal the wound. This concept is reflected in the therapeutic index (TI), which is the ratio of CT50/MBC, that is, the cytotoxic concentration, which is the minimal concentration (μg/ml) of the agent that kills 50% of a test cell line (usually human fibroblasts or keratinocytes) divided by the minimum bactericidal concentration (μM) of the agent that kills 50% of the test bacteria or fungi. 41 Therefore, microbicidal agents that have a high TI against typical wound pathogens should be more effective in reducing bacterial bioburden while not killing wound cells. For example, the TI for hypochlorous acid is typically much higher than the TI for sodium hypochlorite or for hydrogen peroxide for key bacterial pathogens. 47 [Correction added after publication 15 June 2022: in the preceding sentence the reference citation 41 was corrected to 47.] A more complete measurement of TI is needed for commercially available wound antimicrobials, antiseptics, and antibiotics against common wound pathogenic bacteria in both planktonic and biofilm phenotypes. Also, improved delivery systems are needed that provide controlled, sustained release of antimicrobials and antibiotics into wound beds. For example, antibiotics typically require 4–6 h of continuous exposure to planktonic bacteria to achieve full kill, 45 and many antiseptic agents require more than 10 min of exposure to achieve full kill of planktonic bacteria and more than 24 h of exposure to kill mature bacterial biofilms. 46
6. DRESSING MANAGEMENT
6.1. Dressing functions
Dressings or wound coverings need to carry out several different functions, and no single dressing can do this. The main functions are protection, exudate/moisture management, pain reduction, aesthetics, compression, offloading, immobilisation and sometimes provide negative pressure wound therapy (NPWT). Cell‐ and tissue‐based products (CTPs) add matrix and cells that produce growth factors. 50 , 51
6.2. Protection
This is a critical function of a dressing, to avoid re‐injury and to protect the wound from the external environment. All dressings provide some level of protection against trauma. In general multilayer, thicker dressings provide the best protection. They also tend to reduce the heat loss from a wound. Occlusive dressings adherent to surrounding skin can prevent bacteria and toxic material from entering the wounds. Modern dressings create an environment to reduce or eliminate pain and trauma to the wound bed if chosen appropriately to match the exudate levels from the wound. Additionally, newer super absorbent dressings absorb higher levels of exudate further protecting peri‐wound skin and preventing soiling of clothing, bed linens and other materials the patient may contact.
Furthermore, bordered, silicone sacral‐shaped dressings have been used to help prevent pressure injury by deflecting shear, and providing padding and microclimate management.
6.3. Moisture management
Moisture balance in the wound bed is maintained by appropriate choice of dressings. Insufficient moisture in exposed wound tissues causes desiccation and cell death and prevents epithelial migration and matrix deposition. Excessive moisture due to exudate inhibits cell proliferation and breaks down matrix components. 52 , 53 Moist dressings have water vapour transmission rates (WVTRs) varying from close to zero (Winter's polyethylene membrane) to, for instance, hydrated films 12,000 (or more) g/m2/24 h. No moist dressing is completely ideal for all phases of healing because the wound exudate is quite high during the first 48 h and then tapers off. There are literally hundreds of moisture management dressings available and most of them have acceptable WVTR. The absorptive capacity of modern dressings varies greatly based on their composition, from none (films) to great (alginates, gelling fibres and super absorbents). By design, most dressings can absorb large amounts of fluid without altering their WVTR (Figure 2).
FIGURE 2.
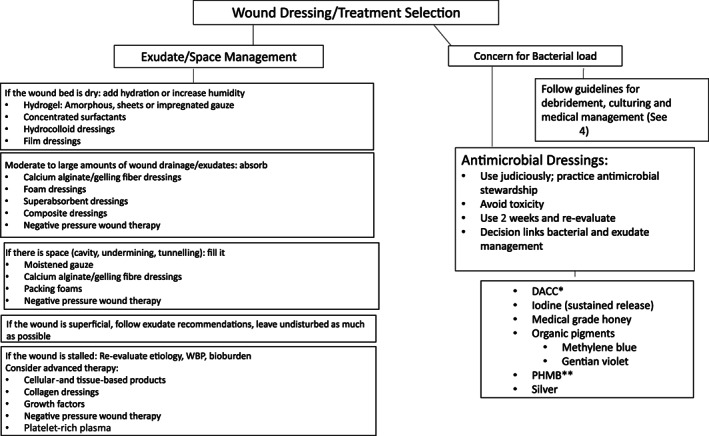
Schematic representation of decision‐making for dressing selection. DACC, dialkylcarbamoyl chloride; PHMB, polyhexamethylene biguanide
6.4. Pain reduction and aesthetics
Applying a moist dressing to a superficial wound will greatly reduce pain by reducing inflammation, evaporation and heat loss. The dressing improves aesthetics by keeping the wound out of sight. A moist dressing also improves the appearance of the healed wound by downregulating inflammatory cytokines and reducing hypertrophic scarring.
6.5. Compression
Essential in venous and lymphatic ulcers and helpful in other types of lower extremity oedema (dependency) and desirable in lower extremity wounds of all causes to enhance venous return is mobilising lymphatic fluids and reversing the effect of gravity. A critical consideration is the presence of a significant arterial insufficiency, preventing compression treatment. Once the volume of the extremity is reduced, compression garments (socks, stockings and juxtaposed strap devices) are important for maintenance of the oedema reduction. These can be exceedingly difficult to apply and take off due to body habitus, loss of flexibility and hand strength. Of importance is to choose a garment that the patient can use, even if it results in less power to the compression.
6.6. Immobilisation
Wounds around joints or with exposed, mobile tendon often need to be immobilised during healing and particularly after a grafting procedure. A plaster cast may be ideal to prevent the patient from removing it, but a splint is often more practical. The patient needs to understand that the splint must be worn continuously to prevent movement of the affected area.
6.7. Offloading
Any wound caused by pressure between a bony prominence and a hard or firm surface (bed, wheelchair and shoe) needs to be offloaded in order to heal and not recur. Pressure injuries require the use of a bed and/or chair cushion which allows the redistribution pressure over a larger body surface area. There are numerous bed and chair devices available, and the choice will depend on the location and stage of the ulcer, the patient's mobility, and insurance coverage or facility contracts and formularies. Similarly, the plantar diabetic foot ulcer must also have the pressure removed or redistributed to heal. The total contact cast (TCC) is still considered the gold standard and works by redistributing weight along the entire plantar aspect of the foot. The challenge with the TCC is that there are patients who are not candidates such as those with concurrent ischemic disease, patients who are claustrophobic, or those who must drive and their ulcer is on the right foot. There are also removable offloading boots and walkers that patients prefer so that they can take them off when not walking, but this also increases the likelihood that they will walk without it even for short distances such as bathroom trips, thus disturbing the healing of the ulcer.
6.8. Negative pressure wound therapy
NPWT is an active wound management system that utilises controlled negative (sub‐atmospheric) pressure which is applied uniformly to the wound through an open cell foam or other interface dressing in a continuous or intermittent fashion. 54 NPWT is used for the removal of exudate from chronic, acute, traumatic, sub‐acute and dehisced wounds, partial‐thickness burns, ulcers (such as diabetic, venous or pressure), surgically closed incisions, flaps and grafts. It speeds healing and reduces the number of complications.
NPWT is contraindicated or should be used with caution with inadequately drained wounds, presence of necrotic tissue such as eschar or adherent slough, exposed blood vessels, anastomotic sites, organs, tendons or nerves, wounds containing malignancy, fistulas, untreated osteomyelitis and actively bleeding wounds.
NPWT has been shown to rapidly improve granulation tissue formation, increase the rate of wound contraction, improve perfusion, remove toxic exudates and likely impact surface biofilm. The devices available allow for the optimum patient management with hospital devices that are used for larger wounds, dressings and devices allowing for instillation and cleansing of wounds, and management of larger volumes of exudate. Available outpatient devices are smaller, more portable and available in single use delivery systems that employ mechanical or battery powered collection devices and dressings. 54 One important aspect is that retained foam or gauze after NPWT is a relatively common complication and is reportable as an adverse event. 55
6.9. Cell‐ and tissue‐based products
There is a variety of CTPs consisting of cellular and acellular matrices that are derived from human and non‐human sources. 6 Replacing damaged and missing connective tissue in the wound, some also provide signalling to reverse a non‐healing phenotype and speed healing. Decision making should depend on the level of evidence for any specific product and any specific wound type. In deep wounds particularly with exposed bone, tendon or nerve, these products may stimulate the formation of granulation tissue over these structures to allow skin grafting or healing without a skin graft. In general, a particulate product would be used on an uneven wound surface and a sheet on an even surface.
7. GRAFTING
Grafting refers to a surgical procedure to move tissue from one site to another on the body, or from another creature, without bringing its own blood supply with it. In some instance, a graft can be an artificially manufactured device. 56 , 57 , 58
7.1. Difference between a skin graft and a flap
A graft does not have its own blood supply and must be revascularised or the metabolic needs must be exogenously supplied. Flaps bring their own blood supply; they may be local rotation, transposition flaps or free flaps but are distinct from grafting. 59 The Consensus Panel Members unanimously agreed that rapid coverage of the wound is preferred and skin grafting represents an option to achieve that goal.
7.2. Autologous skin grafts
Autologous (from another place on the patient's body) skin grafts are commonly used for acute wounds, but are often overlooked for treatment of chronic wounds due to concerns for poor healing. 60 Split‐thickness skin grafts have better ‘take’ than full‐thickness grafts and are therefore more commonly used for chronic wounds. 61 Indications for grafting chronic wounds include large shallow wounds and wounds that have not reduced in surface area by at least 30% during 4 weeks of optimal treatment. 60 , 62 , 63 In either case, the wound bed must be well prepared, highly vascularised with no exposed tendon or bone and no infection prior to grafting. Optimising the patient's condition includes oedema reduction, limiting anticoagulation at the time of grafting, smoking cessation and glucose regulation. Acute wounds may be grafted immediately if the above conditions are met. When the patient and wound bed are optimised, many small open wounds will heal without grafting. Advanced age is not a contraindication to grafting. Pre‐operative planning includes determining how the graft site will be immobilised to allow revascularisation without disruption.
There are four types of autologous skin grafts.
1. Split‐thickness skin grafts are indicated when simpler methods of wound closure are not adequate, such as healing by secondary intention, primary closure or NPWT. 61
2. Full‐thickness grafts are primarily used for small defects when colour match is important and scar contracture needs to be avoided such as the palm of the hand or facial wounds.
3. Epidermal skin grafts offer an alternative to traditional autografts when only the epidermis is needed (typically not in full‐thickness wounds) and may also be used to promote healing similar to the application of allografts/xenografts. 64
4. Last, composite grafts comprising more than one layer of tissue are employed when there is a need for structural support or volume replacement that cannot be supplied by skin alone. They usually include cartilage for nasal defects and possibly muscle or bone for deeper defects. Fat may be left on a composite graft if contour defects are being addressed, as in the nose or lips; however, thick grafts should be avoided on compromised wound beds. The methods for each are described in more detail as follows.
7.2.1. Split‐thickness skin grafts (consisting of epidermis and dermis and usually 0.008–0.016 inch, or .2–.4 mm thick).65,66
- Method/technology:
- Dressing techniques: All grafts heal faster in a moist environment. A non‐adherent layer next to the graft is mandatory. Consider bolster with negative pressure if large surface area or highly mobile area. 64 Immobilisation with a splint on upper or lower extremities is often advisable.
- Donor site: May treat with fibrin spray in anticoagulated patients. Moist‐wound healing principles will promote most rapid healing. Non‐adherent dressing close to the wound and covered by clear film initially, and cover with dry gauze or foam that can be changed as needed. After 4–7 days, keep non‐adherent dressing in place and keep moist with bordered foam dressing until healed. Moisturise daily when healed.
Advantages: replace like with like, donor site heals within 2–3 weeks despite comorbid illnesses in host. Secondary contraction may reduce total surface area. Minced grafts require small donor site compared to the size of the recipient bed and can be done under local anaesthesia as an outpatient. Consider minced grafts for patients who have very large open wounds and/or contraindications to surgical procedures under general anaesthesia.
Disadvantages: Risk of morbidity with creation of a new wound/donor site especially in patients with heightened inflammatory response such as large body surface area burns; infection; donor site pain and scarring, limited donor sites; potential for graft loss with delayed healing. Graft must be immobilised which may be difficult in some anatomical locations. Secondary contraction may lead to contracture. Minced grafts accelerate healing but may still require prolonged healing of the recipient site.
7.2.2. Full‐thickness skin grafts
- Method/technology:
- Harvest: With scalpel, or pinch grafts, or biopsy to create suspension of autologous, homologous skin construct. 65
- Donor site: Closed primarily with sutures.
Advantages: Good colour match, minimal contraction, minimal donor site morbidity (primarily closed vs. small biopsy).
Disadvantages: Limited donor sites, poor take with high risk for graft loss with delayed healing, relatively small grafts; hair growth is possible and may be inappropriate for recipient site.
7.2.3. Epidermal grafts 64
- Method/technology:
- Harvest: Suction blister, biopsy for cultured epidermal autografts.
- Donor site: Non‐adherent dressing.
Advantages: Minimal donor site morbidity, may cover large surface area.
Disadvantage: Instability after healing, requires immobilisation; Cultures require weeks to grow. No dermal component, high rates of infection and graft loss. Usually not indicated for full thickness defects.
7.2.4. Composite grafts
Method/technology: Freehand with scalpel.
Advantages: Provides structure when more than skin has been excised, primarily nasal reconstruction with skin and cartilage.
Disadvantages: Very limited in size, poor vascularity with high rate of graft loss.
7.2.5. Autologous fat grafts
Fat is a source of adipose‐derived stem cells and a stromal vascular fraction that may improve healing, vascularity and scar quality. 67
Method/technology: Liposuction or direct excision with implantation via small cannulas or in a thin layer.
Advantages: Benefits well documented in depressed and adherent scars and may promote healing of chronic wounds but data are inconclusive.
Disadvantages: Does not provide wound cover, requires skilled surgeon familiar with technique,
7.2.6. Non‐autologous grafts
Except for dermal constructs, allografts and xenografts do not persist in the wound bed, are not revascularised by the host and therefore are not replacing ‘like with like’. There are some limited cases of patients with large burns and/or immunosuppression in which allografts persist long term. 68 Although sometimes referred to as skin substitutes, these products are now appropriately labelled as CTPs. 69 , 70 , 71 Cellular allografts are laminated constructs with dermal/epidermal layers from another human. 70 Decellularised or acellular allografts consist of collagen substrates and purportedly act as a template for cellular ingrowth. Some consist of only the dermal substrate and are human reticular allografts. Allografts derived from human placentas may come from the amnion, chorion or umbilical cord. These are thought to act as delivery vehicles for various growth factors. Xenografts are derived from other species, and there are currently porcine, bovine, equine, and fish products on the market. Synthesised and composite allografts are also employed. General principles regarding the entire category are summarised below:
Method/technology: May be frozen or lyophilised requiring reconstitution with sterile application to wound bed.
Advantages: No donor site, off‐the‐shelf, may be applied in clinic or operating room; promote recipient bed healing through induction of cell migration and reduction of inflammation. Provide wide variety of growth factors, cytokines and immunomodulatory molecules. May reduce pain. Dermal allografts will reduce insensible water loss in large wounds, and provide temporary cover until the patient is stable or wound bed is prepared for autografting.
Disadvantages: Usually requires repeated applications.Combined allografting and autografting may also be performed as a staged procedure, with application of dermal allograft first followed by thin‐split‐thickness or epidermal graft. This technique has the advantage of being able to cover large surface areas quickly, reduce insensible water loss, reduce pain and provide thicker, more durable cover over areas vulnerable to friction or when grafting directly on muscle or large scalp defects. The chief disadvantage is that multiple procedures delay final grafting and definitive wound coverage.
7.3. Future technologies
The use of grafting techniques and the development of new technologies is rapidly expanding. The future may hold micro‐ and pixel‐grafts, spray on cells and the use of 3D printing to prefabricate vascularised grafts to assist in wound coverage. 63 , 64
8. PAIN MANAGEMENT
The Consensus Panel Members unanimously agreed that wounds and wound procedures are often associated with pain, and managing the pain is critical to the patient's experience. When pain persists long‐term, referral to a pain specialist may benefit the patient.
Many and possibly most individuals with open wounds present with some level of pain. 72 , 73 , 74 , 75 The exception to this of course would be those in whom innervation to a limb or area of the body has been interrupted due to injury (e.g., spinal cord injury) or the metabolic consequence of diabetes resulting in loss of protective sensation, which is often the precipitating factor for the presence of the wound. Initial and ongoing assessment of pain is important as a sudden increase or change in a patient's pain may indicate a change in the wound condition such as infection.
Wound‐related pain can have a significant impact on a patient's ability to adhere to a prescribed treatment plan, and such patients may often be labelled as non‐compliant when, in actuality, the treatment plan should be altered to be better tolerated and followed. 76 , 77 Treating wound‐related pain is essential for optimal wound healing. The European Wound Management Position Statement explained the complexity of wound pain and its effect on treatment, emphasising the use of a combination of techniques that focussed not only on physiological factors, but also on psychological and emotional factors. 78
Wound pain can be due to four causes: presence of the wound, the disease process (neuropathy, inflammation, ischemia and infection), treatment procedures (including debridement, dressing changes and cleansing) or a very low pain tolerance. 78 The source of the pain can be described as nociceptive 75 or neuropathic and its occurrence as noncyclic, cyclic or chronic. 76 , 77 , 78 , 79 Nociceptive pain involves pain receptors in the area of injury, whereas neuropathic pain is processed by the central nervous system, as in for instance phantom limb pain, or in the peripheral nervous system as in peripheral neuropathy.
Pain should be assessed at each patient encounter and should always be taken seriously. 80 In addition to a pain scale (for instance a visual analogue scale), patients should be queried on the effects of pain on their quality of life. Does it interfere with sleep? Does it influence appetite, activities of daily living? Does it prevent work, family, and social life? Does it limit mobility or exercise? Findings should be documented in the medical record. 73
Treating the pain depends on treating the aetiology. Addressing, infection and inflammation, limb ischaemia, venous disease, areas of pressure in immobile and in diabetic patients, wound desiccation and peri wound dermatitis, should be done in conjunction with treatment of the pain.
Procedural pain may be anticipated and treated with premedication, either topical local anaesthetics or systemic analgesics. Talking through the procedure may help, as well as explaining what efforts are being employed to minimise pain. Try to use a contact layer next to the wound surface. Relaxation techniques are helpful. 78
The World Health Organisation developed a three‐step approach to pain relief in cancer patients, and conceptually this can be applied to wound care. 79
Step 1: A non‐opioid analgesic (NSAID, non‐steroidal anti‐inflammatory drug) with or without an analgesic adjuvant. Adjuvants include tricyclic antidepressants, anticonvulsants, antihistamines, benzodiazepines, steroids and phenothiazines. Adjuvants are given for their indirect benefits in pain management.
Step 2: If pain is not controlled: Continue the initial medication and add an opioid, such as codeine or tramadol, and an adjuvant.
Step 3: When a patient does not respond to second‐step medications, these should be discontinued and a more potent oral narcotic initiated. 1 , 20 , 22
Systemic pain management should start with over‐the‐counter agents such as aspirin, acetaminophen and NSAIDs. Side effects, co‐morbid conditions and concurrent medications may limit their use. If these prove inadequate, short‐term synthetic opioids are given, followed by escalation to short‐term opioids. Referral to a pain management service should occur before resorting to long‐term opioids.
A wide range of topical anaesthetics, oral analgesics and other modalities are available. Non‐invasive therapies such as electrical stimulation, ultrasound and pulsed radio frequency energy have all shown benefit in managing chronic pain. Comprehensive management and pain control are critical for patients with chronic wounds to improve outcomes and speed healing. 81 , 82
9. HYPERBARIC OXYGEN
Hyperbaric oxygen therapy (HBOT) has been used to treat a wide variety of wound disorders and is currently provided at over 1300 locations in the United States. The concept of using enriched oxygen in a pressurised environment is relatively ancient from a medical perspective, having been initially described in the late 17th century. 83 , 84 However, the widespread usage of HBOT did not occur until the mid‐20th century when it was popularised for a variety of medical conditions including cardiac surgery, necrotizing infections and chronic wounds. 85 , 86 , 87 Its adoption was accelerated in the United States when the Centers for Medicare and Medicaid Services (CMS) began reimbursing for HBOT treatment in 2002.
Despite this long history, the use of HBOT remains controversial, especially in the context of chronic wounds. There are various reasons for this controversy, but perhaps, the most compelling is the overall financial cost of HBOT, which typically runs $50,000 and up for a course of this therapy in the United States. 88 , 89 This has led some unscrupulous practitioners to use HBOT as a revenue generator by ‘guiding’ inappropriate patients towards HBOT to act as profit centres for their practices. This is obviously unacceptable from an ethical perspective and third‐party payors have become increasing sceptical of HBOT as it generates significant costs. This mercantile aspect of HBOT has extended at times into outright overuse such as the application of HBOT into areas with no clinical evidence or biologic mechanism of action such as in the treatment of autism, dementia and other disorders. 90
Before considering the clinical evidence for HBOT in wound healing, we should ask whether HBOT has been demonstrated to work in any human disease state. Fortunately, the answer here is that HBOT has proven efficacious for a variety of clinical conditions with a high level of evidence. For example, there is level 1 evidence, published in leading medical journals for carbon monoxide poisoning, air embolism and decompression sickness. 91 , 92 , 93 In addition, comprehensive meta‐analyses have demonstrated that HBOT is helpful in treating the late effects of radiation in a variety of settings. 94 HBOT is authorised and reimbursed by CMS for this and 12 other clinical indications including cyanide poisoning, osteoradionecrosis and actinomycosis. 88
Since HBOT has proven to work in a variety of human disease states, it may be helpful to examine any potential biologic mechanisms of action for HBOT that may be beneficial in the context of chronic wounds. From the clinical and pre‐clinical literature, there appear to be multiple potential mechanisms that might be beneficial in impaired wound healing. The simplest mechanism is governed by the increased dissolution of oxygen in an enriched pressurised environment, which is governed by the gas equation known as Henry's Law. This supersaturation of blood with dissolved oxygen would be expected to increase oxygen delivery to the wound with potentially beneficial effects. 88 Other potentially beneficial mechanisms demonstrated in the clinical and preclinical literature include local wound vasodilation, increased neutrophil bactericidal activity due to the altered redox state, and an overall modulation of wound inflammation. 95 , 96 Thus, given our understanding of the pathophysiology of chronic wounds, there is ample reason to believe that, theoretically, HBOT may be beneficial in their treatment.
However, in reviewing the literature examining the effectiveness of HBOT in the treatment of chronic wounds, no definitive conclusion can yet be drawn. In a meta‐analysis of the relevant literature, two separate Cochrane reviews conducted 10 years apart came to significantly different conclusions regarding the usefulness of HBOT in diabetic foot ulcers. The 2004 meta‐analysis concluded that HBOT decreased the incidence of major amputations and improved healing at 1 year. 92 However, an updated 2015 metanalysis contradicted this conclusion and found that HBOT improved healing in the short term (6 weeks), but not at 1 year. 93 Since this last Cochrane review, numerous new studies have been conducted, including both randomised controlled studies and newer ‘big data’ studies based on large data bases derived from the electronic medical record. These newer techniques again yielded conflicting results with one study showing no impact on amputation outcomes 97 while a later study on the same data base demonstrated efficacy in a subset of more severe DFUs (Wagner 3 and 4) in terms of wound closure. 98 , 99 Clearly, how the analysis is performed and how the questions are asked can skew results in different directions. Unfortunately, since none of these studies is perfect or definitive, this allows the controversy to continue ad infinitum. However, when considering the totality of the existing data, among the potential indications for HBOT in the field of chronic wounds, the strongest evidence exists for ischemic, infected DFUs, that is, Wagner Grade 3 or worse. 88
Most wound care professionals are not interested in the myriad potential applications for HBOT, which is different from the perspective of the Undersea and Hyberbaric Medical Society. 90 For wound professionals, HBOT is one potential tool to use in patients with non‐healing chronic wounds that have failed to improve with standard of care. At present, there is a limited armamentarium for these patients who are at risk of amputation or worse. Given the absence of definitive data demonstrating a lack of efficacy, the high level of evidence for clinical benefit in other clinical indications and the substantial experimental data suggesting biological efficacy, we believe that HBOT should remain a therapeutic option for a narrow set of wound patients, namely complicated Wagner 3 and greater DFUs. The favourable safety profile of HBOT (primarily ear barotrauma) makes it attractive for end stage patients who have no other options. We strongly condemn the practice of using HBOT as a revenue generator but believe it should remain available for Wagner 3 DFUs or DFUs with concurrent infection. We believe that further research should focus not only on clinical outcomes but on developing predictive models 100 , 101 , 102 , 103 to help guide therapy so that this expensive treatment will only be offered to patients likely to benefit from it. We are optimistic that more targeted and focused clinical trials will be able to prove definitely that HBOT works (or does not) in an appropriately selected and stratified patient population.
10. OUTCOMES
The Consensus Panel Members unanimously agreed that defining patient expectations is critical to designing a successful treatment. Patient outcomes might include wound specific clinical outcomes such as time to heal, and wound size reduction, as well as improvement in quality of life. For those patients with expectations of healing, an aggressive approach to achieving that outcome is recommended. When healing is not an expectation, such as in patients receiving palliative wound care, outcomes might include pain reduction, exudate management, odour management and/or other quality of life benefits to wound care.
The goal of systematic and uniform evaluation and delivery of care is to reach a desired outcome. For instance, a King's physician was paid based on the outcome. In wound care, that desired outcome has historically been a healed wound. However, recent appreciation of other outcomes, either as intermediate, surrogates, or of desired endpoints that by themselves have value, has emerged. 104 , 105 , 106 , 107 , 108 Outcomes or endpoints have also become a focal point in regulatory aspects of wound care. Recognition of the impact of wounds and how other fields approach drug and device development led to holistic evaluations of providers' and patients' opinions regarding important outcomes. A detailed literature search for supporting evidence of which outcomes might be important enough to allow for drug and device approval was performed. 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 As a result several outcomes were identified and validated through an extensive literature search. 109
To summarise, providers divided important outcomes into five major categories which included healing outcomes (including quality of healing), healing precedents (perfusion, oedema, etc), wound complications to be reduced (infection, amputation, pain and others), patient function and cost. 108 Patients, beyond clinical and quality of life outcomes, also voiced the importance of logistical matters such as dealing with insurance companies, the wound care staff and affordability of treatment. 110 Eight outcomes were identified by both providers and patients as being important beyond complete healing of the wound. These are: time to healing, wound size reduction, infection reduction, pain reduction, prevention of recurrence, amputation reduction, improved ambulation functionality and reduced isolation. While this work was part of a process helping the FDA to evaluate new drugs, biologics and devices for approval, understanding the value put on several outcomes for clinical use, especially by patients, is a critical step forward for wound healers providing optimal care. 102
Extrapolating to clinical care, identifying shared recognition of the outcome of care is an important step in providing individualised and personalised care. 111 Some patients may not have the potential to heal and their care may be considered palliative. Nonetheless wound care will still be essential to the patient and a variety of other outcomes may be pursued. For example, outcomes of importance might include pain reduction, exudate management, odour management and/or other quality of life benefits of providing wound care. Thus, honest and earnest communication is essential. Clinicians may rely on a variety of quality of life (QOL) tools to assess the impact on QOL and how certain interventions might change QOL. Such tools may measure overall QOL, or may be disease specific (e.g., to venous leg ulcer, diabetic foot ulcer or pressure ulcers). 104 , 105 , 106 , 107 , 108 Depending on individual patient needs, QOL assessment may not be needed for every patient; choosing the best assessment tool might differ between patients. For example, wound pain may be a critical driver of diminished QOL in patients with venous leg ulcers but less important for a patient with a neuropathic insensate diabetic foot ulcer. 108 It is also likely new tools will emerge over time. Providers should work aggressively to achieve the desired outcomes that have been agreed upon with patients. It must be recognised that all outcomes may not be dependent on each other. For example, healing a wound may or may not achieve other desired outcomes such as reduction in pain or improved QOL, and specific interventions aimed at achieving these goals may be needed. 109 , 110
As was noted previously, cost of care was identified by providers as an important outcome of interest. Reducing cost and/or improving cost effectiveness may help reduce patient and health care system burdens. 111 This raises the importance of acknowledging health care system outcomes as another important, but not primary, outcome of care. For example, reducing readmission rates from wound recurrence and/or infection might be another outcome that goes beyond any one individual patient and may identify systemic trends of care that can serve to model and/or improve care. 112
11. FUTURE NEEDS
High‐quality research together with wound care practice that leads to the best outcomes and produce consensus‐based knowledge will remain a top priority. This knowledge should be easily available to all wound care practitioners. The presentation of the knowledge should also lend itself to be easily expressed in treatment algorithms for various types of wounds. The future of wound care is dependent on achieving marked progress in successful outcomes, namely more healed wounds. As many participants in this consensus panel have pointed out, this needs to be economically feasible. Barring a paradigm shift in the health care marketplace, this future will be centred on using specialised wound centres collaborating with home care and greater participation by patients and their families. Use of visiting nurse association (VNA) care, especially in rural areas, may not be feasible, and may spur greater reliance on tele/video medicine practices.
Coupling advances in basic science research to filling clinical needs seems like an obvious and ongoing requirement. 113 , 114 Yet, the ‘valley of death’ has often precluded successful bench to bedside translation. 115 Many of the failures stem from lack of monetary resources, including investment in funding wound science grants and bench research by biopharma. 116 There is a way forward. One example is illustrated by a collaborative effort spearheaded by some of the co‐authors of this paper. Jung et al. mined a large dataset of patient and wound data collected at 68 outpatient wound care centres in 26 states between 2009 and 2013. The dataset included basic demographic information on 59,953 patients, and both quantitative and categorical information on 180,696 wounds. They used the data to enhance accuracy of predicting which wounds would lapse into chronicity, to better focus more aggressive techniques on those wounds. 117 A similar effort could be mounted to collect tissue from those wounds to generate molecular data from what would amount to a huge wound tissue bank. Correlating tissue with demographic data and disease context, genomic mapping, proteomic correlation, and even mathematical modelling of wounds to enable in silico trials of potential therapeutics, could yield new possibilities for care. 118 This would take a page from our colleagues in cancer care who have made tremendous progress in the last couple of decades by applying advances in genomics, proteomics and metabolomics and coupling them to powerful, newly developed big data analytical methods and modelling. Personalised medicine has led to targeted therapies for solid tumours and blood‐borne dyscrasias that have yielded tremendous successes and triumphant stories of cure. 119
Many of the sections included in this panel have specified future directions and the main ones are as follow.
11.1. Smart dressings
The development of remotely monitored ‘smart dressings’ together with telemonitoring might help improve patient care. Flexible sensors that measure oxygen, pH, temperature and other parameters could be monitored remotely and directly or indirectly report the condition of the wound. If the sensors are optical they could also potentially detect the specific auto fluorescence of common bacteria and diagnose wound infections. The goal would be to limit patient visits to the initial one and only subsequent visits that require debridement or other invasive treatment. These ‘smart dressings’ would also help developing a precise diagnosis at the first visit. The dressings could also be used to determine when a dressing change or other intervention would be needed.
11.2. Imitation dressings and devices
Another goal would be to reduce the number of ‘imitation dressings and devices’ and instead encourage the wound care companies to put more effort into innovation and creating new products that would provide better treatments.
11.3. Imaging to determine extent of debridement
The methodology and depth of debridement would benefit from imaging devices that could help determine the amount of tissue to be removed. One promising technology correlating depth of injury and repair is optical coherence tomography (OCT). 120
11.4. More precise diagnosis of wound bioburden
All chronic wounds are contaminated or infected. With the goal of reducing bioburden in the chronic wound it is critical to determine type and relative numbers of bacteria in the wound. 121 , 122 , 123 , 124 Routine microbial sequencing (PCR) to identify and quantify microorganisms in the wound is becoming standard of care technology. Bioinformatics that helps distinguish pathogenic from non‐pathogenic bacteria is also very useful. Indicator species analysis which considers species' fidelity and specificity will help determine the relative influence of a microorganism on healing.
11.5. Better methods for delivery of antimicrobials and antibiotics
There is a growing need for better antimicrobials as well as antibiotics for topical wound treatment. Many of the currently available antimicrobials have relatively high cytotoxicity in combination with a low MIC. The opposite, that is, low cytotoxicity in combination with a high MIC, are typical characteristics of common antibiotics. The concern about developing antibiotic resistance is limiting the use of topical antibiotics in wound care. Better devices for delivery of antimicrobials and antibiotics would greatly enhance efficacy of both and reduce the potential for antimicrobial or antibiotic resistance. The same delivery devices could also be used for delivery of topical analgesics, thus reducing the need for systemic opioids.
11.6. Autologous skin grafting
There is a great need for better outcomes studies to determine when autologous skin grafting should enter the treatment algorithm. Currently, many wound care practitioners consider it a default option, but introducing autologous skin grafting as a strategic option early in the treatment algorithm could potentially make many chronic wounds heal faster. Improved methods for wound bed preparation and autologous skin grafting should facilitate this option. 61 , 63 , 64
11.7. Topical pain treatment
There is a strong need for better pain treatment in chronic wounds. Local treatment with sustained release bupivacaine is showing promise. Particularly in the patients with severe wound pain, any treatment that would eliminate or delay the need for systemic opioids would be very beneficial.
11.8. Outcome studies
There is a great need for outcomes studies of different chronic wounds to determine if and when oxygen and other treatments might be deployed successfully.
In summary, a significant progress has been made in understanding the science and clinical responses of chronic wounds. Consensus, when reached, has been presented. Much work remains to be done!
Eriksson E, Liu PY, Schultz GS, et al. Chronic wounds: Treatment consensus. Wound Rep Reg. 2022;30(2):156-171. doi: 10.1111/wrr.12994
Funding information Chromologic, LLC, Grant/Award Number: Unrestricted Educational Grant; ConvaTec; David Zamierowski MD; Medline
DATA AVAILABILITY STATEMENT
Not relevant
REFERENCES
- 1. White‐Chu EF, Conner‐Kerr TA. Overview of guidelines for the prevention and treatment of venous leg ulcers: a US perspective. J Multidiscip Healthc. 2014;7:111‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyko TV, Longaker MT, Yang GP. Review of the current management of pressure ulcers. Adv Wound Care. 2018;7(2):57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 4. Järbrink K, Ni G, Sönnergren H, et al. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst Rev. 2016;5(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Assumptions, Goal, Objectives, Problems, Prevention. ACS/ASE Medical Student Core Curriculum Non‐Healing Wounds . https://www.facs.org/-/media/files/education/core-curriculum/nonhealing_wounds.ashx. Accessed February 3, 2022.
- 6. Snyder D, Sullivan N, Margolis D, et al. Skin Substitutes for Treating Chronic Wounds [Internet]. Agency for Healthcare Research and Quality (US); 2020. https://www.ncbi.nlm.nih.gov/books/NBK554220/ [PubMed] [Google Scholar]
- 7. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560‐582. https://pubmed.ncbi.nlm.nih.gov/26339534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care. 2003;26(6):1879‐1882. [DOI] [PubMed] [Google Scholar]
- 10. Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol. 2000;142(5):960‐964. [DOI] [PubMed] [Google Scholar]
- 11. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62‐S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736‐1743. [DOI] [PubMed] [Google Scholar]
- 13. Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract. 2010;25(1):61‐68. [DOI] [PubMed] [Google Scholar]
- 14. Kavalukas SL, Barbul A. Nutrition and wound healing: an update. Plast Reconstr Surg. 2011;127(Suppl):38S‐43S. [DOI] [PubMed] [Google Scholar]
- 15. Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8(6):775‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drugs that delay wound healing. Prescrire Int. 2013. Apr;22(137):94‐98. [PubMed] [Google Scholar]
- 17. Hirano Y, Kojima T, Kanayama Y, et al. Influences of anti‐tumour necrosis factor agents on postoperative recovery in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29(5):495‐500. [DOI] [PubMed] [Google Scholar]
- 18. Kakagia DD, Papanas N, Karadimas E, Polychronidis A. Warfarin‐induced skin necrosis. Ann Dermatol. 2014;26(1):96‐98. https://europepmc.org/articles/PMC3956802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tautenhahn J, Lobmann R, Koenig B, Halloul Z, Lippert H, Buerger T. The influence of polymorbidity, revascularization, and wound therapy on the healing of arterial ulceration. Vasc Health Risk Manag. 2008;4(3):683‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xia N, Morteza A, Yang F, Cao H, Wang A. Review of the role of cigarette smoking in diabetic foot. J Diabetes Investig. 2019;10(2):202‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tay WL, Lo ZJ, Hong Q, Yong E, Chandrasekar S, Tan GWL. Toe pressure in predicting diabetic foot ulcer healing: a systematic review and meta‐analysis. Ann Vasc Surg. 2019;60:371‐378. [DOI] [PubMed] [Google Scholar]
- 22. Anwar MA, Georgiadis KA, Shalhoub J, Lim CS, Gohel MS, Davies AH. A review of familial, genetic, and congenital aspects of primary varicose vein disease. Circ Cardiovasc Genet. 2012;5(4):460‐466. [DOI] [PubMed] [Google Scholar]
- 23. Crawford JM, Lal BK, Durán WN, Pappas PJ. Pathophysiology of venous ulceration. J Vasc Surgery Venous Lymphat Disord. 2017;5(4):596‐605. [DOI] [PubMed] [Google Scholar]
- 24. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333‐346. [DOI] [PubMed] [Google Scholar]
- 25. Bunke N, Brown K, Bergan J. Phlebolymphemeda: usually unrecognized, often poorly treated. Perspect Vasc Surg Endovasc Ther. 2009;21(2):65‐68. [DOI] [PubMed] [Google Scholar]
- 26. Smeltzer DM, Stickler GB, Schirger A. Primary lymphedema in children and adolescents: a follow‐up study and review. Pediatrics. 1985;76(2):206‐218. [PubMed] [Google Scholar]
- 27. Khan K, Schafer C, Wood J. Marjolin ulcer: a comprehensive review. Adv Skin Wound Care. 2020;33(12):629‐634. [DOI] [PubMed] [Google Scholar]
- 28. Rogers LC, Armstrong DG, Capotorto J, et al. Wound center without walls: the new model of providing care during the COVID‐19 pandemic. Wounds a Compend Clin Res Pract. 2020;32(7):178‐185. [PMC free article] [PubMed] [Google Scholar]
- 29. Sibbald RG, Elliott JA, Persaud‐Jaimangal R, et al. Wound bed preparation 2021. Adv Skin Wound Care. 2021;34(4):183‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cardinal M, Eisenbud DE, Armstrong DG, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen. 2009;17(3):306‐311. [DOI] [PubMed] [Google Scholar]
- 31. Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149(9):1050‐1058. [DOI] [PubMed] [Google Scholar]
- 32. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb‐threatening ischemia. J Vasc Surg. 2019;69(6S):3S‐125S.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mills JLS, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220‐222. [DOI] [PubMed] [Google Scholar]
- 34. Falanga V, Brem H, Ennis WJ, Wolcott R, Gould LJ, Ayello EA. Maintenance debridement in the treatment of difficult‐to‐heal chronic wounds. Recommendations of an expert panel. Ostomy Wound Management. 2008;(Suppl):2‐13. [PubMed] [Google Scholar]
- 35. Legemate CM, Goei H, Gostelie OFE, Nijhuis THJ, van Baar ME, van der Vlies CH. Application of hydrosurgery for burn wound debridement: an 8‐year cohort analysis. Burns. 2019;45(1):88‐96. [DOI] [PubMed] [Google Scholar]
- 36. Lantis J, Paredes J. Permissive maintenance debridement—the role of enzymatic debridement in chronic wound care. Wounds. 2017;8(2):7‐13. [Google Scholar]
- 37. Spichler A, Hurwitz BL, Armstrong DG, Lipsky BA. Microbiology of diabetic foot infections: from Louis Pasteur to 'crime scene investigation'. BMC Med. 2015;13(1):2. doi: 10.1186/s12916-014-0232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolcott RD, Hanson JD, Rees EJ, et al. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016;24(1):163‐174. [DOI] [PubMed] [Google Scholar]
- 39. Atkin L, Bućko Z, Conde Montero E, et al. Implementing TIMERS: the race against hard‐to‐heal wounds. J Wound Care. 2019;23(Sup3a):S1‐S50. [DOI] [PubMed] [Google Scholar]
- 40. Dowd SE, Wolcott RD, Kennedy J, Jones C, Cox SB. Molecular diagnostics and personalised medicine in wound care: assessment of outcomes. J Wound Care. 2011;20(5):232, 234–239‐239. [DOI] [PubMed] [Google Scholar]
- 41. Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time‐dependent therapeutic window. J Wound Care. 2010;19(8):320‐328. [DOI] [PubMed] [Google Scholar]
- 42. Travis J, Malone M, Hu H, et al. The microbiome of diabetic foot ulcers: a comparison of swab and tissue biopsy wound sampling techniques using 16S rRNA gene sequencing. BMC Microbiol. 2020;20(1):163. doi: 10.1186/s12866-020-01843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakagami G, Schultz G, Kitamura A, et al. Rapid detection of biofilm by wound blotting following sharp debridement of chronic pressure ulcers predicts wound healing: a preliminary study. Int Wound J. 2020;17(1):191‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu Y‐F, Lee T‐Y, Liao W‐T, Chuan H‐H, Cheng N‐C, Cheng C‐M. Rapid detection of biofilm with modified alcian blue staining: in‐vitro protocol improvement and validation with clinical cases. Wound Repair Regen. 2020;28(6):834‐843. https://onlinelibrary.wiley.com/doi/abs/10.1111/wrr.12845 [DOI] [PubMed] [Google Scholar]
- 45. Schultz G, Bjarnsholt T, James GA, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25(5):744‐757. [DOI] [PubMed] [Google Scholar]
- 46. Percival SL, Suleman L. Slough and biofilm: removal of barriers to wound healing by desloughing. J Wound Care. 2015;24(11):498, 500–503, 506–510‐510. [DOI] [PubMed] [Google Scholar]
- 47. Wang L, Bassiri M, Najafi R, et al. Hypochlorous acid as a potential wound care agent: part I. stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;6(e5):e5‐17. [PMC free article] [PubMed] [Google Scholar]
- 48. Saillard J, Spiesser‐Robelet L, Gohier P, Briot T. Bacterial keratitis treated by strengthened antibiotic eye drops: an 18 months review of clinical cases and antibiotic susceptibilities. Ann Pharm Fr. 2018;76(2):107‐113. [DOI] [PubMed] [Google Scholar]
- 49. Japaridze S, Lomidze L, Nakhutsrishvili I, Davituliani V, Kekelidze I. Application of antibiotic‐containing ear drops in treatment of acute otitis media. Georgian Med News. 2021;313:41‐44. [PubMed] [Google Scholar]
- 50. Ovington LG. Wound care products: how to choose. Home Healthc Nurse. 2001;19(4):224‐231. 240; quiz 232. [DOI] [PubMed] [Google Scholar]
- 51. Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293‐294. [DOI] [PubMed] [Google Scholar]
- 52. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv Wound Care. 2013;2:348‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care. 2021;10:685‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kirsner R, Dove C, Reyzelman A, Vayser D, Jaimes H. A prospective, randomized, controlled clinical trial on the efficacy of a single‐use negative pressure wound therapy system, compared to traditional negative pressure wound therapy in the treatment of chronic ulcers of the lower extremities. Wound Repair Regen. 2019;27(5):519‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anagnostakos K, Thiery A, Sahan I. Retained negative pressure wound therapy foams as a cause of infection persistence. Adv Wound Care. 2020;10:699‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hallock GG, Morris SF. Skin grafts and local flaps. Plast Reconstr Surg. 2011;127(1):5e‐22e. [DOI] [PubMed] [Google Scholar]
- 57. Yammine K, Assi C. A meta‐analysis of the outcomes of split‐thickness skin graft on diabetic leg and foot ulcers. Int J Low Extrem Wounds. 2019;18(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 58. Braza ME, Fahrenkopf MP. Split‐Thickness Skin Grafts. StatPearls [Internet]. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK551561/ [PubMed] [Google Scholar]
- 59. Anderson JJ, Wallin KJ, Spencer L. Split thickness skin grafts for the treatment of non‐healing foot and leg ulcers in patients with diabetes: a retrospective review. Diabet Foot Ankle. 2012;3:3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Serra R, Rizzuto A, Rossi A, et al. Skin grafting for the treatment of chronic leg ulcers – a systematic review in evidence‐based medicine. Int Wound J. 2017;14(1):149‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kirsner RS, Bernstein B, Bhatia A, et al. Clinical experience and best practices using epidermal skin grafts on wounds. Wounds. 2015;27(11):282‐292. [PubMed] [Google Scholar]
- 62. Snyder RJ, Doyle H, Delbridge T. Applying split‐thickness skin grafts: a step‐by‐step clinical guide and nursing implications. Ostomy Wound Manage. 2001;47(11):20‐26. [PubMed] [Google Scholar]
- 63. Singh M, Nuutila K, Kruse C, Robson MC, Caterson E, Eriksson E. Challenging the conventional therapy: emerging skin graft techniques for wound healing. Plast Reconstr Surg. 2015;136(4):524e‐530e. [DOI] [PubMed] [Google Scholar]
- 64. Singh M, Nuutila K, Kruse C, Dermietzel A, Caterson EJ, Eriksson E. Pixel grafting: an evolution of mincing for transplantation of full‐thickness wounds. Plast Reconstr Surg. 2016;137(1):92e‐99e. [DOI] [PubMed] [Google Scholar]
- 65. Mundinger GS, Armstrong DG, Smith DJ, et al. Autologous homologous skin constructs allow safe closure of wounds: a retrospective, noncontrolled, multicentered case series. Plast Reconstr Surg Glob Open. 2020;8(5):e2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim W‐S, Park B‐S, Sung J‐H, et al. Wound healing effect of adipose‐derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15‐24. [DOI] [PubMed] [Google Scholar]
- 67. Nyame TT, Chiang HA, Leavitt T, Ozambela M, Orgill DP. Tissue‐engineered skin substitutes. Plast Reconstr Surg. 2015;136(6):1379‐1388. [DOI] [PubMed] [Google Scholar]
- 68. Rezaei E, Beiraghi‐Toosi A, Ahmadabadi A, et al. Can skin allograft occasionally act as a permanent coverage in deep burns? A pilot study. World J Plast Surg. 2017;6(1):94‐99. [PMC free article] [PubMed] [Google Scholar]
- 69. Johnson MB, Wong AK. Integra‐based reconstruction of large scalp wounds: a case report and systematic review of the literature. Plast Reconstr SurgeryGlob Open. 2016;4(10):e1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shakir S, Messa CA 4th, Broach RB, et al. Indications and limitations of bilayer wound matrix‐based lower extremity reconstruction: a multidisciplinary case‐control study of 191 wounds. Plast Reconstr Surg. 2020;145(3):813‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Phua QH, Han HA, Soh B‐S. Translational stem cell therapy: vascularized skin grafts in skin repair and regeneration. J Transl Med. 2021;19(1):83. doi: 10.1186/s12967-021-02752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maier, M. Treatment of painful cutaneous wounds. Pract Pain Manag, 10(4). 2012. https://www.practicalpainmanagement.com/pain/other/treatment-painful-cutaneous-wounds. Accessed July 12, 2012. [Google Scholar]
- 73. Briggs M, Ferris FD, Glynn C, et al. Assessing pain at wound dressing‐related procedures. Nurs Times. 2004;100(41):56‐57. [PubMed] [Google Scholar]
- 74. Price PE, Fagervik‐Morton H, Mudge EJ, et al. Dressing‐related pain in patients with chronic wounds: an international patient perspective. Int Wound J. 2008;5(2):159‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Paice JA. Understanding nociceptive pain. Nursing (Lond). 2002;32(3):74‐75. [DOI] [PubMed] [Google Scholar]
- 76. Woo KY, Sibbald RG. Chronic wound pain: a conceptual model. Adv Skin Wound Care. 2008;21(4):175‐190. [DOI] [PubMed] [Google Scholar]
- 77. Price P, Fogh K, Glynn C, Krasner DL, Osterbrink J, Sibbald RG. Managing painful chronic wounds: the wound pain management model. Int Wound J. 2007;4(Suppl 1):4‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leren L, Johansen E, Eide H, Falk RS, Juvet LK, Ljoså TM. Pain in persons with chronic venous leg ulcers: a systematic review and meta‐analysis. Int Wound J. 2020;17(2):466‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. WHO ; 2018. “WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. WHO. https://apps.who.int/iris/bitstream/handle/10665/279700/9789241550390-eng.pdf [PubMed] [Google Scholar]
- 80. Bechert K, Abraham SE. Pain management and wound care. J Am Col Certif Wound Spec. 2009;1(2):65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Treadwell T, Walker D, Nicholson BJ, Taylor M, Alur H. Treatment of pain in wounds with a topical long acting lidocaine gel. Chronic Wound Care Manag Res. 2019;6:117‐121. [Google Scholar]
- 82. Wound pain. Use of lidocaine in managing wounds . Accessed July 14, 2021. https://www.woundsource.com/blog/use-lidocaine-in-managing-wounds. Accessed July 14, 2021.
- 83. Hajhosseini B, Kuehlmann BA, Bonham CA, Kamperman KJ, Gurtner GC. Hyperbaric oxygen therapy: descriptive review of the technology and current application in chronic wounds. Plast Reconstr SurgeryGlob Open. 2020;8(9):e3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Löndahl M. Hyperbaric oxygen therapy as adjunctive treatment of diabetic foot ulcers. Med Clin North Am. 2013;97(5):957‐980. [DOI] [PubMed] [Google Scholar]
- 85. Lipsky BA, Berendt AR. Hyperbaric oxygen therapy for diabetic foot wounds: has hope hurdled hype? Diabetes Care. 2010;33:1143‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Glauser W. Unregulated hyperbaric oxygen therapy clinics assailed. Can Med Assoc J. 2010;182:1950‐1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mechem C, Manaker S. In: Traub SJ, ed. Hyperbaric Oxygen Therapy. UpToDate; 2019. https://www.uptodate.com [Google Scholar]
- 88. Bennett MH, Feldmeier J, Hampson NB, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016;4(4):CD005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Camporesi EM, Bosco G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41(3):247‐252. [PubMed] [Google Scholar]
- 90. Ishihara A. Mild hyperbaric oxygen: mechanisms and effects. J Physiol Sci. 2019;69(4):573‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347(14):1057‐1067. [DOI] [PubMed] [Google Scholar]
- 92. Kranke P, Bennett M, Roeckl‐Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004;2015(2):CD004123. [DOI] [PubMed] [Google Scholar]
- 93. Kranke P, Bennett MH, Martyn‐St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;2015(6):CD004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care. 2013;36(7):1961‐1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ennis WJ, Huang ET, Gordon H. Impact of hyperbaric oxygen on more advanced Wagner grades 3 and 4 diabetic foot ulcers: matching therapy to specific wound conditions. Adv Wound Care. 2018;7(12):397‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gill AL, Bell CNA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97(7):385‐395. [DOI] [PubMed] [Google Scholar]
- 97. Hajhosseini B, Chiou GJ, Virk SS, et al. Hyperbaric oxygen therapy in management of diabetic foot ulcers: Indocyanine green angiography may be used as a biomarker to analyze perfusion and predict response to treatment. Plast Reconstr Surg. 2021;147(1):209‐214. [DOI] [PubMed] [Google Scholar]
- 98. Snyder RJ, Hanft JR. Diabetic foot ulcers‐‐effects on QOL, costs, and mortality and the role of standard wound care and advanced‐care therapies. Ostomy Wound Manage. 2009;55(11):28‐38. [PubMed] [Google Scholar]
- 99. Armstrong DG, Boulton AJM, Andros G, et al. Defining success in clinical trials of diabetic foot wounds: the Los Angeles DFCon consensus. Int Wound J. 2009;6(3):211‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Maida V, Ennis M, Kuziemsky C, Corban J. Wounds and survival in cancer patients. Eur J Cancer. 2009;45(18):3237‐3244. [DOI] [PubMed] [Google Scholar]
- 101. Escandon J, Vivas AC, Tang J, Rowland KJ, Kirsner RS. High mortality in patients with chronic wounds. Wound Repair Regen. 2011;19:526‐528. [DOI] [PubMed] [Google Scholar]
- 102. Eaglstein WH, Kirsner RS, Robson MC. Food and Drug Administration (FDA) drug approval end points for chronic cutaneous ulcer studies. Wound Repair Regen. 2012;20(6):793‐796. [DOI] [PubMed] [Google Scholar]
- 103. Driver VR, Gould LJ, Dotson P, et al. Identification and content validation of wound therapy clinical endpoints relevant to clinical practice and patient values for FDA approval. Part 1. Survey of the wound care community. Wound Repair Regen. 2017;25(3):454‐465. [DOI] [PubMed] [Google Scholar]
- 104. Driver VR, Gould LJ, Dotson P, Allen LL, Carter MJ, Bolton LL. Evidence supporting wound care end points relevant to clinical practice and patients' lives. Part 2. Literature survey. Wound Repair Regen. 2019;27(1):80‐89. [DOI] [PubMed] [Google Scholar]
- 105. Gould LJ, Liu J, Wan R, Carter MJ, Dotson MP, Driver VR. Evidence supporting wound care end points relevant to clinical practice and patients' lives. Part 3: the patient survey. Wound Repair Regen. 2021;29(1):60‐69. [DOI] [PubMed] [Google Scholar]
- 106. Weigelt MA, Lev‐Tov HA, Tomic‐Canic M, et al. Advanced wound diagnostics: toward transforming wound care into precision medicine. Adv Wound Care. 2021;1319‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Finlayson K, Miaskowski C, Alexander K, et al. Distinct wound healing and quality‐of‐life outcomes in subgroups of patients with venous leg ulcers with different symptom cluster experiences. J Pain Symptom Manage. 2017;53(5):871‐879. [DOI] [PubMed] [Google Scholar]
- 108. Spanos K, Saleptsis V, Athanasoulas A, et al. Factors associated with ulcer healing and quality of life in patients with diabetic foot ulcer. Angiology. 2017;68(3):242‐250. [DOI] [PubMed] [Google Scholar]
- 109. Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and management of lower‐extremity ulcers. N Engl J Med. 2017;377(16):1559‐1567. [DOI] [PubMed] [Google Scholar]
- 110. Stechmiller JK, Lyon D, Schultz G, et al. Biobehavioral mechanisms associated with nonhealing wounds and psychoneurologic symptoms (pain, cognitive dysfunction, fatigue, depression, and anxiety) in older individuals with chronic venous leg ulcers. Biol Res Nurs. 2019;21(4):407‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Al‐Gharibi KA, Sharstha S, Al‐Faras MA. Cost‐effectiveness of wound care: a concept analysis. Sultan Qaboos Univ Med J. 2018;18(4):e433‐e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tapking C, Boson AL, Rontoyanni VG, et al. A systematic review and meta‐analysis of 30‐day readmission rates following burns. Burns. 2020;46(5):1013‐1020. [DOI] [PubMed] [Google Scholar]
- 113. Thanigaimani S, Singh T, Golledge J. Topical oxygen therapy for diabetes‐related foot ulcers: a systematic review and meta‐analysis. Diabet Med. 2021;38(8):e14585. [DOI] [PubMed] [Google Scholar]
- 114. Dhall S, Do DC, Garcia M, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. Tilton RG, editor. J Diabetes Res. 2014;2014:562625. doi: 10.1155/2014/562625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Butler D. Translational research: crossing the valley of death. Nature. 2008;453:840‐842. [DOI] [PubMed] [Google Scholar]
- 116. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jung K, Covington S, Sen CK, et al. Rapid identification of slow healing wounds. Wound repair Regen. 2016;24(1):181‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bowden LG, Maini PK, Moulton DE, et al. An ordinary differential equation model for full thickness wounds and the effects of diabetes. J Theor Biol. 2014;361:87‐100. [DOI] [PubMed] [Google Scholar]
- 119. Tsimberidou A‐M. Initiative for molecular profiling and advanced cancer therapy and challenges in the implementation of precision medicine. Curr Probl Cancer. 2017;41(3):176‐181. [DOI] [PubMed] [Google Scholar]
- 120. Tsai M‐T, Yang C‐H, Shen S‐C, Lee Y‐J, Chang F‐Y, Feng C‐S. Monitoring of wound healing process of human skin after fractional laser treatments with optical coherence tomography. Biomed Opt Express. 2013;4(11):2362‐2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Le L, Baer M, Briggs P, et al. Diagnostic accuracy of point‐of‐care fluorescence imaging for the detection of bacterial burden in wounds: results from the 350‐patient fluorescence imaging assessment and guidance trial. Adv Wound Care. 2021;10(3):123‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kalan LR, Meisel JS, Loesche MA, et al. Strain‐ and species‐level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe. 2019;25(5):641‐655.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kim JH, Ruegger PR, Lebig EG, et al. High levels of oxidative stress create a microenvironment that significantly decreases the diversity of the microbiota in diabetic chronic wounds and promotes biofilm formation [internet]. Front Cell Infect Microbiol. 2020;10:259. https://www.frontiersin.org/article/10.3389/fcimb.2020.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Armstrong DG, Lew EJ, Hurwitz BL, Wild T. The quest for tissue repair's holy grail: the promise of wound diagnostics or just another fishing expedition? Wound Medicine. 2015;1(8):1‐5. doi: 10.1016/j.wndm.2015.03.010 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not relevant


